Abstract
Glutathione S-transferases (GSTs) are polymorphic enzymes that catalyze the glutathione conjugation of alkylating agents, platinum compounds, and free radicals formed by radiation used to treat medulloblastoma. We hypothesized that GST polymorphisms may be responsible, in part, for individual differences in toxicity and responses in pediatric medulloblastoma. We investigated the relationship between GSTM1 and GSTT1 polymorphisms and survival and toxicity in 42 children with medulloblastoma diagnosed and treated at the Texas Children’s Cancer Center. We conducted Kaplan-Meier analyses to determine if the GST polymorphisms were related to progression-free survival (PFS) and performed logistic regression to explore associations between GST polymorphisms and occurrence of grade 3 or greater (≥Gr 3) myelosuppression, ototoxicity, nephrotoxicity, neurotoxicity, and intellectual impairment. Patients with at least one null genotype had a 4.3 (95% confidence interval, 1.1–16.8), 3.7 (1–13.6), and 6.4 (1.2–34) times increased risk for any ≥Gr 3 toxicity, any ≥Gr 3 toxicity excluding peripheral neuropathy, and any ≥Gr 3 toxicity requiring omission or cessation of chemotherapy, respectively. Compared with all others, patients with at least one null genotype had, on average, 27.2 (p = 0.0002), 29 (p = 0.0004), and 21.7 (p = 0.002) lower full-scale, performance, and verbal intelligence quotient (IQ) scores, respectively. GSTM1 and GSTT1 polymorphisms may predict adverse events, including cognitive impairment after therapy, in patients with medulloblastoma. A larger study to validate these findings is under way.
Keywords: glutathione S-transferase polymorphisms, intellectual impairment, medulloblastoma, pharmacogenetics, toxicity
Medulloblastoma is the most common CNS malignancy in childhood and adolescence, accounting for approximately 20% of all primary pediatric brain tumors.1 Surgical resection followed by craniospinal radiation and chemotherapy is an effective standard of care.2–6 Overall, 60%–80% of these patients achieve long-term cure; however, many suffer from varying degrees of significant morbidity secondary to therapy, including intellectual impairment, hearing loss, and renal failure.5–8 However, patients who will benefit from therapy and those who will experience significant side effects cannot be distinguished in advance, giving no opportunity for tailoring treatment to maximize outcome.
Glutathione S-transferases (GSTs) belong to a family of isoenzymes that catalyze the glutathione conjugation of a variety of electrophilic compounds, including carcinogens, mutagens, cytotoxic drugs and their metabolites, and products of reactive oxidation.9 They catalyze detoxification of alkylating agents and platinum compounds that are used in medulloblastoma chemotherapy.10–13 Furthermore, GSTs also detoxify free oxygen radicals formed spontaneously or by chemotherapy drugs and radiation and can sequester alkylating agents and steroids by direct binding.9 They are highly heterogeneous proteins expressed in virtually all tissues, including the brain.14 Polymorphism in GSTM1 was reported by Board,15 who described three alleles: GSTM1*0, GSTM1*A, and GSTM1*B. In the common GSTM1*0 allele, GSTM1 is deleted, and homozygotes (null genotype), comprising 42%–60% of the white population, do not express GSTM1 protein.16,17 GSTT1 is polymorphic, and 13%–26% of the white population has a homozygous deletion and thus lacks function.16,18 Polymorphisms in the GST family of enzymes have been associated with survival and occurrence of toxicity in children and adults who have leukemia, lymphoma, or glioma; breast, lung, ovarian, gastric, or colorectal cancers; or germ cell tumors.19–38
In this pilot study, we examined the relationship between polymorphisms in the GST genes and clinical outcomes in 42 patients with medulloblastoma. We hypothesized that patients who have GST genotypes that encode for high-activity enzymes would have poorer survival and decreased incidence of adverse effects compared with patients with genetically determined, low or nonfunctioning detoxification activity. We measured the association between GSTM1 and GSTT1 polymorphisms and the following clinical outcomes: progression-free survival (PFS), development of grade 3 or greater (≥Gr 3) toxicity requiring dose modification, and intellectual impairment. We observed a significant relation between combined GSTM1T1 polymorphisms and development of any ≥Gr 3 toxicity requiring dose modification and intellectual decline. In the future, after further testing in a larger population, findings from this study and others are expected to form the foundation to develop tailored individualized treatment regimens and prevention strategies for adverse effects.
Materials and Methods
Patient Population and Data Collection
We identified 42 patients who were consecutively diagnosed and treated at the Texas Children’s Cancer Center between 1996 and 2005 who were younger than 19 years at diagnosis, who had an available DNA sample isolated from peripheral blood mononuclear cells, and who consented to participate in the study, which had been approved by the Institutional Human Subjects Review Committee. All patients who were older than 3 years at diagnosis were treated with craniospinal radiation followed by systemic chemotherapy. Patients who were younger than 3 years were given systemic chemotherapy first, followed by craniospinal radiation when they reached 3 years of age or experienced relapse. Most patients received four cycles of high-dose chemotherapy followed by stem cell rescue (SJMB96 protocol, n = 24) or eight cycles of cisplatin, cyclophosphamide, and vincristine (A9961 regimen B protocol, n = 9). The details of each protocol have been previously published.5,6 The remaining nine patients were treated with PBTC-001 (n = 2), POG9031 (n = 3), or POG9233 (n = 4).
We reviewed the medical records of participating patients and abstracted the following information: demographic characteristics at diagnosis; risk group (high risk, >1.5 cm2 residual disease on MRI scan 24–72 h after surgery and/or presence of disseminated disease; average risk, ≤1.5 cm2 residual disease on MRI scan 24–72 h after surgery and absence of dissemination by MRI or by cerebrospinal fluid cytology), treatment characteristics, date of progression, and date of death or date of last follow-up. In 41 of 42 patients, data were available for occurrence of ≥Gr 3 hematopoietic, audiological, neurological (peripheral neuropathy), and renal toxicity requiring dose modification. In 34 patients, consecutive hearing assessment results were available with a baseline completed prior to initiation of chemotherapy with cisplatin. Full-scale, verbal, and performance intelligence quotient (IQ) scores measured on the Wechsler Intelligence Scale for Children (WISC-III)39 were also available in 21 patients ≥3 years of age, with at least two assessments including a baseline assessment prior to radiotherapy present in 10. (Only one assessment in 11, two assessments in 4, three assessments in 3, four assessments in 2, and five assessments in 1 patient.) Age requirement of ≥3 years was used because WISC-III is not an appropriate test to use in younger patients.
Multiplex GSTM1 and GSTT1 Genotyping
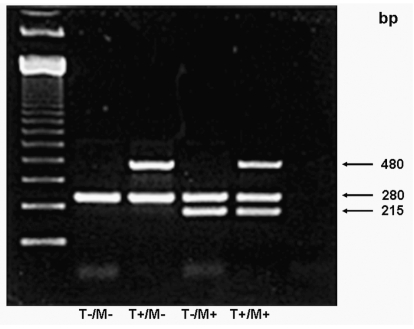
We used a multiplex polymerase chain reaction (PCR) technique to amplify both GSTM1 and GSTT1 simultaneously in a single PCR reaction.17 Briefly, we amplified isolated DNA using GSTM1 primers 5′-GAA CTC CCT GAA AAG CTA AAG C-3′ and 5′-GTT GGG CTC AAA TAT ACG GTG G-3′ and GSTT1 primers corresponding to the 3′ coding region of human cDNA: 5′-TTC CTT ACT GGT CCT CAC ATC TC-3′ and 5′-TCA CCG GAT CAT GGC CAG CA-3′. As an internal control, we coamplified the dihydrofolate reductase gene (DHFR) using the primers 5′-GCA TGT CTT TGG GAT GTG GA-3′ and 5′-GGA ATG GAG AAC CAG GTC TT-3′. The PCR conditions consisted of an initial melting temperature of 95°C (5 min) followed by 35 cycles of melting (95°C, 30 sec), annealing (58°C, 45 sec), and extension (72°C, 1 min). We then viewed the PCR products from coamplification of GSTT1 (480 bp), DHFR (280 bp), and GSTM1 (215 bp) with an ethidium bromide–stained 2% agarose gel for the presence or absence of GSTM1 and GSTT1 genes (Fig. 1). In 10% of the samples, for quality control, PCR was repeated. This is a robust technique that readily identifies presence or absence of the gene of interest. DHFR is used as an internal control to ensure that there is amplifiable DNA in the sample in the case of a double-null genotype. We compared patients with null genotypes with patients with nonnull genotypes. For combined GSTM1T1 genotype, we compared patients with at least one null genotype with patients null for neither.
Fig. 1.
Representative PCR products from coamplification of glutathione S-transferase T1 polymorphism (GSTT1; 480 bp), dihydrofolate reductase gene (DHFR; 280 bp), and glutathione S-transferase M1 polymorphism (GSTM1; 215 bp) viewed with an ethidium bromide–stained 2% agarose gel. Abbreviations: T−/+, absence or presence of GSTM1; M−/+, absence or presence of GSTT1.
Statistical Analyses
We computed basic descriptive statistics for demographic and treatment characteristics and GSTM1 and GSTT1 variants. We used chi-square or Fisher’s exact test for categorical variable comparisons. We applied the Kaplan-Meier procedure to estimate PFS and used the log-rank test for comparison. There is no effective salvage therapy for recurrent medulloblastoma; therefore, for assessing the effect of the genetic polymorphisms on survival, PFS is a more appropriate marker than overall survival. We calculated survival time from the date of registration to date of disease progression or relapse, date of death from any cause, or date of last follow-up visit. All survival estimates are reported in years ± 1 SEM unless otherwise noted. We included age at diagnosis as a continuous variable and used median age to dichotomize the group of patients who were ≥3 years of age at diagnosis: <8 or ≥8 years of age. Patients younger than 3 years of age are well known to have a significantly inferior survival because they are unable to receive the standard upfront radiation therapy. We performed bivariate analyses, adjusting for one variable at a time, for age at diagnosis, gender, race/ethnicity, and risk group. We did not perform multivariable analyses because our study sample is too small to adjust for all the necessary variables.
We calculated odds ratios (ORs) using logistic regression analysis to evaluate the relation between the genotypes and occurrence of ≥Gr 3 treatment toxicity requiring dose modification, omission, or cessation, according to Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE), for the following outcomes: myelosuppression, neurotoxicity, ototoxicity, and nephrotoxicity. We also used the Kaplan-Meier method to compare elapsed time to ≥Gr 3 ototoxicity during chemotherapy among the GST variants.
For verbal, performance, and full-scale IQ comparisons, we performed t-tests to compare the within-subject slopes and mixed linear modeling to compare mean scores across time among the genotype groups. The within-subject slope was calculated by regression using the multiple measurements in each subject. Mixed linear modeling was performed with strong assumption that covariance structure was compound symmetry. If unstructured covariance was specified, analyses stopped because of too many likelihood evaluations. We compared IQ scores at single time points among the genotypes using the Wilcoxon rank sum test. We selected p < 0.05 as a statistical significance value, and all tests of statistical significance were two sided.
Results
Demographic and Clinical Characteristics of the Study Population
The mean and median ages for the overall group were 7.3 and 6.8 years, respectively (range, 1.6–18 years). Six (14%) patients were younger than 3 years at diagnosis. Thirty-four (81%) patients were male, and 21 (50%) were non-Hispanic white. Table 1 summarizes descriptive data for all patients, patients who were older than 3 years of age at diagnosis, and patients who were treated with high-dose chemotherapy followed by stem cell rescue. The proportions of the selected GST polymorphisms were similar to published population values, suggesting that GST polymorphisms were not potential risk factors for development of medulloblastoma.
Table 1.
Demographic and clinical characteristics, including glutathione S-transferase genotype, for all patients, patients older than 3 years at diagnosis, and patients treated with high-dose chemotherapy and stem cell rescue (HDCSCR)
| All Patients (n = 42)
|
Patients >3 years of Age at Diagnosis (n = 36)
|
Patients Treated with HDCSCR (n = 24)
|
||||
|---|---|---|---|---|---|---|
| Variable | n | % | n | % | n | % |
| Age at diagnosis | ||||||
| <8 years | 23 | 55 | 17 | 47 | 11 | 46 |
| >8 years | 19 | 45 | 19 | 53 | 13 | 54 |
| Gender | ||||||
| Male | 34 | 81 | 28 | 77.8 | 19 | 79.2 |
| Female | 8 | 19 | 8 | 22.2 | 5 | 20.8 |
| Ethnicity/race | ||||||
| Hispanic | 14 | 33.3 | 14 | 38.9 | 10 | 41.7 |
| Non-Hispanic white | 21 | 50.0 | 16 | 44.4 | 10 | 41.7 |
| African American | 5 | 11.9 | 4 | 11.1 | 3 | 12.5 |
| Other | 2 | 4.8 | 2 | 5.6 | 1 | 4.2 |
| Risk group (age >3 years) | ||||||
| Average risk | NA | NA | 22 | 61.1 | 15 | 62.5 |
| High risk | NA | NA | 14 | 38.9 | 9 | 37.5 |
| GSTM1 | ||||||
| Null | 17 | 40.5 | 13 | 36.1 | 9 | 37.5 |
| Nonnull | 25 | 59.5 | 23 | 63.9 | 15 | 62.5 |
| GSTT1 | ||||||
| Null | 12 | 28.6 | 11 | 30.6 | 5 | 20.8 |
| Nonnull | 30 | 71.4 | 25 | 69.4 | 19 | 79.2 |
| GSTM1T1 combined | ||||||
| ≥1 null | 25 | 59.5 | 20 | 55.6 | 12 | 50 |
| Nonnull | 17 | 40.5 | 16 | 44.4 | 12 | 50 |
Abbreviations: GSTM1, glutathione S-transferase M1 polymorphism; GSTT1, glutathione S-transferase T1 polymorphism.
Survival Analyses
The median follow-up for the 29 survivors was 3.7 years (range, 2–8.6 years; Table 2). Fourteen patients had progressed, and 13 died, resulting in an estimated 4-year PFS of 68% ± 7% and 4-year overall survival rate of 76% ± 7%. In univariate analyses, patients <8 years of age (p = 0.12) and with high-risk disease at diagnosis (p = 0.01) had shorter PFS. In patients >3 years of age at diagnosis, patients with average-risk disease (p = 0.16) and GSTM1 nonnull genotype (p = 0.21) had longer PFS. In patients treated with high-dose chemotherapy followed by stem cell rescue (n = 24), those with the GSTM1 null genotype had an estimated 4-year PFS of 44% ± 16% compared with 79% ± 10% in patients with the GSTM1 nonnull genotype (p = 0.12). Adjustment for age at diagnosis, gender, ethnicity, or risk group did not change this result.
Table 2.
Univariate comparisons for the study variables for progression-free survival in patients >3 years old at diagnosis and patients treated with high-dose chemotherapy and stem cell rescue (HDCSCR)
| Patients >3 Years Old at Diagnosis (n = 36)
|
Patients Treated with HDCSCR (n = 24)
|
|||||
|---|---|---|---|---|---|---|
| Variable | n (Event) | 4-Year PFS (SE) | HR (95% CI) | n (Event) | 4-Year PFS (SE) | HR (95% CI) |
| Age at diagnosis | ||||||
| <8 years | 17 (5) | 71 (11) | 1.5 (0.4–5.6) | 11 (5) | 55 (15) | 2.4 (0.6–10) |
| >8 years | 19 (4) | 76 (11) | 1 | 13 (3) | 71 (14) | 1 |
| Gender | ||||||
| Male | 28 (6) | 78 (8) | 1 | 19 (6) | 68 (11) | 1 |
| Female | 8 (3) | 50 (22) | 2.3 (0.6–9.3) | 5 (2) | 40 (30) | 1.6 (0.3–8) |
| Risk group | ||||||
| High risk | 14 (5) | 64 (13) | 2.5 (0.7–9.3) | 9 (4) | 56 (17) | 2.2 (0.6–8.9) |
| Average risk | 22 (4) | 80 (9) | 1 | 15 (4) | 70 (13) | 1 |
| GSTM1 | ||||||
| Null | 13 (5) | 61 (14) | 2.3 (0.6–8.5) | 9 (5) | 44 (17) | 3 (0.7–13) |
| Nonnull | 23 (4) | 82 (8) | 1 | 15 (3) | 79 (11) | 1 |
| GSTT1 | ||||||
| Null | 11 (2) | 81 (12) | 1 | 5 (1) | 75 (22) | 1 |
| Nonnull | 25 (7) | 70 (10) | 1.6 (0.3–7.6) | 19 (7) | 62 (12) | 1.8 (0.2–15) |
| GSTM1T1 combined | ||||||
| ≥1 null | 20 (6) | 68 (11) | 1.7 (0.4–6.8) | 12 (5) | 55 (15) | 1.8 (0.4–7.7) |
| Nonnull | 16 (3) | 81 (10) | 1 | 12 (3) | 75 (13) | 1 |
Abbreviations: PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; GSTM1, glutathione S-transferase M1 polymorphism; GSTT1, glutathione S-transferase T1 polymorphism. “Event” refers to relapse or progression.
Treatment-Related Toxicity and GST Genotypes
Twenty-seven (66%) of the 41 patients (data not available for one patient) experienced ≥Gr 3 treatment toxicity according to CTCAE, requiring dose modification, omission, or cessation of therapy. Patients with GSTT1 null were 8.9 times (95% confidence interval, 1.1–79) more likely to have any ≥Gr 3 toxicity than were patients with the GSTT1 nonnull genotype (Table 3). In combined genotype analyses, patients with at least one null genotype had a 4.3 (1.1–16.8), 3.7 (1–13.6), and 6.4 (1.2–34) times increased risk for any ≥Gr 3 toxicity, any ≥ Gr 3 toxicity excluding peripheral neuropathy, and any ≥ Gr 3 toxicity requiring omission or cessation of chemotherapy, respectively.
Table 3.
Distribution of glutathione S-transferase M1 (GSTM1) and glutathione S-transferase T1 (GSTT1) genotypes and calculated odds ratios (ORs) for occurrence of grade 3 or greater (≥Gr 3) treatment toxicity requiring dose modification, omission, or cessation for myelosuppression, neurotoxicity, ototoxicity, and nephrotoxicity
|
GSTM1 |
GSTT1 |
GSTM1T1 Combined
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Toxicity | Null (n) | Nonnull (n) | OR (95% CI) | Null (n) | Nonnull (n) | OR (95% CI) | ≥1 Null (n) | Nonnull (n) | OR (95% CI) |
| Any ≥Gr 3 toxicity | |||||||||
| Yes | 11 | 16 | 1.2 (0.3–4.7) | 11 | 16 | 8.9 (1.1–79.0) | 19 | 8 | 4.3 (1.1–16.8) |
| No | 5 | 9 | 1 | 13 | 5 | 9 | |||
| Any ≥Gr 3 toxicity excluding peripheral neuropathy | |||||||||
| Yes | 9 | 13 | 1.2 (0.3–4.1) | 10 | 12 | 7.1 (1.3–38.0) | 16 | 6 | 3.7 (1–13.6) |
| No | 7 | 12 | 2 | 17 | 8 | 11 | |||
| Any ≥Gr 3 toxicity requiring omission or cessation of chemotherapy | |||||||||
| Yes | 7 | 6 | 2.5 (0.6–9.6) | 5 | 8 | 1.8 (0.5–7.5) | 11 | 2 | 6.4 (1.2–34.0) |
| No | 9 | 19 | 7 | 21 | 13 | 15 | |||
| Any ≥Gr 3 toxicity requiring omission or cessation of chemotherapy excluding peripheral neuropathy | |||||||||
| Yes | 5 | 3 | 3.3 (0.7–17.0) | 4 | 4 | 3.1 (0.6–15.4) | 8 | 0 | —a |
| No | 11 | 22 | 8 | 25 | 16 | 17 | p = 0.01 | ||
| Any ≥Gr 3 ototoxicity requiring dose modification | |||||||||
| Yes | 8 | 11 | 0.9 (0.2–3.6) | 6 | 13 | 1.1 (0.3–4.6) | 13 | 6 | 1.9 (0.5–7.7) |
| No | 6 | 9 | 5 | 10 | 8 | 7 | |||
Abbreviation: CI, confidence interval.
OR cannot be calculated with one cell containing 0 value.
Serial hearing evaluations (at least two, including a pretreatment assessment) were available in 34 patients. Nineteen (56%) developed ≥Gr 3 ototoxicity, requiring hearing aids. There was no relation between development or time to development of ≥Gr 3 ototoxicity and the study variables, including the GST polymorphisms. There was also no relation between any toxicity outcome and PFS.
GST Genotypes and Intellectual Impairment
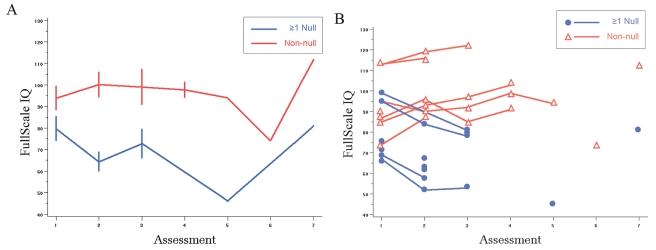
Data were available from 21 patients for at least one time point after diagnosis. At baseline comparisons, there were no statistically significant differences for all three IQ scores for the selected polymorphisms (Table 4). The mean slopes for full-scale, performance, and verbal IQ scores were, −8.4, −11.1, and −4, respectively, in patients with the GSTM1 null genotype, compared with mean slopes of 4.5, 6.6, and 2.6, respectively, in patients with the GSTM1 nonnull genotype (p = 0.001, 0.004, 0.009, respectively; Table 5). Combined GSTM1T1 genotype analyses showed statistically significant differences for comparisons of average slope values (t-test) and mean IQ scores (linear mixed model). IQ scores for the patients with at least one null genotype significantly declined in time (negative slope) compared with patients with no null genotypes (Fig. 2, Table 5). Age at diagnosis (<8 years vs. >8 years) and risk group did not correlate significantly with the genotypes and were not associated significantly with IQ change in time. All patients with at least one null genotype had a negative slope for all three IQ scales after radiation.
Table 4.
Average full-scale, performance, and verbal intelligence quotient (IQ) scores by GSTM1 and combined GSTM1T1 genotypes for baseline (prior to radiation) and first follow-up assessment
|
GSTM1 |
GSTM1T1 Combined
|
||||||
|---|---|---|---|---|---|---|---|
| IQ Scores | Assessment | Null | Nonnull | p-Value | ≥1 Null | Nonnull | p-Value |
| Full-scale IQ | 1 | 81.2 | 91.1 | 0.4 | 79.7 | 93.9 | 0.15 |
| 2 | 65.3 | 90.8 | 0.048 | 64.3 | 100.2 | 0.002 | |
| Performance IQ | 1 | 80.6 | 85.6 | 0.5 | 78.5 | 88.5 | 0.2 |
| 2 | 65.5 | 92.9 | 0.08 | 63.8 | 103.7 | 0.004 | |
| Verbal IQ | 1 | 84.8 | 92.4 | 0.4 | 84.2 | 94.3 | 0.2 |
| 2 | 70.8 | 90.6 | 0.046 | 70.5 | 97.5 | 0.002 | |
Abbreviations: GSTM1, glutathione S-transferase M1 polymorphism; GSTM1T1, glutathione S-transferase T1 and M1 polymorphisms combined.
Table 5.
Comparison of scores for full-scale, performance, and verbal intelligence quotient (IQ) (Wechsler Intelligence Scale for Children) over time following radiation therapy in 21 children with medulloblastoma by glutathione S-transferase genotype
| Full-Scale IQ
|
Performance IQ
|
Verbal IQ
|
||||
|---|---|---|---|---|---|---|
| Genotype | Score | p-Value | Score | p-Value | Score | p-Value |
| GSTM1 | ||||||
| Mean slope | −8.4 | 0.001a | −11.1 | 0.004a | −4.0 | 0.009a |
| Null | 4.5 | 0.16b | 6.6 | 0.18b | 2.6 | 0.3b |
| Nonnull mean differenceb | −12.5 | −12.4 | −8.7 | |||
| GSTM1T1 combined | ||||||
| Mean slope | −8.9 | 0.01a | −11.5 | 0.02a | −4.5 | 0.02a |
| ≥1 null | 4.5 | 0.0002b | 6.6 | 0.0004b | 2.6 | 0.002b |
| Nonnull mean differenceb | −27.2 | −29.0 | −21.7 | |||
Abbreviations: GSTM1, glutathione S-transferase M1 polymorphism; GSTM1T1, glutathione S-transferase T1 and M1 polymorphisms combined.
Comparison of slopes by t-test.
Comparison by linear mixed model.
Fig. 2.
Comparisons for serial full-scale intelligence quotient (IQ) measurements by GSTM1T1 combination genotype. (A) Mean slope comparison. (B) All observations. The first assessment was conducted before radiation therapy.
Discussion
In an attempt to discover potential markers to identify patients with medulloblastoma who are at risk of treatment failure and development of adverse effects, we conducted a pilot study in 42 children and adolescents. Most remarkable was the association between GSTM1T1 polymorphism and multiple adverse events, including intellectual impairment and ≥Gr 3 toxicity due to treatment. Neurocognitive deficits usually become evident within 1–2 years after radiotherapy and are progressive in nature. Affected children may experience information-processing deficits and attention and memory impairment, leading to academic failure in the areas of reading, mathematics, and language. Progressive loss of IQ scores in children diagnosed with brain tumors and leukemia following cranial radiotherapy has been reported in a number of studies.8,40–42 In medulloblastoma, the rate of decline for full-scale, performance, and verbal IQ scores ranged from 2 to 4.3 points per year in three separate reports.8,40,41 While the most likely cause for neurocognitive impairment is radiation therapy, chemotherapy also has been associated with this outcome.43
While young age at diagnosis and higher dose of radiation have been associated with development of intellectual impairment after radiation therapy, we were not able to adjust for these markers appropriately in our small study sample. However, neither factor was statistically significantly related to cognitive impairment. In current care of children with brain tumors, we are unable to predict who will develop significant intellectual impairment after radiation therapy. Our findings suggest that GSTM1 and GSTT1 polymorphisms may be a potential marker to identify such patients in advance. The mean scores for full-scale, performance, and verbal IQ at the baseline evaluation (prior to radiation) were slightly lower than expected in patients with no null (combined GSTM1T1) genotype, but this difference was not statistically significant. Three patients had all three scores less than 80 points at this evaluation. Their tests were repeated within 1 month of their initial evaluation, and the repeat scores were very similar. The neuropsychologist who performed the tests commented that these scores reflected the patients’ true performance and were not influenced by any health impairment due to surgery or medication at the time. It is also important to emphasize that our analyses compared the slopes of cognitive evaluations at consequent time points, not at one single time point. There are no data regarding whether GST polymorphisms may be related to cognitive function differences in the normal population.
GST enzymes are known for their free-radical– scavenging function in addition to xenobiotic metabolism. In a recent study exploring the relation between homocysteine metabolism polymorphisms and changes in IQ scores over the 4 years following diagnosis of pediatric acute lymphoblastic leukemia, endothelial nitric oxide synthase (eNOS) 894T homozygosity was associated with a change in IQ scores (p = 0.007).44 Almost two-thirds of the patients were treated with cranial radiation. Together with our finding, this result further supports the premise that enzymes such as GSTs and eNOS that are involved in cellular protection from free radicals may help predict which patients will develop neurocognitive toxicity from radiation.
In addition to neurocognitive adverse events, the combined GSTM1T1 polymorphism was also significantly related to other adverse events after medulloblastoma therapy. These consistent observations support the potentially important implication of this genotype in modifying the effects of cancer therapy. The trends for the main genotype effect for the toxicity outcome were in the direction of our hypotheses for each gene individually. Existence of synergy and substrate overlap between the GST genotypes have been described before and are a possible explanation for why the statistical comparisons became significant with the combined genotype analyses.9
We observed a trend for a possible relation with GSTM1 polymorphisms and PFS. In contrast to our hypothesis, patients with the GSTM1 null genotype who did not have a functional enzyme had outcomes inferior to outcomes of patients with the GSTM1 non-null genotype. This could occur simply because of the small sample size. While patients with GSTM1 null genotype experienced more frequent, but statistically insignificant, toxicity requiring omission or cessation of a chemotherapy agent compared with patients with the GSTM1 nonnull genotype, this difference was not related to PFS. In a larger study, we will explore whether this observation persists, and if it does, we will investigate whether excess toxicity and dose modification may have led to inferior outcome in the GSTM1 null patients. The GSTM1 null genotype has been related to increased toxicity requiring dose reduction in adults with glioma treated with nitrosourea-based regimens.26
Clearly, our study is small and our results should be interpreted with caution. These preliminary findings are promising but require validation in a larger study that will enable us to perform multivariate analyses, adjusting for age at diagnosis, radiation dose, and other potential confounding variables. A follow-up study in a larger patient group treated with high-dose chemotherapy followed by stem cell rescue is under way. GSTs are not the only mechanisms that modify chemotherapy and radiation therapy effects. Alkylating agents are initially activated by phase 1 cytochrome P450 enzymes. Free radicals created by radiation can possibly be cleared by other enzymes, including superoxide dismutase, glutathione peroxidase, and eNOS. All of these enzymes have polymorphisms, and a large study is needed to explore whether combined effects of these polymorphisms can predict survival and the toxicities examined in our study better than GSTs alone.
Individual variation in the responses to chemotherapeutic agents at the host level is an understudied clinical problem. Studies have found that this variability contributes to widely disparate outcomes, including complete responsiveness, toxic effects (which can be severe), drug withdrawal, and, in the worst cases, therapeutic failure.45,46 This variability in drug response is in part determined genetically.45–47 Combined modality treatment with surgery, radiation, and chemotherapy achieves reasonable survival rates in children and adolescents with medulloblastoma. However, cure rates are still unacceptably low for high-risk patients, and such permanent adverse effects as intellectual impairment significantly reduce quality of life. Because there is no successful salvage therapy for patients who relapse, identification of patients at risk of treatment failure in advance could perhaps promote development of more aggressive treatment options. Similarly, if we were able to identify patients who were at increased risk for intellectual impairment or other toxicities, we could examine whether they could be treated with lower doses of radiation and or chemotherapy. The current national Children’s Oncology Group medulloblastoma protocol is indeed investigating whether reduced-dose craniospinal radiation therapy (18 Gy instead of 24 Gy) is effective in average-risk medulloblastoma patients. Moreover, in patients who are at increased risk of cognitive impairment, early implementation of prevention strategies such as cognitive remediation or methylphenidate therapy could be explored.
Acknowledgments
This work was supported by the Gillson Longenbaugh Foundation, American Brain Tumor Association, and Hope Street Foundation. These data were presented at the Thirteenth International Symposium of Pediatric Neuro-Oncology (Chicago, IL, USA), June 29 through July 2, 2008. We thank Shu Qin for assistance in genotyping, Jingrong Yan for assistance in data management, and Cindy Power for administrative assistance.
References
- 1.Blaney SM, Kun L, Hunter J, et al. Tumors of the central nervous system. In: Pizzo PA, Poplack D, editors. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott Williams and Wilkins; 2006. pp. 786–864. [Google Scholar]
- 2.Albright AL, Wisoff JH, Zeltzer PM, Boyett JM, Rorke LB, Stanley P. Effects of medulloblastoma resections on outcome in children: a report from the Children’s Cancer Group. Neurosurgery. 1996;38(2):265–271. doi: 10.1097/00006123-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 3.Evans AE, Jenkin RD, Sposto R, et al. The treatment of medulloblastoma. Results of a prospective randomized trial of radiation therapy with and without CCNU, vincristine, and prednisone. J Neurosurg. 1990;72(4):572–582. doi: 10.3171/jns.1990.72.4.0572. [DOI] [PubMed] [Google Scholar]
- 4.Packer RJ, Sutton LN, Elterman R, et al. Outcome for children with medulloblastoma treated with radiation and cisplatin, CCNU, and vincristine chemotherapy. J Neurosurg. 1994;81(5):690–698. doi: 10.3171/jns.1994.81.5.0690. [DOI] [PubMed] [Google Scholar]
- 5.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 6.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multi-centre trial. Lancet Oncol. 2006;7(10):813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 7.McNeil DE, Cote TR, Clegg L, Rorke LB. Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: a SEER update. Surveillance Epidemiology and End Results. Med Pediatr Oncol. 2002;39(3):190–194. doi: 10.1002/mpo.10121. [DOI] [PubMed] [Google Scholar]
- 8.Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children’s Cancer Group study. J Clin Oncol. 2001;19(15):3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- 9.Hayes JD, Pulford DJ. The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol. 1995;30(6):445–600. doi: 10.3109/10409239509083491. [DOI] [PubMed] [Google Scholar]
- 10.Dirven HA, van Ommen B, van Bladeren PJ. Involvement of human glutathione S-transferase isoenzymes in the conjugation of cyclophosphamide metabolites with glutathione. Cancer Res. 1994;54 (23):6215–6220. [PubMed] [Google Scholar]
- 11.Goto S, Iida T, Cho S, Oka M, Kohno S, Kondo T. Overexpression of glutathione S-transferase pi enhances the adduct formation of cisplatin with glutathione in human cancer cells. Free Radic Res. 1999;31(6):549–558. doi: 10.1080/10715769900301121. [DOI] [PubMed] [Google Scholar]
- 12.Smith MT, Evans CG, Doane-Setzer P, Castro VM, Tahir MK, Mannervik B. Denitrosation of 1,3-bis(2-chloroethyl)-1-nitrosourea by class mu glutathione transferases and its role in cellular resistance in rat brain tumor cells. Cancer Res. 1989;49(10):2621–2625. [PubMed] [Google Scholar]
- 13.Weber GF, Waxman DJ. Denitrosation of the anti-cancer drug 1,3-bis(2-chloroethyl)-1-nitrosourea catalyzed by microsomal glutathione S-transferase and cytochrome P450 monooxygenases. Arch Biochem Biophys. 1993;307(2):369–378. doi: 10.1006/abbi.1993.1602. [DOI] [PubMed] [Google Scholar]
- 14.Terrier P, Townsend AJ, Coindre JM, Triche TJ, Cowan KH. An immunohistochemical study of pi class glutathione S-transferase expression in normal human tissue. Am J Pathol. 1990;137(4):845–853. [PMC free article] [PubMed] [Google Scholar]
- 15.Board PG. Biochemical genetics of glutathione-S-transferase in man. Am J Hum Genet. 1981;33(1):36–43. [PMC free article] [PubMed] [Google Scholar]
- 16.Garte S, Gaspari L, Alexandrie AK, et al. Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev. 2001;10(12):1239–1248. [PubMed] [Google Scholar]
- 17.Arand M, Muhlbauer R, Hengstler J, et al. A multiplex polymerase chain reaction protocol for the simultaneous analysis of the glutathione S-transferase GSTM1 and GSTT1 polymorphisms. Anal Biochem. 1996;236(1):184–186. doi: 10.1006/abio.1996.0153. [DOI] [PubMed] [Google Scholar]
- 18.Pemble S, Schroeder KR, Spencer SR, et al. Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J. 1994;300(pt 1):271–276. doi: 10.1042/bj3000271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stanulla M, Schrappe M, Brechlin AM, Zimmermann M, Welte K. Polymorphisms within glutathione S-transferase genes (GSTM1, GSTT1, GSTP1) and risk of relapse in childhood B-cell precursor acute lymphoblastic leukemia: a case-control study. Blood. 2000;95(4):1222–1228. [PubMed] [Google Scholar]
- 20.Anderer G, Schrappe M, Brechlin AM, et al. Polymorphisms within glutathione S-transferase genes and initial response to glucocorticoids in childhood acute lymphoblastic leukaemia. Pharmacogenetics. 2000;10(8):715–726. doi: 10.1097/00008571-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Davies SM, Bhatia S, Ross JA, et al. Glutathione S-transferase genotypes, genetic susceptibility, and outcome of therapy in childhood acute lymphoblastic leukemia. Blood. 2002;100(1):67–71. doi: 10.1182/blood.v100.1.67. [DOI] [PubMed] [Google Scholar]
- 22.Rocha JC, Cheng C, Liu W, et al. Pharmacogenetics of outcome in children with acute lymphoblastic leukemia. Blood. 2005;105(12):4752–4758. doi: 10.1182/blood-2004-11-4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies SM, Robison LL, Buckley JD, et al. Glutathione S-transferase polymorphisms and outcome of chemotherapy in childhood acute myeloid leukemia. J Clin Oncol. 2001;19(5):1279–1287. doi: 10.1200/JCO.2001.19.5.1279. [DOI] [PubMed] [Google Scholar]
- 24.Naoe T, Tagawa Y, Kiyoi H, et al. Prognostic significance of the null genotype of glutathione S-transferase-T1 in patients with acute myeloid leukemia: increased early death after chemotherapy. Leukemia. 2002;16(2):203–208. doi: 10.1038/sj.leu.2402361. [DOI] [PubMed] [Google Scholar]
- 25.Mertens AC, Mitby PA, Radloff G, et al. XRCC1 and glutathione-S-transferase gene polymorphisms and susceptibility to radiotherapy- related malignancies in survivors of Hodgkin disease. Cancer. 2004;101(6):1463–1472. doi: 10.1002/cncr.20520. [DOI] [PubMed] [Google Scholar]
- 26.Okcu MF, Selvan M, Wang L, et al. Glutathione S-transferase polymorphisms and survival in primary malignant glioma. Clin Cancer Res. 2004;10:2618–2625. doi: 10.1158/1078-0432.ccr-03-0053. [DOI] [PubMed] [Google Scholar]
- 27.Ambrosone CB, Sweeney C, Coles BF, et al. Polymorphisms in glutathione S-transferases (GSTM1 and GSTT1) and survival after treatment for breast cancer. Cancer Res. 2001;61(19):7130–7135. [PubMed] [Google Scholar]
- 28.Stoehlmacher J, Park DJ, Zhang W, et al. Association between glutathione S-transferase P1, T1, and M1 genetic polymorphism and survival of patients with metastatic colorectal cancer. J Natl Cancer Inst. 2002;94(12):936–942. doi: 10.1093/jnci/94.12.936. [DOI] [PubMed] [Google Scholar]
- 29.Lu C, Spitz MR, Zhao H, et al. Association between glutathione S-transferase pi polymorphisms and survival in patients with advanced nonsmall cell lung carcinoma. Cancer. 2005;106(2):441–447. doi: 10.1002/cncr.21619. [DOI] [PubMed] [Google Scholar]
- 30.Holley SL, Rajagopal R, Hoban PR, et al. Polymorphisms in the glutathione S-transferase mu cluster are associated with tumour progression and patient outcome in colorectal cancer. Int J Oncol. 2006;28(1):231–236. [PubMed] [Google Scholar]
- 31.Goekkurt E, Hoehn S, Wolschke C, et al. Polymorphisms of glutathione S-transferases (GST) and thymidylate synthase (TS)—novel predictors for response and survival in gastric cancer patients. Br J Cancer. 2006;94(2):281–286. doi: 10.1038/sj.bjc.6602891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beebe D, Holmes E. Location may not affect IQ and adaptive outcome in pediatric cerebellar tumors. J Int Neuropsychol Soc. 2002;8:293–294. [Google Scholar]
- 33.DeMichele A, Aplenc R, Botbyl J, et al. Drug-metabolizing enzyme polymorphisms predict clinical outcome in a node-positive breast cancer cohort. J Clin Oncol. 2005;23(24):5552–5559. doi: 10.1200/JCO.2005.06.208. [DOI] [PubMed] [Google Scholar]
- 34.Lee JM, Wu MT, Lee YC, et al. Association of GSTP1 polymorphism and survival for esophageal cancer. Clin Cancer Res. 2005;11(13):4749–4753. doi: 10.1158/1078-0432.CCR-04-2333. [DOI] [PubMed] [Google Scholar]
- 35.Sweeney C, Nazar-Stewart V, Stapleton PL, Eaton DL, Vaughan TL. Glutathione S-transferase M1, T1, and P1 polymorphisms and survival among lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2003;12(6):527–533. [PubMed] [Google Scholar]
- 36.Oldenburg J, Kraggerud SM, Cvancarova M, Lothe RA, Fossa SD. Cisplatin-induced long-term hearing impairment is associated with specific glutathione S-transferase genotypes in testicular cancer survivors. J Clin Oncol. 2007;25(6):708–714. doi: 10.1200/JCO.2006.08.9599. [DOI] [PubMed] [Google Scholar]
- 37.Lecomte T, Landi B, Beaune P, Laurent-Puig P, Loriot MA. Glutathione S-transferase P1 polymorphism (Ile105Val) predicts cumulative neuropathy in patients receiving oxaliplatin-based chemotherapy. Clin Cancer Res. 2006;12(10):3050–3056. doi: 10.1158/1078-0432.CCR-05-2076. [DOI] [PubMed] [Google Scholar]
- 38.Hohaus S, Di RA, Di FA, et al. Glutathione S-transferase P1 genotype and prognosis in Hodgkin’s lymphoma. Clin Cancer Res. 2005;11(6):2175–2179. doi: 10.1158/1078-0432.CCR-04-1250. [DOI] [PubMed] [Google Scholar]
- 39.Wechsler D. Manual for the Wechsler Intelligence Scale for Children. 3rd ed. San Antonio, TX: Psychological Corporation; 1991. [Google Scholar]
- 40.Spiegler BJ, Bouffet E, Greenberg ML, Rutka JT, Mabbott DJ. Change in neurocognitive functioning after treatment with cranial radiation in childhood. J Clin Oncol. 2004;22(4):706–713. doi: 10.1200/JCO.2004.05.186. [DOI] [PubMed] [Google Scholar]
- 41.Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23(24):5511–5519. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 42.Waber DP, Tarbell NJ, Fairclough D, et al. Cognitive sequelae of treatment in childhood acute lymphoblastic leukemia: cranial radiation requires an accomplice. J Clin Oncol. 1995;13(10):2490–2496. doi: 10.1200/JCO.1995.13.10.2490. [DOI] [PubMed] [Google Scholar]
- 43.Vardy J, Rourke S, Tannock IF. Evaluation of cognitive function associated with chemotherapy: a review of published studies and recommendations for future research. J Clin Oncol. 2007;25(17):2455–2463. doi: 10.1200/JCO.2006.08.1604. [DOI] [PubMed] [Google Scholar]
- 44.Krajinovic M, Robaey P, Chiasson S, et al. Polymorphisms of genes controlling homocysteine levels and IQ score following the treatment for childhood ALL. Pharmacogenomics. 2005;6(3):293–302. doi: 10.1517/14622416.6.3.293. [DOI] [PubMed] [Google Scholar]
- 45.Maitland-van der Zee AH, de Boer A, Leufkens HG. The interface between pharmacoepidemiology and pharmacogenetics. Eur J Pharmacol. 2000;410(2–3):121–130. doi: 10.1016/s0014-2999(00)00810-4. [DOI] [PubMed] [Google Scholar]
- 46.Vesell ES. Advances in pharmacogenetics and pharmacogenomics. J Clin Pharmacol. 2000;40(9):930–938. doi: 10.1177/00912700022009666. [DOI] [PubMed] [Google Scholar]
- 47.Relling MV, Dervieux T. Pharmacogenetics and cancer therapy. Nat Rev Cancer. 2001;1(2):99–108. doi: 10.1038/35101056. [DOI] [PubMed] [Google Scholar]