Abstract
To investigate the utility of postoperative chemotherapy in delaying radiotherapy and to identify prognostic factors in early childhood medulloblastoma, we studied children younger than 3 years of age registered to the HIT-SKK’87 (Therapieprotokoll für Säuglinge und Kleinkinder mit Hirntumoren [Brain Tumor Radiotherapy for Infants and Toddlers with Medulloblastoma] 1987) trial who received systemic interval chemotherapy until craniospinal radiotherapy was applied at 3 years of age or at relapse, from 1987 to 1993. Children with postoperative residual tumor or metastatic disease received systemic induction chemotherapy prior to interval chemotherapy. Twenty-nine children were eligible for analyses (median age, 1.7 years; median follow-up, 12.6 years). In children without macroscopic metastases, rates (±SEM) for 10-year progression-free survival (PFS) and overall survival (OS) were 52.9% ± 12.1% and 58.8% ± 11.9% (complete resection), and 55.6% ± 16.6% and 66.7% ± 15.7% (incomplete resection), compared with 0% and 0% in children with macroscopic metastases. Survival was superior in nine children with desmoplastic or extensive nodular histology compared with 20 children with classic medulloblastoma (10-year PFS, 88.9% ± 10.5% and 30.0% ± 10.3%, p = 0.003; OS, 88.9% ± 10.5% and 40.0% ± 11.0%, p = 0.006). Eleven of 12 children with tumor progression during chemotherapy had classic medulloblastoma. After treatment, IQ scores were inferior compared with nonirradiated children from the subsequent study, HIT-SKK’92. Classic histology, metastatic disease, and male gender were independent adverse risk factors for PFS and OS in 72 children from HIT-SKK’87 and HIT-SKK’92 combined. In terms of survival, craniospinal radiotherapy was successfully delayed especially in young children with medulloblastoma of desmoplastic/extensive nodular histology, which was a strong independent favorable prognostic factor. Because of the neurocognitive deficits of survivors, the emerging concepts to avoid craniospinal radiotherapy should rely on the histological medulloblastoma subtype.
Keywords: chemotherapy, histology, medulloblastoma, prognosis, radiotherapy
Medulloblastoma, the most common malignant brain tumor of childhood, occurs 25%–35% of the time in children younger than 3 years of age. Compared with older children, the prognosis of early childhood medulloblastoma treated with surgery, radiotherapy, and chemotherapy has been unsatisfactory (20%–45%) until the last decade. This was related to not only the more frequent occurrence of metastases at diagnosis but also to a different biology of medulloblastoma in younger children.2–4 The high susceptibility of the immature brain to radiotherapy-induced neurocognitive deficits5–10 has led to limitations for the delivery of radiotherapy in this age group. Consequently, dose reduction and delay of radiotherapy have been investigated.11–14 Other strategies aimed to avoid radiotherapy, and some patients were cured by postoperative chemotherapy only.15–17 Recently, different strategies to delay or avoid craniospinal radiotherapy have shown evidence of improved survival rates,18–20 so more attention has focused on treatment-related late effects and long-term neurocognitive outcome of survivors.21–23
The trial HIT-SKK’87 (Therapieprotokoll für Säuglinge und Kleinkinder mit Hirntumoren [Brain Tumor Radiotherapy for Infants and Toddlers with Medulloblastoma] 1987) of the German Society of Pediatric Oncology and Hematology was designed to increase survival rates and to reduce long-term neuropsychological deficits by delaying craniospinal radio-therapy with multiagent systemic chemotherapy.24 We have shown that desmoplastic histology is a favorable prognostic factor and that neuropsychological deficits of patients treated by intensive postoperative chemotherapy alone (HIT-SKK’92) were less pronounced compared with patients treated in the HIT-SKK’87 trial.18 Here, we report on the survival and prognostic factors of children treated in the pilot trial HIT-SKK’87 and compare the results with the subsequent HIT-SKK’92 trial.
Patients and Methods
The study was open between August 1987 and July 1993 for children younger than 3 years of age with malignant brain tumors. Thirty-two children with medulloblastoma diagnosed by the local institution were registered. Central reference histopathology was performed in 18 patients, and available samples were reassessed by a reference neuropathologist in 2003 according to the WHO classification for brain tumors.25 Four patients with a different diagnosis in central review were excluded from analysis (two with atypical teratoid rhabdoid tumor, one with anaplastic ependymoma, and one with choroid plexus carcinoma). One patient with local diagnosis of anaplastic ependymoma was included after medulloblastoma was confirmed by central pathology review, leaving 29 patients (16 males, 13 females) from 20 pediatric oncology centers in Germany and Austria for analysis (Table 1). In 25 of these 29 children, lumbar cerebro-spinal fluid (CSF) was analyzed for microscopic dissemination of tumor cells. After evaluation of staging procedures, 26 patients were classified without macroscopic metastases (stage M0/M1: 16 with negative CSF evaluation and M0 stage, 6 with positive CSF and M1 stage, and 4 without CSF analyses), and 3 children had macroscopic stage M2/M3 metastases.
Table 1.
Patient characteristics
| Variable | HIT-SKK’87 | HIT-SKK’92 |
|---|---|---|
| n | 29 | 43 |
| Age (years), median (range) | 1.7 (0.2–2.8) | 1.9 (0.4–2.9) |
| Follow-up of survivors (months), median (range) | 151 (112–195) | 96 (39–143) |
| Sex, n | ||
| Male | 16 | 28 |
| Female | 13 | 15 |
| Staging | ||
| M0/M1, gross total resection | 17 | 17 |
| M0/M1, incomplete resection | 9 | 14 |
| M2/M3 | 3 | 12 |
| Histologya | ||
| Classic medulloblastoma | 20 | 23 |
| Desmoplastic medulloblastoma | 4 | 13 |
| Medulloblastoma with extensive nodularity | 5 | 7 |
Central histopathological review was performed in all patients from HIT-SKK’92 and in 22 patients from HIT-SKK’87.
The study was approved by the ethics committee of the University of Wuerzburg. Informed consent was obtained from legal representatives of all patients.
Surgery and Imaging
Maximal surgical removal of primary tumor, dependent on the anatomical location of the tumor and the condition of the child, was recommended. The extent of resection was assessed by neuroradiology based on results of MRI or CT. Presence of macroscopic solid or laminar metastases was evaluated by initial cranial and spinal MRI (± gadolinium), and lumbar CSF analysis was recommended according to the Chang tumor staging system.26 Complete response was defined as the complete disappearance or absence of residual tumor or macroscopic metastasis during chemotherapy or at the end of treatment. Partial response was defined as a decrease of ⩾50% of area; stable disease, decrease of <50% or increase of <25%; and progressive disease, increase of ⩾25%.
Evaluation Procedures and Follow-up
Before each treatment element, complete blood count, electrolyte levels, creatinine concentration, and liver function parameters were determined. Serum concentrations of methotrexate were measured at standard intervals until levels were lower than 0.25 μmol/l. Imaging was repeated every 10 weeks during therapy and every 3–6 months in the first 2 years after completion of therapy. After treatment, children underwent detailed age-adapted neuropsychological examination, as described previously.18 The intelligence levels of patients were determined by Raven Colored Progressive Matrices (CPM) and Kaufman Assessment Battery for Children (K-ABC). Sensor motor ability was measured by the Visual-Motor Integration Test (VMI).27–29
Treatment
Chemotherapy was to be started within 2–4 weeks after surgery, as described previously.30 Low-risk patients (complete resection and no macroscopically detectable dissemination of disease) received maintenance chemotherapy until radiotherapy at the age of 3 years or until progression (Fig. 1).
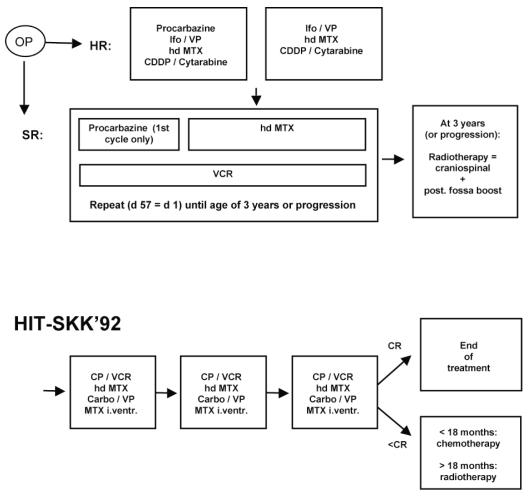
Fig. 1.
Treatment regimens HIT-SKK’87 and HIT-SKK’92. Abbreviations: OP, operation; HR, high risk; Ifo, ifosfamide; VP, etoposide; hd MTX, high-dose methotrexate; CDDP, cisplatinum; SR, standard risk; VCR, vincristine; d, day; post., posterior; CP, cyclophosph-amide; Carbo, carboplatin; I.ventr., intraventricular; CR, complete response.
High-risk patients (after subtotal resection or with metastatic disease) and children between 2.5 and 3.0 years of age at diagnosis received two cycles of a post-operative induction chemotherapy after surgery, followed by the maintenance chemotherapy described for low-risk patients until radiotherapy was administered at 3 years of age. In cases of progression or tumor recurrence, chemotherapy was interrupted and radiotherapy was administered immediately.
One cycle of induction chemotherapy consisted of procarbazine (100 mg/m2/day, days 1–10, given only in the first cycle), ifosfamide (3 g/m2/day, 72-h infusion, days 15–17, with mesna 1 g/m2 before ifosfamide and 3 g/m2/day, days 15–19), etoposide (150 mg/m2/day, 1-h infusion, days 15–17), methotrexate (5 g/m2/day, 24-h infusion, days 29 and 43; after 36-h, citrovorum factor rescue 6 × 15 mg/m2 every 6 h), cisplatinum (40 mg/m2/ day, 1-h infusion, days 57–59), and cytarabine (400 mg/ m2/day, 0.5-h infusion, days 57–59).
One cycle of maintenance chemotherapy consisted of procarbazine (100 mg/m2/day, days 1–8, given only in the first cycle), methotrexate (5 g/m2/day, 24-h infusion, days 15, 29, 43; after 36–42 h, citrovorum factor rescue 6 × 15 mg/m2 every 6 h), and vincristine (1.5 mg/m2/day, days 1, 8, 15, 29, 43). Maintenance cycles were repeated (day 57 = day 1) until radiotherapy was administered at 3 years of age or at tumor progression.
For children older than 3 years, the prescribed total dose to the neuraxis was 35.2 Gy (1.6 Gy per fraction, five times per week). The posterior fossa was to receive a boost dose of 20.0 Gy (2.0 Gy per fraction, five times weekly). For younger children, a neuraxis dose of 24 Gy was prescribed in cases of progressive disease. Meta-static deposits were boosted at the discretion of the local radiation oncologist.
The subsequent HIT-SKK’92 trial for children younger than 3 years of age was designed to avoid radio-therapy by an intensive postoperative systemic and intra-ventricular chemotherapy alone. Patients received three cycles of systemic chemotherapy with cyclophosphamide, methotrexate, vincristine, carboplatin, and etoposide, as previously described.18 To substitute radiotherapy, intraventricular methotrexate was administered in single doses of 2 mg via subcutaneous reservoir. Craniospinal radiotherapy was applied only if a patient was not in complete remission after postoperative chemotherapy, and if older than 18 months by that time. The characteristics of 43 eligible children, used for comparison with HIT-SKK’87 and for a common multivariate analysis, are described in Table 1.
For further analyses and identification of independent risk factors, data from the 29 children from HIT-SKK’87 were pooled with data from the 43 children from HIT-SKK’92 for a total data set of 72 children.
Statistical Analyses
Survival functions were estimated using the method of Kaplan and Meier, and the log-rank test was used for comparison. Progression-free survival (PFS) was defined as time from the date of diagnosis to the date of first progression, relapse, or death of any cause, or to the date of the last contact. Overall survival (OS) was defined as time from the date of diagnosis to death of any cause or last contact. All univariate analyses were done with a local significance level of 5% and were not adjusted for multiple comparisons. Results of intelligence tests are expressed as t-scores (mean = 50, 1-fold SD = 10) and were compared by Mann-Whitney U-test.
Multivariate Cox regression analysis of the data from the studies HIT-SKK’87 and HIT-SKK’92 was used to analyze possible prognostic factors for the risk of recurrence or death. The forward stepwise model selection procedure according to Collett31 was used (p-value of likelihood-ratio test ⩽ 0.05 as inclusion criteria; likelihood-ratio test ⩾ 0.10 as exclusion criteria) to define the final model. The following variables were entered: gender, age at diagnosis, histological subtype (classic medulloblastoma [CMB], desmoplastic medulloblastoma [DMB], and medulloblastoma with extensive nodularity [MBEN]), stage (macroscopic metastases, no macroscopic metastases), extent of resection (gross total resection, incomplete resection), and treatment regimen (HIT-SKK’87, HIT-SKK’92). We regard multivariate analysis as explorative, and p-values are expressed descriptively.
Results
Staging and Follow-up (HIT-SKK’87)
Of 29 eligible patients with a median age of 1.7 years (range, 0.2–2.8 years), 26 children had no macroscopic metastases at diagnosis (stage M0, 16 patients; stage M1, 6 patients; no CSF examination in 4 patients). Among these, 17 children underwent gross total tumor resection, and 9 children had postoperative residual tumor. Macroscopic metastases were detectable at diagnosis in three children. Fifteen children were treated according to the high-risk strategy with two cycles of induction chemotherapy prior to maintenance chemotherapy; 14 children were treated according to the low-risk strategy. Radiotherapy was not applied in eight children based on decisions of the local institutions. The median follow-up of the 14 surviving patients was 12.6 years (range, 9.3–16.2 years). Patient details are given in Table 1.
Progression-Free and Overall Survival
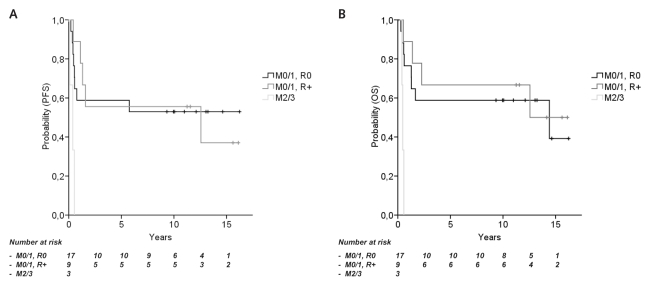
The estimated rates (±SEM) for 10-year PFS and OS for all 29 children from HIT-SKK’87 were 48.3% ± 9.3% and 55.2% ± 9.3%, respectively. Considering postsurgical status, 10-year PFS and OS were 52.9% ± 12.1% and 58.8% ± 11.9% (complete resection, no macroscopic metastases), 55.6% ± 16.6% and 66.7% ± 15.7% (residual tumor, no macroscopic metastases), and 0% and 0% (macroscopic metastases), respectively (Fig. 2). The rates for 10-year PFS and OS of 26 patients without macroscopic metastases (M0/M1) were 53.8% ± 9.8% and 61.5% ± 9.5%. The corresponding rates of 16 patients without microscopic metastases (i.e., lumbar CSF examination done) or macroscopic metastases (M0) were 43.8% ± 12.4% and 50.0% ± 12.5%; those of six patients with exclusively microscopic CSF metastases (M1) were 50.0% ± 20.4% and 66.7% ± 19.2%; and those of patients with microscopic and/or macroscopic metastases were 33.3% ± 15.7% and 44.4% ± 16.6%. There was no survival difference between groups with complete or incomplete surgical resection (10-year PFS rates, 52.9% ± 12.1% and 55.6% ± 16.6%, p = 0.986; 10-year OS rates, 58.8% ± 11.9% and 66.7% ± 15.7%, p = 0.704) in 26 children without macroscopic metastases. PFS and OS differed between children with nonmetastatic (M0/M1) and metastatic (M2/M3) disease (both p-values < 0.001). Results are summarized in Table 2.
Fig. 2.
Survival rates according to staging (HIT-SKK’87). (A) Progression-free survival (PFS) for stage M0/M1 disease with (R+) and without (R0) postoperative residual tumor (10-year PFS, 52.9% and 55.6%, p = 0.986) and in children with macroscopic metastases (M2/M3; PFS, 0%; p < 0.001). (B) Overall survival (OS) in M0/M1 disease with and without postoperative residual tumor (10-year OS, 58.8% and 66.7%, p = 0.704) and in children with macroscopic metastases (0%; p = 0.001).
Table 2.
HIT-SKK’87: Univariate survival analyses (mean ± SEM)
| Group (n) | 10-Year Progression-Free Survival | 10-Year Overall Survival |
|---|---|---|
| All (29) | 48% ± 9% | 55% ± 9% |
| Classic medulloblastoma (20) | 30% ± 10% | 40% ± 11% |
| Desmoplastic medulloblastoma (9) | 89% ± 11% | 89% ± 11% |
| R0 (20) | 45% ± 11% | 50% ± 11% |
| R1 (9) | 56% ± 17% | 67% ± 16% |
| M0 (16) | 44% ± 12% | 50% ± 13% |
| M1 (6) | 50% ± 20% | 67% ± 19% |
| M0/M1 (26)a | 54% ± 10% | 62% ± 10% |
| M0/M1, R0 (17) | 53% ± 12% | 59% ± 12% |
| M0/M1, R1 (9) | 56% ± 17% | 67% ± 16% |
| M2/M3 (3) | 0% | 0% |
| M1–M3 (9) | 33% ± 16% | 44% ± 17% |
Includes four patients without cerebrospinal fluid analysis.
Histological Subtypes
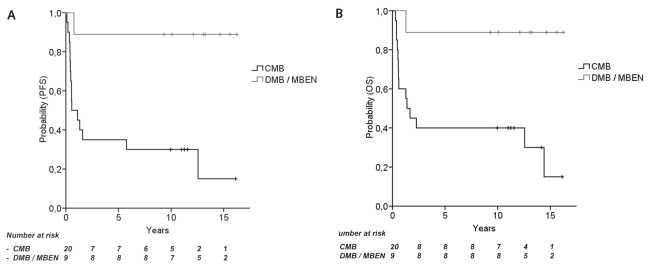
Twenty children from HIT-SKK’87 had CMB, four had DMB, and five had MBEN. One of the four children with DMB and none of the five children with MBEN experienced tumor relapse or progression, and rates for 10-year PFS and OS in all nine children were higher compared with 20 children with CMB (10-year PFS, 88.9% ± 10.5% and 30.0% ± 10.3%, p = 0.003; 10-year OS, 88.9% ± 10.5% and 40.0% ± 11.0%, p = 0.006; Fig. 3). Two of nine children with DMB or MBEN did not receive any radiotherapy upon decision of the local physician and remained in remission. The localization of primary tumors differed between 9 children with DMB or MBEN (4 hemispheric, 4 midline, 1 unspecified) and 20 children with CMB (4 hemispheric, 15 midline, 1 unspecified).
Fig. 3.
Survival rates according to histological subtype (HIT-SKK’87). (A) Progression-free survival (PFS) in children with desmoplastic medulloblastoma (DMB) or medulloblastoma with extensive nodularity (MBEN), compared with classic medulloblastoma (CMB) (10-year PFS, 88.9% and 30.0%, p = 0.003). (B) Overall survival (OS) in children with desmoplastic DMB or MBEN compared with children with CMB (10-year OS, 88.9% and 40.0%, p = 0.006).
Progressive Disease during Therapy and Salvage Therapy
Twelve of 29 children from HIT-SKK’87 (41%) had tumor progression or relapse during chemotherapy at a median time of 0.44 years after diagnosis and before they were to receive radiotherapy at the age of 3 years (1 DMB, 11 CMB). Considering the risk group and corresponding chemotherapy, tumor relapse or progression under chemotherapy was observed in 6 of 17 (35%) standard-risk patients and 6 of 12 high-risk patients (50%). Two children relapsed 0.9 and 5.4 years after radiotherapy.
After relapse or tumor progression, further therapy with or without secondary tumor resection was reported for 10 children: five children received radiotherapy and systemic chemotherapy, two children had systemic chemotherapy alone, two children had radiotherapy alone, and one child received systemic chemotherapy and high-dose chemotherapy. In four children with relapse, no further therapy was applied. One child was salvaged successfully and was still in second continuous remission 14 years after relapse treatment with surgery and radiotherapy.
Toxicity and Death Unrelated to Tumor Progression
No major unexpected toxicity was reported from systemic chemotherapy. One child died from postoperative intracerebral bleeding. No other treatment-related deaths or lethal infections were reported. One patient was diagnosed with glioblastoma as secondary malignancy 12 years after medulloblastoma and was deceased 1 year later.
Comparison with HIT-SKK’92
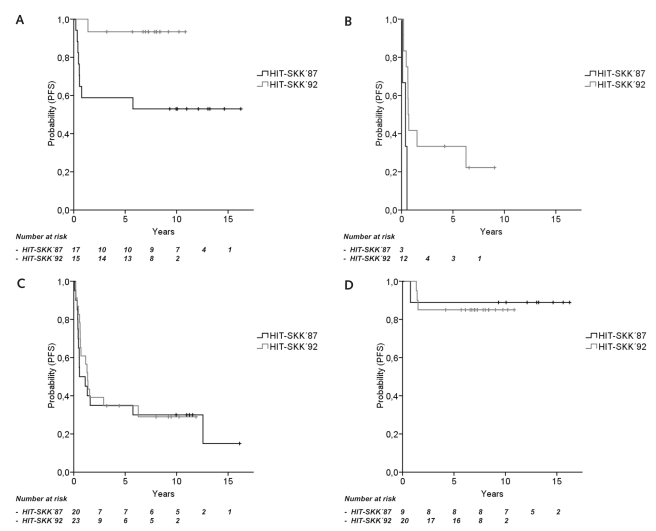
Rates for 10-year PFS and OS did not differ between all 29 patients from HIT-SKK’87 and 43 children from HIT-SKK’9218 (respectively, 10-year PFS, 48.3% ± 9.3% and 55.2% ± 7.7%, p = 0.364; 10-year OS, 55.2% ± 9.2% and 63.6% ± 7.6%, p = 0.274) by univariate analyses. Among children without macroscopic metastases (M0/M1 disease) and complete tumor resection, PFS and OS were higher in HIT-SKK’92 compared with HIT-SKK’87 (10-year PFS, 93.3% ± 6.4% and 52.9% ± 12.1%, p = 0.011; 10-year OS, 100% and 58.8% ± 11.9%, p = 0.006; Fig. 4A). Comparing children with macroscopic metastases, PFS and OS were superior in the HIT-SKK’92 study (8-year PFS, 33.3% ± 13.6% and 0 ± 0%, p = 0.012; 5-year OS, 40.0% ± 14.6% and 0 ± 0%, p = 0.015; Fig. 4B). However, only three children had initial macroscopic metastases in the HIT-SKK’87 trial. In contrast, there was no survival difference between both studies in children with stage M0/M1 and postoperative residual tumor (10-year PFS, 43.8% ± 12.4% and 55.6% ± 16.6%, p = 0.656; 10-year OS, 55.6% ± 12.6% and 66.7% ± 15.7%, p = 0.745). Children without microscopic metastases (i.e., CSF examination done) or macroscopic metastases (M0) had superior survival rates in the HIT-SKK’92 study compared with HIT-SKK’87 (10-year PFS, 72.7% ± 9.5% and 43.8% ± 12.4%, p = 0.032; 10-year OS, 86.4% ± 7.3% and 50.0% ± 12.5%, p = 0.006). Survival rates in children with microscopic and/or macroscopic metastases (M1–M3) did not differ between HIT-SKK’92 and HIT-SKK’87 (10-year PFS, 36.1% ± 11.2% and 33.3% ± 15.7%, p = 0.589; 10-year OS, 39.7% ± 11.7% and 44.4% ± 16.6%, p = 0.835; data not shown). Survival rates did not differ between HIT-SKK’92 and HIT-SKK’87 subgroups of CMB (10-year PFS, 29.0% ± 9.8% and 30.0% ± 10.3%, p = 0.666; 10-year OS, 36.5% ± 10.5% and 40.0% ± 11.0%, p = 0.695) and DMB including MBEN (10-year PFS, 85.0% ± 8.0% and 88.9% ± 10.5%, p = 0.827; 10-year OS, 95.0% ± 4.9% and 88.9% ± 10.5%, p = 0.532; Fig. 4C, D).
Fig. 4.
HIT-SKK’87 compared with HIT-SKK’92: 8-year progression-free survival (PFS). (A) Children with M0/M1 disease and complete tumor resection (93.3% and 52.9%, p = 0.011). (B) Children with macroscopic metastases (M2/M3) (33.3% and 0%, p = 0.012). (C) Children with classic medulloblastoma (29.0% and 30.0%, p = 0.666). (D) Children with desmoplastic medulloblastoma and medulloblastoma with extensive nodularity (85.0% and 88.9%, p = 0.827).
Pooled Analysis of HIT-SKK’87 and HIT-SKK’92 and Multivariate Analysis
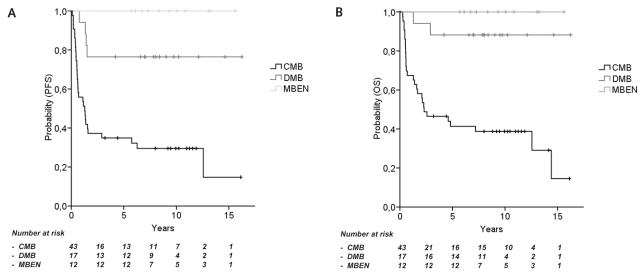
Analyzing 72 patients from both studies, OS and PFS differed among histological subgroups. All 12 children with MBEN remained relapse-free (10-year PFS and OS, 100%; Fig. 5). PFS and OS did not differ between children with DMB and children with MBEN (10-year PFS, 76.5% ± 10.3%; 10-year OS, 88.2% ± 7.8%; p = 0.078 and 0.228, respectively). Children with CMB had inferior survival compared with children with DMB or with MBEN (10-year PFS, 29.5% ± 7.1% and 86.2% ± 6.4%; 10-year OS, 38.8% ± 7.5% and 93.1% ± 4.7%; both p < 0.001; Fig. 5).
Fig. 5.
HIT-SKK’87 and HIT-SKK’92: Pooled analyses according to histology. (A and B) Eight-year progression-free survival (PFS) (A) and 8-year overall survival (OS) (B) of children from both studies with medulloblastoma with extensive nodularity (MBEN; 100% and 100%), with desmoplastic medulloblastoma (DMB; 76.5% and 88.2%; not significant) and classic medulloblastoma (CMB; 29.5% and 38.8%; both p < 00.001).
Multivariate analyses on all 72 patients identified the histological subtype of DMB and MBEN as a strong independent favorable prognostic factor (hazard ratio compared with CMB: PFS, 0.08; OS, 0.05; p < 0.001). Furthermore, metastatic disease, male gender, and treatment according to HIT-SKK’87 regimen were independent adverse prognostic factors for PFS and OS. Age at diagnosis and postoperative residual tumor status were not independent prognostic factors. The results of multivariate analyses are summarized in Table 3.
Table 3.
HIT-SKK’87 and HIT-SKK’92: Multivariate Cox regression analysis on 72 patients
| Progression-Free Survival
|
Overall Survival
|
|||||
|---|---|---|---|---|---|---|
| Parameter | Hazard Ratio | 95% Confidence Interval | p-Value | Hazard Ratio | 95% Confidence Interval | p-Value |
| Desmoplasia and extensive nodularity vs. classic histology | 0.08 | 0.03–0.24 | p < 0.001 | 0.05 | 0.01–0.22 | p < 0.001 |
| Macroscopic metastases: yes vs. no | 6.25 | 2.71–14.39 | p < 0.001 | 6.38 | 2.66–15.27 | p < 0.001 |
| Female vs. male sex | 0.38 | 0.18–0.81 | p = 0.010 | 0.32 | 0.14–0.73 | p = 0.005 |
| Treatment regimen: HIT-SKK’87 vs. HIT-SKK’92 | 2.16 | 1.02–4.59 | p = 0.048 | 2.43 | 1.08–5.48 | p = 0.034 |
Neuropsychological Evaluation
Neuropsychological follow-up was performed in 10 patients from HIT-SKK’87 after a median of 6.1 years (range, 4.0–7.9 years) from diagnosis. The mean IQ scores were lower compared with healthy controls within the same age group (IQ: CPM, 77.7 ± 7.2 vs. 104.1 ± 11.3; K-ABC, 74.2 ± 15.1 vs. 100.0 ± 11.2; VMI, 77.4 ± 9.4 vs. 102.6 ± 19.1; p < 0.001; Table 4). CPM scores for these irradiated children were lower compared with 14 patients from HIT-SKK’92 (77.7 ± 7.2 vs. 92.0 ± 12.7, p < 0.005). In addition, there was a trend toward lower results for K-ABC and VMI in HIT-SKK’87 compared with HIT-SKK’92.
Table 4.
IQ scores of children from HIT-SKK’87, HIT-SKK’92, and healthy controls (median ± SD)
| Test | HIT-SKK’87 (n = 10) | HIT-SKK’92 (n = 14) | Controls (n = 23) |
|---|---|---|---|
| Kaufmann Assessment Battery for Children | 74.2 ± 15.1 | 82.1 ± 15.5 | 100.0 ± 11.2 |
| Colored Progressive Matrices | 77.7 ± 7.2 | 92.0 ± 12.7 | 104.1 ± 11.3 |
| Visual-Motor Integration Test | 77.4 ± 9.4 | 83.9 ± 11.8 | 102.6 ± 19.1 |
Discussion
Our results from the prospective multicenter pilot trial HIT-SKK’87 demonstrate that early childhood medulloblastoma can be treated with considerable efficacy by systemic chemotherapy and deferred craniospinal radiotherapy. With substantial follow-up of patients, the survival rates compare favorably with other studies from the 1980s2,4,8,13 and equal to those of the most efficacious studies from the 1990s.18–20 After identifying the detrimental effects of craniospinal irradiation on the neurocognitive outcome,2,5–9,21,32,33 different strategies aiming to avoid craniospinal radiotherapy have been developed by different study groups. Although craniospinal radiotherapy is no longer part of modern frontline strategies for early childhood medulloblastoma, various findings from the present study have relevant implications for current and future studies.
Our data strongly support results from HIT-SKK’92, where desmoplastic histological subtypes were newly identified as an independent favorable prognostic factor in this age group.18 Interestingly, the OS rates of children with CMB and DMB are similar to the rates observed in HIT-SKK’92. Therefore, the prognostic relevance of desmoplastic histology may not be limited to predominantly methotrexate-based treatment regimen as used in HIT-SKK’92, but may also be relevant in the context of other treatment concepts. The fact that in both studies desmoplastic histology was a favorable risk factor in localized medulloblastoma and in metastatic disease suggests that DMB not only may offer better chances of resectability, but also could be more sensitive to adjuvant treatment modalities.
This is further supported by our observation that tumor progression during chemotherapy was almost exclusively observed in CMB in HIT-SKK’87. Our results from HIT-SKK’87, where 9 of 26 children without macroscopic metastases had tumor progression, are in line with reports of increased risk of neuraxis progression with the duration of chemotherapy.34,35 No tumor progression was observed during chemotherapy in 32 children without initial macroscopic metastases in HIT-SKK’92, suggesting that tumor control may be improved by dose-intensified induction chemotherapy. DMB and MBEN have also been confirmed by other national groups as favorable risk factors in early childhood medulloblastoma.36,37 Although DMB and MBEN are classified in the most recent WHO classification as distinct medulloblastoma variants, they are regarded as closely related histological variants.38
The fact that treatment by HIT-SKK’92 was an independent favorable risk factor for PFS and OS compared with HIT-SKK’87 provides evidence that the prolonged cytotoxic concentrations of repeatedly applied intraventricular chemotherapy may be as efficacious as craniospinal radiotherapy, which is generally considered a highly effective treatment modality in childhood medulloblastoma. However, differences of systemic chemotherapy between both studies have to be considered: ifosfamide, cisplatinum, and cytarabine were replaced by cyclophosphamide, carboplatin, and vincristine in HIT-SKK’92, and procarbazine was omitted.
The concept of using an exclusively chemotherapy-based approach for first-line treatment may contribute to improved salvage strategies at relapse: while 8 of 16 treated children (50%) were successfully salvaged in HIT-SKK’92, only 1 of 10 treated children (10%) was salvaged in HIT-SKK’87.
Despite the relatively high survival rates of this study, the IQ results for fluid intelligence (CPM), considered an indicator of central mental problem-solving ability,39 were lower compared with children treated without radiotherapy in the HIT-SKK’92 study. Our clinical results are in line with a study demonstrating that white matter loss after treatment for medulloblastoma may be the neuroanatomical substrate for the adverse impact of young age at the time of radiotherapy.40
In conclusion, the strategy to defer craniospinal radiotherapy by using postoperative chemotherapy had considerable efficacy in terms of tumor control and survival. Tumor control during chemotherapy may be further improved by intensified chemotherapeutic strategies, and salvage strategies including radiotherapy may be more efficacious after exclusively chemotherapy-based first-line treatment. Desmoplastic histology (DMB and MBEN) is an independent favorable prognostic factor, and stratification according to histological variants may improve survival rates and quality of survival. Children with DMB are candidates for treatment de-escalation in future trials. The underlying biological differences between classic and desmoplastic variants should be investigated further, because specific therapeutic targets may be identified in the future. Brain-sparing treatment concepts avoiding craniospinal radiotherapy need to be further improved, and the neurocognitive outcome of children should be a major study end point in future studies on early childhood medulloblastoma.
Acknowledgments
The HIT trial office and reference centers have been supported by the Deutsche Kinderkrebsstiftung (German Children’s Cancer Foundation) and by the Deutsche Krebshilfe (German Cancer Aid). We thank the members of the German Society of Pediatric Oncology and Hematology; Paul Kleihues, Department of Pathology, University Hospital of Zurich, Switzerland; and pathologists, radiologists, and radiotherapists at cooperating centers for entering patients into the study. Special thanks to Wiebke Treulieb and Julia Becker (HIT data center Wuerzburg) for excellent data management. This work was presented at the 39th annual congress of the International Society of Pediatric Oncology in Mumbai, India, in 2007.
References
- 1.Kaatsch P, Rickert CH, Kuhl J, et al. Population-based epidemiologic data on brain tumors in German children. Cancer. 2001;92:3155–3164. doi: 10.1002/1097-0142(20011215)92:12<3155::aid-cncr10158>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 2.Kellie SJ. Chemotherapy of central nervous system tumours in infants. Childs Nerv Syst. 1999;15:592–612. doi: 10.1007/s003810050548. [DOI] [PubMed] [Google Scholar]
- 3.Evans AE, Jenkin RD, Sposto R, et al. The treatment of medulloblastoma. Results of a prospective randomized trial of radiation therapy with and without CCNU, vincristine, and prednisone. J Neurosurg. 1990;72:572–582. doi: 10.3171/jns.1990.72.4.0572. [DOI] [PubMed] [Google Scholar]
- 4.Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children’s Cancer Group 921 randomized phase III study. J Clin Oncol. 1999;17:832–845. doi: 10.1200/JCO.1999.17.3.832. [DOI] [PubMed] [Google Scholar]
- 5.Packer RJ, Rood BR, MacDonald TJ. Medulloblastoma: present concepts of stratification into risk groups. Pediatr Neurosurg. 2003;39:60–67. doi: 10.1159/000071316. [DOI] [PubMed] [Google Scholar]
- 6.Mulhern RK, Horowitz ME, Kovnar EH, et al. Neurodevelopmental status of infants and young children treated for brain tumors with preirradiation chemotherapy. J Clin Oncol. 1989;7:1660–1666. doi: 10.1200/JCO.1989.7.11.1660. [DOI] [PubMed] [Google Scholar]
- 7.Jenkin D, Danjoux C, Greenberg M. Subsequent quality of life for children irradiated for a brain tumor before age four years. Med Pediatr Oncol. 1998;31:506–511. doi: 10.1002/(sici)1096-911x(199812)31:6<506::aid-mpo7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 8.Kiltie AE, Lashford LS, Gattamaneni HR. Survival and late effects in medulloblastoma patients treated with craniospinal irradiation under three years old. Med Pediatr Oncol. 1997;28:348–354. doi: 10.1002/(sici)1096-911x(199705)28:5<348::aid-mpo4>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 9.Hoppe-Hirsch E, Brunet L, Laroussinie F, et al. Intellectual outcome in children with malignant tumors of the posterior fossa: influence of the field of irradiation and quality of surgery. Childs Nerv Syst. 1995;11:340–346. doi: 10.1007/BF00301666. [DOI] [PubMed] [Google Scholar]
- 10.Mulhern RK, Palmer SL, Merchant TE, et al. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23:5511–5519. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- 11.Palmer SL, Goloubeva O, Reddick WE, et al. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19:2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- 12.Goldwein JW, Radcliffe J, Johnson J, et al. Updated results of a pilot study of low dose craniospinal irradiation plus chemotherapy for children under five with cerebellar primitive neuroectodermal tumors (medulloblastoma) Int J Radiat Oncol Biol Phys. 1996;34:899–904. doi: 10.1016/0360-3016(95)02080-2. [DOI] [PubMed] [Google Scholar]
- 13.Duffner PK, Horowitz ME, Krischer JP, et al. Postoperative chemotherapy and delayed radiation in children less than three years of age with malignant brain tumors. N Engl J Med. 1993;328:1725–1731. doi: 10.1056/NEJM199306173282401. [DOI] [PubMed] [Google Scholar]
- 14.Saran FH, Driever PH, Thilmann C, et al. Survival of very young children with medulloblastoma (primitive neuroectodermal tumor of the posterior fossa) treated with craniospinal irradiation. Int J Radiat Oncol Biol Phys. 1998;42:959–967. doi: 10.1016/s0360-3016(98)00262-4. [DOI] [PubMed] [Google Scholar]
- 15.Geyer JR, Zeltzer PM, Boyett JM, et al. Survival of infants with primitive neuroectodermal tumors or malignant ependymomas of the CNS treated with eight drugs in 1 day: a report from the Children’s Cancer Group. J Clin Oncol. 1994;12:1607–1615. doi: 10.1200/JCO.1994.12.8.1607. [DOI] [PubMed] [Google Scholar]
- 16.White L, Kellie S, Gray E, et al. Postoperative chemotherapy in children less than 4 years of age with malignant brain tumors: promising initial response to a VETOPEC-based regimen. A Study of the Australian and New Zealand Children’s Cancer Study Group (ANZCCSG) J Pediatr Hematol Oncol. 1998;20:125–130. doi: 10.1097/00043426-199803000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Ater JL, van Eys J, Woo SY, et al. MOPP chemotherapy without irradiation as primary postsurgical therapy for brain tumors in infants and young children. J Neurooncol. 1997;32:243–252. doi: 10.1023/a:1005744527443. [DOI] [PubMed] [Google Scholar]
- 18.Rutkowski S, Bode U, Deinlein F, et al. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. N Engl J Med. 2005;352:978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- 19.Geyer JR, Sposto R, Jennings M, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children’s Cancer Group. J Clin Oncol. 2005;23:7621–7631. doi: 10.1200/JCO.2005.09.095. [DOI] [PubMed] [Google Scholar]
- 20.Grill J, Sainte-Rose C, Jouvet A, et al. Treatment of medulloblastoma with postoperative chemotherapy alone: an SFOP prospective trial in young children. Lancet Oncol. 2005;6:573–580. doi: 10.1016/S1470-2045(05)70252-7. [DOI] [PubMed] [Google Scholar]
- 21.Rutkowski S. Current treatment approaches to early childhood medulloblastoma. Expert Rev Neurother. 2006;6:1211–1221. doi: 10.1586/14737175.6.8.1211. [DOI] [PubMed] [Google Scholar]
- 22.Kalifa C, Grill J. The therapy of infantile malignant brain tumors: current status? J Neurooncol. 2005;75:279–285. doi: 10.1007/s11060-005-6752-x. [DOI] [PubMed] [Google Scholar]
- 23.Walker DA, Wilne S. Treatment of medulloblastoma in young children. Lancet Oncol. 2005;6:541–542. doi: 10.1016/S1470-2045(05)70259-X. [DOI] [PubMed] [Google Scholar]
- 24.Kuhl J, Beck J, Bode U, et al. Delayed radiation therapy after postoperative chemotherapy in children less than 3 years of age with medulloblastoma. Results of the trial HIT-SKK’87, and preliminary results of the pilot trial HIT-SKK’92 [abstract] Med Pediatr Oncol. 1995;25:250. [Google Scholar]
- 25.Giangaspero F, Bigner SH, Kleihues P, Pietsch T, Trojanowski JQ. Medulloblastoma. In: Kleihues P, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. Lyon, France: IARC Press; 2000. pp. 129–137. [Google Scholar]
- 26.Chang CH, Housepian EM, Herbert C., Jr An operative staging system and a megavoltage radiotherapeutic technic for cerebellar medulloblastomas. Radiology. 1969;93:1351–1359. doi: 10.1148/93.6.1351. [DOI] [PubMed] [Google Scholar]
- 27.Raven JC. Coloured Progressive Matrices. Revised Order. London: Lewis; 1956. [Google Scholar]
- 28.Melchers P. Preuβ U. Kaufmann Assessment Battery for Children—deutschsprachige Fassung (K-ABC) Frankfurt: Swets; 1994. [Google Scholar]
- 29.Beery KE. Beery Developmental Test of Visual-Motor Integration: Administration, Scoring and Teaching Manual. Cleveland: Modern Curriculum Press; 1989. [Google Scholar]
- 30.Timmermann B, Kortmann RD, Kuhl J, et al. Role of radiotherapy in supratentorial primitive neuroectodermal tumor in young children: results of the German HIT-SKK87 and HIT-SKK92 trials. J Clin Oncol. 2006;24:1554–1560. doi: 10.1200/JCO.2005.04.8074. [DOI] [PubMed] [Google Scholar]
- 31.Collett D. Strategy for model selection. In: Collett D, editor. Modelling Survival Data in Medical Research. London: CRC Press/Chapman and Hall; 1994. pp. 78–83. [Google Scholar]
- 32.Clarke JW, Hadziahmetovic M, Tzou K, et al. What is the best adjuvant treatment for very young patients with medulloblastoma? Expert Rev Neurother. 2007;7:373–381. doi: 10.1586/14737175.7.4.373. [DOI] [PubMed] [Google Scholar]
- 33.Duffner PK. Long-term effects of radiation therapy on cognitive and endocrine function in children with leukemia and brain tumors. Neurologist. 2004;10:293–310. doi: 10.1097/01.nrl.0000144287.35993.96. [DOI] [PubMed] [Google Scholar]
- 34.Attard-Montalto S, Plowman N, Breatnach F, et al. Is there a danger in delaying radiotherapy in childhood medulloblastoma? Br J Radiol. 1993;66:807–813. doi: 10.1259/0007-1285-66-789-807. [DOI] [PubMed] [Google Scholar]
- 35.Hartsell WF, Gajjar A, Heideman RL, et al. Patterns of failure in children with medulloblastoma: effects of preirradiation chemotherapy. Int J Radiat Oncol Biol Phys. 1997;39:15–24. doi: 10.1016/s0360-3016(97)00136-3. [DOI] [PubMed] [Google Scholar]
- 36.Giangaspero F, Perilongo G, Fondelli MP, et al. Medulloblastoma with extensive nodularity: a variant with favorable prognosis. J Neurosurg. 1999;91:971–977. doi: 10.3171/jns.1999.91.6.0971. [DOI] [PubMed] [Google Scholar]
- 37.McManamy CS, Pears J, Weston CL, et al. Nodule formation and desmoplasia in medulloblastomas—defining the nodular/desmoplastic variant and its biological behavior. Brain Pathol. 2007;17:151–164. doi: 10.1111/j.1750-3639.2007.00058.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giangaspero F, Eberhart CG, Haapasalo H, Pietsch T, Wiestler OD, Ellison DW. Medulloblastoma. In: Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, editors. WHO Classification of Tumours of the Central Nervous System. Lyon, France: IARC Press; 2007. pp. 132–140. [Google Scholar]
- 39.Cattell R. Advances in Psychology. Amsterdam: Elsevier; 1987. [Google Scholar]
- 40.Mulhern RK, Palmer SL, Reddick WE, et al. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol. 2001;19:472–479. doi: 10.1200/JCO.2001.19.2.472. [DOI] [PubMed] [Google Scholar]