Abstract
The introduction of methotrexate (MTX)-based chemotherapy has improved median survival for patients with primary CNS lymphoma (PCNSL). Older age is a negative prognostic marker in patients with PCNSL and may increase the likelihood of MTX toxicity. We studied the response and adverse effects of intravenous high-dose MTX in patients who were 70 or more years of age at the time of diagnosis. We identified 31 patients at our institution diagnosed with PCNSL at age ≥ 70 years (median, 74 years) who were treated with high-dose MTX (3.5–8 g/m2) as initial therapy from 1992 through 2006. The best response to MTX was determined by contrast-enhanced MRI. Toxicity was analyzed by chart review. These 31 patients received a total of 303 cycles of MTX (median, eight cycles per patient). Overall, 87.9% of the cycles required dose reduction because of impaired creatinine clearance. In 30 evaluable patients, the overall radiographic response rate was 96.7%, with 18 complete responses (60%) and 11 partial responses (36.7%). Progression-free survival and overall survival were 7.1 months and 37 months, respectively. Grade I–IV toxicities were observed in 27 of 31 patients and included gastrointestinal disturbances in 58% (3.2% grade III), hematological complications in 80.6% (6.5% grade III), and renal toxicity in 29% (0% grade III/IV). High-dose MTX is associated with a high proportion of radiographic responses and a low proportion of grade III/IV toxicity in patients 70 or more years of age. High-dose MTX should be considered as a feasible treatment option in elderly patients with PCNSL.
Keywords: chemotherapy, elderly, high-dose methotrexate, primary CNS lymphoma
Primary CNS lymphoma (PCNSL) is a rare type of extranodal non-Hodgkin’s lymphoma that is confined to the brain, spinal cord, or leptomeninges, with a median age of 60 years at diagnosis. The pathogenesis of PCNSL is unclear, but these tumors appear to be distinct from their systemic counterparts and thus require unique treatment.1
Older age is an independent negative prognostic marker in patients with PCNSL.2–4 Although whole-brain radiotherapy (WBRT) results in a high response proportion (> 90%), this treatment is associated with a high relapse rate and with delayed neurotoxicity, especially in patients older than 60 years of age. The introduction of methotrexate (MTX)-based chemotherapy has improved median survival from 10–16 months with WBRT alone to >30 months with MTX-based chemotherapy regimens.5
As with radiation, the toxicity of multiagent chemotherapy may outweigh the benefits in elderly patients.6 MTX monotherapy may have fewer treatment-associated toxic effects than multiagent regimens. However, high-dose MTX (≥ 3.5 g/m2) is a hospital-based regimen requiring aggressive fluid management and may not be as well tolerated in elderly patients with an increased prevalence of comorbid illnesses. We studied the adverse effects, radiographic responses, and survival of a consecutive cohort of PCNSL patients who were 70 or more years of age at the time of diagnosis and who were treated with MTX at our institution over a 14-year period.
Materials and Methods
We identified 36 consecutive patients with pathologically confirmed PCNSL involving the brain, the eyes, or both who were 70 or more years of age at the time of diagnosis from our institutional review board–approved electronic patient database. Of these 36, 31 patients were treated with MTX (3.5–8 g/m2) as initial therapy from 1992 through 2006 at Massachusetts General Hospital. The use of MTX monotherapy was considered the standard of care at our institution during this time. The five patients excluded from this report were initially treated with radiation (four) or received no treatment (one).
Thirty-one patients were treated with MTX (3.5–8 g/m2) in induction cycles every 10–14 days until a complete response (CR) was obtained or a maximum of eight induction cycles was reached. If a patient achieved a CR during induction (after one to eight cycles of induction therapy), an additional two consolidation cycles were administered every 14 days, followed by 11 maintenance cycles every 28 days. Four patients continued to receive MTX (8 g/m2) every 3 months following the maintenance phase at the discretion of the treating physician. The dose of MTX (either 3.5 g/m2 or 8 g/m2) was adjusted based on renal function as measured by 24-h urine collection for creatinine clearance. The dose of MTX was reduced by the percentage reduction in creatinine clearance less than 100. For example, a creatinine clearance of 75 ml/min mandated a reduction in the dose of MTX by 25%. Radiographic assessments with contrast-enhanced MRI scans were obtained every month during induction therapy and then every 2–3 months thereafter. The best response to MTX was determined by contrast-enhanced MRI scans as previously described.7 Patients had to be off corticosteroids to be classified as CR.
All patients were admitted to the hospital for each cycle of treatment and provided consent for chemotherapy. Prechemotherapy laboratory assessments included a 24-h urine collection, complete blood count, electrolytes, blood urea nitrogen, and creatinine. Patients were administered an antiemetic 30 min prior to each MTX infusion. MTX was administered as a 4-h intravenous infusion followed 24 h later by rescue with calcium leucovorin until the MTX serum level was ≤ 0.20 μmol/liter, as previously described.8–10
Clinical characteristics, laboratory values, and toxicities were retrieved and analyzed by reviewing inpatient and outpatient medical records. Laboratory values were assessed according to institutional normal ranges, and toxicities were classified according to the NCI Common Terminology Criteria for Adverse Events version 3.0 (http://ctep.cancer.gov).
Parameters with potential prognostic value, including KPS, cerebrospinal fluid (CSF) protein, serum lactate dehydrogenase (LDH), and tumor location, were analyzed when these parameters were known.2–4 Univariate analyses of clinical characteristics and outcomes, including radiographic response proportion, progression-free survival (PFS), and overall survival (OS), were performed. Kaplan-Meier survival distributions were estimated to assess PFS and OS. This study was approved by the institutional review board at the Massachusetts General Hospital.
Results
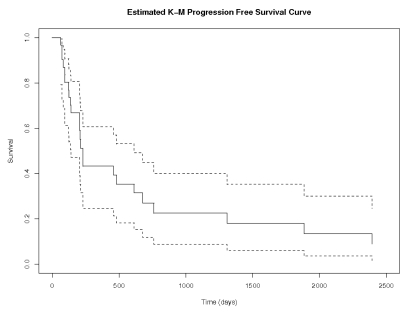
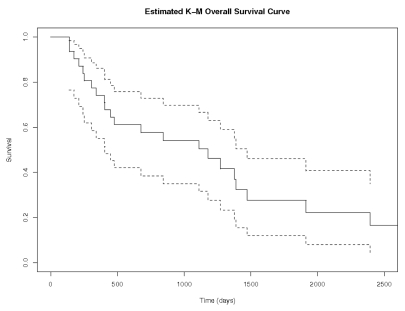
Thirty-one patients with a median age of 74 years (range, 70–85 years), median KPS of 70 (range, 30–70), and a median Mini-Mental Status Examination (MMSE) score of 28 (range, 5–30) received a total of 303 cycles of MTX as initial treatment (Table 1). The median follow-up for all 31 patients was 28 months. Thirty of the patients started treatment with 8 g/m2, while the remaining patient was treated with 3.5 g/m2. In 30 patients who could be evaluated for radiographic response (one patient did not have a follow-up MRI), the overall response proportion was 96.7%, with 18 CR (60%), 11 with partial response (PR; 36.7%), and 1 with progressive disease (PD; 3.3%) (Table 2). The median number of cycles to CR was 4. Median PFS and OS were 212 days (7.1 months) and 1,110 days (37 months), respectively (Figs. 1 and 2). On univariate analysis, only elevated CSF protein approached significance with PFS (p = 0.05). The small sample size precluded multivariate analysis.
Table 1.
Baseline demographics and clinical characteristics
| Characteristic | Patient Data |
|---|---|
| Median age (range), years | 74 (70–85) |
| Male | 10/31 (32.3%) |
| Female | 21/31 (67.7%) |
| Median KPS (range) | 70 (30–70) |
| Median MMSE (range) | 28 (5–30) |
| Median number of MTX cycles received (range) | 8 (1–22) |
| Elevated LDH | 9/23 (39.1%) |
| Elevated CSF protein | 12/22 (54.5%) |
| Location (deep structures) | 19/31 (61.3%) |
| Location (other) | 12/31 (38.7%) |
| Ocular involvement | 4/31 (12.9%) |
| Atypical cells in CSF | 6/22 (27.3%) |
Abbreviations: MMSE, Mini-Mental Status Exam; MTX, methotrexate; LDH, lactate dehydrogenase; CSF, cerebrospinal fluid.
Table 2.
Response to treatment with methotrexate (MTX)
| Response Type | Result |
|---|---|
| Radiographic response | |
| CR | 60% (18/30) |
| PR | 36.7% (11/30) |
| PD | 3.3% (1/30) |
| Median progression-free survival | 212 days (7.1 months) (95% CI 145–356 days) |
| Median overall survival | 1,110 days (37 months) (95% CI, 759–1,849 days) |
Abbreviations: CR, complete response; PR, partial response; PD, progressive disease; CI, confidence interval.
Fig. 1.
Progression-free survival (days). Analysis was performed using the Kaplan-Meier method for all 31 patients. Dotted lines represent 95% confidence intervals.
Fig. 2.
Overall survival (days). Analysis was performed using the Kaplan-Meier method for all 31 patients. Dotted lines represent 95% confidence intervals.
All 31 patients were included in the toxicity analysis. Twenty-two of 31 patients (71%) required dose reduction of the first cycle of MTX due to impaired creatinine clearance prior to any treatment (Table 3). Overall, 87.9% of evaluable MTX cycles required dose reduction because of impaired creatinine clearance. Patients received a median of eight cycles (range, 1–22 cycles). MTX-associated toxicities were observed in 27 of 31 patients (87%), with 74.1% grade I/II and 9.7% grade III/IV toxicities (Table 4). Gastrointestinal disturbances, including mucositis, were observed in 18 of 31 (58%) patients (1 of 31 grade III, 3.2%); hematological complications in 25 (80.6%; 2 of 31 grade III, 6.5%); renal toxicity in 8 (25.8%; 0% grade III/IV); and myocardial infarction in 1 (3.2%). Only 4 of 31 (12.9%) patients had to terminate MTX due to toxicity: three patients because of recurrent infections that were possibly related to chemotherapy, and one because of a myocardial infarction that was unlikely related to chemotherapy. The majority of the toxicities were mild and reversible.
Table 3.
Dose of methotrexate (MTX) given and amount of reduction based on creatinine clearance
| Patient | Starting Dose (mg/m2) | Reduction | Dose Given (mg/m2) |
|---|---|---|---|
| 1 | 8,000 | None | 8,000 |
| 2 | 8,000 | None | 8,000 |
| 3 | 8,000 | None | 8,000 |
| 4 | 8,000 | None | 8,000 |
| 5 | 8,000 | None | 8,000 |
| 6 | 8,000 | None | 8,000 |
| 7 | 8,000 | None | 8,000 |
| 8 | 8,000 | None | 8,000 |
| 9 | 3,500 | None | 3,500 |
| 10 | 8,000 | 3% | 7,760 |
| 11 | 8,000 | 4% | 7,680 |
| 12 | 8,000 | 6% | 7,520 |
| 13 | 8,000 | 11% | 7,120 |
| 14 | 8,000 | 15% | 6,800 |
| 15 | 8,000 | 18% | 6,560 |
| 16 | 8,000 | 19% | 6,480 |
| 17 | 8,000 | 21% | 6,320 |
| 18 | 8,000 | 22% | 6,240 |
| 19 | 8,000 | 28% | 5,760 |
| 20 | 8,000 | 32% | 5,440 |
| 21 | 8,000 | 33% | 5,360 |
| 22 | 8,000 | 33% | 5,360 |
| 23 | 8,000 | 33% | 5,360 |
| 24 | 8,000 | 36% | 5,152 |
| 25 | 8,000 | 38% | 4,960 |
| 26 | 8,000 | 40% | 4,800 |
| 27 | 8,000 | 42% | 4,640 |
| 28 | 8,000 | 42% | 4,640 |
| 29 | 8,000 | 43% | 4,560 |
| 30 | 8,000 | 44% | 4,480 |
| 31 | 8,000 | 50% | 4,000 |
Table 4.
Toxicity associated with methotrexate (MTX)
| Toxicity | Frequency |
|---|---|
| MTX terminated due to toxicity | 4/31 (12.9%) |
| MTX dose reduction based on GFR | 218/248 cycles (87.9%) |
| Grade III or IV toxicity | Total: 3/31 (9.7%) |
| Hematological | 2/31 (6.5%) |
| GI | 1/31 (3.2%) |
| Cardiac | 1/31 (3.2%) |
| Renal | 0/31 |
Abbreviations: GFR, glomerular filtration rate; GI, gastrointestinal.
Among the 18 patients who developed PD, five had local recurrence at the site of the original tumor, four had both local recurrence and involvement of other parts of the brain, and nine had only distant recurrence in other parts of the CNS. No patient experienced systemic recurrence of PCNSL. Salvage therapy at the time of progression for these 18 patients included reinduction with MTX (3 of 18 patients), WBRT (7 of 18 patients), stereotactic radiosurgery (1 of 18) followed by continued MTX, and chemotherapy plus WBRT (2 of 18 patients). Five of the 18 patients elected not to receive further treatment. Response to salvage therapy could not be assessed because many patients had salvage treatment at their community medical centers.
Discussion
Elderly PCNSL patients are perceived to be at higher risk of treatment-related complications. The high risk of neurotoxicity in this patient population has led to deferral of WBRT in patients 60 or more years of age.11 Moreover, it has been observed that MTX alone or MTX-based multiagent chemotherapy is associated with comparable response rates and OS compared to regimens that include WBRT.5,8,9 However, the use of higher doses of MTX regimens in elderly patients may be associated with a higher risk of systemic toxicity because of the increased prevalence of comorbidities.
Although most patients with PCNSL are older than 60 years at the time of diagnosis, few studies have focused on elderly patients with this disease.12–18 In one study, 13 patients 50 or more years of age (mean, 74 years; 12 were ≥ 66 years) were treated with an MTX-based multiagent regimen (MTX dose, 1–3.5 g/m2).15 The response rate was 92%, and the OS was 30.5 months. An alternate multiagent regimen that included MTX (3.5 g/m2) resulted in a median OS of 29 months in 26 patients (although four of these were > 60 years old).18 In a multicenter study of 50 PCNSL patients with a median age of 72 years, subjects were treated with MTX-based multiagent chemotherapy, and the response rate was 48%, with an OS of 14.3 months, but the intravenous dose of MTX was only 1 g/m2.13 One patient died during chemotherapy, and grade III/IV toxicity (neutropenia) was reported in 19% of patients. In another study, temozolomide and MTX (3 g/m2) were administered to patients 60 or more years of age (median, 63 years).16 The radiographic response proportion was 55%, and the PFS and OS were 8 months and 35 months, respectively. In a study of MTX (4 g/m2) in 154 PCNSL patients with a median age of 61 years, including 21 patients 70 or more years of age, toxicity from MTX was analyzed as a function of age (< 60, 60–70, and > 70 years).12 A significant proportion of patients required a reduction in their MTX dose based on their diminished creatinine clearance. Dose reduction for this reason was required in 70% of patients > 70 years, 44% of patients > 60 years, and 18% of patients < 60 years of age. However, no statistically significant difference in the incidence of toxicity was detected between patients ≤ 60 and > 60 years, suggesting that MTX (4 g/m2) is well tolerated in the elderly population.
The present study is the largest cohort of elderly patents 70 or more years of age at the time of diagnosis of PCNSL analyzed for response and toxicities while receiving MTX (3.5–8 g/m2) alone as the initial treatment. Most patients (27 of 31, 87%) received 8 g/m2 (dose reduced based on creatinine clearance), unlike many of the studies described above in which lower doses were initially prescribed. We observed that MTX is associated with a high proportion of radiographic responses in elderly patients with PCNSL, which is similar to results of prior studies in the general PCNSL population. Median PFS and OS are also similar to prior results for MTX-based regimens in patients 60 or more years of age (Table 5). However, few reports have restricted analysis of PFS and OS to patients older than 70 years, so there are no large cohorts of comparable patients with which to compare our outcome. Dose reductions required for decreased creatinine clearance in the elderly do not appear to limit the efficacy of MTX and may make the treatment more tolerable. In fact, lower creatinine clearance may improve OS because of improved MTX pharmacokinetics.19 The discrepancy between median PFS and OS suggests that salvage therapies, including reinduction with MTX, are effective in this population.
Table 5.
Studies of methotrexate (MTX)-based chemotherapy without radiation in elderly primary CNS lymphoma patients
| Measure | Hoang-Xuan et al.13 | Omuro et al.16 | Freilich et al.15 | Ng et al.14 | Gavrilovic et al.18,a | Present Study |
|---|---|---|---|---|---|---|
| Number of patients | 50 | 23 | 13 | 10 | 26 | 31 |
| Dose of MTX | 1 g/m2 | 3 g/m2 | 1–3.5 g/m2 | 3.5–8 g/m2 | 3.5 g/m2 | 3.5–8 g/m2 |
| Median age (range), years | 72 (60–81) | 68 (60–79) | 74 (54–89) | 72.5 (66–75) | 65 (22–89) | 74 (70–85) |
| Radiographic response | 48% | 55% | 92% | 90% | NA | 96.7% |
| Median progression-free survival (months) | 10.6 | 8 | NA | NA | 7 | 7.1 |
| Median overall survival (months) | 14.3 | 35 | 30.5 | 36 | 29 | 37 |
Abbreviation: NA, not reported. Only Ng et al.14 and our study used MTX monotherapy. Most patients (87%) received 8 g/m2 of MTX in our study.
Four of the 26 patients treated with chemotherapy alone were <60 years old.
Treatment-related complications of hospital-based MTX monotherapy in our patient population were modest and consisted mainly of mild azotemia (usually reversible), nausea, and diarrhea. Grade III or IV toxicities in patients 70 or more years of age were uncommon (9.7% of patients) over 303 cycles of chemotherapy and less than with multiagent chemotherapy regimens. Only 4 of 31 patients (12.9%) discontinued MTX because of toxicity. This study provides further support that MTX at initial doses of 8 g/m2 (adjusted for creatinine clearance) is feasible as first-line treatment of PCNSL patients 70 or more years of age. The response proportion remains high while the risk of toxicity is likely lower than with WBRT or multiagent chemotherapy.20–23 Our data serve as the basis for developing less toxic combination regimens that include MTX (8 g/m2) as a component of therapy for elderly patients.
Acknowledgments
This work was supported by the Richard and Nancy Simches Endowed Fund for Brain Tumor Research at the Massachusetts General Hospital and by National Institutes of Health grant KCA125440A (T.T.B.).
References
- 1.Rubenstein J, Fridlyand J, Shen A, et al. Gene expression and angiotropism in primary CNS lymphoma. Blood. 2006;107:3716–3723. doi: 10.1182/blood-2005-03-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferreri AJ, Reni M, Pasini F, et al. A multicenter study of treatment of primary CNS lymphoma. Neurology. 2002;58:1513–1520. doi: 10.1212/wnl.58.10.1513. [DOI] [PubMed] [Google Scholar]
- 3.Ferreri A, Blay J, Reni M, et al. Prognostic scoring system for primary CNS lymphomas: the International Extranodal Lymphoma Study Group experience. J Clin Oncol. 2003;21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 4.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24:5711–5715. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 5.Plotkin SR, Batchelor TT. Primary nervous-system lymphoma. Lancet Oncol. 2001;2:354–365. doi: 10.1016/S1470-2045(00)00390-9. [DOI] [PubMed] [Google Scholar]
- 6.Pels H, Schmidt-Wolf IG, Glasmacher A, et al. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21:4489–4495. doi: 10.1200/JCO.2003.04.056. [DOI] [PubMed] [Google Scholar]
- 7.Abrey L, Batchelor T, Ferreri A, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034–5043. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- 8.Cher L, Glass J, Harsh GR, et al. Therapy of primary CNS lymphoma with methotrexate-based chemotherapy and deferred radiotherapy: preliminary results. Neurology. 1996;46:1757–1759. doi: 10.1212/wnl.46.6.1757. [DOI] [PubMed] [Google Scholar]
- 9.Batchelor T, Carson K, O’Neill A, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiation therapy: a report of the NABTT 96-07. J Clin Oncol. 2003;21:1044–1049. doi: 10.1200/JCO.2003.03.036. [DOI] [PubMed] [Google Scholar]
- 10.Batchelor TT, Kolak G, Ciordia R, et al. High-dose methotrexate for intraocular lymphoma. Clinical Cancer Research. 2003;9:711–715. [PubMed] [Google Scholar]
- 11.Correa DD, DeAngelis LM, Shi W, et al. Cognitive functions in survivors of primary central nervous system lymphoma. Neurology. 2004;62:548–555. doi: 10.1212/01.wnl.0000109673.75316.d8. [DOI] [PubMed] [Google Scholar]
- 12.Jahnke K, Korfel A, Martus P, et al. High-dose methotrexate toxicity in elderly patients with primary central nervous system lymphoma. Ann Oncol. 2005;16:445–449. doi: 10.1093/annonc/mdi075. [DOI] [PubMed] [Google Scholar]
- 13.Hoang-Xuan K, Taillandier L, Chinot O, et al. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: a multicenter phase II study (26952) of the European Organization for Research and Treatment of Cancer Brain Tumor Group. J Clin Oncol. 2003;21:2726–2731. doi: 10.1200/JCO.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 14.Ng S, Rosenthal M, Ashley D, Cher L. High-dose methotrexate for primary CNS lymphoma in the elderly. Neuro-Oncology. 2000;2:40–44. doi: 10.1215/15228517-2-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freilich RJ, Delattre JY, Monjour A, et al. Chemotherapy without radiation therapy as initial treatment for primary CNS lymphoma in older patients. Neurology. 1996;46:435–439. doi: 10.1212/wnl.46.2.435. [DOI] [PubMed] [Google Scholar]
- 16.Omuro A, Taillandier L, Chinot O, Carnin C, Barrie M, Hoang-Xuan K. Temozolomide and methotrexate for primary central nervous system lymphoma in the elderly. J Neurooncol. 2007;85:207–211. doi: 10.1007/s11060-007-9397-0. [DOI] [PubMed] [Google Scholar]
- 17.Laack NN, Ballman KV, Brown PB, et al. Whole-brain radiotherapy and high-dose methylprednisolone for elderly patients with primary central nervous system lymphoma: results of North Central Cancer Treatment Group (NCCTG) 96-73-51. Int J Radiat Oncol Biol Phys. 2006;65:1429–1439. doi: 10.1016/j.ijrobp.2006.03.061. [DOI] [PubMed] [Google Scholar]
- 18.Gavrilovic IT, Hormigo A, Yahalom J, DeAngelis LM, Abrey LE. Long-term follow-up of high-dose methotrexate-based therapy with and without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006;24:4570–4574. doi: 10.1200/JCO.2006.06.6910. [DOI] [PubMed] [Google Scholar]
- 19.Ferreri AJ, Guerra E, Regazzi M, et al. Area under the curve of methotrexate and creatinine clearance are outcome-determining factors in primary CNS lymphomas. Br J Cancer. 2004;90:353–358. doi: 10.1038/sj.bjc.6601472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O’Brien P, Roos D, Pratt G, et al. Combined-modality therapy for primary central nervous system lymphoma: long-term data from a phase II multicenter study (Trans-Tasman Radiation Oncology Group) Int J Radiat Oncol Biol Phys 200664408–413.16198065 [Google Scholar]
- 21.Abrey LE, DeAngelis LM, Yahalom J, Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol. 1998;16:859–863. doi: 10.1200/JCO.1998.16.3.859. [DOI] [PubMed] [Google Scholar]
- 22.Fliessbach K, Helmstaedter C, Urbach H, et al. Neuropsychological outcome after chemotherapy for primary CNS lymphoma: a prospective study. Neurology. 2005;647 doi: 10.1212/01.WNL.0000156350.49336.E2. [DOI] [PubMed] [Google Scholar]
- 23.Neuwelt EA, Guastadisegni PE, Varallyay P, et al. Imaging changes and cognitive outcome in primary CNS lymphoma after enhanced chemotherapy delivery. AJNR Am J Neuroradiol. 2005;26:258–265. [PMC free article] [PubMed] [Google Scholar]