Abstract
The epidemiology and natural history of adult gliosarcomas (GSMs), as well as patient and treatment factors associated with outcome, are ill defined. Patients over 20 years of age with GSM diagnosed from 1988 to 2004 were identified in the Surveillance, Epidemiology, and End Results (SEER) database. Kaplan-Meier survival analysis and Cox models were used to examine outcomes. Similar analyses were conducted for patients diagnosed with glioblastoma (GBM) over the same time period. GSM represented 2.2% of the 16,388 patients identified with either GSM or GBM. No significant differences between GSM and GBM were identified with respect to age, gender, race, tumor size, or use of adjuvant radiation therapy (RT). Patients with GSM were more likely to have temporal lobe involvement and undergo some form of tumor resection. The most important analyzed factors influencing GSM overall survival were age, extent of resection, and use of adjuvant RT. After adjusting for factors impacting overall survival, the prognosis for GSM appears slightly worse than for GBM (HR = 1.17, 95% CI, 1.05–1.31). GSM is a rare malignancy that presents very similarly to GBM with a slightly greater propensity for temporal lobe involvement. Optimal treatment remains to be defined. However, these retrospective findings suggest tumor excision, as opposed to biopsy only, and adjuvant RT may improve outcome. Despite therapy, prognosis remains dismal and outcomes may be inferior to those seen in GBM patients.
Keywords: glioblastoma, gliosarcoma, radiation, SEER
Gliosarcoma (GSM) is a primary tumor of the central nervous system composed of both malignant glial and sarcomatous elements. GSM was first reported by Strobe in 1895 but did not gain wide acceptance until 1955 when Feigen and Gross described, in detail, three patients with this malignancy.1,2 The incidence of GSM is between 1% and 8% of all malignant gliomas and thus represents an exceptionally rare neoplasm.3,4 Consequently, our knowledge about this entity is limited to small retrospective case series and case reports. In general, the epidemiology and natural history of GSM appears similar to glioblastoma (GBM).3–10 No patient or treatment factors have been unequivocally identified that distinguish outcomes of GSM from GBM.
Due to small patient numbers, available case series are not sufficiently powered to precisely characterize GSM. Modest, yet clinically meaningful, differences between GSM and GBM may surface with examination of a larger series. To refine our understanding of GSM, we used the Surveillance, Epidemiology, and End Results (SEER) database to identify and analyze more than 300 adult GSM patients and compare them to adult GBM patients.
Materials and Methods
Study Population
Data were obtained from the SEER database of the U.S. National Cancer Institute using the SEER 17–Registries 1973–2004 data set.11 All patients with GSM and GBM diagnosed between January 1988 and December 2004 were identified. Histological classification was based on the International Classification of Diseases for Oncology (ICD) code (gliosarcoma: 9442, glioblastoma: 9440).12 Only intracranial tumors were considered. Patients were excluded if this was not their first site of malignant disease and if pathologic confirmation was not obtained. Only patients over 20 years of age were included (5 GSM and 224 GBM patients were excluded for age). The age restriction was used to exclude gliofibroma, a desmoplastic tumor of childhood, which shares the same ICD code with GSM.13,14 A total of 353 GSM and 16,035 GBM patients met criteria for inclusion in the initial analysis. Because all information from the SEER database is deidentified, informed consent by the study participants and approval of an ethics committee were unnecessary to perform the analyses in this study.
Statistical Analysis
The patient populations were evaluated with respect to categorical variables corresponding to multiple patient, tumor, and treatment characteristics (age, sex, race, tumor location, tumor size, extent of surgery, and use of adjuvant radiation therapy [RT]). Unadjusted associations of variables of interest were evaluated using Pearson’s χ2 test. The primary end point in this study was overall survival, with patients censored either at death or at date of last follow-up. Overall survival was assessed using the Kaplan-Meier survival analysis, and the Mantel-Cox log-rank test was used to assess the significance of differences between survival curves. Both univariate and multivariate Cox proportional hazards models were used to calculate hazard ratios (HRs) and their 95% confidence intervals (CIs) to allow further analysis of individual variables with respect to overall survival. For multivariate models, forward stepwise procedures were chosen in an a priori fashion wherein variables were entered into the model with p < 0.05 and removed if the significance of that variable subsequently exceeded p = 0.10. The validity of the proportional hazards assumption was tested for each variable incorporated into the final Cox models using log-log survival curves. No instances in which this assumption was not met were identified.
SEER*STAT version 6.3.5 (Surveillance Research Program, NCI, Bethesda, MD, USA) was used to extract case level data from the SEER public-use databases. All analyses were conducted using the Statistical Package for the Social Sciences (SPSS, V14.0).
Results
The study population consisted of 16,388 patients diagnosed with GSM or GBM. GSM accounted for 353 patients, or 2.2% of this population. Patient, tumor, and treatment characteristics are displayed in Table 1. GSM and GBM are quite similar; both malignancies tend to occur in the elderly and show a slight male predominance. Compared to GBM, GSM has an apparent proclivity for the temporal lobe. Patients with GBM tended to receive less aggressive surgical resection, with nearly a quarter undergoing no cancer-directed surgery (i.e., biopsy only) compared to less than 10% of GSM patients.
Table 1.
Patient, tumor, and treatment characteristics
| GSM (n = 353) | GBM (n = 16,035) | p | |
|---|---|---|---|
| Patient characteristics | |||
| Median age (range) | 63 (21–90) | 62 (21–96) | |
| Age in years (%) | 0.98 | ||
| <40 | 6.8 | 6.5 | |
| 40–49 | 14.2 | 13.2 | |
| 50–59 | 21.8 | 22.8 | |
| 60–69 | 26.3 | 26.4 | |
| ≥70 | 30.9 | 31.1 | |
| Sex (%) | 0.27 | ||
| Male | 61.2 | 58.2 | |
| Female | 38.8 | 41.8 | |
| Race (%) | 0.67 | ||
| White | 91.5 | 91.1 | |
| Black | 5.4 | 4.6 | |
| American Indian/Alaska native | 0.3 | 0.2 | |
| Asian/Pacific islander | 2.8 | 3.8 | |
| Other unspecified | 0.0 | 0.3 | |
| Tumor characteristics | |||
| Location (%) | <0.001 | ||
| Temporal | 34.0 | 23.3 | |
| Frontal | 18.7 | 24.2 | |
| Parietal | 15.0 | 17.3 | |
| Occipital | 5.1 | 4.2 | |
| Ventricle | 0.6 | 0.4 | |
| Cerebellum | 1.4 | 0.6 | |
| Brain stem | 0.0 | 0.5 | |
| Overlapping/NOS | 25.2 | 29.5 | |
| Median tumor size, cm (range)* | 4.7 (0.2–10.0) | 4.5 (0.1–10.0+) | 0.39 |
| Treatment characteristics | |||
| Extent of resection (%) | <0.001 | ||
| No cancer-directed surgery | 7.6 | 24.1 | |
| Local tumor destruction | 0.0 | 0.4 | |
| Subtotal tumor excision | 22.4 | 18.8 | |
| Gross total tumor excision | 17.0 | 14.1 | |
| Partial excision of primary site (i.e., partial lobectomy) | 23.2 | 20.8 | |
| Total excision of primary site (i.e., total lobectomy) | 28.3 | 20.5 | |
| Surgery NOS | 1.4 | 1.1 | |
| Unknown | 0.0 | 0.2 | |
| Radiation therapy (%) | 0.17 | ||
| Yes | 74.5 | 73.5 | |
| No | 24.1 | 23.4 | |
| Unknown | 1.4 | 3.2 | |
Abbreviation: NOS, not otherwise specified.
Tumor size known for 246 patients with gliosarcoma and 10,074 patients with glioblastoma.
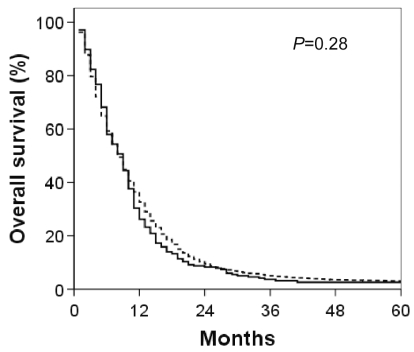
Overall survival for both cohorts is shown in Fig. 1. Clearly, the prognosis for both GSM and GBM is poor, with median survivals of 9 and 8 months, respectively. Factors impacting overall survival for both cohorts were examined, and the univariate analysis is shown in Table 2. Unsurprisingly, age at presentation, extent of resection, and adjuvant RT were significantly associated with GBM survival. Similarly, these three factors impacted GSM survival. Age at presentation also remained a significant predictor of overall survival when analyzed as a continuous variable (data not shown). A small influence of gender on overall survival was observed in both GSM and GBM, with males faring slightly better than females. Similarly, larger tumor size had a small, but statistically significant, impact on overall survival in the GBM cohort. The influence of tumor size on overall survival in GSM patients was of comparable, small magnitude. However, given the much smaller sample size, this did not achieve statistical significance. Tumor location did influence overall survival in both GSM and GBM patients, but this impact was generally of small magnitude and limited to uncommon sites of presentation (e.g., ventricle). Kaplan-Meier overall survival curves for both GBM and GSM, reflecting the three variables most closely associated with survival on univariate analysis (age, extent of resection, and adjuvant RT use), are shown in Figs. 2–4. To correct for postoperative mortality, the impact of adjuvant RT was reanalyzed with the exclusion of patients who survived less than 2 months after diagnosis (Fig. 4C and D). Exploratory analyses using alternate exclusion time points (1 month and 3 months) produced similar results (data not shown).
Fig. 1.
Kaplan-Meier overall survival curves for GSM (solid line) and GBM (dashed line) patients.
Table 2.
Univariate analysis of the impact of patient, tumor, and treatment factors on overall survival (hazard ratio ± 95% confidence intervals)
| GSM | GBM | |
|---|---|---|
| Patient characteristics | ||
| Age in years | p < 0.001 | p < 0.001 |
| <40* | 1.00 | 1.00 |
| 40–49 | 1.61 (0.91–2.84) | 1.59 (1.46–1.73) |
| 50–59 | 1.67 (0.98–2.84) | 2.02 (1.86–2.19) |
| 60–69 | 2.15 (1.27–3.67) | 2.89 (2.67–3.13) |
| ≥70 | 3.37 (1.99–5.71) | 4.53 (4.19–4.91) |
| Sex | p = 0.02 | p = 0.002 |
| Male* | 1.00 | 1.00 |
| Female | 1.32 (1.05–1.66) | 1.05 (1.02–1.09) |
| Race | p = 1.00 | p < 0.001 |
| White* | 1.00 | 1.00 |
| Black | 0.99 (0.61–1.62) | 1.01 (0.93–1.09) |
| American Indian/Alaska native | 0.89 (0.13–6.39) | 0.83 (0.59–1.17) |
| Asian/Pacific islander | 0.97 (0.48–1.96) | 0.84 (0.77–0.92) |
| Other unspecified | — | 0.59 (0.41–0.85) |
| Tumor characteristics | ||
| Location | p = 0.01 | p < 0.001 |
| Temporal* | 1.00 | 1.00 |
| Frontal | 0.86 (0.62–1.20) | 0.99 (0.95–1.04) |
| Parietal | 0.96 (0.68–1.36) | 1.10 (1.04–1.15) |
| Occipital | 0.74 (0.43–1.30) | 1.03 (0.94–1.12) |
| Ventricle | 4.83 (1.18–19.8) | 1.56 (1.22–1.99) |
| Cerebellum | 0.68 (0.25–1.85) | 0.89 (0.72–1.11) |
| Brain stem | — | 1.29 (1.02–1.64) |
| Overlapping/NOS | 1.41 (1.06–1.88) | 1.30 (1.24–1.36) |
| Tumor size† | p = 0.42 | p < 0.001 |
| <Median size* | 1.00 | 1.00 |
| ≥Median size | 1.12 (0.85–1.47) | 1.10 (1.05–1.14) |
| Treatment characteristics | ||
| Extent of resection†† | p = 0.001 | p < 0.001 |
| No cancer-directed surgery* | 1.00 | 1.00 |
| Local tumor destruction | — | 0.62 (0.48–0.80) |
| Subtotal tumor excision | 0.43 (0.27–0.68) | 0.54 (0.51–0.57) |
| Gross total tumor excision | 0.49 (0.31–0.78) | 0.51 (0.48–0.53) |
| Partial excision of primary site (i.e., partial lobectomy) | 0.52 (0.33–0.81) | 0.58 (0.55–0.61) |
| Total excision of primary site (i.e., total lobectomy) | 0.36 (0.23–0.57) | 0.40 (0.38–0.43) |
| Surgery NOS | 0.38 (0.14–0.98) | 0.48 (0.41–0.57) |
| Radiation therapy††† | p < 0.001 | p < 0.001 |
| Yes* | 1.00 | 1.00 |
| No | 2.83 (2.18–3.69) | 2.74 (2.63–2.85) |
Abbreviation: NOS, not otherwise specified.
Reference category.
Only tumors with known size are included in the analysis (n = 246 GSM patients; n = 10,074 GBM patients).
Patients with unknown extent of surgery were excluded (n = 29 GBM patients).
Patients with unknown use of RT were excluded (n = 5 GSM patients; n = 509 GBM patients).
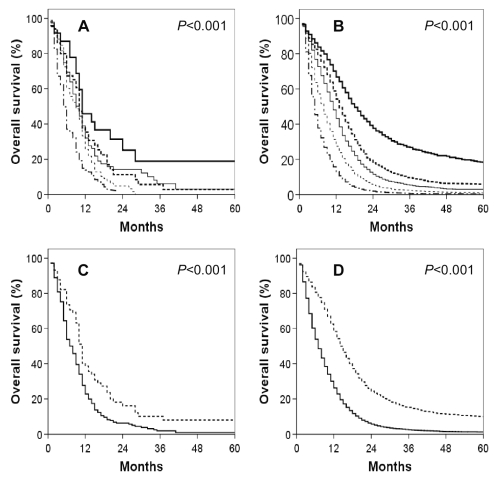
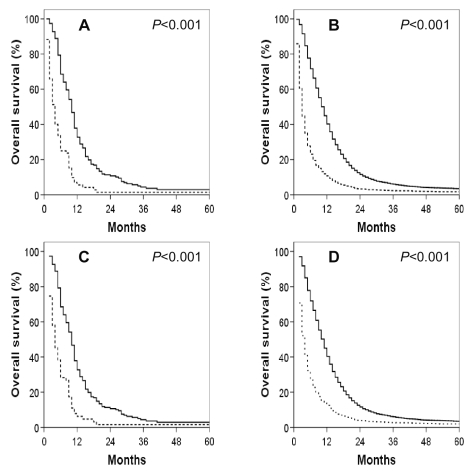
Fig. 2.
Kaplan-Meier overall survival curves for GSM and GBM patients by age. (A) GSM patients. (B) GBM patients. (A, B) Thick solid line: ≤ 40 years; thick dashed line: 40–49 years; thin solid line: 50–59 years; thin dashed line: 60–69 years; broken medium line: >70 years. (C) GSM patients. (D) GBM patients. (C, D) Dashed line: <50 years of age; solid line ≥50 years of age.
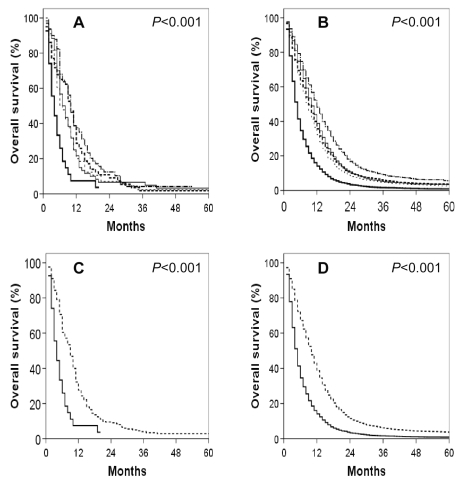
Fig. 4.
Kaplan-Meier overall survival curves for (A) GSM and (B) GBM patients who received (solid line) and who did not receive (dashed line) adjuvant radiation therapy. Comparable Kaplan-Meier overall survival curves for (C) GSM and (D) GBM patients who received (solid line) and who did not receive (dashed line) adjuvant radiation therapy with the exclusion of patients who did not survive a minimum of 2 months following diagnosis.
Multivariate Cox proportional hazards models for GSM and GBM are shown in Table 3. Age, extent of resection, and adjuvant RT remained the most significant predictors of overall survival for both GSM and GBM. In this model, tumor size was excluded because it (1) was not significant in GSM patients on univariate analysis, (2) had a small, albeit statistically significant, influence on overall survival in GBM patients, and (3) resulted in the exclusion of more than 6,000 patients because tumor size was unknown. In additional multivariate analyses, including tumor size in only those patients where it was available, the results paralleled those found on univariate analysis: larger tumor sizes correlated with poorer survival in GBM patients (HR = 1.15 [95% CI, 1.10–1.20] for larger tumors compared to tumors smaller than median tumor size), but was not significantly associated with worse survival in GSM patients. Gender was not significantly associated with overall survival for either histology on multivariate analysis. Similarly, race did not influence overall survival of GSM patients, and it was modestly associated with the overall survival of GBM patients. A multivariate Cox model was also constructed with the entire cohort using histology as an additional variable. Age, race, tumor location, extent of surgery, and adjuvant RT were again significantly associated with overall survival. Additionally, histology proved to be a significant predictor of survival with worse prognosis for GSM (HR = 1.17, 95% CI, 1.05–1.31 compared to GBM).
Table 3.
Multivariate analysis of the impact of patient, tumor, and treatment factors on overall survival (hazard ratio ± 95% confidence intervals)†
| GSM | GBM | |
|---|---|---|
| Patient characteristics | ||
| Age in years | p < 0.001 | p < 0.001 |
| <40* | 1.00 | 1.00 |
| 40–49 | 1.83 (0.99–3.40) | 1.66 (1.52–1.81) |
| 50–59 | 2.47 (1.37–4.47) | 2.07 (1.90–2.24) |
| 60–69 | 2.90 (1.63–5.17) | 2.91 (2.68–3.16) |
| ≥70 | 3.93 (2.20–7.02) | 4.13 (3.80–4.48) |
| Race | p = 0.003 | |
| White* | 1.00 | |
| Black | 1.05 (0.97–1.14) | |
| American Indian/Alaska native | 0.96 (0.67–1.37) | |
| Asian/Pacific islander | 0.85 (0.78–0.93) | |
| Tumor characteristics | ||
| Location | p = 0.03 | p = 0.001 |
| Temporal* | 1.00 | 1.00 |
| Frontal | 0.85 (0.60–1.19) | 1.07 (1.02–1.12) |
| Parietal | 1.00 (0.70–1.44) | 1.08 (1.02–1.13) |
| Occipital | 0.55 (0.31–0.99) | 1.04 (0.95–1.13) |
| Ventricle | 4.43 (1.02–19.28) | 1.74 (1.35–2.23) |
| Cerebellum | 1.52 (0.53–4.33) | 1.01 (0.81–1.25) |
| Brain stem | — | 1.25 (0.98–1.60) |
| Overlapping/NOS | 1.23 (0.91–1.66) | 1.22 (1.16–1.28) |
| Treatment characteristics | ||
| Extent of resection | p = 0.004 | p < 0.001 |
| No cancer-directed surgery* | 1.00 | 1.00 |
| Local tumor destruction | — | 0.68 (0.53–0.88) |
| Subtotal tumor excision | 0.47 (0.29–0.77) | 0.63 (0.60–0.67) |
| Gross total tumor excision | 0.56 (0.34–0.92) | 0.62 (0.58–0.65) |
| Partial excision of primary site (i.e., partial lobectomy) | 0.55 (0.34–0.89) | 0.68 (0.65–0.71) |
| Total excision of primary site (i.e., total lobectomy) | 0.38 (0.23–0.62) | 0.50 (0.47–0.52) |
| Surgery NOS | 0.34 (0.11–0.98) | 0.53 (0.46–0.62) |
| Radiation therapy | p < 0.001 | p < 0.001 |
| Yes* | 1.00 | 1.00 |
| No | 2.75 (2.06–3.67) | 2.36 (2.27–2.45) |
Abbreviation: NOS, not otherwise specified.
Reference category.
Age, sex, race, tumor location, surgery, and RT were used initially as categoric variables to construct a Cox model of overall survival for both GSM and GBM patients. Patients with unknown race, use of RT, or surgery were excluded from the analysis (GSM: n = 5; GBM: n = 568).
Discussion
Previous attempts to characterize adult GSM have been limited by the rarity of this malignancy. For example, Meis et al. examined 1,479 patients enrolled in five consecutive Radiation Therapy Oncology Group GBM clinical trials between 1974 and 1983, identifying 26 cases of GSM.3 Similarly, Galanis et al. reviewed 748 patients in four consecutive North Central Cancer Treatment Group phase III GBM trials and identified 18 cases of GSM.7 Additional retrospective single and multi-institutional series have been reported, but all have yielded less than 40 cases of GSM.3–10 Using the large patient numbers offered by the SEER database, we have attempted to clarify the epidemiology, natural history, and factors associated with the outcome of adult GSM.
Our results suggest that GSM represents approximately 2% of adult GBM. This finding is consistent with most reviews of GSM3,6,7 but in conflict with the 8% and 4.9% incidences observed by Morantz et al. and Sarkar et al., respectively.4,8 GSM, like GBM, shows a propensity to affect the elderly, with a median age at diagnosis over 60 years. Similarly, both histologies demonstrate comparable male predominance. We found no significant difference in the size of GSMs compared to GBMs, consistent with previous observations.3 A modest temporal lobe predilection was observed for GSM. This observation clarifies a subject of debate. Several authors have reported a tendency for temporal lobe involvement by GSM,4–8,10,15 whereas others have found no such anatomic selectivity.3,9
Our survival analyses confirm previous reports that GSM survival is at least as dismal as that observed with GBM. The median survival among all GSM patients was 9 months. Several factors were associated with overall survival in GSM patients including age at diagnosis, extent of surgery, and adjuvant RT. Patients diagnosed with GSM prior to 50 years of age had a median survival of 15 months compared to 7 months for those diagnosed after age 50. For GSM patients who underwent no cancer- directed surgery (i.e., biopsy only), the median survival was a mere 4 months. With excision, median survival increased to 7–11 months. On multivariate analysis, no significant differences in survival were observed based on the extent of surgery beyond biopsy only. Similarly, in GSM patients who did not receive adjuvant RT, median survival was also 4 months. With RT, median survival increased to 10 months. These same factors influenced GBM survival, consistent with the well-established prognostic significance of age, tumor excision, and adjuvant RT in this malignancy.16 Interestingly, the prognostic significance of the extent of tumor excision in GBM has been the subject of considerable debate.17–19 Our data suggest not only that excision is superior to biopsy in GBM, but also that the extent of excision may influence overall survival. The SEER database allows the extent of surgery to be coded with respect to either the tumor or the primary site. On multivariate analysis, total excision of the primary site was associated with a significantly superior overall survival when compared to partial excision of the primary site. However, there was no difference between gross total tumor excision and subtotal tumor excision. Although not the focus of the present study, these observations highlight the pressing need to define the ideal surgical management of GBM.
There are several limitations to this analysis. Use of the SEER database provides large patient numbers and, consequently, striking statistical power to detect factors influencing outcome. However, several candidate factors are not available through this database, including RT details, the use of chemotherapy, performance status, and presenting symptom severity and duration. These factors have been implicated in GBM outcome and may, by analogy, impact the overall survival of GSM patients.16,20 As in any retrospective review, unknown differences in our patient groups may bias results and suggest associations with outcomes where causal relationships do not exist. The lack of randomized data for rare tumors therefore creates a void that retrospective analyses can only partially fill. Given the rarity of GSM, pathologic classification may also be less secure than for more common malignancies. Centralized pathologic review would certainly add to the veracity of our findings but is clearly not plausible. Finally, due to coding limitations, the SEER database includes patients diagnosed with gliofibroma in the same category as GSM. Gliofibroma is an exceptionally rare neoplasm first described in 1978 by Friede.21 Gliofibroma differs from GSM in that the mesenchymal component is benign in gliofibroma. Furthermore, gliofibroma is generally diagnosed in the first 2 decades of life. To our knowledge, only 30 cases have been reported, and of those, only four have involved intracranial lesions in patients older than 20.22,23 In our analysis, the exclusion of patients 20 years and younger minimizes the small possibility of bias introduced by the inclusion of any gliofibroma patient. Our observation that the GSM cohort has an inferior prognosis compared to the GBM cohort supports the conclusion that no significant bias was introduced because patients with gliofibroma can have prolonged survival.
Despite these limitations, this study represents the most comprehensive analysis of adult GSM providing clinically relevant prognostic and therapeutic insights. GSM presents similarly to GBM with a slightly greater propensity for temporal lobe involvement. The prognosis for GSM patients appears slightly worse than that observed for GBM patients. Despite dismal outcomes, tumor resection, as opposed to biopsy only, and adjuvant RT are warranted in the management of GSM because patients who undergo these interventions have improved overall survival.
Fig. 3.
Kaplan-Meier overall survival curves for GSM and GBM patients segregated by extent of tumor resection. (A) GSM patients. (B) GBM patients. (A, B) Thick solid line: no cancer-directed surgery; thick dashed line: subtotal tumor excision; thin solid line: gross total tumor excision; thin dashed line: partial excision of primary site; medium broken line: total excision of primary site. (C) GSM patients. (D) GBM patients. (C, D) Solid line: no cancer-directed surgery; dashed line: other.
References
- 1.Stroebe H. Uber Entstehung und Bau der Gehirngliome. Beitr Pathol Anat Allg Pathol. 1895;18:405–486. [Google Scholar]
- 2.Feigen IH, Gross SW. Sarcoma arising in glioblastoma of the brain. Am J Pathol. 1955;31:633–653. [PMC free article] [PubMed] [Google Scholar]
- 3.Meis JM, Martz KL, Nelson JS. Mixed glioblastoma multiforme and sarcoma. A clinicopathologic study of 26 Radiation Therapy Oncology Group cases. Cancer. 1991;67:2342–2349. doi: 10.1002/1097-0142(19910501)67:9<2342::aid-cncr2820670922>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Morantz RA, Feigen I, Ransohoff J. Clinical and pathological study of 24 cases of gliosarcoma. J Neurosurg. 1976;45:398–408. doi: 10.3171/jns.1976.45.4.0398. [DOI] [PubMed] [Google Scholar]
- 5.Salvati M, Caroli E, Raco A, Giangaspero F, Delfini R, Ferrante L. Gliosarcomas: Analysis of 11 cases do two subtypes exist? J Neurooncol. 2005;74:59–63. doi: 10.1007/s11060-004-5949-8. [DOI] [PubMed] [Google Scholar]
- 6.Lutterbach J, Guttenberger R, Pagenstecher A. Gliosarcoma: a clinical study. Radiother Oncol. 2001;61:57–64. doi: 10.1016/s0167-8140(01)00415-7. [DOI] [PubMed] [Google Scholar]
- 7.Galanis E, Buckner JC, Dinapoli RP, et al. Clinical outcome of gliosarcoma compared with glioblastoma multiforme: North Central Cancer Treatment Group results. J Neurosurg. 1998;89:425–430. doi: 10.3171/jns.1998.89.3.0425. [DOI] [PubMed] [Google Scholar]
- 8.Sarkar M, Sharma MC, Sudha K, Gaikwad S, Varma A. A clinico-pathological study of 29 cases of gliosarcoma with special reference to two unique variants. Indian J Med Res. 1997;106:229–235. [PubMed] [Google Scholar]
- 9.Perry JR, Ang LC, Bilbao JM, Muller PJ. Clinicopathologic features of primary and postirradiation cerebral gliosarcoma. Cancer. 1995;75:2910–2918. doi: 10.1002/1097-0142(19950615)75:12<2910::aid-cncr2820751219>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Parekh HC, O’Donovan DG, Sharma RR, Keogh AJ. Primary cerebral gliosarcoma: report of 17 cases. Br J Neurosurg. 1995;9:171–178. doi: 10.1080/02688699550041511. [DOI] [PubMed] [Google Scholar]
- 11.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence—SEER 17 Regs Limited-Use, Nov 2006 Sub 1973–2004 varying)—Linked to County Attributes—Total U.S., 1969—2004 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Cancer Statistics Branch, released April 2007, based on November 2006 submission.
- 12.Piercy C, Holt VV, Muir C. Geneva, Switzerland: World Health Organization; 1990. International Classification of Diseases for Oncology. 2nd ed. [Google Scholar]
- 13.Sharma MC, Gaikwad S, Mehta VS, Dhar J, Sarkar C. Gliofibroma: Mixed glial and mesenchymal tumour. Report of three cases. Clin Neurol Neurosurg. 1998;100:153–159. doi: 10.1016/s0303-8467(98)00028-6. [DOI] [PubMed] [Google Scholar]
- 14.Rushing EJ, Rorke LB, Sutton L. Problems in the nosology of desmo-plastic tumors of childhood. Pediatr Neurosurg. 1993;19:57–62. doi: 10.1159/000120701. [DOI] [PubMed] [Google Scholar]
- 15.Jack CR, Jr, Bhansali DT, Chason JL, et al. Angiographic features of gliosarcoma. AJNR Am J Neuroradiol. 1987;8:117–122. [PMC free article] [PubMed] [Google Scholar]
- 16.Curran WJ, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 17.Quigley MR, Maroon JC. The relationship between survival and the extent of the resection in patients with supratentorial malignant gliomas. Neurosurgery. 1991;29:385–389. doi: 10.1097/00006123-199109000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Devaux BC, O’Fallon JR, Kelly PJ. Resection, biopsy, and survival in malignant glial neoplasms. A retrospective study of clinical parameters, therapy, and outcome. J Neurosurg. 1993;78:767–775. doi: 10.3171/jns.1993.78.5.0767. [DOI] [PubMed] [Google Scholar]
- 19.Laws ER, Parney IF, Huang W, et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: data from the Glioma Outcomes Project. J Neurosurg. 2003;99:467–473. doi: 10.3171/jns.2003.99.3.0467. [DOI] [PubMed] [Google Scholar]
- 20.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 21.Friede RL. Gliofibroma. A peculiar neoplasia of collagen forming glia-like cells. J Neuropathol Exp Neurol. 1978;37:300–313. doi: 10.1097/00005072-197805000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Deb P, Sarkar C, Garg A, Singh VP, Kale SS, Sharma MC. Intracranial gliofibroma mimicking a meningioma: a case report and review of the literature. Clin Neurol Neurosurg. 2006;108:176–186. doi: 10.1016/j.clineuro.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 23.Goyal S, Puri T, Gunabushanam G, et al. Gliofibroma: a report of three cases and review of the literature. Acta Oncol. 2007;46:1202–1204. doi: 10.1080/02841860701316081. [DOI] [PubMed] [Google Scholar]