Abstract
The benefit of cytoreductive surgery for glioblastoma multiforme (GBM) is unclear, and selection bias in past series has been observed. The 5-aminolevulinic acid (ALA) study investigated the influence of fluorescence-guided resections on outcome and generated an extensive database of GBM patients with optimized resections. We evaluated whether the Radiation Therapy Oncology Group recursive partitioning analysis (RTOG-RPA) would predict survival of these patients and whether there was any benefit from extensive resections depending on RPA class. A total of 243 per-protocol patients with newly diagnosed GBM were operated on with or without ALA and treated by radiotherapy. Postoperative MRI was obtained in all patients. Patients were allocated into RTOG-RPA classes III–V based on age, KPS, neurological condition, and mental status (as derived from the NIH Stroke Scale). Median overall survival among RPA classes III, IV, and V was 17.8, 14.7, and 10.7 months, respectively, with 2-year survival rates of 26%, 12%, and 7% (p = 0.0007). Stratified for degree of resection, survival of patients with complete resections was clearly longer in RPA classes IV and V (17.7 months vs. 12.9 months, p = 0.0015, and 13.7 months vs. 10.4 months, p = 0.0398; 2-year rates: 21.0% vs. 4.4% and 11.1% vs. 2.6%, respectively), but was not in the small subgroup of RPA class III patients (19.3 vs. 16.3 months, p = 0.14). Survival of patients from the ALA study is correctly predicted by the RTOG-RPA classes. Differences in survival depending on resection status, especially in RPA classes IV and V, support a causal influence of resection on survival.
Keywords: aminolevulinic acid, glioblastoma multiforme, recursive partitioning analysis, resection, survival
The benefit from cytoreductive surgical therapy in the treatment of glioblastoma multiforme is still under discussion. Recently, a large, prospectively randomized, controlled phase III trial was concluded, which demonstrated that a significantly larger number of “complete” resections (defined as absence of contrast-enhancing tumor on early postoperative MRI) could be achieved using fluorescence-guided resections with 5-aminolevulinic acid (ALA)–induced tumor fluorescence, compared to conventional microsurgery (65% vs. 36 %, p < 0.001).1 Historically, complete resections of contrast-enhancing tumor (for the sake of brevity “complete” resection in this paper signifies complete resection of contrast-enhancing tumor on MRI) had only been reported in approximately 20% of cases in surgical series with postoperative imaging.2–6 In contrast, the fraction of patients with complete resections within the ALA study was 50%. Therefore, the ALA study afforded an unprecedented number of patients with complete resections in a highly controlled surgical trial investigating malignant gliomas and provided a unique background for reassessing the influence of resection on survival. To this end, stratification of patients by resection status did demonstrate longer survival in patients with complete resection of contrast-enhancing tumor; however, an influence of age was observed on the degree of resection,1 confounding the interpretation concerning the causal influence of resection on survival.7 Differences in important pretreatment prognostic factors among patients with varying resection status and resulting selection bias have also been observed in a smaller, older series.2
The necessity for considering pretreatment prognostic variables when designing and interpreting therapy studies on malignant gliomas was clearly demonstrated by the Radiation Therapy Oncology Group (RTOG), which published a nonparametric, recursive partitioning analysis (RPA) of the prognostic variables determined from three randomized therapy trials on malignant gliomas.8 This analysis stratified patients into six treatment classes based on incremental improvements of survival. The authors concluded that survival differences among the resulting patient classes required that clinical trial design for malignant glioma patients be tailored to prognostically homogenous patient subpopulations. The RTOG-RPA classification has since been validated in a separate database and has been used for validation of efficacy of various therapies.9–18 It has also successfully predicted the efficacy of concomitant therapy using radiotherapy and temozolomide,19 as later confirmed by the European Organisation for Research and Treatment of Cancer (EORTC) 26981/22981-NCIC CE3 study.20 Recently, it was demonstrated that RPA retained its prognostic power for patients from the EORTC 26981/22981-NCIC CE3 study when these patients were restratified by RTOG-RPA classes.21
To assess the benefits of complete resections we reanalyzed the data from the patients from the ALA study using the criteria proposed by the RTOG-RPA. We hypothesized that stratification according to the prognostic criteria, such as age or KPS, would correct selection bias that might have shown a positive influence of resection on survival. Survival was calculated for each treatment class in both groups of patients. Within the respective classes, this analysis should have minimized the contribution of patient selection in determining survival in patients with incomplete and complete resections and allowed firm conclusions regarding the value of complete resections in the treatment of malignant glioma patients.
Patients and Methods
All patients with WHO grade IV lesions from the ALA study were restratified according to the results of early postoperative MRI, irrespective of their initial study arm. Design and results of the ALA study are described elsewhere.1 In brief, the ALA study was a parallel, randomized, balanced, group-sequential, two-armed, controlled multicenter phase III study of a diagnostic procedure (i.e., a procedure facilitating tumor discrimination during surgery by comparing fluorescence-guided resections using ALA with conventional microsurgery). Primary study aims were the rate of complete resection and the rate of progression-free survival at 6 months. The study was not designed to determine the influence of fluorescence-guided resection on survival, although survival was recorded as a secondary study aim.
Study Population
Patients were enrolled in the ALA study at 17 German centers. Patients 18–72 years of age with suspected or newly diagnosed untreated malignant gliomas and eligible for surgery were randomized to either the ALA or the white light (WL) control group. Eligible patients were required to have tumors with a distinct ring-like pattern of contrast enhancement with thick irregular walls on MRI and a core area of reduced signal suggestive for tumor necrosis. Patients with tumors of the midline, basal ganglia, cerebellum, or brainstem; patients with more than one contrast-enhancing lesion; and patients with significant, non–contrast-enhancing tumor areas indicating low-grade gliomas with malignant transformation were not included. Further inclusion criteria were a KPS >60, no signs for renal or hepatic insufficiency, and no history of other malignant tumors. All patients provided written informed consent, and the study was approved by the ethics committees of the participating centers.
Of a total of 322 patients with suspected malignant glioma that were randomized, 16 of 161 patients in the ALA group, and 18 of 161 patients in the WL group had to be discontinued due to ineligible histology (e.g., metastasis, abscess, and vasculitis). Thus, 139 patients in the ALA group and 131 patients in the WL group qualified for the full analysis set. An additional 19 patients were excluded from the per-protocol set due to missing postoperative MRI, second resection with 1 week after study surgery, loss to follow-up immediately after surgery, or withdrawal of study consent immediately after surgery. Per-protocol patients formed the basis for the present explorative analysis. Furthermore, histological examination showed only eight patients with WHO grade III gliomas, so the present analysis was restricted to the remaining 243 patients with WHO grade IV gliomas. Baseline characteristics of the study are summarized in Table 1.
Table 1.
Original Radiation Therapy Oncology Group recursive partitioning analysis (RTOG-RPA) classes and adapted classes from the 5-aminolevulinic acid (ALA) study
| RPA Class | RTOG Class | ALA RPA Study Class |
|---|---|---|
| III | ||
| Age (years) | <50 | Not included |
| Tumor type | Anaplastic astrocytoma | |
| Mental status | Abnormal | |
| or | ||
| Age (years) | <50 | <50 |
| Tumor type | GBM | GBM |
| KPS | 90–100 | 90–100 |
| IV | ||
| Age (years) | <50 | <50 |
| Tumor type | GBM | GBM |
| KPS | <90 | <90 |
| or | ||
| Age (years) | ≥50 | Not included |
| Tumor type | Anaplastic astrocytoma | |
| KPS | 70–100 | |
| Symptom time | Symptoms ≤3 months | |
| or | ||
| Age (years) | ≥50 | ≥50 |
| Tumor type | GBM | GBM |
| Treatment status | Resection | Resection |
| Neurological function | Good neurological function | NIH SS ≤2 |
| V | ||
| Age (years) | ≥50 | ≥50 |
| Tumor type | GBM | GBM |
| KPS | 70–100 | 70–100 |
| Treatment status | Resection | Resection |
| Neurological function | Unfavourable | NIH-SS ≥2 |
| or | ||
| Age (years) | ≥50 | Not included |
| Tumor type | GBM | |
| KPS | 70–100 | |
| Treatment status | Biopsy only with radiotherapy ≥54.4 Gy | |
| or | ||
| Age (years) | ≥50 | ≥50 |
| KPS | <70 | <70 |
| Mental status | Normal | NIH-SS LOC + LOC questions + LOC commands = 0 |
Abbreviations: GBM, glioblastoma multiforme; NIH-SS, NIH Stroke Scale score; LOC, level of consciousness.
MRI Assessments
MRIs were performed preoperatively and within 72 h after surgery. The presence of residual tumor was assessed centrally (Department of Neuroradiology, University of Frankfurt) by postoperative MRI (within 72 h after surgery). Assessors were blinded to treatment group. Residual tumor was defined as contrast enhancement with a volume >0.175 cm3. The cutoff volume represented the size of one voxel in the T1 image and signified the minimal resolution obtained on MRI. The cutoff was defined to prevent interpretation problems when distinguishing between tumor and nonspecific enhancement (e.g., small vessels or enhancing pia mater). The volumes of compact tumors with spherical geometry were calculated by fitting a rotational ellipsoid defined by the maximum tumor diameters in the available three dimensions. The volumes of cup-shaped, residual tumors were calculated by subtracting the volumes of the central resection defect from the space defined by the outer boundaries of tumors. The volumes of residual tumors with complex configuration were segmented on individual scans, and the individual volumes summed for the final volume.
Treatments
Pretreatment with a dose of 3 × 4 mg/d of dexamethasone was obligatory for at least 2 days prior to surgery and until early MRI had been obtained (within 72 h after surgery). Patients randomized to the ALA group received freshly prepared solutions of 5-ALA (20 mg/kg body weight) orally for 3 h (range, 2–4 h) preceding induction of anesthesia.
The study required tumor resections to be as complete as considered safely possible by the responsible study surgeon. In all patients the tumor was resected using only an NC 4 OPMI Neuro FL surgical microscope (Zeiss, Oberkochen, Germany), which enabled switching from conventional standard xenon light to filtered, violet-blue excitation light for visualizing fluorescence. In the control group the tumor was resected as thoroughly as possible under standard white light. In the ALA group violet-blue light could be used intermittently to visualize the tumor marker (red tissue fluorescence). In all patients surgery was required to be followed by standard fractionated radiotherapy with a recommended lesion dose of 60 Gy (30 × 2 Gy) and no chemotherapy prior to radiological progression, as this was not the standard of treatment in centers participating in the study during the recruitment period. No restrictions were imposed upon therapy after progression.
Recursive Partitioning Analysis
Patients from the ALA study were redistributed based on the criteria put forth by the RTOG-RPA classification.8 As mentioned, patients with grade III tumors in the ALA database were excluded due to their small number. Thus, no assumptions had to be made for duration of symptoms. “Duration of symptoms” was a patient characteristic included in the RTOG-RPA analysis for identifying class III patients but was not available from the ALA database.
The characteristics “mental status” and “neurological status” used in the RTOG-RPA analysis were derived from the NIH Stroke Scale (NIH-SS) score data collected in the ALA study. In the ALA study the NIH-SS was used for assessing individual items of neurological function and acute deterioration of these functions as a consequence of surgery. The NIH-SS score assesses 15 neurological functions, grading the severity of impairment for each function individually,22–24 including data on the level of consciousness (LOC) per se and the LOC as assessed by responses to questions or commands. The latter data were essential to assess “mental status” as used in the RTOG-RPA nomenclature. LOC per se was evaluated as the following levels: 0 = alert, keenly responsive; 1 = not alert, but arousable by minor stimulation to obey, answer, or respond; 2 = not alert, requires repeated stimulation to attend or was obtunded and required strong or painful stimulation to make movements (not stereotyped); and 3 = responded only with reflex motor or autonomic effects or totally unresponsive, flaccid, areflexic. To assess LOC responses to questions, the patient was asked the month and his or her age: 0 = answered both questions correctly; 1 = answered one question correctly; and 2 = answered neither question correctly. To assess LOC responses to commands, the patient was asked to open and close the eyes and then to grip and release the nonparetic hand: 0 = performed both tasks correctly; 1 = performed one task correctly; and 2 = performed neither task correctly.
Assumptions had to be made concerning the covariate “neurological function” as used in the RTOG-RPA classification. In the RTOG-RPA classification, neurologic function is defined as either “good” in RPA class III or “neurologic function that inhibits the ability to work” in RPA class IV. For the present evaluation it was assumed that an NIH-SS score ≤2 would be an indicator for “good neurological function” or the ability to work. Data on “the ability to work” were not available from the ALA study database.
Statistical Analysis
The Kaplan-Meier method was used for calculating survival for the patients from the ALA study stratified by their RPA class (III, IV, and V). For each class, patients were further stratified by their resection status (complete vs. incomplete resection) and again compared using the Kaplan-Meier method to assess the influence of resection. Chi-square, Cochran-Armitage trend, and log-rank tests were used for comparing patient groups.
Results
Patient Characteristics
Table 2 gives an overview of patient characteristics stratified by degree of resection. Patients were well balanced regarding the following factors: sex, preoperative tumor volume, KPS, grade IV glioma subgroup, preoperative NIH-SS score, preoperative NIH-SS score ≤2, and NIH-SS score of LOC and substrata LOC questions and commands. There was a significant age difference between patients with complete resection and patients with incomplete resection. Restratification of patients into RPA classes demonstrated patients to be balanced in RPA classes IV and V regarding resection status.
Table 2.
Preoperative patient characteristics
| Characteristic | n | Complete Resection No. (%) | Incomplete Resection No. (%) | pValue |
|---|---|---|---|---|
| All Patients | 243 | 122 (50.2) | 121 (49.8) | |
| RTOG-RPA Relevant Covariates | ||||
| Age (years) | ||||
| ≤ 60 | 123 | 72 (58.5) | 51 (41.5) | 0.0123* |
| > 60 | 120 | 50 (41.7) | 70 (58.3) | |
| KPS | ||||
| 60 | 1 | 1 (100.0) | 0 | 0.1714** |
| 70 | 24 | 10 (41.7) | 14 (58.3) | |
| 80 | 30 | 10 (33.3) | 20 (66.7) | |
| 90 | 109 | 59 (54.1) | 50 (45.9) | |
| 100 | 79 | 42 (53.2) | 37 (46.8) | |
| Preoperative NIH-SS | ||||
| < 2 (favorable neurological function) | 155 | 80 (51.6) | 75 (48.4) | 0.560* |
| ≥ 2 (unfavorable neurological function) | 88 | 42 (47.7) | 46 (42.3) | |
| Preoperative NIH-SS LOC (LOC, LOC questions, LOC commands) | ||||
| < 1 (normal mental status) | 130 | 114 (94.2) | 116 (95.1) | 0.7843 |
| ≥ 1 (abnormal mental status) | 13 | 7 (5.8) | 6 (4.9) | |
| RTOG-RPA class | ||||
| III | 38 | 24 (63.2) | 14 (36.8) | 0.579* |
| IV | 130 | 62 (47.7) | 68 (52.3) | |
| V | 75 | 36 (48.0) | 39 (52.0) | |
| General Covariates | ||||
| Sex | ||||
| F | 90 | 48 (53.3) | 42 (46.7) | 0.4545* |
| M | 153 | 74 (48.4) | 79 (51.6) | |
| Preoperative tumor volume*** | ||||
| Median (cm3) | 241 | 30.2 | 36.4 | 0.1306**** |
| Missing | 2 | 1 (50.0) | 1 (50.0) | |
| Postoperative tumor volume*** | ||||
| 0 cm3 | 122 | 122 (100.0) | 0 | <0.0001** |
| Median (cm3) | 241 | 0 | 1.5 | |
| Histology (WHO grade IV) | ||||
| Gliosarcoma | 15 | 7 (46.7) | 8 (53.3) | 0.1455* |
| Glioblastoma giant cell | 7 | 1 (14.3) | 6 (85.7) | |
| Glioblastoma multiforme | 221 | 114 (51.6) | 107 (48.4) | |
Abbreviations: RTOG-RPA, Radiation Therapy Oncology Group recursive partitioning analysis; NIH-SS, NIH Stroke Scale score; LOC, level of consciousness; F, female; M, male.
Chi-square test;
Cochran-Armitage trend test;
Gadolinium diethylenetriamine pentaacetic acid enhancing tumor on T1 sequence;
Log-rank test.
Survival Stratified by RTOG-RPA Class
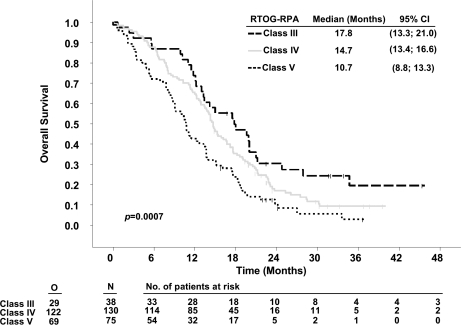
When comparing glioblastoma patients with complete versus incomplete resections, survival was 16.7 versus 11.8 months (p < 0.0001).7 Overall survival was calculated for patients restratified according to RTOG-RPA classes III, IV, and V. Median survival times were 17.8 months (95% confidence interval [CI], 13.3–21), 14.7 months (13.4–16.6), and 10.7 months (8.8–13.3), respectively (p = 0.0007). These values were associated with 24-month survival rates of 26.3%, 12.3%, and 6.6% (Table 3 and Fig. 1).
Table 3.
Overall survival data among recursive partitioning analysis (RPA) classes (Curran et al.8) and patients from the 5-aminolevulinic acid (ALA) study
| RPA Class |
RTOG Database |
ALA Study Database (All Patients)a |
||||
|---|---|---|---|---|---|---|
| Median (Months) | 95% CI | 2-Year Rate (%) | Median (Months) | 95% CI | 2-Year Rate (%) | |
| III | 17.9 | NA | 35 | 17.8 | 13.3–21 | 26.3 |
| IV | 11.1 | NA | 15 | 14.7 | 13.4–16.6 | 12.3 |
| V | 8.9 | NA | 6 | 10.7 | 8.8–13.3 | 6.6 |
Abbreviations: RTOG, Radiation Therapy Oncology Group; CI, confidence interval; NA, not available.
Favorable neurological function = NIH ≤2; unfavorable = NIH >2.
Fig. 1.
Kaplan-Meier estimates of overall survival of 5-aminolevulinic acid (ALA) study patients according to Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis (RPA) classes. Abbreviation: CI, confidence interval; O, observed events; N, total number of patients.
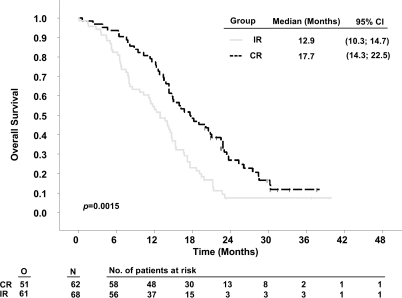
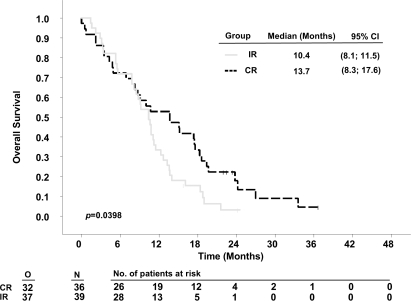
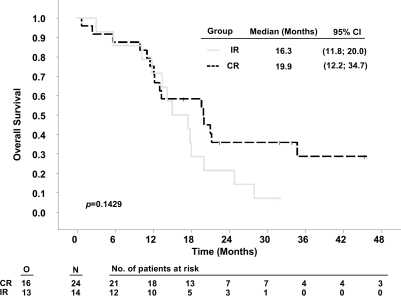
Stratifying by resection status demonstrated that patients with complete resections survived significantly longer in RTOG-RPA classes IV and V than patients with incomplete resections. Median survival times in patients with complete resections compared to incomplete resections were 17.7 months (95% CI, 14.3–22.5) versus 12.9 months (10.3–14.7) for class IV (p = 0.0015, Fig. 2) and 13.7 months (8.3–17.6) versus 10.4 months (8.10–11.5) for class V patients (p = 0.0398, Fig. 3). The respective 2-year survival rates were 21.0% versus 4.4% (class IV) and 11.1% versus 2.6 % (class V, Table 4). For the small group of class III patients (Fig. 4), numerical differences did not reach statistical significance (complete vs. incomplete resections: 19.9 months [12.2–34.7] vs. 16.3 months [11.8–20.0]; 2-year survival rate: 29.1% vs. 21.4%, p = 0.1429).
Fig. 2.
Kaplan-Meier estimates of overall survival of 5-aminolevulinic acid (ALA) study patients according to Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis (RPA) class IV, stratified by resection. Abbreviations: O, observed events; N, total number of patients; CR, complete resection; IR, incomplete resection; CI, confidence interval.
Fig. 3.
Kaplan-Meier estimates of overall survival of 5-aminolevulinic acid (ALA) study patients according to Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis (RPA) class V, stratified by resection. Abbreviations: O, observed events; N, total number of patients; CR, complete resection; IR, incomplete resection; CI, confidence interval.
Table 4.
Overall survival data among recursive partitioning analysis (RPA) classes for patients from the 5-aminolevulinic acid (ALA) study
| RPA Class |
ALA Study Databasea |
|||||
|---|---|---|---|---|---|---|
|
Complete Resection |
Incomplete Resection |
|||||
| Median (Months) | 95% CI | 2-Year Rate (%) | Median (Months) | 95% CI | 2-Year Rate (%) | |
| III | 19.9 | 12.2–34.7 | 29.1 | 16.3 | 11.8–20.0 | 21.4 |
| IV | 17.7 | 14.3–22.5 | 21.0 | 12.9 | 10.3–14.7 | 4.4 |
| V | 13.7 | 8.3–17.6 | 11.1 | 10.4 | 8.10–11.5 | 2.6 |
| All | 16.7 | 14.3–19 | 15.6 | 11.8 | 10.4–13.7 | 3.3 |
Abbreviation: C I, confidence interval.
Favorable neurological function = NIH ≤1; unfavorable = NIH >1.
Fig. 4.
Kaplan-Meier estimates of overall survival of 5-aminolevulinic acid (ALA) study patients according to Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis (RPA) class III, stratified by resection. Abbreviations: O, observed events; N, total number of patients; CR, complete resection; IR, incomplete resection; CI, confidence interval.
Discussion
To date, only one randomized study has compared biopsy to resection,25 whereas no prospectively randomized studies are available that substantiate an impact of the degree of resection of glioblastoma multiforme on survival. One nonrandomized study concerning the influence of resection on survival noted several preoperative prognostic factors, such as age, to be unevenly distributed between resection groups.2 This observation was repeated in a prospectively randomized ALA study,1 which investigated the usefulness of ALA-induced fluorescence for improving resection and outcome in patients with malignant glioma. Lacroix et al.26 have presented a patient series utilizing extensive multivariate analysis as a tool to account for such preoperative factors that influence prognosis.26 On the other hand, some available retrospective studies on the influence of resection on survival6 are confounded by the lack of information on the preoperative distribution of such factors. Such studies establish resection status as a prognostic factor for survival but only provide limited evidence regarding the causal influence of resection on survival. Factors influencing the extent of resection, such as age, might simultaneously affect outcome. Ideally, therefore, a prospectively randomized trial leading to patient collectives with varying resection status but balanced distributions of prognostic factors would be necessary to evaluate the causal influence of resection. Such a study is unlikely to ever be performed, however, because ethical and practical reasons would render such an endeavor questionable.
The ALA study was a surgical trial on a well-defined collection of patients with restricted entry criteria (resectable tumors, KPS 70–100, age 18–75). Primary study aims were the frequency of complete resections and 6-month progression-free survival. The study was not designed for demonstrating influences on survival. In retrospect, the study was underpowered to demonstrate such differences and suffered from a bias in augmental therapies that favored the control arm; because 35% of control patients also had complete resections, the increase to 65% complete resections in the study arm might not have been large enough to elicit statistical differences in survival, if the effect of resection on survival was not overwhelming. Nevertheless, the trial contained a large population of patients with complete resections (50%), as confirmed by early postoperative MRI, which was used in the present analysis. Unfortunately, simple restratification of patients in this study by resection status led to two patient populations that differed in age.7 Patients with complete resections were significantly younger than those without. This imbalance confounded the interpretation of resection as being a causal factor for determining survival.
To overcome heterogeneities in known prognostic factors we then stratified patients according to the prognostic classes constructed by Curran et al.8 These classes model pretreatment and treatment variables in over 1,500 patients and have been taken to provide reliable “historical” controls, with which results of phase I/II and II studies have been compared. To our knowledge, the present analysis is the first attempt at categorizing patients with varying degrees of resection according to the RTOG-RPA classes. We wanted to demonstrate that survival of patients observed in the original RTOG-RPA classification could be mirrored with the surgical collective of patients from the ALA study. Our survival results showed differences among RPA classes III, IV, and V, demonstrating the feasibility of our approach. Interestingly, 2-year survival rates were very comparable between the older RTOG-RPA classes and the present patient collective. Treatment or selection bias was also confirmed using RPA. Class III patients tended to have a higher frequency of complete resections than class IV and V patients (63% vs. 48%).
The main finding of our analysis, however, was that stratification of RPA survival classes showed significant survival differences depending on resection status (complete vs. incomplete resection) in the prognostically unfavorable classes IV and V. Nominal differences in class III patients were also noted (19.9 vs. 16.3 months), but due to small patient numbers (n = 24 and 14, respectively) the comparison was underpowered and did not reach statistical significance (p = 0.1). Given that RTOG-RPA classification alleviates misbalanced prognostic factors in the groups treated by complete or incomplete resection and in view of the otherwise highly controlled setting in which patients were treated, including centralized neuropathological and neuroradiological review and perioperative care, the RTOG-RPA classification of patients in the ALA study gives strong evidence that complete resections influence survival. Compared to the RTOG collective of patients, survival appeared much enhanced in ALA study patients with complete resections when these were allocated to the RTOG-RPA classes (class III: 17.9 vs. 19.9 months; class IV: 11.1 vs. 17.7 months; and class V: 8.9 vs. 13.7 months), whereas patients without complete resection were comparable to the expected values observed in the original RTOG collective.
In this regard it is noteworthy that median residual volumes of tumors in patients with incomplete resections were small (1.5 cm3). This observation signifies that patients in the group with incomplete resection had a high standard of surgical care, even though their resections were incomplete. Furthermore, it suggests that even small volumes of residual tumor are associated with a worse prognosis compared to no visible and residual contrast-enhancing tumor.
Conclusions
Survival of patients from the ALA study was correctly predicted by the RTOG-RPA classes. Differences in survival depending on resection status, especially in RPA classes IV and V, strongly support a causal influence of resection on survival.
Acknowledgments
ALA Glioma Study Group: F. Oppel, A. Brune (Krankenanstalten Gilead gGmbH, Bielefeld), W. Lanksch, C. Woiciechowsky (Virchow-Klinikum der HU Berlin); M. Brock, J. Vesper (Universitätsklinikum Benjamin Franklin, Berlin); J.-C. Tonn, C. Goetz (Universitätsklinikum München); J.M. Gilsbach, L. Mayfrank, M.F. Oertel (Universitätsklinikum der RWTH, Aachen); V. Seifert, K. Franz, A. Bink (University of Frankfurt Medical Center); G. Schackert, T. Pinzer (Carl Gustav Carus University, Dresden); W. Hassler, A. Bani (Klinikum Duisburg gGmbH, Duisburg); H.-J. Meisel, B.C. Kern (Bergmannstrost Krankenhaus, Halle); H.M. Mehdorn, A. Nabavi (Universitätsklinikum Kiel); A. Brawanski, O.W. Ullrich (Klinikum der Universität Regensburg); D.K. Böker, M. Winking (Universitätsklinikum Giessen); F. Weber, U. Langenbach (Klinikum Saarbrücken); M. Westphal, U. Kähler (Universitätsklinikum Hamburg-Eppendorf); H. Arnold, U. Knopp (Med. Universität zu Lübeck); T. Grumme, T. Stretz (Zentralklinikum Augsburg); D. Stolke, H. Wiedemayer (Universitätsklinikum Essen); B. Turowski (Universitätsklinikum Düsseldorf); T. Pietsch (Universitätsklinikum Bonn); O.D. Wiestler (Deutsches Krebsforschungszentrum, Heidelberg). Review Institutions: Institute of Neuroradiology, University of Frankfurt, Frankfurt am Main. Principal Investigator: H.-J. Reulen. Coordinating Investigator: W. Stummer.
References
- 1.Stummer W, Pichlmeier U, Meinel T, Wiestler OD, Zanella F, Reulen HJ, ALA Glioma Study Group Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7:392–401. doi: 10.1016/S1470-2045(06)70665-9. [DOI] [PubMed] [Google Scholar]
- 2.Albert FK, Forsting M, Sartor K, Adams HP, Kunze S. Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery. 1994;34:45–60. doi: 10.1097/00006123-199401000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Barker FG, II, Prados MD, Chang SM, et al. Radiation response and survival time in patients with glioblastoma multiforme. J Neurosurg. 1996;84:442–448. doi: 10.3171/jns.1996.84.3.0442. [DOI] [PubMed] [Google Scholar]
- 4.Kowalczuk A, Macdonald RL, Amidei C, et al. Quantitative imaging study of extent of surgical resection and prognosis of malignant astrocytomas. Neurosurgery. 1997;41:1028–1036. doi: 10.1097/00006123-199711000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Vecht CJ, Avezaat CJ, van Putten WL, Eijkenboom WM, Stefanko SZ. The influence of the extent of surgery on the neurological function and survival in malignant glioma: a retrospective analysis in 243 patients. J Neurol Neurosurg Psychiatry. 1990;53:466–471. doi: 10.1136/jnnp.53.6.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simpson JR, Horton J, Scott C, et al. Influence of location and extent of surgical resection on survival of patients with glioblastoma multiforme: results of three consecutive Radiation Therapy Oncology Group (RTOG) clinical trials. Int J Radiat Oncol Biol Phys. 1993;26:239–244. doi: 10.1016/0360-3016(93)90203-8. [DOI] [PubMed] [Google Scholar]
- 7.Stummer W, Reulen HJ, Meinel T, et al. Extent of resection and survival in glioblastoma multiforme: identification of and adjustment for bias. Neurosurgery. 2008;62:564–576. doi: 10.1227/01.neu.0000317304.31579.17. [DOI] [PubMed] [Google Scholar]
- 8.Curran WJ, Jr, Scott JB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85:690–691. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 9.Scott CB, Scarantino C, Urtasun R, et al. Validation and predictive power of Radiation Therapy Oncology Group (RTOG) recursive partitioning analysis classes for malignant glioma patients: a report using RTOG 90–60. Int J Radiat Oncol Biol Phys. 1998;40:51–55. doi: 10.1016/s0360-3016(97)00485-9. [DOI] [PubMed] [Google Scholar]
- 10.Videtic GMM, Gaspar LE, Zamorano L, et al. Use of the RTOG recursive partitioning analysis to validate the benefit of iodine-125 implants in the primary treatment of malignant gliomas. Int J Radiat Oncol Biol Phys. 1999;45:687–692. doi: 10.1016/s0360-3016(99)00244-8. [DOI] [PubMed] [Google Scholar]
- 11.Videtic GMM, Gaspar LE, Zamorano L, et al. Implant volume as a prognostic variable in brachytherapy decision-making for malignant gliomas stratified by the RTOG recursive partitioning analysis. Int J Radiat Oncol Biol Phys. 2001;51:963–968. doi: 10.1016/s0360-3016(01)01746-1. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, Won M, Macdonald D, et al. Phase II study of topotecan plus cranial radiation for glioblastoma multiforme: results of Radiation Therapy Oncology Group 9513. Int J Radiat Oncol Biol Phys. 2002;53:980–986. doi: 10.1016/s0360-3016(02)02817-1. [DOI] [PubMed] [Google Scholar]
- 13.Coughlin C, Scott C, Langer C, et al. Phase III two-arm RTOG trial (94-11) of bischloroethylnitrosourea plus accelerated hyperfractionated radiotherapy (64.0 or 70.4 Gy) based on tumor volume (> 20 or ≤ 20 cm2, respectively) in the treatment of newly-diagnosed radiosurgery ineligible glioblastoma multiforme patients. Int J Radiat Oncol Biol Phys. 2000;48:1351–1358. doi: 10.1016/s0360-3016(00)01412-7. [DOI] [PubMed] [Google Scholar]
- 14.Langer CJ, Ruffer J, Rhodes H, et al. Phase II: Radiation Therapy Oncology Group trial of weekly paclitaxel and conventional external beam radiation therapy for supratentorial glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;51:113–119. doi: 10.1016/s0360-3016(01)01597-8. [DOI] [PubMed] [Google Scholar]
- 15.Sultanem K, Patrocinio H, Lambert C, et al. The use of hypofractionated intensity-modulated irradiation in the treatment of glioblastoma multiforme: preliminary results of a prospective trial. Int J Radiat Oncol Biol Phys. 2004;58:247–252. doi: 10.1016/s0360-3016(03)00819-8. [DOI] [PubMed] [Google Scholar]
- 16.Lamborn KR, Chang SM, Prados MD. Prognostic factors for survival of patients with glioblastoma: recursive partitioning analysis. Neuro-Oncol. 2004;6:227–235. doi: 10.1215/S1152851703000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Del Rowe J, Scott C, Werner-Wasik M, et al. Single-arm openlabel phase II study of intravenously administered tirapazamine and radiation therapy for glioblastoma multiforme. J Clin Oncol. 2000;18:1254–1259. doi: 10.1200/JCO.2000.18.6.1254. [DOI] [PubMed] [Google Scholar]
- 18.Miralbell R, Mornex F, Greiner R, et al. Accelerated radiotherapy, carbogen and nicotinamide in glioblastoma multiforme: report of European Organization for Research and Treatment of Cancer Trial 22933. J Clin Oncol. 1999;17:3143–3149. doi: 10.1200/JCO.1999.17.10.3143. [DOI] [PubMed] [Google Scholar]
- 19.Stupp R, Dietrich P-Y, Ostermann Kraljevic S, et al. Promising survival for patients with newly diagnosed glioblastoma multiforme treated with concomitant radiation plus temozolomide followed by adjuvant temozolomide. J Clin Oncol. 2002;20:1375–1382. doi: 10.1200/JCO.2002.20.5.1375. [DOI] [PubMed] [Google Scholar]
- 20.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 21.Mirimanoff RO, Gorlia T, Mason W, et al. Radiotherapy and temozolomide for newly diagnosed glioblastoma: recursive partitioning analysis of the EORTC 26981/22981-NCIC CE3 phase III randomized trial. J Clin Oncol. 2006;24:2563–2569. doi: 10.1200/JCO.2005.04.5963. [DOI] [PubMed] [Google Scholar]
- 22.Albanese MA, Clarke WR, Adams HP, Jr, Woolson RF. Ensuring reliability of outcome measures in multicentre clinical trials of treatments for acute ischemic stroke: the program developed for the Trial of Org 10172 in Acute Stroke Treatment (TOAST) Stroke. 1994;25:1746–1751. doi: 10.1161/01.str.25.9.1746. [DOI] [PubMed] [Google Scholar]
- 23.Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol. 1998;46:660–662. doi: 10.1001/archneur.1989.00520420080026. [DOI] [PubMed] [Google Scholar]
- 24.Wityk RJ, Pessin MS, Kaplan RF, Caplan LR. Serial assessment of acute stroke using the NIH Stroke Scale. Stroke. 1994;25:362–365. doi: 10.1161/01.str.25.2.362. [DOI] [PubMed] [Google Scholar]
- 25.Vuorinen V, Hinkka S, Farkkila M, Jaaskelainen J. Debulking or biopsy of malignant glioma in elderly people: a randomised study. Acta Neurochir (Wien) 2003;145:5–10. doi: 10.1007/s00701-002-1030-6. [DOI] [PubMed] [Google Scholar]
- 26.Lacroix M, Abi-Said D, Fourney DR, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. [DOI] [PubMed] [Google Scholar]