Abstract
The aim of this study was to determine the efficacy of sagopilone (ZK-EPO), a novel epothilone, compared with other anticancer agents in orthotopic models of human primary and secondary brain tumors. Autoradiography and pharmacokinetic analyses were performed on rats and mice to determine passage across the blood–brain barrier and organ distribution of sagopilone. Mice bearing intracerebral human tumors (U373 or U87 glioblastoma, MDA-MB-435 melanoma, or patient-derived non-small-cell lung cancer [NSCLC]) were treated with sagopilone 5–10 mg/kg, paclitaxel 8–12.5 mg/kg (or temozolomide, 100 mg/kg) or control (vehicle only). Tumor volume was measured to assess antitumor activity. Sagopilone crossed the blood–brain barrier in both rat and mouse models, leading to therapeutically relevant concentrations in the brain with a long half-life. Sagopilone exhibited significant antitumor activity in both the U373 and U87 models of human glioblastoma, while paclitaxel showed a limited effect in the U373 model. Sagopilone significantly inhibited the growth of tumors from CNS metastasis models (MDA-MB-435 melanoma and patient-derived Lu7187 and Lu7466 NSCLC) implanted in the brains of nude mice, in contrast to paclitaxel or temozolomide. Sagopilone has free access to the brain. Sagopilone demonstrated significant anti-tumor activity in orthotopic models of both glioblastoma and CNS metastases compared with paclitaxel or temozolomide, underlining the value of further research evaluating sagopilone in the treatment of brain tumors. Sagopilone is currently being investigated in a broad phase II clinical trial program, including patients with glioblastoma, NSCLC, breast cancer, and melanoma.
Keywords: blood-brain barrier, brain tumor models, sagopilone
Both primary and secondary malignant brain tumors are associated with profound physical and cognitive impairments. Almost 80% of primary brain tumors are gliomas, and the most common form, glioblastoma, is associated with very poor survival.1 Similarly, median 1-year survival for breast cancer patients with brain metastases is approximately 20%,2,3 and median survival for non-small-cell lung cancer (NSCLC) or melanoma patients with CNS metastases is 3 and 3.8 months, respectively.4,5 New, effective treatment options are clearly required for patients with CNS tumors.
The incidence of CNS metastases varies between tumor types, being most commonly seen in patients with breast cancer, lung cancer, and melanoma.6–9 This incidence, however, is often underestimated due to the general absence of brain-imaging studies in neurologically asymptomatic cancer patients. Additionally, the occurrence of CNS metastases may be increasing because of improvements in survival due to earlier detection and better treatment of primary tumors,10 as illustrated in patients with human epidermal growth factor receptor 2 (HER2)-positive breast cancer11 or those receiving multimodality treatment for NSCLC.12
Although the pathophysiology of metastatic brain tumor growth is not yet fully understood, the lack of accurate early detection and of effective systemic therapies are certainly important contributing factors for the poor prognosis. The blood–brain barrier, a unique, highly specialized structure created by the interaction between astrocytes and vascular endothelium,13 is also thought to have significant implications for the effective treatment of brain tumors or brain metastases. Astrocytic foot processes surround blood vessels, producing tight junctions with decreased pinocytic vessel activity. Along with the lack of a lymphatic system, this creates an immunologically privileged space that prevents entry of certain macromolecules. In breast and ovarian cancer patients treated with paclitaxel, the CNS may become a sanctuary site for metastases,14–16 and the incidence of CNS metastases in breast cancer is thought to be as high as 30%–34% in patients treated with therapies such as taxanes or trastuzumab.17 Similarly, CNS metastases have been reported to be present in 37% of patients with distant-stage primary melanoma.18
The reasons underlying the poor or selective efficacy of agents against CNS tumors are complex. For example, paclitaxel appears to be recognized and excluded from the CNS by cellular efflux mechanisms such as P-glycoprotein (P-gp), which is highly expressed in cerebral capillaries.19 Unlike paclitaxel, temozolomide can cross the blood–brain barrier20 and is effective in treating glioblastoma; however, it has been associated with the development of drug resistance21,22 and appears to have little activity in the treatment of brain metastases in patients with breast cancer or NSCLC.23,24
Epothilones are microtubule-stabilizing agents currently in development for the treatment of cancer. Sagopilone (ZK-EPO), the first fully synthetic epothilone in clinical development, was strategically designed to combine high efficacy and a balanced side-effect profile for the treatment of solid tumors. Sagopilone is not recognized by P-gp efflux mechanisms and has demonstrated higher in vitro activity compared with conventional agents or other epothilones across a range of human tumor types, with a mean 50% inhibitory concentration (IC50) of <1 nM in >60 cell lines tested.25 In vivo studies have confirmed the efficacy of sagopilone in multiple models of human tumors, including those that are normally not sensitive to chemotherapy.26,27
The aim of this study was to determine whether sagopilone could cross the blood–brain barrier and reduce brain tumor growth more effectively than other anti-cancer agents in clinically relevant models of human tumors.
Materials and Methods
Compounds
Sagopilone was produced by total synthesis in the Bayer Schering laboratories. A 6 mg/ml solution of paclitaxel (Bristol-Myers Squibb, Princeton, NJ, USA) containing 50.2% (vol) ethanol was used in experiments. Temozolomide was purchased from ABCR GmbH & Co. KG (Karlsruhe, Germany).
Animals
Severe combined immunodeficiency (SCID) mice and Wistar rats were used to investigate how effectively sagopilone penetrated the blood–brain barrier. Distribution of tritiated sagopilone was evaluated in rats, and tumor-bearing NMRI nude mice (HeLa/MaTu cervical cancer xenograft model) were used to determine the organ distribution of intact sagopilone.
All adult NMRI:nu/nu mice (Taconic, Ry, Denmark) used in the brain tumor model studies were maintained in pathogen-free, controlled conditions. Female NMRI nude mice were used in the U373 and U87 glioblastoma models and the MDA-MB-435 melanoma brain metastasis model; male NMRI nude mice were used in the Lu7187 and Lu7466 patient-derived NSCLC brain metastasis models. All animal experiments were performed according to the German Animal Welfare Act of 1998 and with approval from the responsible authorities.
Distribution of Tritiated Sagopilone
The distribution of [3H]-sagopilone was investigated in rats. [3H]-sagopilone was administered intravenously at 2.5 mg/kg (corresponding to 100 MBq) to a treatment group of seven animals. Animals were sacrificed at various time points, and the distribution of radioactivity was measured using whole-body autoradiography. Animals were sectioned at −20°C using a Leica CM 3600 cryomicrotome (Leica GmbH, Nussloch, Germany) with a single-use knife (Feather, no. 45L, Leica GmbH) at an angle of 22°. Images were analyzed with the bioimaging analyzer BAS 2000 (Fuji, Tokyo, Japan) using an ultra-sensitive imaging plate (BAS-TR2040, 20 × 40 cm2, Fuji) that accumulates and stores energy resulting from tritium decay.
Pharmacokinetics
To evaluate brain and plasma concentrations of sagopilone and paclitaxel over time, mice were treated with 5 mg/kg [3H]-sagopilone or [3H]-paclitaxel (equivalent to 7.4 MBq/mg), and the brain and plasma concentrations of either agent were determined at 10, 20, and 40 min for three sagopilone-treated mice and one paclitaxel-treated mouse. Concentrations were analyzed using a liquid scintillation counter and high-performance liquid chromatography radioflow.
The pharmacokinetics of intact sagopilone were evaluated in mice bearing the HeLa/MaTu cervical cancer xenograft model. Cells (1.5 × 106) were implanted subcutaneously at baseline, and sagopilone was administered as a single 8 mg/kg i.v. injection on day 20. Animals were sacrificed and autopsied at various time points after sagopilone administration. Organ samples were evaluated at five time points (0.5, 1, 3, 7, and 24 h), and plasma samples were evaluated at an additional five time points (5, 15, 45, and 90 min and 5 h) after sagopilone treatment. Organs were weighed, followed by shock-freezing in liquid nitrogen and storage at −80°C. Sagopilone was quantified by liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS) using an XTerra MS C18 column (2.1 × 50 mm, 3.5 μm) with a 2.1 × 10 mm precolumn. Injection volume was 5 μl, and flow rate was 300 μl/min, using a gradient program of 75% A/25% B (0–4 min), 5% A/95% B (4–5.4 min), 75% A/25% B (5.4–6.7 min). MS/MS detection was performed using a positive turbo ion spray.
All pharmacokinetic parameters, including half-life (t1/2) and area under the curve (AUC), were calculated from derivations of the equation [c(t) = a·e−bt· + d·egt· ], where c = concentration (μmol/liter), t = time (h), and a, b, d, and g were determined by SigmaPlot (Systat Software Inc., San Jose, CA, USA) nonlinear regression analysis based on the experimental data. Experimental time-dependent concentration data could be described using the equation with high accuracy.
Tumor Cell Culture Conditions
HeLa/MaTu human cervical cancer and MDA-MB-435 melanoma cells were cultured in RPMI 1640 (Gibco Invitrogen Lifescience, Carlsbad, CA, USA) with 10% fetal calf serum. The human glioma cell lines U373 and U87 were grown in Eagle’s minimum essential medium and supplemented with 10% fetal calf serum and sodium pyruvate. Tumor cells were harvested at 60%–80% confluency using 0.05% trypsin plus 0.02% EDTA in phosphate-buffered saline.
Human Tumor Xenograft Models
U373 and U87 human glioblastoma models
Animals (10 mice per group) received both a subcutaneous (1 × 107) and an orthotopic intracerebral inoculum of U373 (2 × 105) or U87 (1 × 104) tumor cells at baseline. U373 animals were treated with control (vehicle only), 9 mg/kg sagopilone i.v. on days 7 and 14, or 8 mg/kg paclitaxel i.v. on days 7–11. U87 animals were treated with control, 10 mg/kg sagopilone i.v. on days 3 and 20, or 12.5 mg/kg paclitaxel i.v. on days 3–7. Animals were sacrificed on day 21 (U87) or 32 (U373). The growth of subcutaneous tumors was measured twice per week with calipers. Tumor volumes (TVs) were calculated using the equation TV = (width2 × length) × 0.5. Brain tumors were measured microscopically.
MDA-MB-435 human melanoma brain metastasis model
Animals (10 mice per group) received an intra-cerebral inoculum of 1 × 104 tumor cells at baseline followed by treatment with control, 10 mg/kg sagopilone i.v. on days 3 and 14, or 8 mg/kg paclitaxel i.v. on days 3–7. Mice were sacrificed on day 25. Brain tumors were measured microscopically.
Lu7187 and Lu7466 human NSCLC brain metastasis models
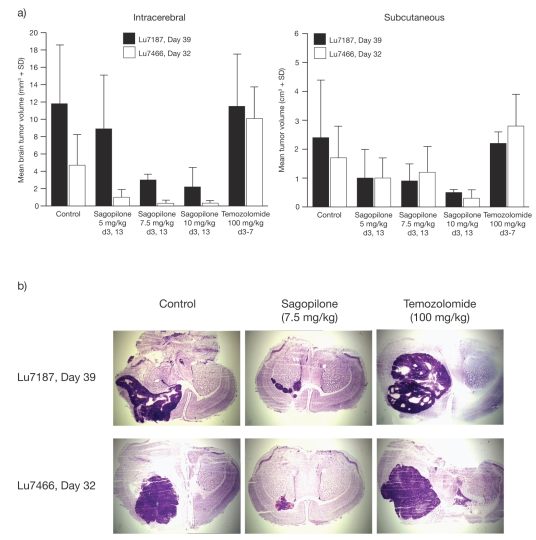
Each of the 10 mice per treatment group received Lu7187 or Lu7466 human NSCLC tumors both as a subcutaneous tumor fragment and as an intracerebral inoculation (as a single-cell suspension of 104 tumor cells prepared by mechanical dissection of a solid tumor from serial passage). Animals were treated with control, 5, 7.5, or 10 mg/kg sagopilone i.v. on days 3 and 13, or 100 mg/kg temozolomide (the maximum tolerated dose; I. Fichtner, unpublished data) via intraperitoneal injection on days 3–7. Animals were sacrificed on day 39 (Lu7187) or 32 (Lu7466). Brain tumors were measured microscopically.
Histology
Tumor growth of the intracerebral tumors was confirmed using histologic evaluation. When the first mice in the control group became moribund, animals from all treatment groups were sacrificed, and brains were snap-frozen in 2-methylbutane. Sequential cryosections (10 μm) were prepared and stained with cresyl violet. The area of largest tumor diameter was determined with the help of a microscope (Zeiss Axio Scope, Carl Zeiss MicroImaging GmbH, Göttingen, Germany), and TVs and perimeters were calculated.
Results
Sagopilone Crosses the Blood–Brain Barrier More Effectively Than Paclitaxel
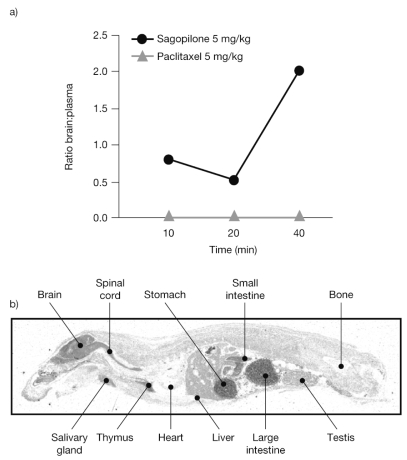
Pharmacokinetic studies showed that sagopilone crosses the blood–brain barrier in both rat and mouse models. As early as 10 min after application, sagopilone was detected in the brain of mice in significant concentrations, with an AUCbrain:AUCplasma ratio of 0.8, indicating that sagopilone has free access to the brain (Fig. 1a). Similar concentrations of sagopilone in the brain (0.9 μg/g) and plasma (1.2 μg/ml) were detected 10 min after intravenous application. In contrast, the concentration of paclitaxel remained below the limit of detection (0.1 μmol/ml) in all brain samples (AUCbrain:AUCplasma ratio of 0) but was found to be in concentrations comparable with sagopilone in plasma (e.g., 0.24 μg/ml paclitaxel vs. 0.30 μg/ml sagopilone at 40 min).
Fig. 1.
Sagopilone penetration of the blood–brain barrier. (a) Brain:plasma AUC ratios for sagopilone and paclitaxel in severe combined immunodeficiency (SCID) mice. (b) Rat whole-mount showing the presence of radiolabeled sagopilone (2.5 mg/kg) in the brain 3 days after application.
When administered to rats, the highest concentrations of tritiated sagopilone were detected in the lung and excretory organs from 5 min postdose, and in the brain, glandular tissue, spleen, bone marrow, and excretory organs from 1 h after application. Sagopilone was clearly detectable in the brain after 3 days using whole-body autoradiography (Fig. 1b). Together, these results clearly indicate an unrestricted access to the brain, with a similar concentration of sagopilone found in the brain and in the plasma.
Intact Sagopilone Is Present at High Concentrations and Has a Long Half-Life in the Brain
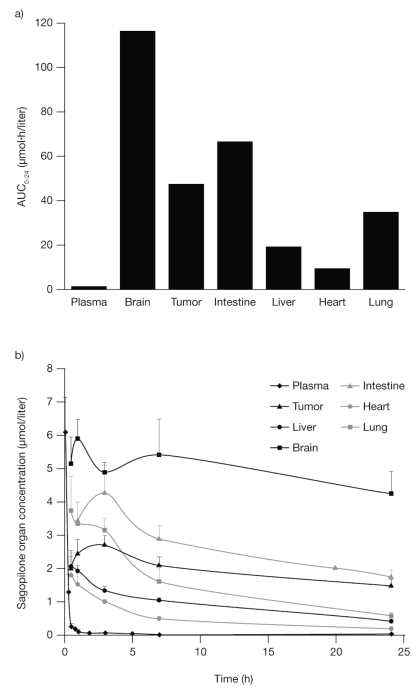
Although tritiated sagopilone was shown to have free access to the brain and other organs, the above methods of detection do not distinguish between the intact drug and inactive metabolites. In order to investigate the in vivo pharmacokinetics of intact sagopilone, the quantity of drug in the plasma, tumor, and several organs was measured using LC/MS/MS quantification in mice carrying HeLa/MaTu tumors. Calculations of different pharmacokinetic parameters over time indicate that the AUC for the first 24 h after sagopilone (AUC0–24) was highest in the brain (Fig. 2a), a result also found for AUC48–72 (66.3 μmol·h/liter in the brain vs. 12.1 μmol·h/liter in the tumor and <6.9 μmol·h/liter in other organs), and that the highest concentration of sagopilone after that found in the plasma (13.0 μM) was in the brain (5.5 μM). The half-life of sagopilone was also longer in the brain compared with other organs (Fig. 2b). These data confirm that intact sagopilone is freely distributed to the brain at high concentrations with an attractive half-life for tumor therapy.
Fig. 2.
Sagopilone has a long half-life in the brain. (a) Area under the curve for the first 24 h after sagopilone (AUC0–24) in NMRI mice bearing a HeLa/MaTu cervical tumor after treatment of 8 mg/kg sagopilone. (b) Mean + SD concentration of sagopilone in selected organs and tissues over time in the same treatment model.
Sagopilone Significantly Inhibits Brain Tumor Growth in Two Models of Human Glioblastoma Compared with Paclitaxel
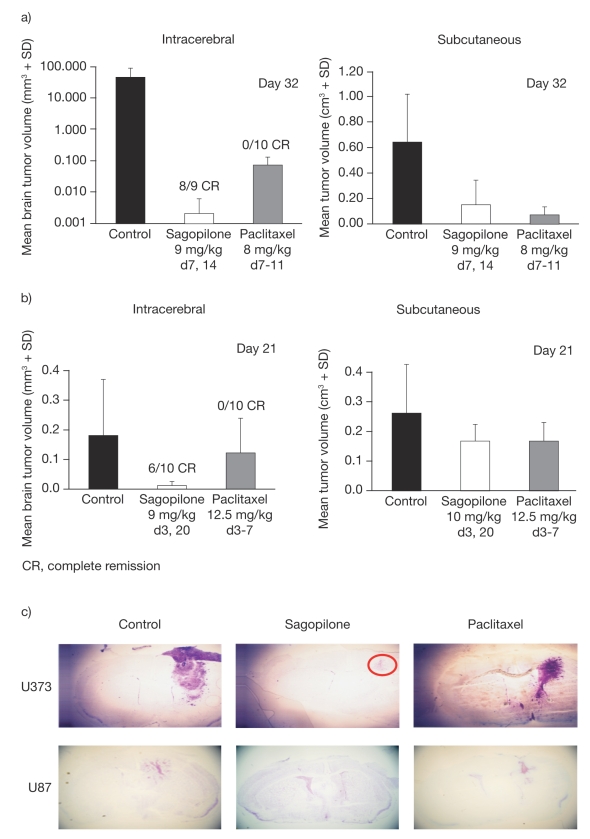
In two orthotopically implanted intracerebral models of human glioblastoma in nude mice (U373 and U87), sagopilone significantly reduced TV versus control (p < 0.05 [U87] and p < 0.0005 [U373]) and resulted in 89% complete remission in the U373 model (Fig. 3a, c) and 60% complete remission in the U87 model (Fig. 3b, c). There was also a significantly greater (p < 0.05) inhibition of brain lesions by sagopilone compared with paclitaxel. In contrast, paclitaxel resulted in a limited effect in the U373 model only: decrease in TV compared with control (p < 0.05) with no complete remission (Fig. 3). The control tumor size in U373 was much greater than that in U87 (62 mm3 vs. 0.18 mm3), suggesting that in animal models of this fast and aggressive tumor, the blood–brain barrier may have become impaired at an early stage. Both agents had activity against subcutaneous tumors in the same models (Fig. 3), demonstrating that the dose and regimen used for paclitaxel were not responsible for its limited activity in brain tumors. Additionally, only moderate weight loss was observed (body weight change [BWC] of −7% for sagopilone in both models and −2% [U373] or −5% [U87] for paclitaxel). This confirms the different pharmacokinetic profiles of paclitaxel and sagopilone and indicates that only sagopilone was able to penetrate the blood–brain barrier to effectively inhibit the growth of intracerebral tumors.
Fig. 3.
Inhibition of tumor growth by sagopilone compared with paclitaxel in the orthotopic and subcutaneous human glioma models U373 (a) and U87 (b), and corresponding histologic sections. (c) red circle denotes the extent of tumor in sagopilone-treated animals. d, day.
An experiment was also performed to compare the activity of temozolomide and sagopilone in the U87 model (data not shown), with the results demonstrating that the two agents exhibited comparable efficacy.
Sagopilone Inhibits Tumor Growth in Models of Human Brain Metastases More Effectively Than Paclitaxel or Temozolomide
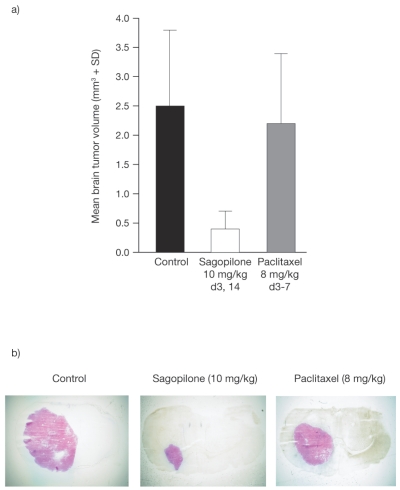
Histologic and TV analyses of female nude mice with intracerebrally orthotopically implanted cancer cells demonstrated that treatment with sagopilone (10 mg/kg, days 3 and 14) significantly inhibited the growth of the intracerebral MDA-MB-435 (p = 0.0007) meta-static sites compared with control (Fig. 4). In contrast, paclitaxel (8 mg/kg, days 3–7) had no significant effect on MDA-MB-435 brain lesions. Weight loss was moderate in both treatment groups, with a BWC of −6% for sagopilone and −5% for paclitaxel.
Fig. 4.
Mean tumor volume (a) and histologic sections (b) showing inhibition of brain tumor growth in the MDA-MB-435 model induced by sagopilone, compared with paclitaxel. d, day.
Sagopilone was compared with temozolomide, an alkylating agent used in the treatment of gliomas, in two patient-derived NSCLC models of brain metastases, Lu7187 and Lu7466 (Fig. 5). When sagopilone treatment at three different dose levels was compared with temozolomide (100 mg/kg, days 3–7), sagopilone showed superior brain tumor inhibition at all dose levels (Fig. 5a). This reduction in tumor size was significantly lower at the 7.5 and 10 mg/kg sagopilone doses versus control in the Lu7187 model (p < 0.05) and at all doses in the Lu7466 model (p < 0.05). Similarly, sagopilone showed a higher antitumor activity against subcutaneous tumors in the same xenograft models (Fig. 5a), with all doses demonstrating a significant decrease versus control in the Lu7187 model. These results were histologically confirmed (Fig. 5b). In the Lu7187 model, the 7.5 mg/kg dose was the maximum tolerated dose for sagopilone, with a BWC of +1%, compared with −13% (which was fatal in many cases) for the 10 mg/kg dose. In the Lu7466 model, both doses showed a similar moderate BWC of −5%. The temozolomide dose resulted in considerable lethality in both models, due to BWC in Lu7187 (−16%) and in LU7466 (BWC −7%), confirming that the dose used was the maximum tolerated dose. Temozolomide completely lacked any influence on brain tumor growth in these two models.
Fig. 5.
Inhibition of brain tumor growth in intracerebral and subcutaneous patient-derived Lu7187 and Lu7466 non-small-cell lung cancer brain metastasis models: mean tumor volume (a) and histologic sections (b) after sagopilone or temozolomide treatment. d, day.
Sagopilone appears to be generally well tolerated and highly active against both intracerebral and subcutaneous tumors in animal models of human brain metastases.
Discussion
Effective treatment options for patients with primary or secondary brain tumors are required because of the limited ability of systemic therapies to cross the blood–brain barrier and the increased number of patients developing brain metastases due to prolonged survival of patients with breast, lung, and other cancers.
The preclinical studies presented here provide direct evidence that sagopilone accumulates in brain tissue, leading to highly effective, well-tolerated levels of the drug in the brain tissue, which are maintained for several days. This means that sagopilone is able to cross the blood–brain barrier, which normally prevents the entry of many molecules into the brain and CNS. The high sagopilone concentrations in the brain have not been associated with any CNS side effects in animal models. In contrast, paclitaxel does not cross the blood–brain barrier in detectable levels, which confirms findings from a previous study.28 The mechanism of sagopilone penetration of the blood–brain barrier and its retention in the brain tissue is currently unclear. However, the ability of sagopilone to evade P-gp pumps29 may provide protection from efflux by the brain capillary endothelium because the P-gp transporter is considered to be the most important element maintaining the blood–brain barrier in the case of therapeutic drugs.30 Despite the expectation that sagopilone will also reach high concentrations in the human brain, CNS toxicity has been reported only in single cases in phase I clinical trials,31,32 and no CNS toxicity has been reported to date in phase II clinical trials.33,34
In vivo studies demonstrated that sagopilone was well tolerated and resulted in significant inhibition of tumor growth in both the subcutaneous and intracerebral glioblastoma xenograft models, whereas paclitaxel showed consistent activity in the subcutaneous models only. The moderate activity seen with paclitaxel in the U373 intracerebral glioblastoma model (reduced tumor size but no complete remission) is interesting, particularly in light of the pharmacokinetic data showing that paclitaxel was below the level of detection in the brain and is most likely to have been due to blood–brain barrier impairment as the disease advanced. Similarly, in models of brain metastases, including a melanoma model and patient-derived models of NSCLC, sagopilone showed superior antitumor activity against brain tumors compared with paclitaxel (MDA-MB-435), which appears to be excluded from the CNS, or temozolomide (Lu7187, Lu7466), which can enter the CNS but shows efficacy only in selected tumor types. These experiments suggest that sagopilone has the potential to provide benefit in several indications of great need.
Studies of other epothilones have shown some activity against human brain tumors. Epothilone D reduced viable glioma cell numbers in vitro with a drug concentration of 10 nM,35 and ixabepilone, recently approved by the FDA for treatment of patients with metastatic breast cancer, has shown efficacy in subcutaneous implantation models of brain tumors, despite being a substrate for P-gp efflux pumps.36 However, neither study examined the activity of these agents in tumors actually located in the brain and so did not consider whether they could cross the blood–brain barrier. The natural product, epothilone B (patupilone), has been reported to significantly reduce intracranial tumors in a human lung brain metastasis model.37 The present study is the first to use intracerebral models of both glioma and CNS metastases to show that an epothilone, sagopilone, is active in clinically relevant models of primary and secondary brain tumors.
In conclusion, this study shows that sagopilone crosses the blood–brain barrier in rats and mice, reaching effective concentrations in the brain with a long half-life. In orthotopic CNS tumor models of melanoma, NSCLC, and glioblastoma, sagopilone was well tolerated and significantly inhibited brain tumor growth compared with paclitaxel or temozolomide. These data indicate that further research is warranted to investigate the potential of sagopilone in the treatment of both glioblastoma and CNS metastases. Sagopilone is currently being investigated in a broad phase II trial program, including trials in patients with glioblastoma, melanoma, breast cancer, and NSCLC.
Acknowledgments
This study was supported by a grant from Bayer Schering Pharma AG. We thank Laura McMahon for editorial assistance during the preparation of the manuscript.
References
- 1.Schwartzbaum JA, Fisher JL, Aldape KD, Wrensch M. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2:494–503. doi: 10.1038/ncpneuro0289. [DOI] [PubMed] [Google Scholar]
- 2.Engel J, Eckel R, Aydemir U, et al. Determinants and prognoses of locoregional and distant progression in breast cancer. Int J Radiat Oncol Biol Phys. 2003;55:1186–1195. doi: 10.1016/s0360-3016(02)04476-0. [DOI] [PubMed] [Google Scholar]
- 3.Shaffrey ME, Mut M, Asher AL, et al. Brain metastases. Curr Probl Surg. 2004;41:665–741. doi: 10.1067/j.cpsurg.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 4.Huang YJ, Wu YL, Xie SX, Yang JJ, Huang YS, Liao RQ. Weekly gemcitabine as a radiosensitiser for the treatment of brain metastases in patients with non-small cell lung cancer: phase I trial. Chin Med J (Engl) 2007;120:458–462. [PubMed] [Google Scholar]
- 5.Sampson JH, Carter JH, Jr, Friedman AH, Seigler HF. Demographics, prognosis, and therapy in 702 patients with brain metastases from malignant melanoma. J Neurosurg. 1998;88:11–20. doi: 10.3171/jns.1998.88.1.0011. [DOI] [PubMed] [Google Scholar]
- 6.Schouten LJ, Rutten J, Huveneers HAM, Twijnstra A. Incidence of brain metastases in a cohort of patients with carcinoma of the breast, colon, kidney, and lung and melanoma. Cancer. 2002;94:2698–2705. doi: 10.1002/cncr.10541. [DOI] [PubMed] [Google Scholar]
- 7.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 8.Weil RJ, Palmieri DC, Bronder JL, Stark AM, Steeg PS. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005;167:913–920. doi: 10.1016/S0002-9440(10)61180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wen PY, Schiff D. Neurologic complications of solid tumors. Neurol Clin. 2003;21:107–140. doi: 10.1016/s0733-8619(02)00029-4. [DOI] [PubMed] [Google Scholar]
- 10.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75:5–14. doi: 10.1007/s11060-004-8093-6. [DOI] [PubMed] [Google Scholar]
- 11.Clayton AJ, Danson S, Jolly S, et al. Incidence of cerebral metastases in patients treated with trastuzumab for metastatic breast cancer. Br J Cancer. 2004;91:639–643. doi: 10.1038/sj.bjc.6601970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ceresoli GL, Reni M, Chiesa G, et al. Brain metastases in locally advanced nonsmall cell lung carcinoma after multimodality treatment: risk factors analysis. Cancer. 2002;95:605–612. doi: 10.1002/cncr.10687. [DOI] [PubMed] [Google Scholar]
- 13.Abbott N. Astrocyte-endothelial interactions and blood–brain barrier permeability. J Anat. 2002;200:527. doi: 10.1046/j.1469-7580.2002.00064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kastritis E, Efstathiou E, Gika D, et al. Brain metastases as isolated site of relapse in patients with epithelial ovarian cancer previously treated with platinum and paclitaxel-based chemotherapy. Int J Gynecol Cancer. 2006;16:994–999. doi: 10.1111/j.1525-1438.2006.00596.x. [DOI] [PubMed] [Google Scholar]
- 15.Seewaldt VL, Figge DC, Greer BE, Tamimi HK, Brown WS, Cain JM. Primary central nervous system recurrence after paclitaxel therapy for epithelial ovarian malignancy. Gynecol Oncol. 1994;55:456–458. doi: 10.1006/gyno.1994.1322. [DOI] [PubMed] [Google Scholar]
- 16.Freilich RJ, Seidman AD, DeAngelis LM. Central nervous system progression of metastatic breast cancer in patients treated with paclitaxel. Cancer. 1995;76:232–236. doi: 10.1002/1097-0142(19950715)76:2<232::aid-cncr2820760212>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 17.Crivellari D, Pagani O, Veronesi A, et al. High incidence of central nervous system involvement in patients with metastatic or locally advanced breast cancer treated with epirubicin and docetaxel. Ann Oncol. 2001;12:353–356. doi: 10.1023/a:1011132609055. [DOI] [PubMed] [Google Scholar]
- 18.Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 19.Fine RL, Chen J, Balmaceda C, et al. Randomized study of paclitaxel and tamoxifen deposition into human brain tumors: implications for the treatment of metastatic brain tumors. Clin Cancer Res. 2006;12:5770–5776. doi: 10.1158/1078-0432.CCR-05-2356. [DOI] [PubMed] [Google Scholar]
- 20.Patel M, McCully C, Godwin K, Balis FM. Plasma and cerebrospinal fluid pharmacokinetics of intravenous temozolomide in non-human primates. J Neurooncol. 2003;61:203–207. doi: 10.1023/a:1022592913323. [DOI] [PubMed] [Google Scholar]
- 21.Yung WK, Prados MD, Yaya-Tur R, et al. Multicenter phase II trial of temozolomide in patients with anaplastic astrocytoma or anaplastic oligoastrocytoma at first relapse. Temodal Brain Tumor Group. J Clin Oncol. 1999;17:2762–2771. doi: 10.1200/JCO.1999.17.9.2762. [DOI] [PubMed] [Google Scholar]
- 22.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 23.Trudeau ME, Crump M, Charpentier D, et al. Temozolomide in meta-static breast cancer (MBC): a phase II trial of the National Cancer Institute of Canada—Clinical Trials Group (NCIC-CTG) Ann Oncol. 2006;17:952–956. doi: 10.1093/annonc/mdl056. [DOI] [PubMed] [Google Scholar]
- 24.Dziadziuszko R, Ardizzoni A, Postmus PE, et al. Temozolomide in patients with advanced non-small cell lung cancer with and without brain metastases: a phase II study of the EORTC Lung Cancer Group (08965) Eur J Cancer. 2003;39:1271–1276. doi: 10.1016/s0959-8049(03)00234-x. [DOI] [PubMed] [Google Scholar]
- 25.Winsel S, Hammer S, Eschenbrenner J, et al. Subcellular distribution and cellular activity of the novel epothilone sagopilone (ZK-EPO). Presented at: ECCO; September 24–27 2007; Barcelona, Spain. [Google Scholar]
- 26.Klar U, Buchmann B, Schwede W, Skuballa W, Hoffmann J, Lichtner RB. Total synthesis and anti-tumor activity of ZK-EPO: the first fully synthetic epothilone in clinical development. Angew Chem Int Ed Engl. 2006;45:7942–7948. doi: 10.1002/anie.200602785. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann J, Vitale I, Buchmann B, et al. Improved cellular pharmacokinetics and pharmacodynamics underlie the wide anticancer activity of sagopilone. Cancer Res. 2008;68:5301–5308. doi: 10.1158/0008-5472.CAN-08-0237. [DOI] [PubMed] [Google Scholar]
- 28.Gangloff A, Hsueh W-A, Kesner AL, et al. Estimation of paclitaxel biodistribution and uptake in human-derived xenografts in vivo with 18 F-fluoropaclitaxel. J Nucl Med. 2005;46:1866–1871. [PubMed] [Google Scholar]
- 29.Klar U, Buchmann B, Schwede W, Skuballa W, Hoffmann J, Lichtner RB. Total synthesis and anti-tumor activity of ZK-EPO: the first fully synthetic epothilone in clinical development. Angew Chem Int Ed Engl. 2006;45:7942–7948. doi: 10.1002/anie.200602785. [DOI] [PubMed] [Google Scholar]
- 30.Bauer B, Hartz AMS, Fricker G, Miller DS. Modulation of p-glycoprotein transport function at the blood–brain barrier. Exp Biol Med (Maywood) 2005;230:118–127. doi: 10.1177/153537020523000206. [DOI] [PubMed] [Google Scholar]
- 31.Schmid P, Kiewe P, Kuhnhardt D, et al. A phase I study of the novel third-generation epothilone ZK-EPO in patients with advanced solid tumors. Presented at: 41st ASCO Annual Meeting; May 13–17, 2005; Orlando, FL. [Google Scholar]
- 32.Arnold D, Voigt W, Kiewe P, et al. Weekly administration of ZK-EPO, a novel third-generation epothilone, in patients with refractory solid tumors: results of a phase I trial [abstract] Ann Oncol. 2006;17:ix138. [Google Scholar]
- 33.Rustin GJ, Reed NS, Jayson G, et al. Phase II trial of the novel epothilone ZK-EPO in patients with platinum resistant ovarian cancer [abstract] J Clin Oncol. 2007;25:280s. [Google Scholar]
- 34.Gatzemeier U, von Pawel JV, Eschbach C, et al. Phase II trial of the novel epothilone ZK-EPO as second-line therapy in patients with stage IIIB or stage IV non-small-cell lung cancer [abstract] Eur J Cancer. 2007;5:378. [Google Scholar]
- 35.Dietzmann A, Kanakis D, Kirches E, Kropf S, Mawrin C, Dietzmann K. Nanomolar concentrations of epothilone D inhibit the proliferation of glioma cells and severely affect their tubulin cytoskeleton. J Neurooncol. 2003;65:99–106. doi: 10.1023/b:neon.0000003679.40609.63. [DOI] [PubMed] [Google Scholar]
- 36.Peterson JK, Tucker C, Favours E, et al. In vivo evaluation of ixabepilone (BMS247550), a novel epothilone B derivative, against pediatric cancer models. Clin Cancer Res. 2005;11:6950–6958. doi: 10.1158/1078-0432.CCR-05-0740. [DOI] [PubMed] [Google Scholar]
- 37.McSheehy PM, Allegrini PR, Becquet M, Ferretti S, Stumm M. Patupilone, the novel microtubule stabilizer, inhibits growth of intra-cranial human lung tumors in athymic mice [abstract] Proc Am Assoc Cancer Res. 2006;47:489. [Google Scholar]