Abstract
The treatment of malignant gliomas with current therapies remains a challenge in neurooncology. Our recent work showed that embryonic stem cell (ESC)-derived astrocytes conditionally expressing genes can be used to induce apoptosis in malignant glioma cells in vitro. The tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) gene has been shown to induce apoptosis in a variety of tumor cells, including gliomas. The aim of this study was to assess the proapoptotic effects of transgenic TRAIL delivered by ESC-derived astrocytes on malignant gliomas in vivo. Malignant glioma A172 cells were used to induce heterotopic xenografts in nude mice. ESC-derived astrocytes conditionally expressing TRAIL were injected into the xenografts. TRAIL expression was documented in the malignant glioma xenografts by reverse transcription PCR and immunohistochemistry after external gene induction. A significant reduction in tumor volume occurred 48 h after a single injection (14%) and double injections (31%) in the experimental groups. Terminal dUTP nick end labeling (TUNEL) revealed abundant apoptotic tumor cells in the experimental groups. Seven days after injection, the tumor had undergone severe necrosis, with only scattered residual tumor cells at the periphery. Death receptor DR4 expression increased significantly in the experimental groups compared with controls. Our data suggest that ESC-derived astrocytes conditionally expressing TRAIL should be considered as vectors to deliver gene therapy for malignant gliomas.
Keywords: astrocytes, embryonic stem cell, gene therapy, malignant glioma, TRAIL
Currently, there is no optimal treatment for malignant gliomas, the most common malignant brain tumors in adults. Patients with these tumors have a dismal prognosis, with a median survival of less than 1 year.1 Although chemotherapy has been incorporated into the treatment of various intracranial neoplasms, the resistance and invasive nature of these tumors call for new treatment strategies. These include targeting brain tumor cells while sparing normal tissue and the use of gene therapy approaches. For brain tumors, the success of gene therapy depends on the ability to deliver genes to the infiltrating tumor cells interspersed within the normal cells.
Viral vectors have been considered the most effective in vivo gene delivery reagents. Retrovirus, adenovirus (Adv), and herpes simplex virus-1 (HSV-1) are the best-studied viral brain tumor therapy vectors.2 Replication-competent retroviruses have infection rates of 97%, with tumor specificity and lack of spread to nontumor tissue. Potential disadvantages, however, include insertional mutagenesis.3 Adv and HSV-1, used in both replicating and replication-deficient forms, offer numerous advantages, including high transgenic capacity (Adv) and persistent gene expression (HSV-1).4,5 Potential disadvantages, however, include short-term transgene expression and potential toxicity for Adv, and neuro-virulence and recombination with wild type for HSV-1. Oncolytic viruses have also been used successfully for clinical trials,6 because they replicate selectively within tumor cells, leading to increased intratumoral viral titers. To date, however, their clinical effectiveness for brain tumors remains to be substantiated.7,8
Recent evidence suggests that stem cells can be used as delivery vehicles for brain tumor therapy.9,10 In vitro migration assays confirm the ability of isolated stem cells to migrate toward factors produced by glioma cells.11 There is, however, concern that these undifferentiated stem cells can interfere with baseline functions.12 Thus, ideally, differentiated cells such as astrocytes should be considered as vectors. Astrocytes are native to the CNS, which should maximize their survival and function after transplantation. Astrocytes are highly secretory cells and can generate large amounts of proteins.13 Therefore, transplanted astrocytes should be protective within the host brain. Finally, normal astrocytes can migrate along white matter tracts after transplantation into the brain.14,15 This migratory capacity may be useful for delivery of gene therapy to infiltrative tumors such as malignant gliomas.
Autologous astrocytes can be derived from the pluripotent neural precursor cells in the periventricular area or from fetal tissue.16,17 These approaches are hazardous, with limited capability of generating large numbers of cells. Our recent work shows that we can generate a pure population of astrocytes from mouse embryonic stem cells (ESCs).18 Thus, it is our hypothesis that ESC-derived astrocytes conditionally expressing transgenes can be used as an alternative source for gene delivery in malignant glioma therapy.
Tumor necrosis factor (TNF)–related apoptosis-inducing ligand (TRAIL), a member of the TNF family, has been shown to induce apoptosis in a variety of transformed cell lines but to spare most normal cells.19,20 Systemic administration of recombinant TRAIL protein was associated with minimal toxicity in recent clinical trials with cancer patients.21 Recombinant TRAIL, however, does not cross the blood–brain barrier and has a short half-life.22 To obviate these limitations, convection-enhanced delivery (CED) of TRAIL has been used in a malignant glioma xenograft model with good results.23 Although CED is a promising technique to bypass the blood–brain barrier, side effects in the clinical setting include invasive implant of multiple catheters and difficult calculation of drug delivery.24 To circumvent these side effects, alternative delivery methods have been sought. Cellular gene delivery methods are gaining importance as stem cells have shown to have migratory and homing capacity.25 Our recent work shows that we can generate a pure population of transgenic ESC-derived astrocytes with a highly regulated, robust conditional expression of TRAIL under the control of a tetracycline (tet)-inducible promoter.26 Furthermore, we have shown that transgenic delivery of TRAIL by ESC-derived astrocytes induced apoptosis in malignant glioma cells in vitro.26 Based on our recent work, we hypothesize that ESC-derived astrocytes conditionally expressing TRAIL may represent an effective and novel approach for treatment of human malignant gliomas in vivo. Using a heterotopic xenograft model in the mouse, we have found that ESC-derived astrocytes conditionally expressing TRAIL can be used as a vector for gene therapy in malignant gliomas in vivo.
Materials and Methods
Cell Lines and Cultures
Human malignant glioma A172 cells were used for the in vivo heterotopic malignant glioma tumor xenografts. Cells were cultured in standard condition. In brief, human malignant glioma A172 cell lines were obtained from the Neurosurgery Tissue Bank (San Francisco, CA, USA). Cells were cultured in Iscove’s modified Dulbecco’s medium (Cellgro, CA, USA) supplemented with 10% fetal calf serum at 37ºC in an incubator supplemented with 5% CO2 Mouse ESCs were passaged and maintained according to previously published protocols.18 Mouse Ainv-18 ESCs (a gift of Michael Kyba) constitutively express a doxycycline (Dox)-binding transcriptional activator fusion protein and include a Dox-responsive promoter upstream of a Lox (locus of X-over P1) cloning site.27 Genes cloned into the Lox site using Cyclization Recombination (Cre)-assisted recombination27 are then expressed when the cells are exposed to Dox. ESCs were directed to differentiate into astrocytes using protocols recently developed in our lab.26
Human Brain Tumor Xenografts in Athymic Nude Mice
The experimental protocol was approved by the Institute of Animal Care and Use Committee of Mount Sinai School of Medicine and met all federal guidelines. A172 cells (3 × 106 cells/200 μl phosphate-buffered saline [PBS]) were injected subcutaneously in the right flank of athymic NCr-nu/nu nude mice (5-week-old females, 30 g body weight; Taconic Farms, Germantown, NY, USA). Visible tumors (~7.5 mm3) occurred in 7–14 days. Intratumoral injections of ESC-derived astrocytes conditionally expressing TRAIL (103 cells/20 μl PBS) were administered to the right flank when the xenograft reached a volume of 7.5 mm3. Prior to intratumoral injection, ESC-derived astrocytes conditionally expressing TRAIL were labeled with the vital red dye PKH2628 using the Cell Linker Kit (Sigma, St. Louis, MO, USA) following the manufacturer’s protocol.
Groups Studied and Tumor Volume
Experimental animals (n = 6 per group) received ESC-derived astrocytes conditionally expressing TRAIL once (group 1) or twice for 2 consecutive days (group 2). These animals were sacrificed 48 h after the last astrocyte injection. Other animals (group 3) underwent daily intratumoral injections of ESC-derived astrocytes conditionally expressing TRAIL for 7 days and were then sacrificed. To study “homing,” group 4 mice received systemic delivery of ESC-derived astrocytes conditionally expressing TRAIL in the tail vein and were sacrificed after 7 days.
In the experimental groups, TRAIL was induced by giving animals free access to water containing Dox (20 μg/ml). Water bottles were wrapped with aluminum foil to protect Dox from light degradation. Control groups (n = 6 in each group) received no treatment (control 1), ESC astrocytes with Dox treatment (control 2), or ESC astrocytes engineered with TRAIL without Dox induction (control 3). Tumor volume (V) was calculated as V = π/6 × largest diameter (D) × (small D)2.29
Reverse Transcription PCR Analysis of Transgene Expression
Gene expression was quantified by reverse transcription (RT)-PCR. Total RNA was extracted and RT-PCR performed as previously described. In brief, tumor tissue was harvested for RT-PCR. Total cellular RNA from the tumor tissue was purified using the RNeasy kit (Qiagen, Valencia, CA, USA), with on-column DNase treatment. All RNA preparations were spectrophotometrically quantified and examined for degradation using gel electrophoresis prior to RT. RT was performed on 1 μg of total RNA using the Omniscript Reverse Transcriptase Kit (Promega, Madison, WI, USA) and PDN6 random hexamer primers (Pharmacia, Piscataway, NJ, USA) in a total volume of 20 μl. A total volume of PCR mixture was 12.5 μl; each contained 0.5 μl cDNA template. All PCR amplifications were carried out for 35 cycles. PCR products were examined on 1.8% agarose gels and photographed using an Eagle Eye II imager (Stratagene, La Jolla, CA, USA).
Histology, Immunohistochemistry, and TUNEL
Forty-eight hours after the last ESC-derived astrocyte injection, animals were anesthetized with intraperitoneal ketamine/xylazine, and subcutaneous tumors were excised and snap-frozen into liquid nitrogen. Tissue blocks were embedded in Tissue Tak and sliced in the coronal plane in 10-μm-thick frozen sections. Serial sections were stained with hematoxylin and eosin, or left unstained for immunofluorescent labeling and terminal dUTP nick end labeling (TUNEL) detection. Slides were washed with PBS, fixed in 4% paraformaldehyde in PBS for 30 min at room temperature, washed, and preincubated in 10% normal goat serum (Sigma) for 30 min at room temperature and then in primary antibody for 1 h at room temperature (monoclonal anti-TRAIL antibody, 1:100; Chemicon International, Temecula, CA, USA). Alexa 488 antimouse antibody (1:400) was used as a secondary antibody. Cells were then washed and mounted in Vectashield with added 4',6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlingame, CA, USA), and examined under fluorescence microscopy using the Openlab imaging system (Improvision, Lexington, MA, USA). Omission of the primary antibody served as negative control. TUNEL staining was performed using the ApopTag Plus Fluorescein In Situ Apoptosis Detection kit (Chemicon International). Experimental sections were then mounted on slides with Vectashield mounting medium (Vector Laboratories), and images were taken with a Leica microscope (Leica, Bannockburn, IL, USA).
Western Blot Analysis
Briefly, tumor tissue was harvested in RIPA buffer (1% Nonidet P-40, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS] in PBS) containing protease inhibitor cocktail (Protease Arrest™; Calbiochem, San Diego, CA, USA; 50 mM NaF and 1 mM Na3VO4), homogenized, and centrifuged at 12,000 rpm for 10 min at 4°C. The supernatant was used as total cell lysate. Protein lysates (20 μg) were denatured in 2% SDS, 10 mM dithiothreitol, 60 mM Tris-HCl (pH 6.8), and 0.1% bromophenol blue and loaded onto a 15% polyacrylamide/SDS gel (Bio-Rad). The separated proteins were then transferred by electroblot (80 mA, 1 h) onto a nitrocellulose membrane (Bio-Rad). The membrane was blocked for 1 h in room temperature in Tris-buffered saline/Tween 20 (TBS-T; 0.1 M Tris-HCl [pH 7.6], 1.37 M NaCl, 0.1% Tween 20) containing 5% nonfat dry milk and incubated overnight at 4°C in TBS-T containing the primary antibody. The membrane was washed in TBS-T, incubated with the secondary antibody conjugated to horseradish peroxidase for 1 h at room temperature, and then washed in TBS-T. The ECL nonradioactive detection system (Amersham Pharmacia Biotech, Buck-inghamshire, UK) was utilized to detect the antibody-protein complexes by exposure of the membrane to an X-ray film.
Statistical Analysis
The data are expressed as mean ± SD. Statistical analysis was performed by using Student’s t-test. Intergroup analysis was performed with analysis of variance and post hoc Bonferroni test. The criterion for statistical significance was taken as p < 0.05.
Results
In Vivo Transgenic TRAIL Expression by ESC-Derived Astrocytes after Dox Induction
To study the in vivo effects of transgene expression, we used ESC-derived astrocytes carrying TRAIL in the context of a “tet-on” system (reverse tet transactivator [rtTA]), upstream of Lox, activated by Dox. A schematic representation of the insertional cassettes is shown in Fig. 1A. ESC-derived astrocytes conditionally expressing TRAIL were injected in malignant glioma xenograft in a mouse subcutaneous model. Transgenic TRAIL expression was detected in the explanted heterotopic xenograft by RT-PCR analysis 48 h and 7 days after Dox induction in all experimental animals (Fig. 1B). TRAIL was not detected in the control groups. Immunocytochemistry for the TRAIL protein showed in situ expression in the experimental animals (Fig. 2A). TRAIL expression was colocalized with the ESC-derived astrocytes conditionally expressing TRAIL labeled with PKH26 dye (Fig. 2A). At higher magnification, membrane localization of TRAIL protein was evident (Fig. 2B). Systemic administration of ESC-derived astrocytes (tail vein, experimental group 4) resulted in “homing” within the tumor nodule (Fig. 2C).
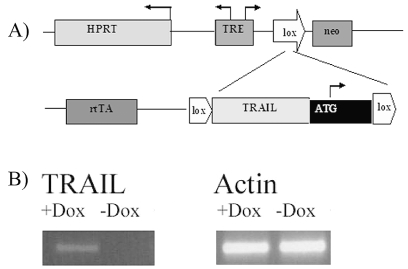
Fig. 1.
Engineering of embryonic stem cell (ESC)-derived astrocytes and expression of transgenic TRAIL. (A) Schematic representation of integrated expression cassettes. The reverse tetracycline transactivator (rtTA) is integrated into the constitutive ROSA 26 locus on chromosome 6. Cre-mediated recombination of targeting vectors into the homing site on the X chromosome restores resistance to the antibiotic G418 (neomycin [neo]), thereby facilitating efficient isolation of transgenic cells. HPRT, hypoxanthine-guanine phosphoribosyltransferase; TRE, tetracycline-responsive element; ATG, methionine initiation codon; TRAIL, tumor necrosis factor–related apoptosis-inducing ligand. (B) Reverse transcription PCR gel of malignant astrocytoma xenografts after injection of ESC-derived astrocytes conditionally expressing TRAIL. TRAIL was detected in the experimental group after doxycycline induction (+Dox). This was not observed in the control groups (− Dox). Actin was used as internal control.
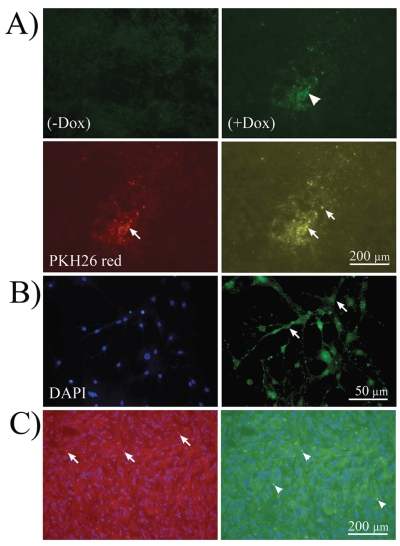
Fig. 2.
In vivo ESC-mediated conditional TRAIL expression. (A) Immunofluorescence microphotographs of A172 tumor xenografts injected with embryonic stem cell (ESC)-derived astrocytes conditionally expressing TRAIL after immunohistochemistry for TRAIL antibody in the absence of doxycycline (− Dox; control) or presence of Dox (+Dox). TRAIL expression was present in experimental animals (arrowhead, upper right) and not in control. TRAIL protein was visualized by Alexa 488 antibody. Lower left, ESC-derived astrocytes tracked with PKH26 vital red (arrow; 565-wavelength filter). Lower right, Computer-overlapped images of PKH26-tracked ESC-derived astrocytes and TRAIL immunolabeling. Note the colocalization (yellow) of both within the same cells (arrows). (B) At higher magnification, ESC-derived astrocytes expressing TRAIL show membrane localization of the protein (arrows). Left, 4’,6-diamidino-2-phenylindole (DAPI) staining; right, TRAIL immunolabeling. (C) “Homing” after systemic injection was seen in experimental animals. Left, PKH26-labeled astrocytes are shown as red punctuate labeling embedded in tumor cells (arrows). Right, astrocytes expressing TRAIL in green embedded within the tumor (arrowheads).
ESC-Derived Astrocyte-Mediated Gene Delivery Results in Decreased Volume and Necrosis of Malignant Astrocytoma Xenografts
A 14% reduction in tumor size was found 48 h after single injections of ESC-derived astrocytes conditionally expressing TRAIL after Dox induction (experimental group 1) compared with controls: 6.25 ± 0.4 mm3 versus 7.6 ± 1.2 mm3 in controls (p < 0.05). Two injections (experimental group 2) resulted in 31% reduction in tumor size compared with controls: 4.50 ± 0.2 mm3 versus 7.6 ± 1.2 mm3 in controls (p < 0.01). Long-term treated animals (experimental group 3) showed a 40% reduction in tumor volume (Fig. 3A, B). Histology performed at this time point showed severe central necrosis with only scattered residual tumor cells at the periphery (Fig. 3C, D).
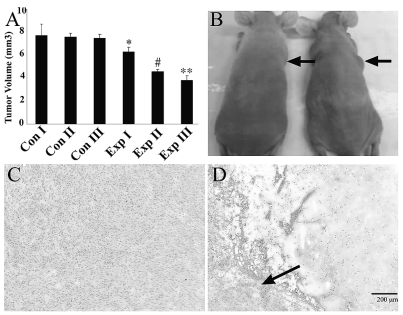
Fig. 3.
Embryonic stem cell (ESC)-derived astrocyte-mediated TRAIL delivery caused decreased tumor volume and necrosis in in vivo malignant astrocytoma xenografts. (A) Bar graph showing significant changes in xenograft volume after transgene delivery in the experimental groups. p-Value for groups: *p < 0.05, #p < 0.01, **p < 0.001, analysis of variance with Bonferroni post hoc test. Groups are defined in “Materials and Methods.” (B) Photograph of experimental (left) and control (right) mice showing decreased tumor size (arrows) 48 h after injection of ESC-derived astrocytes conditionally expressing TRAIL, and doxycycline administration. (C and D) Microphotograph of explanted xenograft in control animal (C) and experimental animal (D) 7 days after transgene administration. Note the severe necrosis in the experimental animal. Scattered tumor cells are seen only at the tumor periphery (arrow). Scale bar, 200 μm.
ESC-Derived Astrocytes Conditionally Expressing TRAIL Induce Apoptosis in Human Malignant Glioma Xenografts In Vivo
Apoptosis was detected by TUNEL in A172 cell xenografts in all experimental animals (Fig. 4A). Apoptosis was not observed in control animals (Fig. 4B). TUNEL-positive cells (apoptotic tumor cells) did not colocalize with ESC-derived astrocytes tracked by PKH26 red dye (Fig. 4C).
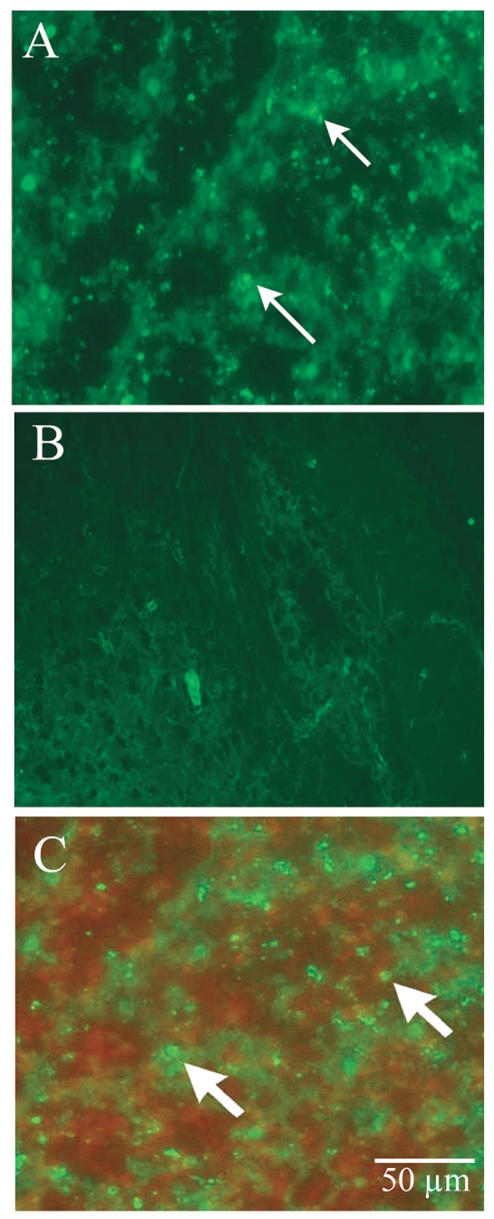
Fig. 4.
Embryonic stem cell (ESC)-derived astrocytes conditionally expressing TRAIL induce apoptosis in human malignant glioma xenografts in vivo. (A and B) Apoptosis occurred in vivo after ESC-mediated gene delivery: fluorescent microphotographs of A172 malignant glioma xenografts in experimental (A) and control (B) animals after TUNEL labeling. Numerous TUNEL-positive cells are seen in the experimental animal (arrows) and not in the controls. (C) Superimposed microphotograph of TUNEL-positive cells (green, arrows) and ESC-derived astrocytes (red). The lack of overlap shows that apoptosis occurred only in tumor cells. Scale bar, 50 μm.
In Vivo Transgenic TRAIL Induces Apoptosis by Increased Expression of Death Receptors
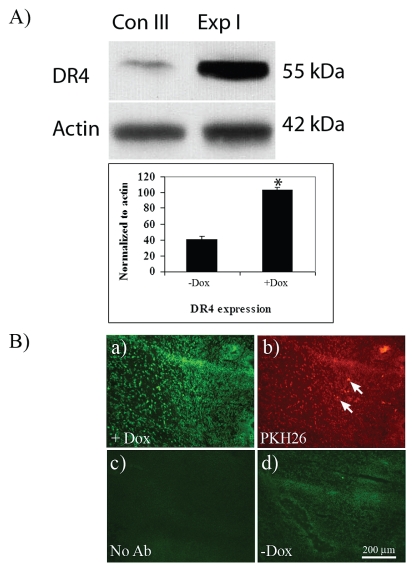
To further study the molecular events underlying the observed enhanced apoptosis caused by ESC-derived astrocytes conditionally expressing TRAIL after Dox induction in in vivo human gliomas, we assessed the expression of the death receptor TRAIL-R1 (DR4) by Western blotting. A 2.5-fold increase in DR4 expression was noted in the experimental groups compared with controls (Fig. 5A). Immunohistochemistry showed marked expression of DR4 in malignant glioma xenografts after Dox induction (Fig. 5B) but not in control groups. DR5 expression was not significantly changed (data not shown).
Fig. 5.
Expression of death receptor DR4 in malignant glioma tumor xenografts. (A) Western blot and bar graph of quantified data from homogenized malignant glioma xenografts. A significant increase (2.5-fold) in DR4 expression was seen in experimental group 1 (Exp I) compared with control group 3 (Con III). Data are normalized to actin. *p < 0.05, Student’s t-test. (B) In situ immunohistochemistry for DR4 in malignant glioma xenografts. (a) DR4 is detected after doxycycline (Dox) induction within the xenograft. (b) PKH26 labeled embryonic-stem-cell–derived TRAIL-expressing astrocytes inside the tumor (arrows). (c and d) Negative control without antibody (c) and without Dox induction (d). Scale bar, 200 μm.
Discussion
In this study, we investigated the application of in vivo gene delivery using ESC-derived astrocytes conditionally expressing TRAIL. ESC-derived astrocytes conditionally expressing TRAIL previously tested in vitro26 were used to induce apoptosis in vivo in a heterotopic brain tumor xenograft model. The finding of tumor reduction and apoptotic tumor cells after administration of ESC-mediated gene delivery supports the hypothesis that ESC-derived astrocytes should be considered as vectors for gene therapy of malignant astrocytomas.
Only a few agents are truly cancer-cell specific in terms of efficacy and cell death induction. TRAIL is a rare example because it kills cancer cells but not normal cells.19,20 Recent studies have also demonstrated that targeting TNF death receptors is a promising strategy for cancer treatment because it induces powerful apoptosis.30 In this study, we used engineered mouse ESC-derived astrocytes to conditionally express TRAIL after Dox induction, as previously published for in vitro experiments.26 We hypothesized that the ESC-derived astrocytes conditionally expressing TRAIL in vivo would retain tet control. Our results showed that transgenic expression of TRAIL after Dox induction was maintained, as demonstrated by RT-PCR results and in situ immunohistochemistry. These data show that the “tet-on” system used to engineer our ESC-derived astrocytes maintains in vivo transcription Dox inducibility. These results, taken together with previously published data,26 support the safety and feasibility of using the “tet-on” system in vivo and suggest that this system can be considered for clinical trials.
Gene therapy provides promising hope for treatment of malignant gliomas. Encouraging results in vitro and in vivo have stemmed from clinical trials using viral vectors.31,32 However, these have had less therapeutic effects than desired, probably due to low levels of gene transduction.2 Alternatively, CED can be used, but this requires the invasive implantation of catheters and precise calculations of drug delivery.24 Improving gene delivery methods and increasing transgene potency are currently being explored to overcome these limitations.31 Alternatively, the use of genetically modified cells to deliver gene therapy to the CNS may avoid some of the limitations of conventional viral therapy. ESCs have tremendous potential in the field of tissue engineering and regenerative medicine because they have both unlimited proliferative ability and the capacity to produce every type of cell and tissue in the body.33 ESCs can propagate indefinitely but retain the capacity to differentiate into all mature somatic phenotypes when induced by appropriate signals.33 Moreover, with a Dox-responsive promoter, the gene can be kept silent when it is no longer required by omitting administration of Dox.18,26
In our study, we showed a significant decrease in tumor burden 48 h after a single injection of ESC-derived astrocytes conditionally expressing TRAIL. The increased reduction in tumor burden seen after multiple injections is most likely secondary to a better distribution of ESC within the tumor itself. Because TRAIL is a cell-membrane-bound protein,34 cell-to-cell interaction is important to increase its proapoptotic effects. The histological finding of minimal residual tumor cells 7 days after transgene administration supports the concept that transgenic delivery by ESC-derived astrocytes should be considered for malignant gliomas. For a clinical trial, human ESC-derived cells should be used to avoid a potential severe immunogenic response with allotransplant. In our laboratory, we are currently working with a human ESC line to replicate our published work with murine ESC.26,27,34
Results from the in vivo investigations reported here suggest two modes of actions for the transgenic TRAIL. First, the increased apoptotic rate using transgenic TRAIL in vivo seems to be independent of p53 status. The malignant glioma cells used for our xenograft model, A172, have mutant p53.34,35 Second, our data suggest that the increased apoptosis might be linked to an increase in DR4 receptor expression. The mechanisms of upregulation of DR4 receptors after TRAIL exposure in vitro are well documented.36–38 However, the mechanisms of TRAIL-R2 (DR4) upregulation and of apoptosis activation pathways (extrinsic vs. intrinsic) are not yet fully elucidated.39 In our study, we found no significant change in DR5 expression, which has been shown to be a p53-inducible gene.40
In conclusion, as the field of gene delivery for malignant gliomas advances, vectors other than viral might be considered. Our study is the first to report in vivo conditional transgenic delivery using ESC-derived astrocytes for malignant gliomas. Conditionally transgenically expressed TRAIL remained under external control of antibiotic administration (“tet-on” system) and resulted in reduced tumor burden, necrosis, and apoptosis. Based on our results, we suggest that ESC-derived astrocyte-mediated gene delivery should be pursued as a new anti-cancer therapeutic approach for patients with malignant gliomas.
Acknowledgment
The work presented in this article was funded in part by a Goldhirsh Foundation research award to I.M.G.
References
- 1.Surawicz TS, Davis F, Freels S, Laws ER, Jr, Menck HR. Brain tumor survival: results from the National Cancer Data Base. J Neurooncol. 1998;40:151–160. doi: 10.1023/a:1006091608586. [DOI] [PubMed] [Google Scholar]
- 2.Chiocca EA, Aghi M, Fulci G. Viral therapy for glioblastoma. Cancer J. 2003;9:167–179. doi: 10.1097/00130404-200305000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Rainov NG, Kramm CM. Recombinant retroviruses for treatment of malignant brain tumors. Int Rev Neurobiol. 2003;55:185–203. doi: 10.1016/s0074-7742(03)01008-0. [DOI] [PubMed] [Google Scholar]
- 4.Hampl JA, Camp SM, Mydlarz WK, et al. Potentiated gene delivery to tumors using herpes simplex virus/Epstein-Barr virus/RV tribrid amplicon vectors. Hum Gene Ther. 2003;14:611–626. doi: 10.1089/104303403321618137. [DOI] [PubMed] [Google Scholar]
- 5.Germano IM, Fable J, Gultekin SH, Silvers A. Adenovirus/herpes simplex-thymidine kinase/ganciclovir complex: preliminary results of a phase I trial in patients with recurrent malignant gliomas. J Neurooncol. 2003;65(3):279–289. doi: 10.1023/b:neon.0000003657.95085.56. [DOI] [PubMed] [Google Scholar]
- 6.Chiocca EA. Oncolytic viruses. Nat Rev Cancer. 2002;2:938–950. doi: 10.1038/nrc948. [DOI] [PubMed] [Google Scholar]
- 7.Martuza RL, Malick A, Markert JM, Ruffner KL, Coen DM. Experimental therapy of human glioma by means of a genetically engineered virus mutant. Science. 1991;252:854–856. doi: 10.1126/science.1851332. [DOI] [PubMed] [Google Scholar]
- 8.Fulci G, Dmitrieva N, Gianni D, et al. Depletion of peripheral macrophages and brain microglia increases brain tumor titers of oncolytic viruses. Cancer Res. 2007;67:9398–9406. doi: 10.1158/0008-5472.CAN-07-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J, Elkhaloun AG, Messina SA, et al. Cellular and genetic characterization of human adult bone marrow-derived neural stem-like cells: a potential antiglioma cellular vector. Cancer Res. 2003;63:8877–8889. [PubMed] [Google Scholar]
- 10.Nakamura K, Ito Y, Kawano Y, et al. Antitumor effect of genetically engineered mesenchymal stem cells in a rat glioma model. Gene Therapy. 2004;11:1155–1164. doi: 10.1038/sj.gt.3302276. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava AS, Sheaouda S, Mishra R, Carrier E. Transplanted embryonic stem cells successfully survive, proliferate, and migrate to damaged regions of the mouse brain. Stem Cells. 2006;24:1689–1694. doi: 10.1634/stemcells.2005-0531. [DOI] [PubMed] [Google Scholar]
- 12.Heese O, Disko A, Zirkel D, Westphal-Lazmus M. Neural stem cell migration toward gliomas in vitro. Neuro-Oncology. 2005;7:476–484. doi: 10.1215/S1152851704000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- 14.Eriksson C, Ericson C, Gates MA. Long-term, EGF-stimulated cultures of attached GFAP-positive cells derived from the embryonic mouse lateral ganglionic eminence: in vitro and transplantation studies. Exp Neurol. 2000;164:184–199. doi: 10.1006/exnr.2000.7424. [DOI] [PubMed] [Google Scholar]
- 15.Selkirk SM, Greenberg SJ, Plunkett RJ, Barone TA, Lis A, Spence PO. Syngeneic central nervous system transplantation of genetically transduced mature, adult astrocytes. Gene Ther. 2002;9:432–443. doi: 10.1038/sj.gt.3301643. [DOI] [PubMed] [Google Scholar]
- 16.Jandial R, Singec I, Ames CP, Snyder EY. Genetic modification of neural stem cells. Mol Ther. 2008;16(3):450–457. doi: 10.1038/sj.mt.6300402. [DOI] [PubMed] [Google Scholar]
- 17.Shih CC, Fu L, Zhu L, Huang Y, Lee TD, Forman SJ. Derivation of neural stem cells from mesenchymal stem cells: evidence for a bipotential stem cell population. Stem Cells Dev. 2008 doi: 10.1089/scd.2008.0068. (online April 21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benveniste RJ, Keller G, Germano IM. Embryonic stem cell-derived astrocytes expressing drug-inducible transgenes: differentiation and transplantation into the mouse brain. J Neurosurg. 2005;103:115–123. doi: 10.3171/jns.2005.103.1.0115. [DOI] [PubMed] [Google Scholar]
- 19.Grataroli R, Vindrieux D, Selva J, Felsenheld C, Ruffion A, Decaussin M. Characterization of tumor necrosis factor alpha-related apoptosis inducing ligand and its receptors in the adult human testis. Mol Hum Reprod. 2004;10:123–128. doi: 10.1093/molehr/gah016. [DOI] [PubMed] [Google Scholar]
- 20.Kagawa S, He C, Gu J, Koch P, Rha SJ, Roth JA. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis inducing ligand (TRAIL) gene. Cancer Res. 2001;61:3330–3338. [PubMed] [Google Scholar]
- 21.Voortman J, Resende TP, Abou El Hassan MA, Giaccone G, Kruyt FA. TRAIL therapy in non-small cell lung cancer cells: sensitization to death receptor-mediated apoptosis by proteasome inhibitor bortezomib. Mol Cancer Ther. 2007;6:2103–2112. doi: 10.1158/1535-7163.MCT-07-0167. [DOI] [PubMed] [Google Scholar]
- 22.Kelley SK, Harris LA, Xie D, et al. Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther. 2001;299:31–38. [PubMed] [Google Scholar]
- 23.Saito R, Bringas JR, Panner A, et al. Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res. 2004;64(19):6858–6862. doi: 10.1158/0008-5472.CAN-04-1683. [DOI] [PubMed] [Google Scholar]
- 24.Linninger AA, Somayaji MR, Mekarski M, Zhang L. Prediction of convection-enhanced drug delivery to the human brain. J Theor Biol. 2008;250(1):125–138. doi: 10.1016/j.jtbi.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 25.Laird DJ, von Andrian UH, Wagers AJ. Stem cell trafficking in tissue development, growth, and disease. Cell. 2008;22(4):132. 612–630. doi: 10.1016/j.cell.2008.01.041. [DOI] [PubMed] [Google Scholar]
- 26.Germano IM, Uzzaman M, Benveniste RJ, Zaurova M, Keller G. Apoptosis in human glioblastoma cells produced using embryonic stem cell-derived astrocytes expressing tumor necrosis factor-related apoptosis-inducing ligand. J Neurosurg. 2006;105:88–95. doi: 10.3171/jns.2006.105.1.88. [DOI] [PubMed] [Google Scholar]
- 27.Uzzaman M, Benveniste RJ, Keller G, Germano IM. Embryonic stem cell-derived astrocytes: a novel approach as a gene therapy vector for brain tumors. Neurosurg Focus. 2005;19(3):E6. doi: 10.3171/foc.2005.19.3.7. [DOI] [PubMed] [Google Scholar]
- 28.Poon Poon RY. PKH fluorescent cell linker dyes. In: Diamond RA, DeMaggio S, editors. Living color: flow cytometry and cell sorting protocols. 2002. pp. 302–352. [Google Scholar]
- 29.Sarkar D, Su ZZ, Vozhilla N, Park ES, Gupta P, Fisher PB. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci U S A. 2005;102(39):14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehtesham M, Kabos P, Gutierrez MA, et al. Induction of glioblastoma apoptosis using neural stem cell-mediated delivery of tumor necrosis factor- related apoptosis inducing ligand. Cancer Res. 2002;62:7170–7174. [PubMed] [Google Scholar]
- 31.Lawler SE, Peruzzi PP, Chiocca EA. Genetic strategies for brain tumor therapy. Cancer Gene Therapy. 2006;13:225–233. doi: 10.1038/sj.cgt.7700886. [DOI] [PubMed] [Google Scholar]
- 32.Lang FF, Bruner JM, Fuller GN, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21:2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 33.Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19:1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- 34.Uzzaman M, Keller G, Germano IM. Enhanced pro-apoptotic effects of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) on temozolomide-resistant glioma cells. J Neurosurg. 2007;106:646–651. doi: 10.3171/jns.2007.106.4.646. [DOI] [PubMed] [Google Scholar]
- 35.Badie B, Goh CS, Klaver J, Herweijer H, Boothman DA. Combined radiation and p53 gene therapy of malignant glioma cells. Cancer Gene Ther. 1999;6(2):155–162. doi: 10.1038/sj.cgt.7700009. [DOI] [PubMed] [Google Scholar]
- 36.Shankar S, Srivastava RK. Enhancement of therapeutic potential of TRAIL by cancer chemotherapy and irradiation: mechanisms and clinical implications. Drug Resist Update. 2004;7:139–156. doi: 10.1016/j.drup.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 37.MacFarlane M. TRAIL-induced signalling and apoptosis. Toxicol Lett. 2003;139(2–3):89–97. doi: 10.1016/s0378-4274(02)00422-8. [DOI] [PubMed] [Google Scholar]
- 38.LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- 39.Kyritsis AP, Tachmazoglou F, Rao JS, Puduvalli VK. Temozolomide and resistant glioma cells. J Neurosurg. 2008;108:197. doi: 10.3171/JNS/2008/108/01/0197. [DOI] [PubMed] [Google Scholar]
- 40.Puduvalli VK, Sampath D, Bruner JM, Nangia J, Xu R, Kyritsis AP. TRAIL-induced apoptosis in gliomas is enhanced by Akt-inhibition and is independent of JNK activation. Apoptosis. 2005;10(1):233–243. doi: 10.1007/s10495-005-6078-3. [DOI] [PMC free article] [PubMed] [Google Scholar]