Abstract
The flavonoid quercetin has been reported to inhibit the proliferation of cancer cells, whereas it has no effect on nonneoplastic cells. U87-MG, U251, A172, LN229, and U373 malignant glioma cells were treated with quercetin (50–200 μM). Quercetin did not cause cytotoxicity 24 h after treatment. Combining quercetin with tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) strongly augmented TRAIL-mediated apoptosis in U87-MG, U251, A172, and LN229 glioma cells; U373 cells could not be sensitized by quercetin to TRAIL-mediated apoptosis. TRAIL-induced apoptosis was enhanced by quercetin-induced reduction of survivin protein levels. Upon treatment with quercetin, the protein level of survivin was strongly suppressed in U87-MG, U251, and A172 but not in U373 glioma cells. Quercetin exposure resulted in proteasomal degradation of survivin. TRAIL-quercetin–induced apoptosis was markedly reduced by overexpression of survivin. In addition, upon treatment with quercetin, downregulation of survivin was also regulated by the Akt pathway. Taken together, the results of the present study suggest that quercetin sensitizes glioma cells to death-receptor–mediated apoptosis by suppression of inhibitor of the apoptosis protein survivin.
Keywords: apoptosis, glioma, quercetin, survivin, TRAIL
Tumor necrosis factor α (TNF-α)-related apoptosis-inducing ligand (TRAIL, or Apo2L) belongs to the TNF cytokine family and is capable of inducing apoptosis in a variety of cancer cells, while producing negligible effects on normal cells.1 TRAIL binds to the death receptors DR4/DR5, which subsequently interact with the adaptor protein FADD (Fas-associated death domain) and procaspase-8, forming the death-inducing signaling complex (DISC). Procaspase-8 activation in DISC leads to cleavage of procaspase-3 and engagement of the cellular machinery associated with the type I extrinsic apoptotic pathway.2,3 Activation of the intrinsic, mitochondrial-associated type II apoptotic pathway is another hallmark of TRAIL-induced cell death because TRAIL, through caspase-8, activates Bid, a proapoptotic bcl-2 family member, and synergizes with agents that induce apoptosis exclusively through a type II mechanism.4 In type I cells, stimulation of the extrinsic pathway is sufficient for commitment of apoptotic cell death. In type II cells, this commitment requires further signal amplification through the intrinsic pathway.5 The intrinsic apoptotic pathway is regulated by the proteins of the bcl-2 family.6,7 Studies have shown that intracranial delivery of native human TRAIL suppresses the growth of human glioma xenografts in mice without host toxicity.8 Clinical phase 1 and phase 2 studies with compounds directed at TRAIL receptors are ongoing with recombinant human TRAIL (Genentech, South San Francisco, CA, USA), with an agonistic TRAIL-DR4 antibody, and with two agonistic TRAIL-DR5 antibodies.9 Data from the studies with recombinant human TRAIL have not yet been published.
Previous reports have shown that most glioma cell lines are more or less resistant to the apoptotic effects of TRAIL.10,11 However, glioma cell lines can be sensitized to TRAIL-induced apoptosis with different chemotherapeutic substances. Thus, identification of novel drugs to sensitize glioma cells toward TRAIL-mediated apoptosis has gained much attention in experimental cancer therapy. Overexpression of inhibitors of apoptosis proteins (IAPs), including survivin and the chromosome X-linked IAP (XIAP), has been reported to confer resistance to TRAIL-mediated apoptosis in several cancer cells.12,13 IAPs contain one or more conserved regions termed baculoviral IAP-repeat (BIR) N-terminal domains and a C-terminal RING (really interesting gene) domain.14,15 The BIR domains block caspase-3 and caspase-9, whereas the RING domain acts as an ubiquitin ligase to facilitate proteasomal degradation of caspases, survivin, and XIAP.15,16 To date, three identified proteins (Smac/DIABLO, Omi/HtrA2, and XAF1) antagonize the anti-apoptotic function of XIAP.17 Both survivin and XIAP are upregulated in gliomas, and their expression positively correlates with poor patient survival, unfavorable prognosis, resistance to therapy, and accelerated rates of recurrence.18
Epidemiological studies in humans have shown that regular consumption of fruits and vegetables is associated with reduced risk of cancer.19–21 One possible explanation is the content of flavonoids, which exert anticarcinogenic activities.21–23 Quercetin is an abundant flavonoid in many fruits and vegetables. To date, several biological activities of flavonoids have been identified. Quercetin induces cell death by apoptosis in leukemia and lung, hepatoma, oral, and colon cancer cell lines.24–26 In addition, quercetin modulates proliferation by affecting mitogen-activated protein kinases and Akt.
In this study, we investigated the effects of quercetin on the expression level of survivin and XIAP. We hypothesized that treatment with quercetin enhances death-receptor–mediated apoptosis induced by TRAIL.
Materials and Methods
Cell Culture and Reagents
Human glioblastoma cell lines U87-MG, A172, and LN229 were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Cell lines U251 and U373 were obtained from ATCC and propagated in our laboratory. Cells were cultured in Dulbecco’s modified Eagle’s medium Glutamax-I (4,500 g/l glucose; Invitrogen, Karlsruhe, Germany) with 10% fetal bovine serum and 1% penicillin/streptomycin (Invitrogen) and incubated at 37°C in a humidified atmosphere containing 10% carbon dioxide. Quercetin was obtained from Sigma, and malignant glioma cell lines were treated with the indicated amounts of quercetin for 24 h or shorter times. Recombinant human TRAIL/Apo2L was purchased from Peprotech (Rocky Hill, NY, USA). MG132 was purchased from Sigma (Schnelldorf, Germany).
Measurement of Cell Viability
Cells were seeded into 24-well plates at a density of 5 × 104 cells/well in 500 μl tissue culture medium, in triplicate. After 24 h of incubation to allow cells to adhere, cells were treated for 24 h with quercetin and TRAIL either separately or in different combinations, as described in individual experiments. Cell viability was determined by trypan blue exclusion assay.
Flow Cytometry
For cell cycle analysis, the glioma cells were cultured for the indicated times, washed, incubated with trypsin for 3 min at 37°C, harvested, washed, and fixed with 75% ice-cold ethanol. Cells (106 per condition) were stained with propidium iodide (50 μg/ml) in phosphate-buffered saline, washed, and subjected to flow cytometric analysis of DNA content using a Becton Dickinson FACSCalibur cytometer (Becton Dickinson, Heidelberg, Germany). The percentage of dead cells was also assessed by flow cytometry.
Transfections
Cells were transfected by electroporation either with control vector or with different plasmids using the Nucleofector device (protocol U29; Amaxa Biosystems, Gaithersburg, MD, USA). Transfection efficiency using nucleofection was between 70% and 90%. Transient transfection of U87 cells was achieved by Fugene Transfection reagent (Roche Deutschland Holding GmbH, Mannheim, Germany) or by electroporation, using Nucleofector I (program U29; Amaxa AG, Cologne, Germany). With electroporation, up to 80% transfection efficiency was achieved. The surviving wild-type plasmid pcDNA3-survivin has been described previously.27 Empty pcDNA3 was used as a negative control in our experiments. The plasmid pUSE-amp-active Akt (Millipore GmbH, Schwalbach, Germany) containing Myc-tagged active Akt and the empty control (pUSE-amp) were transfected into U87-MG.
Western Blot
Twenty micrograms of protein diluted in NuPAGE sample buffer and reducing reagent (Invitrogen) were denatured at 95°C for 5 min and electrophoretically separated on ready-to-use 4%–12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels (Invitrogen). Proteins were blotted onto nitrocellulose membranes at 1.5 mA/cm2 for 1.5 h (Invitrogen). After blocking in 0.5 M Tris base (pH 7.4), 5% milk powder, 1.5 M NaCl, and 0.05% Tween, the membranes were incubated with rabbit antihuman XIAP antibody diluted 1:1,000 (R&D Systems, Minneapolis, USA), rabbit anti-human survivin antibody diluted 1:100 (CST, Inc., Danvers, MA, USA), rabbit antihuman cleaved poly(ADP-ribose) polymerase (PARP; CST, Inc.), rabbit antihuman caspase-7/-8/-9 (CST, Inc.), and rabbit antihuman Bid (CST, Inc.) overnight at 4°C. Staining with secondary horseradish peroxidase-conjugated antirabbit or anti-mouse antibodies at dilutions of 1:10,000 or 1:1,400, respectively (Amersham Biosciences, Buckinghamshire, UK), was followed by immunodetection with the Western blotting detection system ECL Plus (Amersham Biosciences). Protein signals were analyzed semiquantitatively, using a computer-assisted image analysis system and the NIH Image gel analysis software (http://rsb.info.nih.gov/nihimage/download.html). The sum of gray values of all pixels and the sum of densities were determined for each individual band.28 To normalize the means of sum-of-densities values from different experiments, each value was given as the percentage of the control value. Data are reported as means ± SD of n experiments. Significant differences were assessed by paired Student’s t-test, and p < 0.05 was considered statistically significant.
Statistical Analysis
The data are expressed as mean ± SEM of separate experiments (n > 3) and compared by the two-tailed paired Student’s t-test. Differences between two treatments were considered significant at p < 0.05 and p < 0.01.
Results
Quercetin Augments TRAIL-Induced Apoptosis in U87-MG, U251, A172, and LN229 but Not in U373 Cells
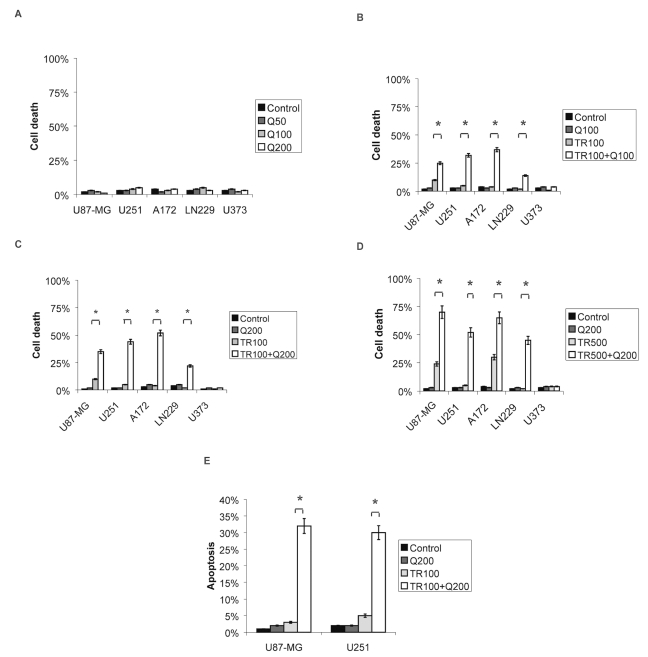
We measured the effects of quercetin with or without addition of TRAIL on cell death by trypan blue exclusion assay. Quercetin alone in various concentrations did not inhibit cell viability 24 h after administration (Fig. 1A). Treatment with 100 ng/ml TRAIL alone had no significant effect on cell death in U87-MG (9% ± 5%), U251 (5% ± 5%), A172 (4% ± 6%), and LN229 (2% ± 2%) cells (Fig. 1B, C), whereas addition of 500 ng/ml TRAIL alone increased cell death in U87-MG (24% ± 5%, p < 0.05) and A172 (30%± 5%, p < 0.05) cells (Fig. 1D). U251, LN229, and U373 cells were resistant to TRAIL treatment (Fig. 1B–D).
Fig. 1.
Trypan blue exclusion assay and flow cytometry 24 h after treatment with tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), quercetin, or the combination of both. (A) Cell death in U87-MG, A172, U251, LN229, and U373 cells upon treatment with quercetin. (B–D) Effect of quercetin on TRAIL-induced cytotoxicity in U87-MG, A172, U251, LN229, and U373 cells. (E) Effect of quercetin on TRAIL-induced apoptosis in U87-MG and U251 cells analyzed by flow cytometry. Control, not treated; Q50/100/200, quercetin 50/100/200 μM; TR100/500, TRAIL 100/500 ng/ml. *Significantly different from the respective control (t-test, *p < 0.05).
Combinations of i) 100 μM quercetin and 100 ng/ml TRAIL, ii) 200 μM quercetin and 100 ng/ml TRAIL, and iii) 200 μM quercetin and 500 ng/ml TRAIL increased cell death in U87-MG (i, 25% ± 5%; ii, 36% ± 2%; iii, 70% ± 4%), U251(i, 32% ± 4%; ii, 44% ± 2%; iii, 52% ± 5%), A172 (i, 14% ± 5%; ii, 52% ± 4%; iii, 65% ± 6%), and LN229 (i, 14% ± 5%; ii, 22% ± 5%; iii, 45% ± 5%) but not in U373 (i, 4% ± 4%; ii, 1% ± 2%; iii, 1% ± 5%) cells (Fig. 1B–D). To confirm that TRAIL-quercetin–mediated cell death occurs by apoptosis, we employed flow cytometry and determined the percentage of apoptotic cells with a subdiploid DNA content. U87-MG and U251 cells treated with either 100 ng/ml TRAIL or 200 μM quercetin alone did not exhibit a significant increase in apoptosis compared with that of untreated controls. Combined treatment with 100 ng/ml TRAIL and 200 μM quercetin resulted in 30%± 6% apoptotic cells in U251 and 32%± 5% in U87-MG (Fig. 1E).
Quercetin Enhances TRAIL-Induced Apoptosis through Activation of Both the Extrinisic and Intrinsic Apoptotic Pathway
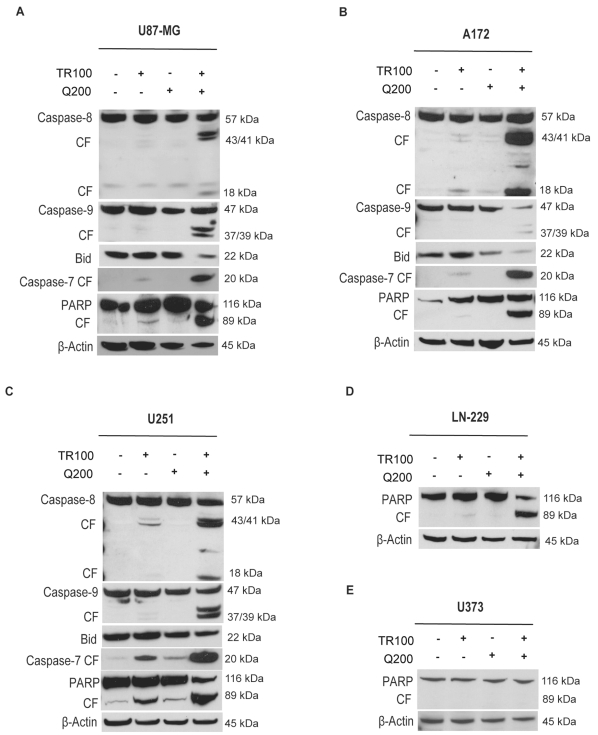
We employed Western blotting to elucidate the proteolytic mechanism in TRAIL-induced and TRAIL-quercetin– induced apoptosis. Because quercetin augments TRAIL-induced apoptosis, we examined the activation/cleavage of caspase-8, -7, and -9, Bid, and PARP.
Treatment of U87-MG, U251, A172, LN229, and U373 cells with 200 μM quercetin alone did not induce cleavage of PARP or effector caspase-7. Exposure of cells to 100 ng/ml TRAIL alone yielded significant signals for the 89-kDa cleaved fragment of PARP and active 20-kDa cleaved caspase-7 in U251 and U87-MG but not A172, LN229, or U373 cells. Combining TRAIL with quercetin led to a significant increase in cleaved fragment of PARP and active cleaved caspase-7 in U87-MG, U251, A172, and LN229 but not U373 cells (Fig. 2A–E).
Fig. 2.
Immunoblot demonstrating the effect of quercetin on tumor necrosis factor–related apoptosis-inducing ligand (TRAIL)-induced proteolytic cleavage of poly(ADP-ribose) polymerase (PARP), caspase-7/-8/-9, and Bid in U87-MG, A172, U251, LN229, and U373 cells. (A) U87-MG, (B) A172, (C) U251, (D) LN229, and (E) U373 cells were treated for 8 h with quercetin (200 μM) in the presence or absence of TRAIL (100 ng/ml). TR100, TRAIL 100 ng/ml; Q200, quercetin 200 μM; CF, cleaved fragment. Immunoblots are representative of at least three independent experiments.
Upon treatment with TRAIL (100 ng/ml) alone we detected 43-kDa and 18-kDa cleavage products of caspase-8 in U87-MG, A172, and U251 cells (Fig. 2A–C). These cleavage products significantly increased after combined treatment with 100 ng/ml TRAIL and 200 μM quercetin (Fig. 2A–C), indicating involvement of the extrinsic apoptotic pathway. To determine whether quercetin enhances the activation of the intrinsic, mitochondrial-associated type II apoptotic pathway by TRAIL-mediated apoptosis, we analyzed the expression of Bid, which is a substrate of active caspase-8. Cleaved Bid participated in the cascade, eventually leading to active cleaved caspase-9, which is a hallmark of mitochondrion-mediated apoptosis (Fig. 2A–C).
The combination of TRAIL with quercetin resulted in a significant decrease of 22-kDa Bid in U87-MG and A172 cells, whereas the reduction of Bid in U251 cells was significant but less pronounced. This treatment resulted in a significant increase in active cleaved 39-kDa and 37-kDa caspase-9 in U87-MG, U251, and A172 cells (Fig. 2A–C). Taken together, these data indicate that combined quercetin-TRAIL treatment induces apoptosis through the extrinsic and intrinsic pathways.
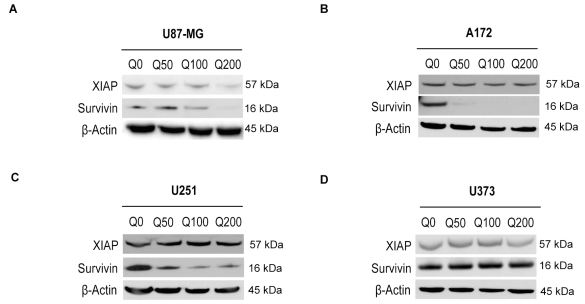
Quercetin Suppresses the Protein Levels of Survivin and XIAP
Because quercetin augments TRAIL-induced apoptosis by activation of caspases, we examined the effects of quercetin on the expression of IAPs XIAP and survivin in U87-MG, U251, A172, and U373 cells (Fig. 3A–D). Expression of IAPs was analyzed by Western blot analysis after treatment with quercetin for 24 h. Survivin levels were suppressed significantly in a concentration-dependent manner by quercetin in U87-MG, U251, and A172 cells, whereas the levels of XIAP were significantly reduced only in U87-MG and not in A172 and U251 cells (Fig. 3A–D). Survivin and XIAP levels were unaltered in U373 glioma cells (Fig. 3D). These data suggest that inhibition/cleavage of IAPs by quercetin may be one of the mechanisms regulating apoptosis and that this effect of quercetin is stronger for survivin than for XIAP.
Fig. 3.
Immunoblot showing expression of chromosome X-linked inhibitor of apoptosis proteins (XIAP) and survivin after treatment with quercetin. (A) U87-MG, (B) A172, (C) U251, and (D) U373 cells were treated for 24 h. Q0/50/100/200, quercetin 0/50/100/200 μM.
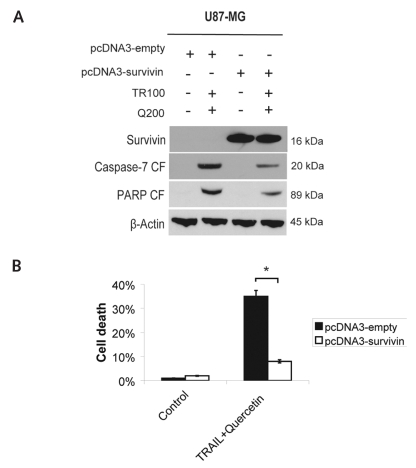
Overexpression of Survivin Suppresses TRAIL-Quercetin–Mediated Cytotoxicity
Survivin is significantly downregulated by quercetin in three of four glioma cells. We therefore investigated whether ectopic overexpression of survivin rescues sensitive U87-MG cells from TRAIL-quercetin–induced apoptosis. Transfection efficiency was 85%, and survivin expression was confirmed by Western blotting (Fig. 4A). Overexpression of survivin in U87-MG cells and treatment with 100 ng/ml TRAIL and 200 μM quercetin for 24 h decreased cell death, from 35% down to 8%, in cells without ectopic survivin expression (p < 0.01; Fig. 4B). As expected, overexpression of survivin after treatment with TRAIL-quercetin resulted in reduced cleavage of PARP and caspase-7 compared with cells without ectopic survivin expression (Fig. 4A).
Fig. 4.
Overexpression of survivin attenuates tumor necrosis factor– related apoptosis-inducing ligand (TRAIL)-quercetin–induced apoptosis in U87-MG cells. (A) Immunoblots demonstrating survivin expression and cleavage products of poly(ADP-ribose) polymerase (PARP) and caspase-7 72 h after transfection with pcDNA3-survivin or empty pcDNA3 and 24 h after combined treatment with TRAIL and quercetin. (B) Trypan blue exclusion assay in U87-MG cells 72 h after transfection and 24 h after treatment with TRAIL and quercetin. Control, not treated; TR100, TRAIL 100 ng/ml; Q200, quercetin 200 μM. *Significantly different from the respective control (t-test, *p < 0.05).
Quercetin Mediates Degradation of Survivin through the Proteasome
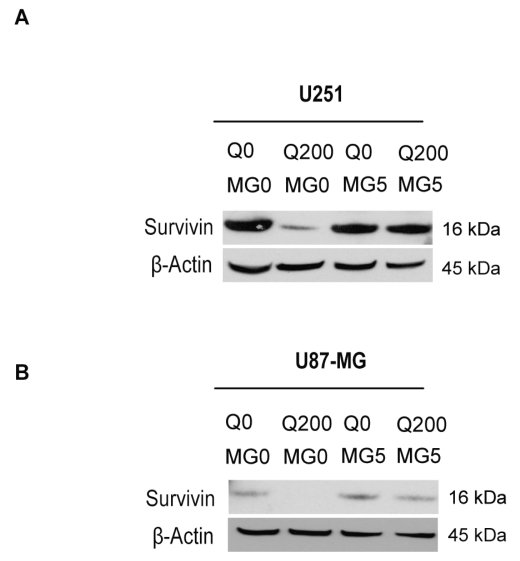
Survivin possesses a short half-life of about 30 min and is readily ubiquitinized, followed by degradation through the proteasome.29 We hypothesized that quercetin affects the prevalence of survivin by activating proteasomal degradation. We treated U251 and U87-MG with combinations of proteasome inhibitor MG132 and 200 μM quercetin for 24 h. Suppression of survivin by quercetin in U251 and U87-MG cells was almost completely abolished by MG132 (Fig. 5A, B; Sigma).
Fig. 5.
Proteasomal degradation of survivin is enhanced in glioma cells upon treatment with quercetin. Immunoblots show inhibition of quercetin-mediated downregulation of survivin by MG132 in U251 (A) and U87-MG (B) cells. Q0/200, quercetin 0/200 μM; MG0/5, MG132 0/5 μM.
Survivin Expression Is Regulated by Phosphoinositide 3-Kinase/Akt after Quercetin Treatment
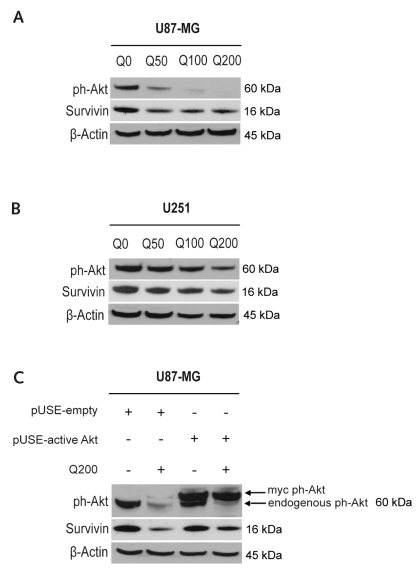
We postulated that quercetin inhibits Akt activity and consecutively enhances TRAIL-induced cytotoxicity through suppression of survivin. We analyzed the expression of Akt phosphorylated at Ser-473 (ph-Akt) in U87-MG and U251 cells after treatment with quercetin for 8 h (Fig. 6A, B). We observed a strong suppression of ph-Akt in U87-MG cells and moderate suppression in U251 cells. Suppression of survivin was also significant in both cell lines (Fig. 6A, B). To confirm the regulation of survivin by Akt upon treatment with quercetin in glioma cells, we ectopically overexpressed ph-Akt (Fig. 6C). After 48 h, the transfected cells were treated with 200 μM quercetin or left untreated for 24 h. Both endogenous Akt and myc-tagged Akt were detected in these cells. The lower band representing endogenous Akt is markedly suppressed by 200 μM quercetin (Fig. 6C). Notably, overexpression of ph-Akt increased survivin levels in quercetin-treated U87-MG glioma cells compared with quercetin-treated cells without ectopic ph-Akt expression (Fig. 6C).
Fig. 6.
Quercetin-mediated suppression of survivin is regulated by Akt. (A and B) Immunoblots show reduced phosphorylated Akt (ph-Akt) and survivin levels in U87-MG (A) and U251 (B) cells following treatment with quercetin for 8 h. (C) Immunoblot shows that over-expression of constitutively active Akt inhibits quercetin-mediated suppression of survivin in U87-MG cells 72 h after transfection and 24 h after treatment with quercetin. Immunoblots are representative of at least three independent experiments. Q0/50/100/200, quercetin 0/50/100/200 μM.
Discussion
In this study, we analyzed effects of the flavonoid quercetin on TRAIL-mediated apoptosis in human glioma cells. Both TRAIL and quercetin are in clinical testing and have been shown to be of minimal toxicity.9,30 Many cancer cells are resistant to TRAIL therapy.1,31,32 We therefore aimed at sensitizing TRAIL-resistant glioma cells with quercetin. In this study, we demonstrated that combined application of TRAIL and quercetin strongly reduced viability of U251, LN229, U87-MG, and A172 glioma cells but failed to do so in U373 cells. All our experiments on cell cultures were conducted with concentrations of quercetin that have been reached in clinical trials up to 400 μM plasma concentration of quercetin was achieved.30
First, we demonstrated that viability of glioma cells was not affected 24 h after a single dose of quercetin. This finding is similar to results in various carcinoma cell lines.33,34 We then determined viability of our set of glioma cells after TRAIL treatment and detected an effect only at a high TRAIL concentration of 500 ng/ml. Our results match those of previously published data that demonstrated moderate TRAIL sensitivity of U87-MG and A172 cells but high resistance to this treatment in U251, LN229, and U373 cells.10,11 Upon combined treatment with quercetin and TRAIL, U87-MG, U251, A172, and LN229 cells exhibited strongly enhanced apoptosis, whereas U373 cells proved completely resistant. Our data parallel those achieved by sensitizing glioma cells to TRAIL with the protein synthesis inhibitor cycloheximide, to which U373 cells were also insensitive.11,35 The strong effect of simultaneous application of TRAIL and quercetin demonstrates a synergistic action of combined treatment. Our results are in line with previous reports because combined treatment with TRAIL and quercetin has been successfully tested in other cell lines.33,34 The resistance of U373 cells is not fully understood. It has been reported that U373 cells express only low levels of the initiator caspase-8, thereby leading to an insufficient activation of DISC, resulting in inhibition of the extrinsic apoptotic pathway.10 Inactivation of p53, which is involved in the intrinsic apoptotic pathway, seems not to be of major importance to quercetin-TRAIL–mediated apoptosis. This is supported by TP53 mutations in both the sensitive U251 and the completely resistant U373 cell lines.36
The IAPs are an important family of apoptosis regulating proteins, with survivin and XIAP as prominent members. Because survivin and XIAP block apoptosis at the level of effector caspases, a point where multiple signaling pathways converge, strategies targeting survivin to remove its inhibitory effect seem to be useful to overcome the resistance of cancer cells. Suppression of survivin has been demonstrated upon quercetin treatment in H460 lung cancer cells.34 We analyzed the effect of quercetin in four glioma cell lines on expression of survivin and XIAP. Survivin expression was strongly reduced in three of four glioma cell lines, whereas XIAP was reduced only in U87-MG. Notably, the U373 glioma cell line, which could not be sensitized to TRAIL-mediated apoptosis, showed suppression neither of survivin nor of XIAP upon treatment with quercetin. The role of survivin in quercetin-TRAIL–mediated apoptosis was demonstrated by ectopic expression. Cells with vector-driven overexpression of survivin had a strongly reduced rate of apoptosis after combined TRAIL-quercetin treatment. These findings indicate that suppression of survivin is a key mechanism through which quercetin enhances TRAIL-mediated apoptosis. The previous demonstration of augmentation of TRAIL-induced apoptosis by cycloheximide may also directly connect to survivin prevalence,10,11 because survivin has a short half-life of 30 min, making this protein very susceptible to inhibition of protein synthesis. In contrast, XIAP expression was reduced in only one of four cell lines upon quercetin treatment. With the exception of U87-MG, the other cell lines did not show XIAP reduction, paralleling the lacking response of such treatment in non-small-cell lung cancer cells.34 Low prevalence of both IAPs, survivin and XIAP, may explain the high rate of apoptosis of U87-MG upon quercetin-TRAIL treatment (Fig. 1E).
We identified two potential mechanisms leading to quercetin-induced changes of expression in survivin. Pretreatment with MG132 significantly inhibited quercetin-induced downregulation of survivin in U251 and U87-MG glioma cells, suggesting that quercetin may promote proteasome-mediated degradation of survivin. Proteasome-mediated degradation of survivin as a mechanism of regulation has already been reported.34,37 It has been demonstrated that survivin protein was unstable (half-life ~ 30 min) and easily ubiquitinized, followed by degradation through the proteasome.29 Akt, also referred to as Rac or protein kinase B, promotes cell survival and blocks apoptosis.38 Activation of phosphoinositide 3-kinase/Akt pathway generates phosphatidylinositol-3,4,5-triphosphate, which in turn binds to a domain of serine/threonine kinase Akt, resulting in recruitment of Akt to the cell membrane. A conformational change of Akt results in phosphorylation of residues Thr-308 and Ser-473 by the upstream kinases 3-phosphoinositide dependent protein kinase-1 (PDK-1) and PDK-2. Akt regulates apoptosis via the direct phosphorylation and inactivation of Bid, caspase 9, the Fork-head transcription factors, and nuclear factor-κB.39,40 The Akt-survivin pathway has been implicated in the resistance of cancer cells to therapeutics and TRAIL.41,42 Quercetin has been shown to inhibit the Akt-1 pathway in HepG2 cancer cells.43 In line with previous reports, in the present study we demonstrated that the inhibitory effect of quercetin on survivin expression appears to result from suppression of Akt activity by quercetin, because overexpression of phosphorylated Akt inhibited quercetin-mediated survivin suppression in U87-MG cells (Fig. 6C). Other research groups have also reported that elevated Akt activity protects cells from TRAIL-induced apoptosis,41,44 which is in line with our results because Akt regulates survivin expression.
In conclusion, we demonstrated that quercetin effectively enhances TRAIL-mediated cytotoxicity by suppressing survivin via increased proteasomal degradation and via repressing phosphorylated Akt known to coregulate survivin protein levels.
Acknowledgments
This work was supported by grants from the postdoctoral program of the University of Heidelberg. We thank Volker Ehemann for performing the flow cytometry and Jana Mucha for technical assistance. The survivin wild-type plasmid pcDNA3-survivin was kindly provided by Dr. Dario Altieri.
References
- 1.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8(8):808–815. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 2.Boldin MP, Goncharov TM, Goltsev YV, Wallach D. Involvement of MACH, a novel MORT1/FADD-interacting protease, in Fas/APO-1- and TNF receptor-induced cell death. Cell. 1996;85(6):803–815. doi: 10.1016/s0092-8674(00)81265-9. [DOI] [PubMed] [Google Scholar]
- 3.Muzio M, Chinnaiyan AM, Kischkel FC, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85(6):817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- 4.Deng Y, Lin Y, Wu X. TRAIL-induced apoptosis requires Bax-dependent mitochondrial release of Smac/DIABLO. Genes Dev. 2002;16(1):33–45. doi: 10.1101/gad.949602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10(1):66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- 6.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 7.Manion MK, Hockenbery DM. Targeting BCL-2-related proteins in cancer therapy. Cancer Biol Ther. 2003;2(4 suppl 1):S105–S114. [PubMed] [Google Scholar]
- 8.Roth W, Isenmann S, Naumann U, et al. Locoregional Apo2L/TRAIL eradicates intracranial human malignant glioma xenografts in athymic mice in the absence of neurotoxicity. Biochem Biophys Res Commun. 1999;265(2):479–483. doi: 10.1006/bbrc.1999.1693. [DOI] [PubMed] [Google Scholar]
- 9.de Vries EG, Gietema JA, de Jong S. Tumor necrosis factor-related apoptosis-inducing ligand pathway and its therapeutic implications. Clin Cancer Res. 2006;12(8):2390–2393. doi: 10.1158/1078-0432.CCR-06-0352. [DOI] [PubMed] [Google Scholar]
- 10.Knight MJ, Riffkin CD, Muscat AM, Ashley DM, Hawkins CJ. Analysis of FasL and TRAIL induced apoptosis pathways in glioma cells. Oncogene. 2001;20(41):5789–5798. doi: 10.1038/sj.onc.1204810. [DOI] [PubMed] [Google Scholar]
- 11.Röhn TA, Wagenknecht B, Roth W, et al. CCNU-dependent potentiation of TRAIL/Apo2L-induced apoptosis in human glioma cells is p53-independent but may involve enhanced cytochrome c release. Oncogene. 2001;20(31):4128–4137. doi: 10.1038/sj.onc.1204534. [DOI] [PubMed] [Google Scholar]
- 12.Ng CP, Bonavida B. X-linked inhibitor of apoptosis (XIAP) blocks Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand- mediated apoptosis of prostate cancer cells in the presence of mitochondrial activation: sensitization by overexpression of second mitochondria-derived activator of caspase/direct IAP-binding protein with low pl (Smac/DIABLO) Mol Cancer Ther. 2002;1(12):1051–1058. [PubMed] [Google Scholar]
- 13.Griffith TS, Fialkov JM, Scott DL, et al. Induction and regulation of tumor necrosis factor-related apoptosis-inducing ligand/Apo-2 ligand-mediated apoptosis in renal cell carcinoma. Cancer Res. 2002;62(11):3093–3099. [PubMed] [Google Scholar]
- 14.Reed JC. Mechanisms of apoptosis avoidance in cancer. Curr Opin Oncol. 1999;11(1):68–75. doi: 10.1097/00001622-199901000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Deveraux QL, Reed JC. IAP family proteins—suppressors of apoptosis. Genes Dev. 1999;13(3):239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 16.Liston P, Young SS, Mackenzie AE, Korneluk RG. Life and death decisions: the role of the IAPs in modulating programmed cell death. Apoptosis. 1997;2(5):423–441. doi: 10.1023/a:1026465926478. [DOI] [PubMed] [Google Scholar]
- 17.Holcik M, Gibson H, Korneluk RG. XIAP: apoptotic brake and promising therapeutic target. Apoptosis. 2001;6(4):253–261. doi: 10.1023/a:1011379307472. [DOI] [PubMed] [Google Scholar]
- 18.Wagenknecht B, Glaser T, Naumann U, et al. Expression and biological activity of X-linked inhibitor of apoptosis (XIAP) in human malignant glioma. Cell Death Differ. 1999;6(4):370–376. doi: 10.1038/sj.cdd.4400503. [DOI] [PubMed] [Google Scholar]
- 19.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3(10):768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 20.Surh YJ, Ferguson LR. Dietary and medicinal antimutagens and anti-carcinogens: molecular mechanisms and chemopreventive potential—highlights of a symposium. Mutat Res. 2003:523–524. 1–8. doi: 10.1016/s0027-5107(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 21.Yang CS, Landau JM, Huang MT, Newmark HL. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu Rev Nutr. 2001;21:381–406. doi: 10.1146/annurev.nutr.21.1.381. [DOI] [PubMed] [Google Scholar]
- 22.Middleton E, Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52(4):673–751. [PubMed] [Google Scholar]
- 23.Watson WH, Cai J, Jones DP. Diet and apoptosis. Annu Rev Nutr. 2000;20:485–505. doi: 10.1146/annurev.nutr.20.1.485. [DOI] [PubMed] [Google Scholar]
- 24.Ong CS, Tran E, Nguyen TT, et al. Quercetin-induced growth inhibition and cell death in nasopharyngeal carcinoma cells are associated with increase in Bad and hypophosphorylated retinoblastoma expressions. Oncol Rep. 2004;11(3):727–733. [PubMed] [Google Scholar]
- 25.Shen SC, Chen YC, Hsu FL, Lee WR. Differential apoptosis-inducing effect of quercetin and its glycosides in human promyeloleukemic HL-60 cells by alternative activation of the caspase 3 cascade. J Cell Biochem. 2003;89(5):1044–1055. doi: 10.1002/jcb.10559. [DOI] [PubMed] [Google Scholar]
- 26.Singhal RL, Yeh YA, Praja N, Olah E, Sledge GW, Jr, Weber G. Quercetin down-regulates signal transduction in human breast carcinoma cells. Biochem Biophys Res Commun. 1995;208(1):425–431. doi: 10.1006/bbrc.1995.1355. [DOI] [PubMed] [Google Scholar]
- 27.Ambrosini G, Adida C, Altieri DC. A novel anti-apoptosis gene, survivin, expressed in cancer and lymphoma. Nat Med. 1997;3(8):917–921. doi: 10.1038/nm0897-917. [DOI] [PubMed] [Google Scholar]
- 28.Siegelin M, Touzani O, Toutain J, Liston P, Rami A. Induction and redistribution of XAF1, a new antagonist of XIAP in the rat brain after transient focal ischemia. Neurobiol Dis. 2005;20(2):509–518. doi: 10.1016/j.nbd.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Zhao J, Tenev T, Martins LM, Downward J, Lemoine NR. The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. J Cell Sci. 2000;113(pt 23):4363–4371. doi: 10.1242/jcs.113.23.4363. [DOI] [PubMed] [Google Scholar]
- 30.Ferry DR, Smith A, Malkhandi J, et al. Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res. 1996;2(4):659–668. [PubMed] [Google Scholar]
- 31.Koschny R, Ganten TM, Sykora J, et al. TRAIL/bortezomib cotreatment is potentially hepatotoxic but induces cancer-specific apoptosis within a therapeutic window. Hepatology. 2007;45(3):649–658. doi: 10.1002/hep.21555. [DOI] [PubMed] [Google Scholar]
- 32.Koschny R, Holland H, Sykora J, et al. Bortezomib sensitizes primary human astrocytoma cells of WHO grades I to IV for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. Clin Cancer Res. 2007;13(11):3403–3412. doi: 10.1158/1078-0432.CCR-07-0251. [DOI] [PubMed] [Google Scholar]
- 33.Kim YH, Lee YJ. TRAIL apoptosis is enhanced by quercetin through Akt dephosphorylation. J Cell Biochem. 2007;100(4):998–1009. doi: 10.1002/jcb.21098. [DOI] [PubMed] [Google Scholar]
- 34.Chen W, Wang X, Zhuang J, Zhang L, Lin Y. Induction of death receptor 5 and suppression of survivin contribute to sensitization of TRAIL-induced cytotoxicity by quercetin in non-small cell lung cancer cells. Carcinogenesis. 2007;28(10):2114–2121. doi: 10.1093/carcin/bgm133. [DOI] [PubMed] [Google Scholar]
- 35.Hao C, Beguinot F, Condorelli G, et al. Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apoptosis in human malignant glioma cells. Cancer Res. 2001;61(3):1162–1170. [PubMed] [Google Scholar]
- 36.Rieger J, Naumann U, Glaser T, Ashkenazi A, Weller M. APO2 ligand: a novel lethal weapon against malignant glioma? FEBS Lett. 1998;427(1):124–128. doi: 10.1016/s0014-5793(98)00409-8. [DOI] [PubMed] [Google Scholar]
- 37.Arora V, Cheung HH, Plenchette S, Micali OC, Liston P, Korneluk RG. Degradation of survivin by the X-linked inhibitor of apoptosis (XIAP)-XAF1 complex. J Biol Chem. 2007;282(36):26202–26209. doi: 10.1074/jbc.M700776200. [DOI] [PubMed] [Google Scholar]
- 38.Franke TF, Yang SI, Chan TO, et al. The protein kinase encoded by the Akt proto-oncogene is a target of the PDGF-activated phosphatidylinositol 3-kinase. Cell. 1995;81(5):727–736. doi: 10.1016/0092-8674(95)90534-0. [DOI] [PubMed] [Google Scholar]
- 39.Cardone MH, Roy N, Stennicke HR, et al. Regulation of cell death protease caspase-9 by phosphorylation. Science. 1998;282(5392):1318–1321. doi: 10.1126/science.282.5392.1318. [DOI] [PubMed] [Google Scholar]
- 40.Brunet A, Bonni A, Zigmond MJ, et al. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96(6):857–868. doi: 10.1016/s0092-8674(00)80595-4. [DOI] [PubMed] [Google Scholar]
- 41.Nesterov A, Lu X, Johnson M, Miller GJ, Ivashchenko Y, Kraft AS. Elevated AKT activity protects the prostate cancer cell line LNCaP from TRAIL-induced apoptosis. J Biol Chem. 2001;276(14):10767–10774. doi: 10.1074/jbc.M005196200. [DOI] [PubMed] [Google Scholar]
- 42.Papapetropoulos A, Fulton D, Mahboubi K, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275(13):9102–9205. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 43.Granado-Serrano AB, Martín MA, Bravo L, Goya L, Ramos S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2) J Nutr. 2006;136(11):2715–2721. doi: 10.1093/jn/136.11.2715. [DOI] [PubMed] [Google Scholar]
- 44.Panner A, James CD, Berger MS, Pieper RO. mTOR controls FLIPS translation and TRAIL sensitivity in glioblastoma multiforme cells. Mol Cell Biol. 2005;25(20):8809–8823. doi: 10.1128/MCB.25.20.8809-8823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]