Abstract
High-grade gliomas release excitotoxic concentrations of glutamate, which has been shown to enhance tumor proliferation and migration. α-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) glutamate receptors are abundantly expressed at the invading edge of glioblastoma specimens, suggesting they may play an important biologic role in tumor invasion. In this study, we examined potential mechanisms by which AMPA receptor (AMPAR) expression and stimulation promote glioma cell migration and invasion. Overexpression of GluR1, the most abundant AMPAR subunit in gliomas, positively correlated with glioma cell adhesion to type I and type IV collagen, which was decreased in cells with knockdown of GluR1 and with blocking antibodies to β1 integrin. Furthermore, stimulation of the AMPAR led to detachment of cells from the extracellular matrix (ECM). Immunoprecipitation studies showed that GluR1 associated with the actin cytoskeleton-linked protein band 4.1B (brain type), which may serve as a link between GluR1 and integrins. Overexpression of GluR1 correlated with increased cell-surface expression of β1 integrin, increased phosphorylation of focal adhesion kinase (FAK-Y397), and enhanced numbers of focal adhesion (FA) complexes. Cells overexpressing GluR1 had increased colocalization of actin and paxillin at FAs and, in several glioma cell lines, significantly increased invasion in an in vitro Matrigel transwell assay. Likewise, in an intracranial xenograft model, overexpression of GluR1 led to perivascular and subependymal glioma cell invasion similar to patterns of tumor dissemination described in human glioblastoma. Together, these results suggest that AMPARs may link signals from the ECM to sites of FA, where signal integration promotes tumor invasion.
Keywords: AMPA receptor, glioblastoma, glutamate, invasion, perivascular
Glioblastomas are the most common type of diffusely infiltrating glioma in adults and are highly aggressive. Despite maximal therapy, glioblastoma tumors regrow rapidly, usually within 1–2 cm of the original tumor, which is a major limitation to current therapy. A prominent characteristic of glioblastomas is their ability to invade and infiltrate normal brain tissue, usually along blood vessels and white matter tracts.1 Because the brain is highly vascular, glioblastoma cells can obtain their blood supply from preexisting vessels via a process termed cooption.2 This implies that glioma cells can spread diffusely throughout the brain without having to recruit new vasculature to support their invasive behavior. Although the patterns of glioma spread are well described, the mechanisms that lead to glioma invasion are not well understood.
Integrins are critical for cell migration and invasion because they mediate interactions between cells and extracellular matrix (ECM) and regulate signaling pathways that control cytoskeletal organization.3 The brain lacks expression of many ECM components that are expressed in other organs, including collagens, laminin, and fibronectin, which are limited to vascular and perivascular areas within the brain.4,5 Glioma cells express several integrin family members thought to have functional significance in glioma invasion. Collagen (integrin subunits α2β1), fibronectin (α5β1), laminin (α3β1), and vitronectin (αvβ3) receptors6,7 have been identified as potential mediators of invasion, with β1 integrin playing a central role because of its ability to bind to multiple subunits.4 Integrins initiate multiple signal transduction cascades upon binding to ECM ligands,8 including activation of cytoplasmic tyrosine kinases such as focal adhesion kinase (FAK), which has been implicated in glioma invasion.9 In addition, cell detachment from the ECM is also highly regulated since a cycle of attachment and detachment of cells to their underlying ECM via integrins is considered essential for cell migration.10 In this way, integrins may mediate a cascade of signaling events through both ligand binding and downstream signaling in response to cell–ECM interactions, thus promoting cellular movement.11
Recent evidence supports the hypothesis that glioblastoma tumors release the neurotransmitter glutamate to enhance their highly malignant behavior. Astrocytic tumors release glutamate at high levels,12,13 which has been shown to enhance tumor proliferation14,15 and invasion.16,17 The α-amino-3-hydroxy-5-methylisoxazole- 4-propionic acid (AMPA) glutamate receptor (AMPAR) appears to play a pivotal role in mediating the biologic effects of glutamate in glioma. GluR1 and GluR4 AMPAR subunits, which are highly expressed in glioblastoma,17 perform critical functions in glioma biology that enhance its malignant phenotype. AMPARs are calcium-permeable ion channels that may augment glioma invasion through regulation of glutamate-induced calcium oscillations.18,19 Thus, excess extracellular glutamate may impart a survival advantage to glioma cells by promoting their migration and invasion.17,18
Glioma cells may recapitulate the migration of progenitor cells that takes place during CNS development. Farin et al.20 described a dynamic analysis of glioma cell migration along blood vessels when injected into rat forebrain slice cultures. These tumor cells closely resembled glial progenitors by demonstrating a unipolar morphology with a leading invasive edge. During CNS development, glutamate stimulates neuronal migration in the developing cerebellum21 and enhances oligodendrocyte precursor cell migration via interactions with a proteolipid protein/αvβ3 integrin complex.22 These observations led us to hypothesize that glutamate and the highly expressed AMPAR may activate glioma migration through interactions with the ECM.
Our study showed that overexpression of GluR1, the most abundant AMPAR subunit in human glioblastomas, enhanced focal adhesion (FA) complex formation, colocalization of actin and paxillin, and cellular polarization. We demonstrated a direct correlation between AMPAR expression and adhesion to the ECM component collagen mediated primarily via β1 integrin. Furthermore, AMPAR stimulation increased tumor cell deadhesion, a feature known to be important for cell migration.23,24 AMPAR expression correlated with higher surface levels of β1 integrin. Cells overexpressing GluR1 had higher levels of activated FAK, and at baseline, glutamate stimulation resulted in increased Rac1 GTPase activity. Our immunoprecipitation studies showed that GluR1 may be linked to integrin receptors via the erythrocyte membrane (ERM) protein 4.1B. Finally, we showed that expression levels of AMPARs directly correlate with the level of glioma cell invasion both in vitro and in vivo. The animal studies demonstrated tumor migration far from the main tumor mass via perivascular and subpial invasion of the brain. Because collagen and other ECM components are abundant in endothelial basement membranes25 and brain meninges,26,27 they may provide a substrate for glutamate-mediated glioma migration.
Materials and Methods
Glioma Cell Lines and Culture Conditions
The human glioblastoma cell lines U87 and U251 were obtained from the American Type Tissue Culture Collection (Manassas, VA, USA). All cell lines were maintained in Dulbecco’s modified Eagle’s medium (GIBCO, Grand Island, NY, USA) supplemented with 10% fetal bovine serum and penicillin-streptomycin in a humidified incubator at 37°C containing 5% CO2.
Generation of Short Hairpin RNA Clones
Stable GluR1 short hairpin RNA (shRNA) and nonsilencing (scrambled) shRNA clones were generated as described previously.28,29 Briefly, shRNA constructs were expressed as human microRNA-30 primary transcripts. The GluR1 shRNA was cloned into the pSHAG-MAGIC2 retroviral vector, which had a murine stem-cell virus backbone.
Retroviral Infections
Once the infection conditions were optimized, human embryonic kidney 293 cells stably expressing vesicular stomatitis virus-G were transfected with the retroviral vector and gag polymerase. At 48 and 72 h, retrovirus-containing medium was collected, filtered through a 0.2-μm filter, and added along with Polybrene (Sigma, St. Louis, MO, USA; 0.8 μg/ml) to U251 and U87 glioma cell lines in culture. At 72 h after infection, stable cell lines were generated by selection with the antibiotic puromycin (selectable marker in viral vector). Clones resistant to puromycin were isolated and assayed for mRNA expression levels using real-time TaqMan reverse transcriptase (RT) PCR and Western blot analysis. Results were compared to those for cells infected with vector expressing nonsilencing control shRNA.
Stable Transfection
The cDNA encoding full-length GluR1 (Origene, Rockville, MD, USA) was subcloned into pcDNA3.1 expression vector. U251 and U87 cells were transfected with GluR1 cDNA with Lipofectamine Plus (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. Stable cell lines overexpressing GluR1 were derived from individually selected clones propagated under selection pressure with G418 (Invitrogen). Cells transfected with empty vector were used as control. Expression levels of GluR1 and GluR4 were determined by quantitative RT-PCR and Western blot analysis.
Western Blot Analysis
Cells in culture were harvested and homogenized by sonication in ice-cold lysis buffer (50 mM Tris-HCl [pH 7.5], 0.1% sodium dodecyl sulfate [SDS], 150 mM sodium chloride, 1% Triton X-100, 1 mM dithiothreitol, proteinase inhibitor cocktail [Sigma], 0.5 mM EDTA, 100 μM sodium orthovanadate, 100 μM sodium pyrophosphate, and 1 mM sodium fluoride). Protein concentrations were determined against control bovine serum albumin levels using a Bio-Rad (Hercules, CA, USA) protein assay kit. Aliquots of protein were separated using SDS-polyacrylamide gel electrophoresis (PAGE) on 8%–12% gels. Proteins were transferred by electro-blotting to polyvinylidene fluoride membranes. Blots were blocked with 5% nonfat dry milk in 50 mM phosphate-buffered saline (PBS) containing 0.1% Tween-20 at room temperature and incubated overnight at 4°C with primary antibodies to GluR1 and GluR4 (Upstate, Billerica, MA, USA), α5 and β1 integrin (Chemicon, Billerica, MA, USA), phosphorylated FAK (FAK-Y397; Cell Signaling, Danvers, MA, USA), and actin (Calbiochem, Gibbstown, NJ, USA). The blots were then washed and incubated for 1 h with horseradish peroxidase– conjugated secondary antibody, and immunoreactive proteins were detected by enhanced chemiluminescence (Bio-Rad). Western blots were digitized and quantified using the ImageJ software version 1.41 for Windows (http://rsb.info.nih.gov/ij).
Immunoprecipitation
Cells were harvested, homogenized by sonication in ice-cold lysis buffer, and centrifuged. Protein concentrations were determined, and equal concentrations of protein were incubated with 1 μg of primary antibody to GluR1 (Chemicon, Temecula, CA, USA) or β1 integrin overnight at 4°C. Protein A/G-conjugated agarose (Pierce Biotechnology, Rockford, IL, USA) was added to each mixture, which was then incubated for 1–2 h at 4°C. Beads were washed three times with lysis buffer and boiled for 5 min in β-mercaptoethanol–containing loading buffer, and the supernatant was separated by SDS-PAGE.
GTPase Activity Assay
To confirm the presence of lamellipodia in GluR1-overexpressing cell lines, we performed a guanosine triphosphate (GTP) molecular affinity pulldown assay to detect the active form of Rac1 (Rac1-GTP). Cells were incubated for 24 h in calcium and glutamate/glutamine-free medium and stimulated with 50 μM AMPA for 10 and 20 min. Rac1 activity was assessed by affinity isolation of GTP-bound Rac1 using binding domains of p21-activated kinase (PAK) as previously described.30 Briefly, cell lysate was incubated with glutathione S-transferase– Rac1 binding domain of PAK immobilized on glutathione-Sepharose beads (Pierce). An aliquot of each sample was reserved to determine total Rac1 expression levels. Following 1 h incubation at 4°C, the agarose beads were collected by centrifugation, the supernatant was discarded, and the agarose beads were washed three times with wash buffer. Bound proteins were solubilized by boiling in SDS sample buffer, resolved by SDS-PAGE, and subjected to immunoblot analysis using anti-Rac1 antibodies. Rac1 activity was quantitated using densitometry (ImageJ software).
Flow Cytometric Analysis
For the flow cytometric analysis, cell suspensions (2.5 × 105 cells) were first treated with FcR blocking reagent (Miltenyi Biotec, Auburn, CA, USA) to block nonspecific antibody binding. To measure expression of β1 integrins on the cell surface, we incubated cells with mouse antihuman integrin β1 (Chemicon, Temecula, CA, USA) at 4°C for 1 h; cells were then incubated with fluorescein isothiocyanate (FITC)-conjugated antimouse antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) for 15 min at room temperature. FITC-conjugated mouse immunoglobulin G1 (IgG1; BD Biosciences, San Jose, CA, USA) was used as a negative control. Samples were analyzed using FACSCalibur flow cytometry (Becton Dickinson, San Jose, CA, USA).
Matrigel Transwell Migration Assay
Cell migration assays were performed on polycarbonate membrane inserts (8-μm pore size; Greiner Bio-One Inc., Longwood, FL, USA). Glioma cells were washed with PBS and serum-free medium twice prior to resuspension in fresh serum-free medium. We placed 2.5 × 105 cells in 250 μl serum-free medium over the inner chamber of Matrigel-coated inserts in a 24-well tissue culture plate and placed 500 μl serum-free medium in the outer chamber of the insert. Plates were incubated at 37°C for 24 h. The cells that had migrated through to the lower surface of the ECM layer were stained with 1% crystal violet and dissolved in 2% deoxycholic acid. Cells were quantified based on the absorbance measured at 595 nm. Each experiment was performed in triplicate.
Immunofluorescence Staining
U251 and U87 short hairpin GluR1 (shGluR1), GluR1-overexpressing, and control cells were plated on glass slides in six-well culture plates at a concentration of 2 × 105 cells per well for 24 h. Cells were fixed with a 3.7% formaldehyde solution in PBS and then permeabilized with 1.0% Triton X-100 in PBS. Primary antibodies were as follows: anti-GluR1 antibodies (clone C3T; Millipore, Billerica, MA, USA), anti-band 4.1B antibodies (courtesy of I. Newsham), and anti-β1 integrin antibodies (Chemicon). FITC-conjugated antimouse antibody (Santa Cruz Biotechnology) was used as a secondary antibody for 1 h at room temperature. To determine FA number, we visualized paxillin by incubating the cells first with mouse antipaxillin monoclonal antibody (BD Biosciences) and then with Texas red–conjugated goat antibody to mouse IgG (Invitrogen). Actin filaments were stained with phalloidin conjugated with Alexa Fluor 488 (Invitrogen). Nuclei were stained with 5.0 ng/ml Hoescht 33258 for 15 min at room temperature. Images were captured under a Zeiss Axioskop 40 microscope equipped with the appropriate filters and aided by AxioVision 4.4 software (Carl Zeiss, Maple Grove, MN, USA).
Cell Adhesion and Deadhesion Assay
For the cell adhesion assay, wells of a 96-well tissue culture plate were coated by exposure to 10 μg/ml types I and IV collagen (BD Biosciences), fibronectin (Sigma), or vitronectin (Sigma) for 2 h at room temperature and blocked with 0.5% bovine serum albumin (heat inactivated at 80°C for 10 min) in PBS for 30 min at room temperature. Cells were detached with trypsin/EDTA and rinsed in serum-free medium. Approximately 1 × 105 cells were plated into the wells, and the plate was incubated at 37°C for 1 h. Wells were gently washed with PBS, and attached cells were fixed in 4% paraformaldehyde and stained with 0.5% crystal violet (Sigma) for 10 min. Cells were solubilized in 2% deoxycholic acid, and the absorbance of each well was read at 570 nm in a microplate reader (Molecular Devices, Sunnyvale, CA, USA). For the blocking experiments, cells were incubated with or without anti-β1 integrin antibody (10 μg/ml, 6S6; Chemicon) for 10 min at 37°C, and cells were plated onto 96-well plates. For the deadhesion experiments, cells were stimulated with AMPA (Tocris Bioscience, Ellisville, MO, USA) at 50 and 100 mM for 10 min at 37°C prior to plating. Dioxobenzo[f]quinoxaline-7-sulfonamide (NBQX; Tocris Bioscience) was used to inhibit the AMPAR. Cyclothiazide, an inhibitor of AMPAR desensitization,31 was obtained from Tocris.
Intracranial Xenograft Animal Studies
U87 cells stably overexpressing GluR1 or empty vector control were grown and counted as described above. Viable cells (2 × 106) in 100 μl serum-containing medium were injected intracranially into 8-week-old female nude mice as previously described.32 Animals were observed daily for signs of tumor growth and were euthanized 35 days after inoculation. The brains were removed, fixed in formalin, and then embedded in paraffin for sectioning. To examine the invasion of neoplastic cells into tissue located along blood vessels, we incubated paraffin-embedded sections with anti-factor VIII antibodies (Dako, Carpinteria, CA, USA) overnight at 4°C, and then with mouse secondary antibodies (Santa Cruz Biotechnology) for 1 h at room temperature. All animal protocols and procedures were performed in accordance with local, state, and federal laws and ethics guidelines and were approved by the M. D. Anderson Cancer Center Institutional Animal Care and Use Committee.
Statistical Analyses
In vitro data were analyzed using a two-tailed Student’s t-test. Data were expressed as the mean ± SD. The differences between control and experimental cell lines were determined using a two-tailed Student’s t-test; p-values less than 0.05 were considered significant.
Results
AMPAR Expression Levels Correlate with In Vitro Transwell Migration
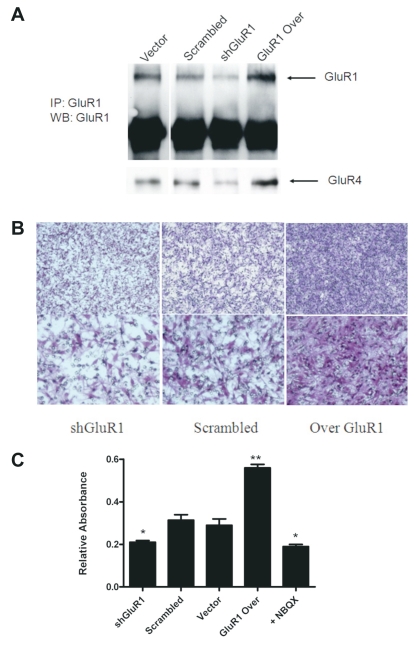
We29 and others17 have recently demonstrated abundant GluR1 and GluR4 AMPAR subunit expression on the leading edge of human glioblastoma specimens.17 To examine the impact of AMPAR expression on glioma invasion, we overexpressed and knocked down the GluR1 subunit of the AMPAR in U251 glioma cells. Coimmunoprecipitation was performed to pull down GluR1 using anti-GluR1 antibodies. The Western blot was stripped and probed for GluR4. Densitometry analysis of the Western blot (Fig. 1A) demonstrates a reduction of 60% and an increase of 80% in GluR1 levels compared to scrambled and vector controls in shGluR1 and GluR1-overexpressing U251 cells, respectively. Similar changes were obtained for levels of GluR4 as shown in Fig. 1A.
Fig. 1.
Glutamate receptor type 1 (GluR1) expression levels correlate with glioma cell invasion. (A) Knockdown of GluR1 via short hairpin RNA (shRNA) targeting human GluR1 and overexpression of GluR1 (Over GluR1) in U251 cells as demonstrated by Western blot (WB) using anti-GluR1 antibodies. Blots were stripped and probed for GluR4. IP, immunoprecipitation. (B) Representative membranes showing U251 scrambled control, shGluR1, and GluR1-overexpressing cells that invaded across Matrigel-coated transwells. (C) Absorbance at 570 nm was used to quantitate levels of invasion relative to control. α-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor inhibition with 50 μM dioxobenzo[f] quinoxaline-7-sulfonamide (NBQX) decreased invasion to a level similar to that of short hairpin GluR1 (shGluR1) cells (*, **p < 0.05, Student’s t-test).
In a Matrigel transwell migration assay, we showed a direct correlation between GluR1 and GluR4 expression and the degree of glioma migration through the transwell. Representative photomicrographs show a significant increase in invasion of U251 cells overexpressing GluR1 in the assay (Fig. 1B). Quantification of these results by dissolving transwell membranes in 2% deoxycholic acid and measuring the absorbance at 570 nm showed a statistically significant association between GluR1 expression level and the number of invading cells. GluR1 overexpression increased invasion by 46% in U251 cells, and shGluR1 induced a decrease in invasion by 35% compared to controls. Glioma cell migration was similarly inhibited by pretreating cells with the AMPAR inhibitor NBQX. Nonsilencing shRNA (scrambled) and pcDNA vector controls had similar degrees of invasion (data not shown). These results suggest that AMPAR expression plays a role in tumor invasion and led us to explore the potential mechanisms for this effect.
AMPAR Expression Alters Glioma Cell Morphology
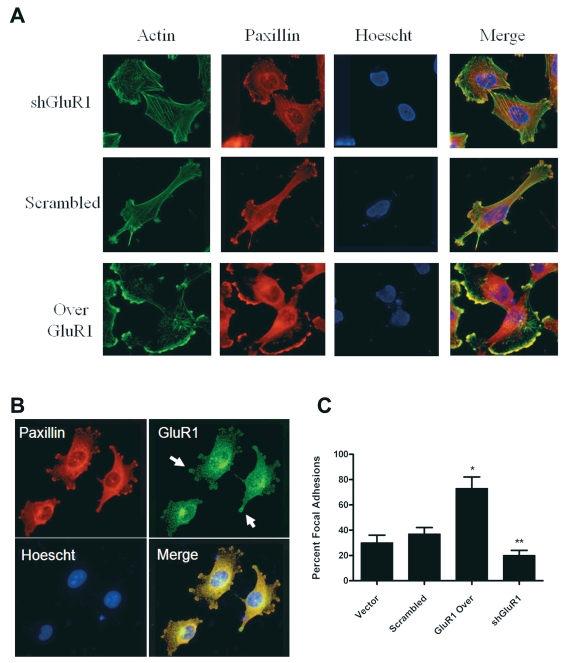
Because of the differences in cell migration, we first evaluated changes in cell morphology. We observed marked changes in the shape of glioma cells depending on the level of GluR1 expression. Staining of F-actin and paxillin showed a marked increase in actin polymerization along with membrane ruffling and the formation of structures similar to lamellipodia or FAs in cells overexpressing GluR1 (Fig. 2A). There were significantly more FA complexes along the edges of the cells overexpressing GluR1 than in control cells, and actin and paxillin colocalized along this edge. Overexpressing cells also had significantly more polarity compared to glioma cells with knockdown of GluR1, which appeared more rounded (Fig. 2A). Immunostaining for GluR1 and paxillin revealed that GluR1 was localized to these FAs (Fig. 2B), suggesting this receptor may be important in FA formation or for FA components interacting with other proteins. We counted the number of cells with observable FAs and confirmed that a significantly higher percentage of cells overexpressing GluR1 had FAs compared to vector and scrambled shRNA controls (Fig. 2C). Scrambled shRNA and vector controls were similar in appearance and had similar numbers of FAs (Fig. 2C). Based on these observations, we hypothesized that cells overexpressing GluR1 may have a greater affinity for binding to the ECM.
Fig. 2.
Increased actin–paxillin colocalization in glutamate receptor type 1 (GluR1) overexpressing cells. (A) U251 short hairpin GluR1 (shGluR1), scrambled control, and GluR1 overexpression (Over GluR1) clones were plated on glass slides and stained with antipaxillin antibodies, Alexa Fluor 488 phalloidin (actin), and Hoescht 33342 nuclear stains. The level of GluR1 expression correlated with the degree of actin and paxillin colocalization and focal adhesion (FA) formation. (B) U251 cells overexpressing GluR1 stained with anti-GluR1 and antipaxillin antibodies demonstrated that GluR1 localized to FAs (arrows), with evidence of paxillin colocalization. (C) The prevalence of FAs after staining was quantified by scoring the incidence of clustered paxillin staining. FA presence was diminished by GluR1 knockdown and increased in GluR1-overexpressing (Over GluR1) cells.
GluR1 Expression Enhances Glioma Adhesion to the ECM via β1 Integrin Surface Expression
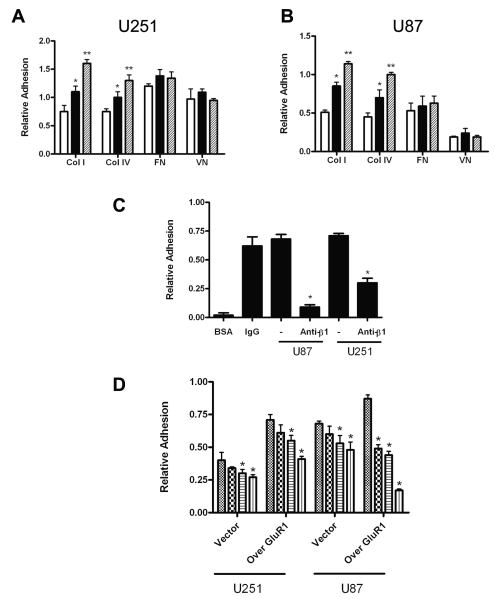
The effect of GluR1 expression on glioma cell adhesion to the ECM was first analyzed by measuring the binding of U251 and U87 cells to different components of the ECM. We showed that expression of GluR1 significantly correlated with binding of both U251 and U87 cells to type I and type IV collagen but not to vitronectin or fibronectin compared to controls. In U251 cells, shGluR1 cells adhered 45% less than did control cells to type I collagen, whereas GluR1-overexpressing cells were 45% more adhesive than were controls (Fig. 3A). In U87 cells, shGluR1 were 35% less adhesive to type I collagen than were controls, while GluR1-overexpressing cells were 30% more adhesive (Fig. 3B).
Fig. 3.
Glioma adhesion correlates with glutamate receptor type 1 (GluR1) expression. (A and B) Glioma cells were plated on 96-well plates precoated with type I collagen (Col I), type IV collagen (Col IV), fibronectin (FN), or vitronectin (VN). Adhesion to both type I and type IV collagen in U251 (A) and U87 (B) cells was significant in controls compared to short hairpin GluR1 (shGluR1; *) and in GluR1-overexpressing (**) cells compared to controls. White columns, shGluR1 cells; black columns, control cells; cross-hatched columns, GluR1-overexpressing cells. (C) Adhesion of U87 and U251 glioma cells overexpressing GluR1 was dependent on β1 integrin. BSA, bovine serum albumin (negative control); IgG, immunoglobulin G (positive control). (D) α-Amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor (AMPAR) stimulation reduced adhesion of glioma cells to collagen. Glioma cells were stimulated with serum-free media (control, hatched bars), 50 μM AMPA (checkered bars), 100 μM AMPA alone (horizontal-striped bars), or AMPAR desensitization inhibitor cyclothiazide (vertical-striped bars) after plating onto collagen. *, ** p < 0.05, Student’s t-test, for C and D. Results are expressed as mean ± SEM. Experiments were conducted in triplicate.
Because β1 integrins may be important for AMPAR function in normal neurons33 and are thought to be important in glioma,4 we hypothesized that β1 integrin may mediate GluR1-enhanced cell adhesion to the ECM. To determine the effect of β1 integrin on cell adhesion, we performed these experiments in the presence of β1 integrin–blocking antibodies. As shown in Fig. 3C, stable GluR1 overexpression increased adhesion of U87 and U251 cells to type I collagen and was effectively blocked with anti-β1 integrin antibodies, suggesting adhesion is at least partly mediated by this integrin. No changes in adhesion were seen using blocking antibodies to α5, αvβ3, or αvβ5 antibodies (data not shown).
Glutamate stimulation of the AMPAR has been shown to increase precursor cell migration in the developing nervous system.22 Because a cycle of cell attachment and detachment to underlying ECM via integrins is essential for cell migration,10 we examined the possibility that AMPAR activation enhances glioma cell invasion by modulating cell detachment from the ECM. We tested the effect of increasing AMPA concentrations on attachment of glioma cells to type I collagen. As shown in Fig. 3D, AMPAR stimulation reduced adhesion to collagen in U251 and U87 cell lines in a dose-dependent manner. Combined treatment with AMPA and the AMPAR desensitization inhibitor cyclothiazine further enhanced glioma detachment from the ECM. Cell detachment was associated with morphologic changes, such as retraction of FAs and directional cellular movement (data not shown). Thus, AMPAR overexpression enhances glioma adhesion to the ECM whereas receptor stimulation decreases cell attachment, linking these potentially important mechanisms underlying glioma invasion.
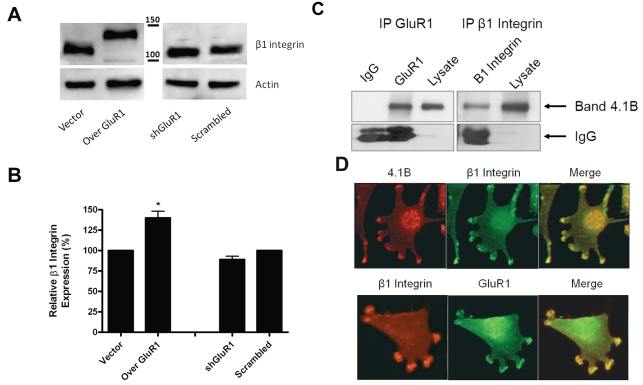
We next studied whether GluR1 levels altered the expression of β1 integrin by performing Western blotting and fluorescence-activated cell sorting analyses. Evaluation of β1 integrin expression levels in cells under- and overexpressing GluR1 revealed that, although total β1 integrin levels were unchanged (Fig. 4A), there was an increase in the apparent molecular weight of β1 integrin from the 105-kDa (incompletely glycosylated) form in the shGluR1 cells to the 125-kDa (p125; completely glycosylated) form in cells overexpressing GluR1. The 125-kDa β1 integrin is able to mediate cellular adhesion and induce signaling due to its surface localization.34–36 To confirm the increase in surface expression of β1 integrin in cells overexpressing GluR1, we performed flow cytometry following incubation with β1 integrin antibodies. Fig. 4B shows a significant increase in the level of surface β1 integrin in cells overexpressing GluR1 compared to vector controls. A nonsignificant decrease was observed in shGluR1 cells compared to scrambled controls. These results suggest that AMPAR expression leads to increased surface expression of β1 integrin.
Fig. 4.
Overexpression of glutamate receptor type 1 (GluR1) in glioma cell lines increases the level of surface β1 integrin. (A) Cell lysates from GluR1 glioma clones were subjected to Western blotting with anti-β1 integrin antibodies. Cells overexpressing GluR1 (Over GluR1) had higher-molecular-weight β1 integrin bands, corresponding to integrin glycosylation. shGluR1, short hairpin GluR1. (B) Expression of surface β1 integrin was determined by flow cytometric analysis. shGluR1, short hairpin GluR1. (C) GluR1, band 4.1 (4.1B), and β1 integrin associate in U251 glioma cells. Anti-GluR1 or anti-β1 integrin antibodies were used for immunoprecipitation (IP). Immunoprecipitated complexes were loaded onto gels, and Western blots were performed using 4.1B antibodies. Isotype immunoglobulin G (IgG) was used as negative control. (D) Band 4.1B and β1 integrin show colocalization at focal adhesions using immunofluorescence. Although β1 integrin and GluR1 do not show significant overlap, they both localize to focal adhesions in close proximity.
To examine the potential association between GluR1 and β1 integrin, we performed immunoprecipitation and immunofluorescence studies. In Fig. 4C, anti-GluR1 antibodies were able to pull down band 4.1B, as might be expected given the known association between band 4.1N and GluR1.37 Interestingly, we were able to show that β1 integrin was able to immunoprecipitate band 4.1B, which suggests that an indirect association may exist between GluR1 and β1 integrin. To further examine this potential association, we performed colocalization studies that demonstrated strong overlap between band 4.1B and β1 integrin. Although there was little overlap, staining showed a very close approximation of β1 integrin with GluR1 within FAs (Fig. 4D), suggesting that there may be an interaction between these two proteins possibly being mediated through band 4.1B.
GluR1 Mediates FAK Phosphorylation and Downstream Rac1 Activation
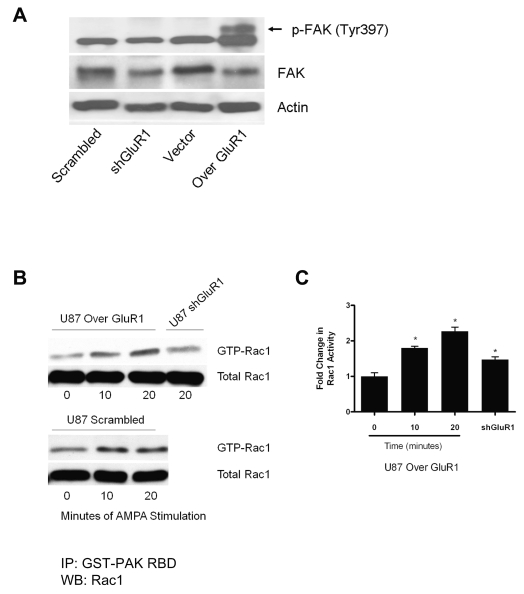
In addition to modulating adhesion, engagement of integrins promotes the formation of signaling complexes that regulate F-actin dynamics. FAK colocalizes with integrins at FAs, where integrin binding results in FAK activation.3 Using shGluR1, control, and GluR1-overexpressing cell lines, we examined autophosphorylation of FAK at tyrosine 397 using Western blot analysis. Cells overexpressing GluR1 had significantly higher levels of phosphorylated FAK (FAK-Y397) than did vector and scrambled control and shGluR1 cells, as demonstrated by the upward shift of the band on the Western blot (Fig. 5A). Stimulation of the AMPAR did not further alter FAK phosphorylation levels.
Fig. 5.
Overexpression of glutamate receptor type 1 (GluR1) enhances focal adhesion kinase (FAK) activation and Rac1 signaling. (A) Overexpression of GluR1 led to increases in FAK phosphorylation (p-FAK) at tyrosine 397 (Tyr397; arrow) in U87 glioma cells overexpressing GluR1 (Over GluR1). (B) Rac1-GTPase activity was evaluated using a glutathione S-transferase–p21-activated kinase (GST-PAK) pulldown assay followed by immunoblotting with anti-Rac1 antibodies. Cells were starved and then stimulated with 50 μM α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) for the designated time. Results showed a time-dependent increase in Rac1 activation following AMPA receptor stimulation in cells overexpressing GluR1 and short hairpin RNA scrambled controls, which was blocked in U87 short hairpin GluR1 (shGluR1) cells. GTP, guanosine triphosphate; IP, immunoprecipitation; RBD, Rac1 binding domain; WB, Western blot. (C) Densitometry revealed the fold changes in Rac1 activity to be significant (*p < 0.05, Student’s t-test).
Integrins regulate the activity of GTPases, such as Rac1, which in turn can regulate cell adhesion, changes in cell morphology, and cell motility by regulating actin cytoskeletal dynamics. We assayed for the activity of Rac1 in cells with high and low GluR1 expression. Overexpression of GluR1 did not change baseline Rac1 levels (data not shown). However, after AMPAR stimulation, Rac1 activity showed a time-dependent increase, which reached a maximum at 20 min after stimulation (Fig. 5B) in both GluR1-overexpressing cells and scrambled controls. Densitometry confirmed a significant increase in Rac1 activity following stimulation with AMPA, which was blocked in cells with knockdown of GluR1 (Fig. 5C), suggesting that AMPA-stimulated increases in Rac1 activity were mediated via the AMPAR.
Overexpression of GluR1 Enhances Glioma Invasion In Vivo
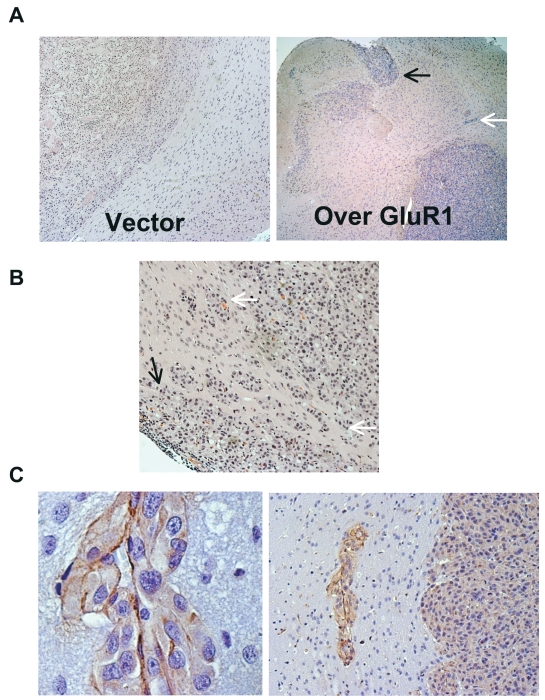
To more adequately evaluate the phenotypic effect of GluR1 overexpression on glioma invasion, we performed an intracranial glioma xenograft experiment. Normally, U87 cells inoculated into the brain of nude mice grow as a noninvasive solid tumor mass (ball-like),32 without any invasion of tumor into the normal brain (Fig. 6A). We implanted U87 cells stably overexpressing GluR1 into the brains of nude mice and followed the mice over time. After approximately 4 weeks, the animals were euthanized and the brains removed for evaluation. Fig. 6A shows brain tissues from control (left panel) and GluR1-overexpressing cells (right panel) following injection into mice, demonstrating dissemination of tumor into the normal brain in the GluR1-overexpressing animals. The tumors’ invasive phenotype mimicked that seen in human glioblastoma tumor specimens. Invasion of normal brain occurred by means of subpial extension along the brain surface (black arrows) and along perivascular spaces similar to that observed when blood vessels are coopted (white arrows), demonstrated by “cuffing” of tumor cells around vessels. A photomicrograph from a different animal implanted with cells overexpressing GluR1 shows subpial invasion (Fig. 6B, black arrow) and extensive perivascular invasion (Fig. 6B, white arrows). Injection of glioma cells with knockdown on GluR1 (shGluR1) did not reliably establish intracranial tumors, consistent with the decrease in cell proliferation and decrease in tumorigenicity established previously (data not shown).29 Immunohistochemical staining of tumor specimens with factor VIII for endothelial basement membrane shows tumor cells migrating along endothelial cells (Fig. 6C). These results confirm the in vitro experiments suggesting that GluR1 expression enhances glioma cell adhesion to ECM components such as collagen, which are prominent components of the endothelial basement membrane25 and the meningeal surfaces,26,27 and support the notion that this interaction leads to enhanced tumor invasion in vivo.
Fig. 6.
Overexpression of glutamate receptor (GluR1) increases glioma invasion in a mouse xenograft model. U87 glioma cells stably overexpressing GluR1 were implanted into the brain of nude mice and allowed to grow for 4 weeks. Tumor was removed for immunohistochemistry analysis. (A) Left: U87 typically grows as a solid tumor mass with no brain invasion. Right: U87 cells overexpressing GluR1 invade normal brain by spreading along the subpial surface (black arrow) and along perivascular spaces (white arrow) away from the main tumor mass. (B) Photomicrograph from a different mouse showing a close-up image of the subpial surface (black arrow) and perivascular spaces (white arrows). (C) Factor VIII staining shows tumor cells adjacent to endothelial cells within the brain parenchyma.
Discussion
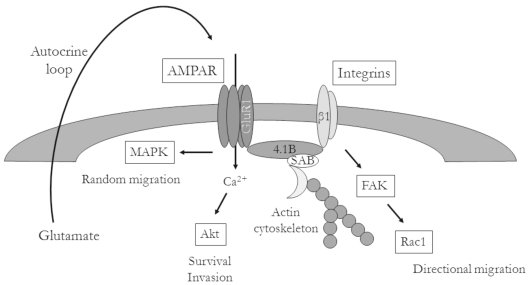
On the basis of our findings, we conclude that glutamate and the highly expressed AMPAR may activate glioma migration through interactions with the ECM. Glutamate has been shown to enhance the migration of progenitor cells during CNS development,21,22 and glutamate-mediated autocrine stimulation of cellular migration was recently shown to be important for glioma invasion via induction of intracellular calcium oscillations.18 Here, we showed that increasing the expression of GluR1, the most abundant subunit of the AMPAR, resulted in enhanced glioma adhesion to ECM components and increased tumor cell migration in vitro. The enhanced adhesion was mediated via increased surface expression of β1 integrin and subsequent activation of FAK, whereas detachment may be mediated through Rac1. Finally, we showed that these effects led to increased invasion in vivo, where overexpression of the AMPAR led to perivascular and subpial tumor invasion, similar to the invasive patterns seen in human glioblastoma specimens as illustrated in 1938 by Scherer,1,38 who described multiple patterns of invasion of glioblastoma into brain parenchyma. These data provide further support for the mechanism by which glutamate promotes glioma invasion (Fig. 7).
Fig. 7.
Hypothetical model of α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor (AMPAR) modulation of glioma invasion. AMPAR expression on the leading edge of cells is stimulated in an autocrine fashion, leading to increases in intracellular calcium. AMPAR-mediated enhancement of integrin surface expression results in activation of downstream signaling events, such as focal adhesion kinase (FAK) autophosphorylation and Rac activation, leading to increased cell motility. Abbreviations: MAPK, mitogen-activated protein kinase; GluR1, glutamate receptor type 1; 4.1B, protein 4.1B; SAB, spectrin-actin–binding domain.
The impact of GluR1 overexpression on levels of surface β1 integrin provides important mechanistic data for how glutamate may stimulate tumor migration and invasion. Integrins are critical for cell migration and invasion because they mediate cell–ECM interactions and regulate signaling pathways that control cytoskeletal organization.3 Gliomas are known to express multiple integrins, including the collagen receptor (α2β1), laminin receptor (α3β1), fibronectin receptor (α5β1), and vitronectin receptor (αvβ3),39,40 which mediate their binding to ECM proteins. Although other integrins may be involved in GluR1-enhanced adhesion to the ECM, our results support the central role of β1 integrin in this process. β1 integrin is known to be an important receptor in glioma4 and is critical for mediating cell adhesion and regulation of adhesion-mediated signaling to Rac1.41 In our experiments, we showed that increased β1 integrin surface expression leads to downstream activation of Rac1, which then induces the formation of lamellipodia, which are required for cell spreading.42 Furthermore, Rac1 has been shown to mediate collagen-dependent matrix metalloproteinase activation,43 an important contributor to tumor invasion in vivo. In our experiments, Rac1 activation confirmed the presence of lamellipodia in GluR1-overexpressing cells as demonstrated on immunofluorescence imaging. Interestingly, an association between β1 integrin and GluR1 has been found to be important for normal neuronal function,33 and glutamate can increase integrin surface expression levels in neurons,44 enhancing learning and memory. In a similar way, glioma cells exploit glutamate contained in the brain and AMPAR expression to enhance binding to the ECM and promote their invasive phenotype.
Ion channels can anchor the cytoskeleton via binding to members of the band 4.1 proteins (4.1/ezrin/radixin/moesin [FERM] protein family). This anchoring provides structural stability, flexibility, and cellular polarity.45 FERM proteins bind F-actin to affect actin-cytoskeletal turnover and, hence, the maintenance of cell shape and motility.46 Band 4.1B is an important membrane cytoskeleton component that is concentrated at the axonal paranode and juxtaparanode regions in neurons, where it restricts adhesion molecules and ion transport proteins to specialized domains of the plasma membrane.47 AMPARs were previously known to bind to the cytoskeletal-linker protein band 4.1N (neuron-type),37 and we showed an association between GluR1 and band 4.1B, which is able to bind to both spectrin and actin (whereas 4.1N is not).48 Our current finding of the association between AMPAR expression and cellular polarity in the context of the observed increase in migration extends our understanding of the potential role of AMPARs in tumor invasion.
One mechanism that may account for the observed increase in cellular movement may be related to the accumulation of AMPARs at FAs and a potential indirect interaction with integrins. AMPAR expression was highly concentrated in regions of FA formation. We showed that GluR1 associates with β1 integrin, possibly through an interaction with band 4.1B via the highly conserved region of the integrin C-terminal domain.49 Taken together, these data suggest that AMPARs can act as membrane-associated cytoskeleton anchors and thus may provide anchors for localized signaling complexes at FAs.
Several mechanisms may be involved in AMPAR modulation of glioma migration, with the most obvious being an increase in cell adhesion to the ECM following AMPAR overexpression. However, we also showed that AMPAR agonist treatment leads to cellular detachment. This detachment may play a role in glioma cell migration. Calcium signaling is important for cell migration, and AMPARs on glioma are known to be calcium permeable. Giannone et al.19 showed that cell movement and FA disassembly are temporally associated with calcium spikes in U87 glioma cells. Furthermore, AMPARs were recently found to mediate calcium oscillations in glioma cells, which affected tumor cell migration.18 Our results build upon these findings, as we have demonstrated the impact of AMPAR expression and stimulation on signaling: AMPARs localized to FAs may amplify local signals via integrin engagement and calcium influx through the AMPAR, leading to cell motility.
In addition to mediating adhesion, integrin engagement promotes the formation of signaling complexes that regulate F-actin accumulation. FAK colocalizes with integrins at FAs, where integrin binding results in FAK activation,3 a process that has been implicated in glioma invasion.9 We observed a baseline increase in FAK activation in cells overexpressing GluR1 compared to control and shGluR1 cells. This finding suggests that overexpression of GluR1 can activate FAK independent of whether the AMPAR is activated. The mechanism through which GluR1 expression can increase FAK activation is unknown, but it may be related to FERM protein or integrin engagement. FERM domain proteins directly interact with the central region of FAK to inhibit its kinase activity. With FA formation, FERM proteins shift their association to higher affinity binding with the cytoplasmic tails of integrins or other integrin-associated proteins, resulting in the release of autoinhibition and subsequent FAK autophosphorylation at Tyr397.50–52 This baseline increase in FAK activation may lead to augmentation and targeting of Rac1.53,54
Several patterns of glioma cell invasion are not explained by the overexpression of GluR1. For example, tumor cell invasion into neuropil and invasion of tumor cells along white matter tracts were not demonstrated in our in vivo model, which has little or no brain invasion at baseline. This may be due to the limitations of a noninvasive xenograft model, but it may also reflect different mechanisms utilized by tumor cells to invade into normal brain. GluR1 overexpression resulted in significantly more tumor invasion and demonstrated several of the classic secondary structures described by Scherer,1,38 including perivascular growth around preexisting vessels and subpial spread. In human glioblastoma, it is likely that many mechanisms of tumor invasion occur simultaneously to promote the profound tumor invasion seen in this disease (reviewed in Hoelzinger et al.55).
In summary, glioma cells express AMPARs that clearly affect tumor cell migration and invasion. Collectively, our results suggest that glutamate could act as an autocrine or paracrine chemoattractant, stimulating glioma cell migration along perivascular and subpial spaces. Our data support the hypothesis that AMPARs play a role in regulating glioma migration and invasion. A better understanding of the mechanisms driving glioma invasion are critical. For example, new anti-vascular endothelial growth factor therapies have recently been shown to increase perivascular tumor spread in glioblastoma.56,57 Drugs that target the AMPAR may be useful in combination with antiangiogenic agents to block this mechanism of tumor invasion.
Acknowledgment
This work was supported by an institutional research grant from the University of Texas M. D. Anderson Cancer Center.
References
- 1.Scherer HD. Cerebral astrocytomas and their derivatives. Am J Cancer. 1940;1:159–198. [Google Scholar]
- 2.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci USA. 2002;99(17):11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2(2):91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 4.Bellail AC, Hunter SB, Brat DJ, Tan C, Van Meir EG. Microregional extracellular matrix heterogeneity in brain modulates glioma cell invasion. Int J Biochem Cell Biol. 2004;36(6):1046–1069. doi: 10.1016/j.biocel.2004.01.013. [DOI] [PubMed] [Google Scholar]
- 5.Gladson CL. The extracellular matrix of gliomas: modulation of cell function. J Neuropathol Exp Neurol. 1999;58(10):1029–1040. doi: 10.1097/00005072-199910000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Riemenschneider MJ, Mueller W, Betensky RA, Mohapatra G, Louis DN. In situ analysis of integrin and growth factor receptor signaling pathways in human glioblastomas suggests overlapping relationships with focal adhesion kinase activation. Am J Pathol. 2005;167(5):1379–1387. doi: 10.1016/S0002-9440(10)61225-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deryugina EI, Bourdon MA. Tenascin mediates human glioma cell migration and modulates cell migration on fibronectin. J Cell Sci. 1996;109(pt 3):643–652. doi: 10.1242/jcs.109.3.643. [DOI] [PubMed] [Google Scholar]
- 8.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 9.Jones G, Machado J, Jr, Tolnay M, Merlo A. PTEN-independent induction of caspase-mediated cell death and reduced invasion by the focal adhesion targeting domain (FAT) in human astrocytic brain tumors which highly express focal adhesion kinase (FAK) Cancer Res. 2001;61(15):5688–5691. [PubMed] [Google Scholar]
- 10.Kassis J, Lauffenburger DA, Turner T, Wells A. Tumor invasion as dysregulated cell motility. Semin Cancer Biol. 2001;11(2):105–117. doi: 10.1006/scbi.2000.0362. [DOI] [PubMed] [Google Scholar]
- 11.Brown E, Hogg N. Where the outside meets the inside: integrins as activators and targets of signal transduction cascades. Immunol Lett. 1996;54(2–3):189–193. doi: 10.1016/s0165-2478(96)02671-5. [DOI] [PubMed] [Google Scholar]
- 12.Ye ZC, Sontheimer H. Glioma cells release excitotoxic concentrations of glutamate. Cancer Res. 1999;59(17):4383–4391. [PubMed] [Google Scholar]
- 13.Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci. 1999;19(24):10767–10777. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rzeski W, Turski L, Ikonomidou C. Glutamate antagonists limit tumor growth. Proc Natl Acad Sci USA. 2001;98(11):6372–6377. doi: 10.1073/pnas.091113598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoshida Y, et al. Serum-dependence of AMPA receptor-mediated proliferation in glioma cells. Pathol Int. 2006;56(5):262–271. doi: 10.1111/j.1440-1827.2006.01954.x. [DOI] [PubMed] [Google Scholar]
- 16.Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M. Glutamate release promotes growth of malignant gliomas. Nat Med. 2001;7(9):1010–1015. doi: 10.1038/nm0901-1010. [DOI] [PubMed] [Google Scholar]
- 17.Ishiuchi S, Tsuzuki K, Yoshida Y, et al. Blockage of Ca(2+)-permeable AMPA receptors suppresses migration and induces apoptosis in human glioblastoma cells. Nat Med. 2002;8(9):971–978. doi: 10.1038/nm746. [DOI] [PubMed] [Google Scholar]
- 18.Lyons SA, Chung WJ, Weaver AK, Ogunrinu T, Sontheimer H. Autocrine glutamate signaling promotes glioma cell invasion. Cancer Res. 2007;67(19):9463–9471. doi: 10.1158/0008-5472.CAN-07-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giannone G, Rondé P, Gaire M, et al. Calcium rises locally trigger focal adhesion disassembly and enhance residency of focal adhesion kinase at focal adhesions. J Biol Chem. 2004;279(27):28715–28723. doi: 10.1074/jbc.M404054200. [DOI] [PubMed] [Google Scholar]
- 20.Farin A, Suzuki SO, Weiker M, Goldman JE, Bruce JN, Canoll P. Transplanted glioma cells migrate and proliferate on host brain vasculature: a dynamic analysis. Glia. 2006;53(8):799–808. doi: 10.1002/glia.20334. [DOI] [PubMed] [Google Scholar]
- 21.Komuro H, Rakic P. Modulation of neuronal migration by NMDA receptors. Science. 1993;260(5104):95–97. doi: 10.1126/science.8096653. [DOI] [PubMed] [Google Scholar]
- 22.Gudz TI, Komuro H, Macklin WB. Glutamate stimulates oligodendrocyte progenitor migration mediated via an alphav integrin/myelin proteolipid protein complex. J Neurosci. 2006;26(9):2458–2466. doi: 10.1523/JNEUROSCI.4054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schutt BS, Langkamp M, Rauschnabel U, Ranke MB, E lmlinger MW. Integrin-mediated action of insulin-like growth factor binding protein-2 in tumor cells. J Mol Endocrinol. 2004;32(3):859–868. doi: 10.1677/jme.0.0320859. [DOI] [PubMed] [Google Scholar]
- 24.Podar K, Tai YT, Lin BK, et al. Vascular endothelial growth factor-induced migration of multiple myeloma cells is associated with beta 1 integrin- and phosphatidylinositol 3-kinase-dependent PKC alpha activation. J Biol Chem. 2002;277(10):7875–7881. doi: 10.1074/jbc.M109068200. [DOI] [PubMed] [Google Scholar]
- 25.Kalluri R. Basement membranes: structure, assembly and role in tumour angiogenesis. Nat Rev Cancer. 2003;3(6):422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 26.Giese A, Laube B, Zapf S, Mangold U, Westphal M. Glioma cell adhesion and migration on human brain sections. Anticancer Res. 1998;18(4A):2435–2447. [PubMed] [Google Scholar]
- 27.Brandsma D, Ulfman L, Reijneveld JC, et al. Constitutive integrin activation on tumor cells contributes to progression of leptomeningeal metastases. Neuro-Oncology. 2006;8(2):127–136. doi: 10.1215/15228517-2005-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paddison PJ, Cleary M, Silva JM, et al. Cloning of short hairpin RNAs for gene knockdown in mammalian cells. Nat Methods. 2004;1(2):163–167. doi: 10.1038/nmeth1104-163. [DOI] [PubMed] [Google Scholar]
- 29.de Groot JF, Piao Y, Lu L, Fuller GN, Yung WK. Knockdown of GluR1 expression by RNA interference inhibits glioma proliferation. J Neurooncol. 2008;88(2):121–133. doi: 10.1007/s11060-008-9552-2. [DOI] [PubMed] [Google Scholar]
- 30.Anastasiadis PZ, Moon SY, Thoreson MA, et al. Inhibition of RhoA by p120 catenin. Nat Cell Biol. 2000;2(9):637–644. doi: 10.1038/35023588. [DOI] [PubMed] [Google Scholar]
- 31.Yamada KA, Tang CM. Benzothiadiazides inhibit rapid glutamate receptor desensitization and enhance glutamatergic synaptic currents. J Neurosci. 1993;13(9):3904–3915. doi: 10.1523/JNEUROSCI.13-09-03904.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lal S, Lacroix M, Tofilon P, Fuller GN, Sawaya R, Lang FF. An implantable guide-screw system for brain tumor studies in small animals. J Neurosurg. 2000;92(2):326–333. doi: 10.3171/jns.2000.92.2.0326. [DOI] [PubMed] [Google Scholar]
- 33.Chan CS, Weeber EJ, Zong L, Fuchs E, Sweatt JD, Davis RL. Beta 1-integrins are required for hippocampal AMPA receptor-dependent synaptic transmission, synaptic plasticity, and working memory. J Neurosci. 2006;26(1):223–232. doi: 10.1523/JNEUROSCI.4110-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akiyama SK, Yamada KM. Biosynthesis and acquisition of biological activity of the fibronectin receptor. J Biol Chem. 1987;262(36):17536–17542. [PubMed] [Google Scholar]
- 35.De Strooper B, Van Leuven F, Carmeliet G, Van Den Berghe H, Cassiman JJ. Cultured human fibroblasts contain a large pool of precursor beta 1-integrin but lack an intracellular pool of mature subunit. Eur J Biochem. 1991;199(1):25–33. doi: 10.1111/j.1432-1033.1991.tb16087.x. [DOI] [PubMed] [Google Scholar]
- 36.Chen X, Jiang J, Yang J, et al. Down-regulation of the expression of beta1,4-galactosyltransferase V promotes integrin beta1 maturation. Biochem Biophys Res Commun. 2006;343(3):910–916. doi: 10.1016/j.bbrc.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 37.Shen L, Liang F, Walensky LD, Huganir RL. Regulation of AMPA receptor GluR1 subunit surface expression by a 4.1N-linked actin cytoskeletal association. J Neurosci. 2000;20(21):7932–7940. doi: 10.1523/JNEUROSCI.20-21-07932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scherer HD. Structural development in gliomas. Am J Cancer. 1938;34:333–351. [Google Scholar]
- 39.Newton HB. Molecular neuro-oncology and the development of targeted therapeutic strategies for brain tumors. Part 3: brain tumor invasiveness. Expert Rev Anticancer Ther. 2004;4(5):803–821. doi: 10.1586/14737140.4.5.803. [DOI] [PubMed] [Google Scholar]
- 40.Uhm JH, Gladson CL, Rao JS. The role of integrins in the malignant phenotype of gliomas. Front Biosci. 1999;4:D188–D199. doi: 10.2741/uhm. [DOI] [PubMed] [Google Scholar]
- 41.Berrier AL, Martinez R, Bokoch GM, LaFlamme SE. The integrin beta tail is required and sufficient to regulate adhesion signaling to Rac1. J Cell Sci. 2002;115(pt 22):4285–4291. doi: 10.1242/jcs.00109. [DOI] [PubMed] [Google Scholar]
- 42.Kjoller L, Hall A. Signaling to rho GTPases. Exp Cell Res. 1999;253(1):166–179. doi: 10.1006/excr.1999.4674. [DOI] [PubMed] [Google Scholar]
- 43.Zhuge Y, Xu J. Rac1 mediates type I collagen-dependent MMP-2 activation: role in cell invasion across collagen barrier. J Biol Chem. 2001;276(19):16248–16256. doi: 10.1074/jbc.m010190200. [DOI] [PubMed] [Google Scholar]
- 44.Lin CY, Lynch G, Gall CM. AMPA receptor stimulation increases alpha-5beta1 integrin surface expression, adhesive function and signaling. J Neurochem. 2005;94(2):531–546. doi: 10.1111/j.l471-4159.2005.03203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Denker SP, Barber DL. Ion transport proteins anchor and regulate the cytoskeleton. Curr Opin Cell Biol. 2002;14(2):214–220. doi: 10.1016/s0955-0674(02)00304-6. [DOI] [PubMed] [Google Scholar]
- 46.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3(8):586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 47.Ohara R, Yamakawa H, Nakayama M, Ohara O. Type II brain 4.1 (4.1B/KIAA0987), a member of the protein 4.1 family, is localized to neuronal paranodes. Brain Res Mol Brain Res. 2000;85(1–2):41–52. doi: 10.1016/s0169-328x(00)00233-3. [DOI] [PubMed] [Google Scholar]
- 48.Gimm JA, An X, Nunomura W, Mohandas N. Functional characterization of spectrin-actin-binding domains in 4.1 family of proteins. Biochemistry. 2002;41(23):7275–7282. doi: 10.1021/bi0256330. [DOI] [PubMed] [Google Scholar]
- 49.McCarty JH, Cook AA, Hynes RO. An interaction between {alpha} v{beta}8 integrin and band 4.1B via a highly conserved region of the band 4.1 C-terminal domain. Proc Natl Acad Sci USA. 2005;102(38):13479–13483. doi: 10.1073/pnas.0506068102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cooper LA, Shen TL, Guan JL. Regulation of focal adhesion kinase by its amino-terminal domain through an autoinhibitory interaction. Mol Cell Biol. 2003;23(22):8030–8041. doi: 10.1128/MCB.23.22.8030-8041.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunty JM, Gabarra-Niecko V, King ML, Ceccarelli DF, Eck MJ, Schaller MD. FERM domain interaction promotes FAK signaling. Mol Cell Biol. 2004;24(12):5353–5368. doi: 10.1128/MCB.24.12.5353-5368.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen LA, Guan JL. Residues within the first subdomain of the FERM-like domain in focal adhesion kinase are important in its regulation. J Biol Chem. 2005;280(9):8197–8207. doi: 10.1074/jbc.M412021200. [DOI] [PubMed] [Google Scholar]
- 53.Siesser PM, Hanks SK. The signaling and biological implications of FAK overexpression in cancer. Clin Cancer Res. 2006;12(11 pt 1):3233–3237. doi: 10.1158/1078-0432.CCR-06-0456. [DOI] [PubMed] [Google Scholar]
- 54.Chang F, Lemmon CA, Park D, Romer LH. FAK potentiates Rac1 activation and localization to matrix adhesion sites: a role for betaPIX. Mol Biol Cell. 2007;18(1):253–264. doi: 10.1091/mbc.E06-03-0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoelzinger DB, Demuth T, Berens ME. Autocrine factors that sustain glioma invasion and paracrine biology in the brain microenvironment. J Natl Cancer Inst. 2007;99(21):1583–1593. doi: 10.1093/jnci/djm187. [DOI] [PubMed] [Google Scholar]
- 56.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]