Abstract
The purpose of this study was to describe the quality of life (QOL) of low-grade glioma (LGG) patients at baseline prior to chemotherapy and through 12 cycles of temozolomide (TMZ) chemotherapy. Patients with histologically confirmed LGG with only prior surgery were given TMZ for 12 cycles. QOL assessments by the Functional Assessment of Cancer Therapy–Brain (FACT-Br) were obtained at baseline prior to chemotherapy and at 2-month intervals while receiving TMZ. Patients with LGG at baseline prior to chemotherapy had higher reported social well-being scores (mean difference = 5.0; p < 0.01) but had lower reported emotional well-being scores (mean difference = 2.2; p < 0.01) compared to a normal population. Compared to patients with left hemisphere tumors, patients with right hemisphere tumors reported higher physical well-being scores (p = 0.01): 44% could not drive, 26% did not feel independent, and 26% were afraid of having a seizure. Difficulty with work was noted in 24%. Mean change scores at each chemotherapy cycle compared to baseline for all QOL subscales showed either no significant change or were significantly positive (p < 0.01). Patients with LGG on TMZ at baseline prior to chemotherapy reported QOL comparable to a normal population with the exception of social and emotional well-being, and those with right hemisphere tumors reported higher physical well-being scores compared to those with left hemisphere tumors. While remaining on therapy, LGG patients were able to maintain their QOL in all realms. LGG patients’ QOL may be further improved by addressing their emotional well-being and their loss of independence in terms of driving or working.
Keywords: brain tumor, chemotherapy, low-grade glioma, quality of life, temozolomide
Low-grade gliomas (LGG) are tumors that comprise a variety of histopathologic subtypes arising from the glial matter surrounding neurons in the brain. The clinical course of these tumors varies widely among patients. Although median survival time is 4.1 years, patients may survive from less than a year to 20 or more years after initial diagnosis.1 Survival is often limited by recurrence and progression of LGG to high-grade gliomas. Factors influencing survival in these patients include histologic subtype, age, extent of surgical resection, and 1p/19q status.2–4
There is no consensus on the optimal management of patients with residual LGG following surgical resection.5 Often conformal external beam radiation therapy is used to treat residual disease; however, LGGs are often diffuse in nature, and the treatment fields for radiation therapy can be large. The potential complications of radiation therapy correlate to the neuro-anatomic location involved and include possible cognitive decline, seizures, endocrinopathies, necrosis, vasculopathies, and secondary malignancies such as meningiomas, gliomas, and sarcomas.
Given the potential for long-term survival of patients with LGG and the potential morbidity associated with radiation therapy, alternative treatment approaches are being evaluated.6 In particular, the role of chemotherapy as up-front postsurgical treatment of LGG continues to be evaluated.6–8 A single-institution phase II study at the University of California, San Francisco (BTRC 9902) was therefore initiated to assess the response rate of temozolomide (TMZ) chemotherapy in patients with LGG who have residual disease but have not yet received radiation therapy. In this study, LGG patients are prospectively evaluated clinically and radiographically every 2 months while on TMZ therapy. Accrual began in February 2000 with a goal to enroll 120 patients. Analysis of the main findings of this trial will await completion of patient accrual.
While awaiting the final results of the trial, adequate data have been captured on baseline and longitudinal quality of life (QOL) for interim analysis. Information on QOL is critical in this population because there have been few investigations of the multidimensional aspect of QOL in LGG patients receiving chemotherapy. Most published QOL studies on LGG have focused on cognitive changes in patients that have received radiation therapy.9–13 The QOL assessments in this study prospectively capture QOL data longitudinally. In addition, because patients who received prior radiation therapy were excluded, this study assesses QOL of patients undergoing chemotherapy without the potential confounding side effects of radiation therapy. Therefore, this is the first study in the literature to prospectively describe the multidimensional longitudinal QOL of LGG patients over 1 year of TMZ chemotherapy.
The objective of the current study was to assess baseline and longitudinal changes in multiple dimensions of QOL in LGG patients who are receiving TMZ as part of the above-mentioned clinical trial. The information obtained from the analysis will reveal areas for further study and identify future interventions aimed at improving QOL. It will also provide a historical control to compare the impact of other therapeutic interventions such as radiotherapy.
The multidimensional aspect of QOL was measured in this study through the Functional Assessment of Cancer Therapy–Brain (FACT-Br).14 FACT-Br is a well-validated instrument that provides a comprehensive assessment of four major QOL domains: physical well-being, social/family well-being, emotional well-being, and functional well-being, as well as a set of QOL indicators specific to brain tumor patients. These domains are generally accepted as the major domains that contribute to overall QOL. The questions have demonstrated reliability, validity, and sensitivity to change.15
With prospective evaluation of FACT-Br, we can investigate the baseline values of each individual QOL domain as well as the effects of changes in these areas over time in LGG patients. In this report, we present an interim analysis of the QOL data as measured by FACT-Br for all patients enrolled from the beginning of the BTRC 9902 study in February 2000 through July 2007.
Materials and Methods
Patient Characteristics
Patients with histologically proven supratentorial LGG (oligodendroglioma, astrocytoma, and oligoastrocytoma) enrolled at the University of California, San Francisco from February 2000 to July 2007 and treated with TMZ between 14 days and 4 months of surgical resection or biopsy were included. Patients must have had no previous treatment for their tumor other than surgery and must have evaluable disease. Patients could have multiple surgeries as long as pathology from the most recent surgery within 4 months of enrollment continued to show LGG. Patients must have been 18 years of age or older with KPS ≥60. Patients must have signed an institutionally approved Committee on Human Research consent form.
Study Design
Patients received TMZ orally at a starting dose of 200 mg/m2/day for 5 consecutive days of a 28-day cycle. Patients continued to receive treatment with TMZ until unacceptable toxicity and/or disease progression for at least 12 cycles. Dose reductions for both hematologic toxicities and nonhematologic toxicities were allowed. Responses, overall survival, and progression were recorded. Other chemotherapy, radiation, or biologic therapy was not allowed while the patient was on study. No other investigational drugs were allowed during the study, but prophylactic antiemetics could be administered at the discretion of the treating physician.
Response was measured by MRI, and clinical status was determined after every two cycles of TMZ chemotherapy. Comparisons of objective assessments, excluding progressive disease, were based on changes in tumor size on the MRI scan compared to the baseline scan. A resection was considered subtotal if between 5% and 95% of tumor tissue was removed. A resection was considered a biopsy if less than 5% of tumor tissue was removed. Determination of progressive disease was based on Macdonald criteria.16
QOL Evaluation
FACT-Br version 3.0 was administered at enrollment prior to chemotherapy and at each subsequent clinic visit. Clinic visits were timed after the completion of every two cycles of TMZ chemotherapy. Caregivers were instructed not to complete the questionnaires for patients.
FACT-Br version 3.0 contains 54 questions divided into five major realms of QOL.17 The realms are physical, social, emotional, functional, and brain tumor–specific well-being. A few questions address patients’ relationship with their doctor and are not part of these subscales and are not typically included in statistical analysis. Questions are based on a Likert scale 0–4, with 0 being described as “not at all,” 1 as “a little bit,” 2 as “somewhat,” 3 as “quite a bit,” and 4 as “very much.” Patients were described as having difficulty with an item if they reported “quite a bit” or “very much” in positively phrased questions or “not at all” or “a little bit” in negatively phrased questions. A subscale score was created by adding up scores from individual questions within the subscale. The inverse of scores was used for questions worded with negative phrasing. Higher scores represent better QOL in each of the subscales. Questionnaires are written at the fourth-grade reading level and are specifically formatted for ease of self-administration. Time to completion is estimated at 5–10 min.
Statistical Analysis for Baseline Data
Descriptive analysis of QOL at time of enrollment as measured by FACT-Br was performed. Differences among subgroups at baseline with respect to each patient characteristic variable were assessed for all self-reported QOL subscales using the Kruskal-Wallis test. The Kendall rank correlation coefficient (tau) was used to examine the correlation between age and each subscale. No adjustment for multiple comparisons was made due to the exploratory nature of these analyses; in all cases, p values <0.01 were considered statistically significant. Significance of this study population compared to other populations was calculated using a two-sample t-test. Adjustment for multiple comparisons was made by choosing p values of <0.01 to indicate significance.
Comparison populations included a normal population, a mixed cancer population, and a mixed brain tumor population. Comparisons between versions of FACT are considered acceptable given the lack of substantial changes between versions.
Statistical Analysis for Longitudinal Data
Descriptive analysis of the change in QOL over 12 cycles, as measured by FACT-Br every two cycles, was performed. Box plots were constructed for each FACT-Br subscale by cycle. Two methods for analyzing mean change in QOL were utilized. First, the significance of changes in scores for each QOL realm at evaluation time points compared to baseline was examined using a paired t-test. Adjustment for multiple comparisons was made by choosing p values of <0.01 to indicate significance.
To directly explore the individual time trajectories in change of QOL, we also employed hierarchical linear models to assess the change in each FACT-Br realm over cycles of chemotherapy while adjusting for baseline covariates including gender, age, extent of resection, hemisphere, and histology subtype. Hierarchical linear models enable one to study changes within individual patients over time by taking into proper account the correlation arising from repeated measurements in each of the individuals. A random intercept model was fitted to the longitudinal QOL data. Dummy variables were created for each post-baseline cycle to allow for nonlinear changes in QOL. Normal errors were assumed for the outcomes with symmetric distributions. The estimation of parameters was based on the maximum likelihood method. The analysis was conducted with the lme4 package in R version 2.6.1 (open-source software, www.r-project.org).
We also looked at the longitudinal pattern in QOL by examining the frequency of clinically significant changes. The minimally important difference (MID) was defined as the smallest score difference that is clinically significant and therefore likely to be meaningful to both patients and clinicians. The MID has been reported for both individual patients and patient groups and for single and multiple time points.18 The MIDs for scores of subscales have been identified using both anchor and distribution-based methods.17 They are 2–3 points for each of the subscales. The MIDs for the brain subscale are 5–7 points.15
Missing Data
Missing data within a questionnaire were handled according to previous validation measures of FACT-Br.17 The prorating of subscale scores was considered acceptable as long as more than 50% of the items were answered. The total score was considered appropriate as long as the overall item response rate was greater than 80%. Due to a clerical error, one question under the social well-being subscale was not included in the majority of the questionnaires. Therefore, the social well-being subscale was rescaled to account for this error.
A chart review was performed to identify reasons why patients went off treatment; therefore, by study protocol, patients were not required to fill out questionnaires at subsequent evaluations. Reasons included tumor progression, intolerable side effects of chemotherapy, development of other cancer or other medical issues, clinical deterioration without progression, and personal reasons. Patients still receiving active therapy and had not yet filled out questionnaires were noted.
Results
Baseline Data
Sixty-six patients were enrolled between February 2000 and July 2007. One patient was not included, as central review of pathology did not confirm LGG. Patient characteristics at enrollment are shown in Table 1. Of 65 patients evaluated, 60% were male, with a median age of 40 years (range, 20–72) and a median KPS score of 90; 83.8% of patients underwent subtotal resection, with the remainder undergoing biopsy; 60% of tumors were located in the left hemisphere.
Table 1.
Patient characteristics at enrollment
| Characteristic | Number |
|---|---|
| Gender | |
| Male | 39 (60.0%) |
| Female | 26 (40.0%) |
| Median age at diagnosis | 40 (range, 20–72) |
| Median KPS at diagnosis | 90 (range, 80–100) |
| Seizures prior to diagnosis | 30 (48.0%) |
| Tumor type | |
| Astrocytoma | 26 (40.0%) |
| Oligodendroglioma | 21 (32.3%) |
| Oligoastrocytoma | 16 (24.6%) |
| Tumor location | |
| Frontal | 27 (41.5%) |
| Temporal | 11 (16.9%) |
| Parietal | 3 (4.6%) |
| Insular | 2 (3.1%) |
| Occipital | 1 (1.5%) |
| Multiple lobes | 18 (27.7%) |
| Other | 3 (4.6%) |
| Tumor hemisphere | |
| Left | 39 (60.0%) |
| Right | 23 (35.4%) |
| Both | 3 (4.6%) |
| Degree of surgery | |
| Biopsy | 17 (26.2%) |
| Subtotal resection | 48 (73.8%) |
There were no differences at baseline in any Functional Assessment of Cancer Therapy–General (FACT-G) or brain tumor–specific subscale between female and male patients, between patients who underwent biopsy and those who underwent subtotal resection, and among patients with different histology subtypes (astrocytoma vs. oligodendroglioma vs. oligoastrocytoma). No statistically significant correlation was found between age and any baseline QOL subscale. Patients with lesions located in the left hemisphere tended to report worse physical well-being scores (p = 0.01), whereas a significant association was not found in social, emotional, functional, brain tumor–specific subscales, or total FACT scores (p = 0.62, 0.03, 0.24, 0.14, and 0.03, respectively).
Baseline scores for FACT-Br version 3.0, in comparison to other populations, are detailed in Table 2. The mean FACT-G score (summation of all subscales except the brain tumor–specific subscale) was 83 (range, 38–106). The mean brain tumor–specific subscale score was 56.9 with a range from 20 to 76. In comparison to a normal population,17 the LGG patient population studied had statistically significant higher reported social well-being scores (mean difference = 5.0, p < 0.01) but statistically significant lower reported emotional well-being scores (mean difference = 2.2, p < 0.01). The difference in social well-being scores between the LGG population studied and the standard population was above the minimally important difference of 2–3 points.
Table 2.
Baseline subscale scores and comparisons to other populations
| Our Population at Baseline
|
Normal Population17 (n = 1,075)a |
Mixed Cancer Population19 (n = 545)b |
Original FACT-Br Sample (Mixed Brain Tumors)14 (n = 101)c |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | SD | n | Mean | SD | p Value | Mean | SD | p Value | Mean | SD | p Value | |
| Physical WB | 23.0 | 5–28 | 5.4 | 61 | 22.7 | 5.3 | 0.69 | 20.5 | 5.5 | <0.01 | 22.3 | 5.1 | 0.37 |
| Social WB | 24.1 | 9–28 | 4.3 | 62 | 19.1 | 6.8 | <0.01 | 21.9 | 4.8 | <0.01 | 21.7 | 5.5 | <0.01 |
| Relationship with MD | 6.8 | 2–8 | 1.4 | 61 | 6.85 | 1.51 | 0.76 | 6.96 | 1.66 | 0.48 | |||
| Emotional WB | 17.7 | 7–24 | 3.9 | 62 | 19.9 | 4.8 | <0.01 | 14.8 | 3.9 | <0.01 | 16.0 | 4.4 | 0.01 |
| Functional WB | 18.2 | 6–28 | 6.0 | 62 | 18.5 | 6.8 | 0.76 | 18.0 | 6.1 | 0.76 | 19.9 | 6.4 | 0.09 |
| FACT-G total score | 83 | 38–106 | 14.0 | 61 | |||||||||
| Other WB (brain tumor specific) | 56.9 | 20–76 | 12.0 | 62 | |||||||||
Abbreviations: FACT-Br, Functional Assessment of Cancer Therapy–Brain; SD, standard deviation; WB, well-being; FACT-G, Functional Assessment of Cancer Therapy–General. Significance of our population compared to other populations was calculated using a two-sample t-test. Adjustment for multiple comparisons was made by choosing p-values of <0.01 to indicate significance. Boldfaced p-values indicate that the mean of the underlying population is statistically different from the LGG population.
Normal population includes mean age of 45.9 years (range, 18–91); 49.4% male and 50.6% female.
Mixed cancer population included 39% breast, 15% lung, 12% colorectal, 8% leukemia/lymphoma, 8% head and neck, 6% prostate, 2% ovarian, and 10% other/unknown cancers. CNS metastasis excluded.
Original FACT-Br sample population characteristics: mean age of 41.2 years, 56% male and 44% female, 27 (27%) glioblastoma multiforme, 47 (47%) grade III gliomas, 13 (13%) meningiomas, 7 (7%) mixed gliomas, and 7 (7%) other; 94% had surgery, and 86% had additional treatment (radiation therapy, chemotherapy, or both).
In comparison to a mixed cancer population,19 the LGG population studied had higher reported physical (mean difference = 2.5, p < 0.01), social (mean difference = 2.2, p < 0.01), and emotional well-being scores (mean difference = 2.9, p < 0.01). Compared to a mixed brain tumor population,14 this patient population had higher reported social (mean difference = 2.4, p < 0.01) and emotional well-being scores (mean difference = 1.8, p = 0.01).
The specific areas that more than 20% of patients reported difficulty with at baseline are presented in Table 3; 44% of patients noted being able to drive “not at all.” Other difficulties noted in greater than 20% of patients included loss of independence and being unable to contribute to the family. Over 20% of patients reported difficulty with being able to work or find work fulfilling or to enjoy the things they usually do for fun. Although less frequent, patients reported difficulty with emotional symptoms (18% feeling sad, and 16% feeling nervous) and physical/functional symptoms (18% with lack of energy, and 15% sleeping poorly).
Table 3.
Patient reported difficulties at baselinea
| Subscale | Question | Percentage (%) with Difficulty |
|---|---|---|
| Brain specific | I get frustrated that I cannot do things I used to. | 22 |
| I am bothered by the drop in my contribution to the family. | 23 | |
| I have been afraid of having a seizure (convulsion). | 26 | |
| I don’t feel independent. | 26 | |
| I am not able to drive a vehicle. | 44 | |
| Functional realm | I am not enjoying the things I usually do for fun. | 21 |
| My work is not fulfilling. | 23 | |
| I am not able to work. | 24 |
At least 85% of patients responded to each question. Questions with positive phrasing were reworded negatively. The questions listed represent those that ≥20% of patients reported some difficulty. Difficulty was defined as the two worst scores for negatively phrased questions and the two best scores for the positively phrased questions.
Longitudinal Data
Table 4 shows the compliance of patients who remained on study at each evaluation. Baseline compliance was 95%. When accounting for patients that went off study and those that had not yet reached the scheduled cycle, compliance ranged from 71% to 85%. Six patients progressed while on therapy, and six patients went off study secondary to intolerable side effects.
Table 4.
Compliancea rate per cycle
| Time Point | Number of Patients on Study | Number of Questionnaires Filled Out | Number of Missing Questionnaires | Compliance (%) | Reasons Off Study |
|---|---|---|---|---|---|
| Baseline | 65 | 62 | 3 | 95 | |
| Cycle 2 | 60 | 51 | 9 | 85 | 2 progressed |
| 1 off for bladder cancer | |||||
| 1 off for unrelated medical issues | |||||
| 1 did not reach cycle | |||||
| Cycle 4 | 56 | 45 | 11 | 80 | 1 progressed |
| 2 intolerable side effects | |||||
| 1 did not reach cycle | |||||
| Cycle 6 | 51 | 41 | 10 | 80 | 2 progressed |
| 2 intolerable side effects | |||||
| 1 did not reach cycle | |||||
| Cycle 8 | 48 | 39 | 9 | 81 | 1 clinical worsening without progression |
| 2 did not reach cycle | |||||
| Cycle 10 | 45 | 32 | 13 | 71 | 1 progressed |
| 2 intolerable side effects | |||||
| Cycle 12 | 42 | 32 | 10 | 74 | 1 personal reasons |
| 1 geographic limitation | |||||
| 1 did not reach cycle |
Compliance was obtained by accounting for those that were not required to fill out questionnaires for the following reasons: tumor progression, intolerable side effects, development of other cancer or other medical issues, clinical deterioration without progression, personal reasons, and patient still actively receiving therapy. Geographic limitation was considered as failure to comply because the protocol still required questionnaire completion in that one case.
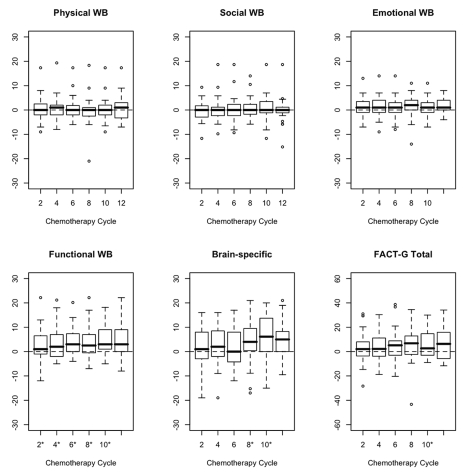
Figure 1 shows box plots of the change score at each chemotherapy cycle compared to baseline for five realms of QOL as well as the FACT-G total scores. A p value of <0.01 was used as the criterion for declaring statistical significance to adjust for multiple comparisons. No significant change in reported physical or social realm scores was noted over the course of 12 cycles of therapy compared to baseline. Mean score changes were significantly positive at every time point for the functional realm; at cycle 12 for the emotional well-being realm; at cycles 8, 10, and 12 for the brain tumor–specific realm; and at cycle 10 for the overall FACT-G total well-being.
Fig. 1.
Box plots of the change score at each chemotherapy cycle compared to baseline for five realms of quality of life and Functional Assessment of Cancer Therapy–General (FACT-G). Abbreviation: WB, well-being. Asterisks indicate cycles for which p-values based on the paired t-test are statistically significant (p<0.01). Note: The values within the box represent the lower quartile (Q1), median, and the upper quartile (Q3) of the distribution. The horizontal bars at the two ends are the smallest and largest non-outlier observations. The circles beyond the horizontal bars represent outlying cases, defined as 1.5 times the interquartile range (Q3–Q1), below Q1 or above Q3.
Results from hierarchical modeling are consistent with those based on descriptive analyses. In particular, adjusting for age, gender, extent of resection, hemisphere, and histology subtype, the hierarchical model including the cycle effect was found to be statistically significant compared with the model without cycle effect in predicting emotional well-being, functional well-being, brain tumor–specific well-being, and the total FACT-G score (likelihood ratio test; p values 0.008, <0.0001, 0.002, and 0.03, respectively). The coefficients with respect to the cycle effect in these models are all positive in magnitude, indicating an overall positive change over baseline in these self-reported FACT-Br subscales. No significant time trend was found in the physical and social realms (p = 0.82 and 0.85, respectively).
Table 5 shows the percentage of patients who reported improved or declined ratings of QOL beyond the minimally important difference at each time point relative to their own baseline. To be conservative, we used a minimally important difference of three points as the threshold for improvement or deterioration in the physical, social, emotional, and functional realms. A difference of seven points was used as the threshold for the brain tumor–specific subscale. Overall, there was a consistent pattern of change in self-reported QOL as demonstrated in the above descriptive and hierarchical modeling analyses. Particularly, over 30% of patients reported improved emotional well-being over baseline at cycles 4, 8, and 12. This positive trend was observed at all cycles for the functional subscale. For the brain tumor–specific subscale, over 30% of patients reported improvement over baseline beyond seven points at the last three cycles. The percentages of patients reporting decline in all subscales were substantially lower, providing little evidence of self-reported deterioration in QOL during the course of the treatment.
Table 5.
Proportion of patients demonstrating minimally important difference (MID) of improvement or decline in QOL relative to baseline
| Percentage (%) with Improvement from Baseline >MIDa |
Percentage (%) with Decline from Baseline >MID
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | S | E | F | Brain | P | S | E | F | Brain | |
| Cycle 2 | 19 | 19 | 26 | 36 | 26 | 13 | 25 | 9 | 11 | 15 |
| Cycle 4 | 15 | 14 | 31 | 36 | 26 | 17 | 19 | 10 | 6 | 7 |
| Cycle 6 | 14 | 21 | 21 | 45 | 26 | 22 | 24 | 11 | 5 | 5 |
| Cycle 8 | 8 | 19 | 39 | 42 | 31 | 17 | 17 | 6 | 8 | 8 |
| Cycle 10 | 14 | 30 | 20 | 40 | 50 | 17 | 17 | 10 | 7 | 13 |
| Cycle 12 | 14 | 10 | 38 | 48 | 31 | 25 | 14 | 3 | 10 | 3 |
Abbreviations: P, physical; S, social; E, emotional; F, functional. Boldfaced values indicate that the percentage of patients who reported improved well-being (over baseline) at that particular cycle exceeds 30%.
MID used for physical, social, emotional, and functional was 3. MID used for brain tumor–specific subscale was 7.
Discussion
Baseline QOL
Due to the long accrual period anticipated for BTRC 9902, we report interim QOL results of patients enrolled in the clinical trial through July 2007. Sixty-five patients were included in the analysis. Overall patient characteristics show that they were representative of LGG populations seen in other large studies of patients without prior radiotherapy.20–22 One major difference is that patients in this study did not receive gross total resection because the study only enrolled patients with evaluable disease. Therefore, assuming that gross total resection improves QOL compared to biopsy or subtotal resection,23 this patient population at baseline may actually have had a worse QOL than standard LGG populations.
The finding that patients with right-hemisphere tumors report higher QOL scores in the physical realm is interesting and deserves further study. It may be that right hemisphere lesions spare the dominant side, allowing patients to have better QOL. In prior studies, tumor location and laterality have been shown to correlate with specific symptoms. For example, depression may arise from left-brain injury,24, 25 and anxious states may arise from right-brain injury.25–27 As patients with tumors in the left hemisphere may have greater problems with communication24,28 and with concentration,29–31 the reliability of conclusions about laterality based on self-reported symptoms and QOL is sometimes in question.
In examining subscale scores, the reported physical and functional well-being scores of this population were comparable to those reported by a normal population. The high median KPS of 90 at baseline supports the finding that this patient population as a whole does not have significant physical and functional limitations.
In comparison to a normal population, LGG patients had a statistically significant increase in reported social well-being scores, and the mean difference was far beyond the minimally important difference. There are several possible explanations for this finding. The patient population enrolling in our clinical trial could have been highly selected, as the study institution is a tertiary care center for brain tumor patients. However, examination of baseline characteristics compared to other LGG trials would suggest that our population is at least comparable to previously studied LGG patients. Another explanation is that people with cancer may receive better social support from friends and family than a normal population. The statistically higher reported social well-being of a mixed cancer population and a mixed brain tumor population compared to a normal population supports this concept. Finally, patients’ perception of their social well-being may change once they are diagnosed with a life-threatening illness. This change in perception may explain the disconnect found in some studies between self-reported symptoms and objective measures of those symptoms.23,30,32
The LGG population studied also had lower reported emotional well-being scores compared to a normal population. The clinical significance of this change is just within the range of the minimally important difference. The reason for the lower emotional well-being scores compared to a normal population may be related to the diagnosis of cancer, which has been shown to lead to emotional distress.33 Indeed, both a mixed cancer population and a mixed brain tumor population had lower reported emotional well-being scores compared to the normal population.
While a subscale score is useful in broadly comparing populations, these scores do not help delineate the specific issues within the QOL realm that may be affecting patients. Table 3 describes each of the questions in the brain tumor–specific subscales. There are four main findings to note. First, a significant minority of patients reported “quite a bit” and “very much” for emotional symptoms such as feeling sad or nervous, which likely corresponds to the decreased emotional well-being scores reported. Second, with the exception of not being able to operate a motor vehicle and working, the majority of patients did not report significant difficulties in any brain tumor–specific areas of QOL. Third, the loss of independence, especially in terms of operating a motor vehicle, and the feeling that patients can no longer contribute to the family are themes represented in a substantial minority of patients. Finally, a substantial minority of patients are afraid of having seizures. Therefore, by supporting patients’ emotional needs and independence and by providing improved monitoring and care for seizures, we may be able to improve QOL for a broad range of LGG patients.
There are several limitations to our comparison of baseline data. The mixed cancer population included a very heterogeneous group of tumors at all stages of treatment. The mixed brain tumor population was also heterogeneous and included mostly malignant gliomas and a minority of other gliomas and meningiomas. Finally, there was limited demographic information in comparison groups to be able to determine if differences in comparisons were due to other patient characteristics. Missing data was not a significant issue with baseline data, as compliance with baseline questionnaires was high (95%).
Longitudinal QOL
Availability of data for analysis declined after the initial time point, although through a chart review we were able to identify reasons for the missing data in cases where patients went off the study protocol and were no longer required to fill out questionnaires. Although the chart review did not identify the reason for not completing questionnaires in the remainder of the cases, discussion with staff providing the questionnaires revealed that the likely primary reason for not providing questionnaires was related to administrative failure. Of the patients who remained on study, the overall compliance rate was between 71% and 85%.
There was no significant change in physical or social realm scores compared to baseline over the course of 12 cycles of therapy. It should be noted, however, that patients already had a high mean score for social well-being, with a mean baseline score of 24 out of a total possible subscale score of 28. Therefore, the scale would not be able to detect a further increase in social well-being. There were mean positive changes in the other realms compared to baseline. These change scores approached the minimally important difference for each of these three realms. The significance of these changes was further confirmed by hierarchical linear modeling.
Based on these analyses, it may be tempting to assume that patients’ QOL is improving over time in terms of emotional, functional, and brain tumor–specific well-being. However, there are several alternative explanations for our findings. First, patients who progressed and those who had intolerable side effects were not included in the analysis because they were no longer required to fill out questionnaires. In addition, despite overall adequate compliance, the sickest patients may have been too ill to fill out follow-up questionnaires. Finally, patients’ perception of their own QOL may rise over time, even when objective measures do not indicate such a change.
An additional limitation of the longitudinal QOL data collected is that although compliance rates were relatively high, missing data over time were not accounted for in a systematic way prior to the beginning of the study. A chart review allowed us to identify reasons for a portion of the missing data. In addition, administrative failure likely contributed to the majority of the remaining missing data points. Administrative failure has been reported as the major reason for decreased compliance in another QOL study in brain tumor patients that specifically addressed this issue.34 We cannot rule out the possibility that there may be other reasons why patients did not fill out questionnaires. Better accounting of this missing data will help improve the statistical validity of conclusions on future longitudinally collected data. In addition, measurement of QOL for those patients who stop protocol therapy due to intolerance or progression is an important goal in future studies, because those patients are the ones whose QOL is likely to be most severely impacted.
The duration of data collection could also have been longer. Measurement of nonprogressing LGG patients’ QOL over approximately 1 year does not describe the long-term survivorship of patients who live for many years. Unfortunately, the completion of active protocol therapy made it difficult to collect meaningful data after the 12-cycle time point.
Despite these limitations, we at least did not see a decline in QOL measures over time when compared to baseline. The maintenance of a median KPS of 90 over time supports the high functional status of those who were able to continue TMZ therapy. Furthermore, multiple analyses including production of box plots and hierarchical modeling showed consistent results, adding strength to our findings.
Future Directions
There are many potential directions for future research. In particular, longer-term QOL outcomes in LGG patients need to be established. Identification of risk factors leading to symptoms and interventions that can improve QOL need to be better studied. Ultimately, the establishment of rigorous historical QOL data in brain tumor patients will allow future investigators to truly assess the impact of other interventions such as radiotherapy. The addition of QOL information to therapeutics will in turn help patients and physicians make truly informed decisions about therapy.
One area that deserves further study is the complex interactions between environment, treatments, tumor characteristics, and the social and emotional context in which patients experience symptoms. A more comprehensive way of studying QOL may be needed as traditional QOL measures have typically only accounted for subjective patient-reported symptoms. Such a model would incorporate the traditional patient-reported realms of QOL as well as objective measures that contribute to a patient’s perception of QOL. By developing and validating such models in future studies, we may begin to understand the complex interactions that explain a patient’s perception of their overall QOL.
Conclusions
To our knowledge, this is the first article that has prospectively described the QOL of LGG patients on chemotherapy, without prior radiotherapy. The QOL of these LGG patients at baseline was comparable to a normal population, with the exception of social and emotional well-being. Patients with right hemisphere tumors reported better QOL scores in the physical realm compared to those with left hemisphere tumors. On evaluating individual questions at baseline, patients’ lower emotional well-being scores compared to a normal population were reflected by feelings of sadness and nervousness. A substantial minority of patients were also concerned about loss of independence and seizures. In addition, patients reported difficulty contributing to the family and working. Few patients had difficulty with energy or sleep, and very few patients had difficulty with nausea or pain. These findings suggest that future studies should examine risk factors and potential interventions to address loss of independence, inability to drive, fear of seizures, and contribution to family and work.
Finally, this study demonstrates that self-reported QOL measured by FACT-Br did not show deterioration over time among LGG patients receiving TMZ as up-front therapy after resection. This finding will provide valuable information for future research aimed at comparing QOL of patients who receive RT after resection.
Acknowledgments
We acknowledge the National Brain Tumor Foundation for providing grant support to study patient QOL. Dr. Liu receives support from the National Brain Tumor Foundation. Karla Solheim receives grant support from the University of California School of Medicine Student Research Training Program. Dr. Chang receives research grant support from the National Brain Tumor Foundation, NeoPharm, and Schering-Plough and has acted as a consultant to Genentech. Dr. Prados receives research grant support from NeoPharm, Genetech, and Eli Lilly. Dr. Butowski receives research support from Schering-Plough, Genentech, and Eli Lilly. We also thank the Center for Outcomes, Research and Education (CORE) at Northwestern University, and in particular Dr. David Cella for his assistance with FACT data.
References
- 1.Claus EB, Black PM. Survival rates and patterns of care for patients diagnosed with supratentorial low-grade gliomas: data from the SEER program, 1973–2001. Cancer. 2006;106:1358–1363. doi: 10.1002/cncr.21733. [DOI] [PubMed] [Google Scholar]
- 2.Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20:2076–2084. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 3.Yeh SA, Ho JT, Lui CC, Huang YJ, Hsiung CY, Huang EY. Treatment outcomes and prognostic factors in patients with supratentorial low-grade gliomas. Br J Radiol. 78:230–235. doi: 10.1259/bjr/28534346. [DOI] [PubMed] [Google Scholar]
- 4.Smith JS, Perry A, Borell TJ, et al. Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol. 2000;18:636–645. doi: 10.1200/JCO.2000.18.3.636. [DOI] [PubMed] [Google Scholar]
- 5.Lang FF, Gilbert MR. Diffusely infiltrative low-grade gliomas in adults. J Clin Oncol. 2006;24:1236–1245. doi: 10.1200/JCO.2005.05.2399. [DOI] [PubMed] [Google Scholar]
- 6.Grier JT, Batchelor T. Low-grade gliomas in adults. Oncologist. 2006;11:681–693. doi: 10.1634/theoncologist.11-6-681. [DOI] [PubMed] [Google Scholar]
- 7.Hoang-Xuan K, Capelle L, Kujas M, et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22:3133–3138. doi: 10.1200/JCO.2004.10.169. [DOI] [PubMed] [Google Scholar]
- 8.Brada M, Viviers L, Abson C, et al. Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol. 2003;14:1715–1721. doi: 10.1093/annonc/mdg371. [DOI] [PubMed] [Google Scholar]
- 9.Laack NN, Brown PD, Ivnik RJ, et al. Cognitive function after radiotherapy for supratentorial low-grade glioma: a North Central Cancer Treatment Group prospective study. Int J Radiat Oncol Biol Phys. 2005;63:1175–1183. doi: 10.1016/j.ijrobp.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Brown PD, Buckner JC, O’Fallon JR, et al. Effects of radiotherapy on cognitive function in patients with low-grade glioma measured by the folstein mini-mental state examination. J Clin Oncol. 2003;21:2519–2524. doi: 10.1200/JCO.2003.04.172. [DOI] [PubMed] [Google Scholar]
- 11.Torres IJ, Mundt AJ, Sweeney PJ, et al. A longitudinal neuropsychological study of partial brain radiation in adults with brain tumors. Neurology. 2003;60:1113–1118. doi: 10.1212/01.wnl.0000055862.20003.4a. [DOI] [PubMed] [Google Scholar]
- 12.Kiebert GM, Curran D, Aaronson NK, et al. Quality of life after radiation therapy of cerebral low-grade gliomas of the adult: results of a randomised phase III trial on dose response (EORTC trial 22844). EORTC Radiotherapy Co-operative Group. Eur J Cancer. 1998;34:1902–1909. doi: 10.1016/s0959-8049(98)00268-8. [DOI] [PubMed] [Google Scholar]
- 13.Gustafsson M, Edvardsson T, Ahlstrom G. The relationship between function, quality of life and coping in patients with low-grade gliomas. Support Care Cancer. 2006;14:1205–1212. doi: 10.1007/s00520-006-0080-3. [DOI] [PubMed] [Google Scholar]
- 14.Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA. The Functional Assessment of Cancer Therapy (FACT) scale: development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75:1151–1161. doi: 10.1002/1097-0142(19950301)75:5<1151::aid-cncr2820750515>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 15.Nickolov A, Beumont JL, Victorson D, et al. Validation of functional assessment of cancer therapy: brain (FACT-Br) questionnaire and FACT-Br symptom index (FBrSI) in patients with recurrent high-grade glioma. Paper presented at: Chicago Supportive Oncology Conference; October 6–8, 2005; Chicago, IL. 2005. [Google Scholar]
- 16.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 17.Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi: 10.1186/1477-7525-1-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ringash J, O’Sullivan B, Bezjak A, Redelmeier DA. Interpreting clinically significant changes in patient-reported outcomes. Cancer. 2007;110:196–202. doi: 10.1002/cncr.22799. [DOI] [PubMed] [Google Scholar]
- 19.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11:570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 20.van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366:985–990. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 21.Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy of low-grade cerebral glioma: European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Int J Radiat Oncol Biol Phys. 1996;36:549–556. doi: 10.1016/s0360-3016(96)00352-5. [DOI] [PubMed] [Google Scholar]
- 22.Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group study. J Clin Oncol. 2002;20:2267–2276. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 23.Klein M, Heimans JJ, Aaronson NK, et al. Effect of radiotherapy and other treatment-related factors on mid-term to long-term cognitive sequelae in low-grade gliomas: a comparative study. Lancet. 2002;360:1361–1368. doi: 10.1016/s0140-6736(02)11398-5. [DOI] [PubMed] [Google Scholar]
- 24.Hahn CA, Dunn RH, Logue PE, King JH, Edwards CL, Halperin EC. Prospective study of neuropsychologic testing and quality-of-life assessment of adults with primary malignant brain tumors. Int J Radiat Oncol Biol Phys. 2003;55:992–999. doi: 10.1016/s0360-3016(02)04205-0. [DOI] [PubMed] [Google Scholar]
- 25.Mainio A, Hakko H, Niemela A, Tuurinkoski T, Koivukangas J, Rasanen P. The effect of brain tumour laterality on anxiety levels among neurosurgical patients. J Neurol Neurosurg Psychiatry. 2003;74:1278–1282. doi: 10.1136/jnnp.74.9.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummings JL. Neuropsychiatric manifestations of right hemisphere lesions. Brain Lang. 1997;57:22–37. doi: 10.1006/brln.1997.1832. [DOI] [PubMed] [Google Scholar]
- 27.Wasserstein J, Stefanatos GA. The right hemisphere and psychopathology. J Am Acad Psychoanal. 2000;28:371–395. doi: 10.1521/jaap.1.2000.28.2.371. [DOI] [PubMed] [Google Scholar]
- 28.Tucha O, Smely C, Preier M, Lange KW. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47:324–333. doi: 10.1097/00006123-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Heimans JJ, Taphoorn MJ. Impact of brain tumour treatment on quality of life. J Neurol. 2002;249:955–960. doi: 10.1007/s00415-002-0839-5. [DOI] [PubMed] [Google Scholar]
- 30.Taphoorn MJ, Heimans JJ, Snoek FJ, et al. Assessment of quality of life in patients treated for low-grade glioma: a preliminary report. J Neurol Neurosurg Psychiatry. 1992;55:372–376. doi: 10.1136/jnnp.55.5.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taphoorn MJ, Schiphorst AK, Snoek FJ, et al. Cognitive functions and quality of life in patients with low-grade gliomas: the impact of radiotherapy. Ann Neurol. 1994;36:48–54. doi: 10.1002/ana.410360111. [DOI] [PubMed] [Google Scholar]
- 32.Reijneveld JC, Sitskoorn MM, Klein M, Nuyen J, Taphoorn MJ. Cognitive status and quality of life in patients with suspected versus proven low-grade gliomas. Neurology. 2001;56:618–623. doi: 10.1212/wnl.56.5.618. [DOI] [PubMed] [Google Scholar]
- 33.Vitek L, Rosenzweig MQ, Stollings S. Distress in patients with cancer: definition, assessment, and suggested interventions. Clin J Oncol Nurs. 2007;11:413–418. doi: 10.1188/07.CJON.413-418. [DOI] [PubMed] [Google Scholar]
- 34.Walker M, Brown J, Brown K, Gregor A, Whittle IR, Grant R. Practical problems with the collection and interpretation of serial quality of life assessments in patients with malignant glioma. J Neurooncol. 2003;63:179–186. doi: 10.1023/a:1023900802254. [DOI] [PubMed] [Google Scholar]