Abstract
Pathological angiogenesis is a hallmark of cancer, specifically of glioblastomas, the most malignant and common primary brain tumor. Vascular endothelial growth factor (VEGF) is the key protein in the regulation of the hypervascular phenotype of primary malignant brain tumors. In this study, we tested VEGF Trap, a soluble decoy receptor for VEGF, in an intracranial glioma model. VEGF Trap was administered in short or prolonged schedules to animals bearing human gliomas at different stages of disease. Of importance, VEGF Trap treatment was efficacious in both initial and advanced phases of tumor development by significantly increasing overall survival. Furthermore, this effect was enhanced in animals treated with more prolonged regimens. In addition, we observed the emergence of a VEGF Trap-resistant phenotype characterized by tumor growth and increased invasiveness. Our results suggest that VEGF Trap will be effective in treating both patients with recurrent or progressive resectable glioblastoma and patients that have undergone extensive initial surgery. Finally, our results indicate that the clinical success of VEGF Trap may depend on a prolonged treatment in combined therapy aiming to simultaneously inhibit angiogenesis and tumor invasion.
Keywords: glioblastoma, therapy, VEGF, VEGF Trap
The striking induction of angiogenesis in glioblastoma multiforme (GBM) has fueled the speculation that progression to GBM requires the activation of angiogenesis, a finding that has stimulated significant efforts to develop angiogenesis-blocking agents. Vascular endothelial growth factor (VEGF) is critical for promoting the earliest stages of vasculogenesis, which includes endothelial cell proliferation, differentiation, migration, and tubular formation. Clinical trials of specific VEGF inhibitors for the treatment of patients with gliomas are ongoing, and preliminary analyses showed beneficial effects in patients with malignant gliomas.1–4 Recently, a new anti-VEGF agent, VEGF Trap/aflibercept (henceforth referred to as VEGF Trap), has been developed by incorporating domains of both VEGF receptor 1 (VEGFR-1) and VEGFR-2 fused to the constant region of human immunoglobulin G1, which acts as a soluble decoy receptor for VEGF. VEGF Trap has very high affinity for all isoforms of VEGF-A (<1 pM), as well as placental growth factor, a closely related angiogenic factor.5 VEGF Trap was engineered to have minimal interactions with the extracellular matrix, and this property apparently accounts for its satisfying pharmacokinetic profile superior to soluble forms of VEGFR-1.5 Its efficacy has been proven in preclinical studies in several types of solid tumor5–9 and in a subcutaneous glioma model.10 Because tumor progression and angiogenesis are greatly dependent on the existent microenvironment of the tumor,11,12 we undertook this study to characterize the effect of VEGF Trap in an orthotopic glioblastoma model in several stages of the disease. We have previously described the development of growth patterns and angiogenesis in an intracranial U-87 MG human glioma model. Vessel cooption and remodeling were present at the early stages of disease, whereas the advanced stages are distinguished by high vascular density.13 These two phases were similar to stages described in other previous reports.14,15 Based on this tumoral angiogenesis and kinetic pattern, we administered VEGF Trap to animals bearing U-87 MG intracranial xenografts at several phases of tumor development. In the present study, we demonstrated that VEGF Trap treatment in animals bearing human gliomas resulted in significant prolonged survival. Of importance, our results indicate that VEGF Trap was equally effective against initial or advanced disease, and that the response was enhanced when VEGF Trap was administered in a prolonged schedule.
Material and Methods
Cell Line
The human glioma cell line U-87 MG was purchased from the American Type Culture Collection (Manassas, VA, USA). Cells were maintained in Dulbecco’s modified Eagle/F12 medium (1:1, vol:vol) (The University of Texas M. D. Anderson Cancer Center Media Core Facility, Houston, TX, USA) supplemented with 10% fetal calf serum and 1% antibiotic/antimycotic agent (Invitrogen, Carlsbad, CA, USA) in a humidified atmosphere containing 5% CO2 at 37°C.
Drugs
VEGF Trap and human Fc (hFc, constant region of human IgG1) were kindly provided by Regeneron Pharmaceuticals (Tarrytown, NY, USA). Stocks of 50 mg/ml in aqueous solution were kept at −80°C.
In Vivo Experiments
The U-87 MG human glioma cells (5 × 105) were engrafted in the caudate nucleus of athymic mice (Harlan Sprague Dawley Inc., Indianapolis, IN, USA), as previously described.13 At 0, 4, and 10 days after cell implantation, we administered VEGF Trap (25 mg/kg subcutaneously, twice a week, for a total of 3 or 6 weeks) to separate groups of 10–15 animals per treatment bearing U-87 MG intracranial xenografts. Either phosphate-buffered saline (PBS) or hFc was blindly administered as a control agent in randomly selected subgroups of glioma-bearing animals. Animals showing generalized or local symptoms of disease were euthanized. Brains were fixed in 4% formaldehyde for 24 h and embedded in paraffin. Slides were stained with hematoxylin and eosin. All animal studies were performed in the veterinary facilities of The M. D. Anderson Cancer Center in accordance with institutional guidelines.
Enzyme-Linked Immunosorbent Assays
Blood was collected from the tail vein of glioma-bearing mice 3 days after the initial dose of VEGF Trap, hFc, or vehicle, and VEGF Trap was quantified in the serum by enzyme-linked immunosorbent assays (ELISA), as previously reported.16
Statistical Analyses
The in vivo anticancer effect of different treatments was assessed by plotting Kaplan-Meier survival curves, and treatment groups were compared using the log-rank test. The effects of VEGF Trap when administered in different treatment schedules were analyzed using a permutation test.
Results and Discussion
Antiglioma Effect of VEGF Trap on Initial Disease
The VEGF Trap-mediated antiglioma effect was assessed in vivo using an intracranial human glioma xenograft model. We selected the U-87 MG cell line for this study because it produces gliomas in nude mice with highly predictable growth kinetics and well-characterized pathological features13; in addition, U-87 MG cells express high levels of VEGF and, when implanted intracranially in immunocompromised mice, develop as highly vascularized tumors.11,13 Our group has previously characterized the kinetics of tumor growth and vascularization of human U-87 MG xenografts implanted intracranially in nude mice. Of interest to the present study, U-87 MG intracranial tumors exhibited initially minimal tumor growth, but changes in the host vessels were evident as soon as day 1 and definitely by day 4 after implantation; these changes included significant vessel co-option, as illustrated by the existence of engorged smooth-muscle actin (SMA)-positive vascular structures in the periphery of the xenograft.13
To test the effect of VEGF Trap in the initial phases of the disease, we planned two different treatment schedules (Figs. 1 and 2) consisting of the subcutaneous administration of 25 mg/kg VEGF Trap twice weekly over 3 weeks, starting on either day 0 (schedule A) or day 4 (schedule B) after the intracranial implantation of human glioma cells in nude mice. Control groups were treated with PBS or hFc at doses and volumes similar to those used for the test drug. The agents were administered in a double-blinded manner; that is, the identity of the test groups was concealed from both the personnel preparing the drugs and the animal caretakers.
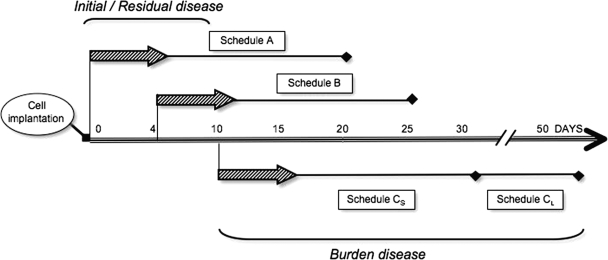
Fig. 1.
Schematic representation of the treatment schedule used with the anti-vascular endothelial growth factor (VEGF) agent VEGF Trap, which was based on our previous studies of the kinetics of growth and vascularization in the U-87 MG intracranial model. U-87 MG cells were implanted in the brains of the animals on day 0, and VEGF Trap was administered starting on day 0 (schedule A), day 4 (schedule B), or day 10 (schedules CS and CL) after cell implantation. The two schedule C subgroups were treated in either a 3-week (schedule CS) or 6-week (schedule CL) schedule. Schedules A and B followed a 3-week treatment regimen. Animals were euthanized when signs of neurological or generalized disease appeared.
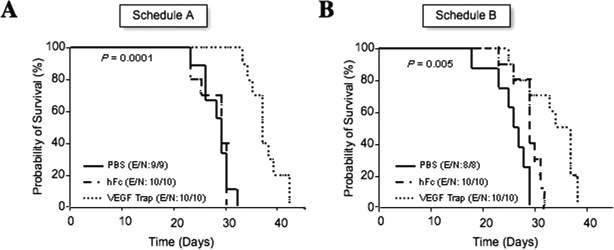
Fig. 2.
Effect of the anti-vascular endothelial growth factor (VEGF) agent VEGF Trap on initial phases of disease: survival analysis of glioma-bearing animals treated with VEGF Trap since day 0 (A) or day 4 (B), as pictured in Fig. 1. Kaplan-Meier survival curves begin on the day of U-87 MG intracranial implantation following the subcutaneous injection of VEGF Trap or of vehicle or human Fc (control). The p-values (determined by log-rank test) show significant overall survival differences between VEGF Trap-treated and control-treated animals. Abbreviations: E, events; N, number of animals.
Animals treated with VEGF Trap starting on day 0 or day 4 after implantation had significantly prolonged survival compared to the hFc- or PBS-treated animals (p < 0.0001 and p < 0.005, respectively). In animals treated with schedule A, the median overall survival of the control-treated animals (treated with either hFc or PBS) was 30 days, with all animals dying by day 33. Treatment with VEGF Trap prolonged the mean survival by 8 days. In animals treated with schedule B, the mean survival in the PBS- and hFc-treated animals was 27.5 and 30 days, respectively, but it was increased to 36 days in the group treated with VEGF Trap. No treatment-schedule-dependent differences in survival duration were observed in animals receiving VEGF Trap, suggesting VEGF Trap is efficacious in initial phases of disease that were characterized by active vessel co-option and remodeling. Analysis performed 3 days after the first VEGF Trap doses were administered revealed high VEGF Trap levels (approximately >50 μg/ml) in the serum of all these animals, suggesting an efficient systemic biodistribution (data not shown).
Antiglioma Effect of VEGF Trap on Disease Burden
To test the effect of VEGF Trap on tumor burden, and based on our previous study of U-87 MG intracranial growth and angiogenesis, we decided to start treatment on day 10 after cell implantation in one subgroup of mice (Fig. 1, schedule CS). According to our previous studies, by day 10, increased microvascular density (MVD) was associated with exponential tumor growth and a decrease in the rate of induced angiogenesis within the host and the tumor periphery.13 Twelve days after implantation, the tumors consisted of spherical masses of cells with a high MVD and large, distorted, SMA-positive vessels. The tumor limits were clearly defined, and the cancer cells did not exhibit the invasive pattern into host tissue seen in preceding days.13
In the present study, glioma cells were implanted intracranially, and 10 days later, VEGF Trap was administered subcutaneously at a dose of 25 mg/kg twice weekly for 3 weeks. Control groups were treated with PBS or hFc at doses and volumes similar to those of the test drug. Treatment of the glioma-bearing animals with VEGF Trap resulted in a significant increase in the survival of these animals (p < 0.005) (Fig. 3A). In particular, the median overall survival of control-treated (PBS or hFc) animals was 31 days, with all the animals dead by day 33, whereas the mean survival of VEGF Trap-treated animals was 45 days. We observed no significant difference in the effect of VEGF Trap on prolonging survival at different stages of the disease (comparing effects of schedules A and B with schedule CS) (p > 0.1, permutation test), suggesting that VEGF Trap can be similarly effective in both the initial and burden disease stage. These data further suggest that targeting circulating levels of VEGF is equally effective in challenging tumor growth under both initial and established tumoral vasculature phases.
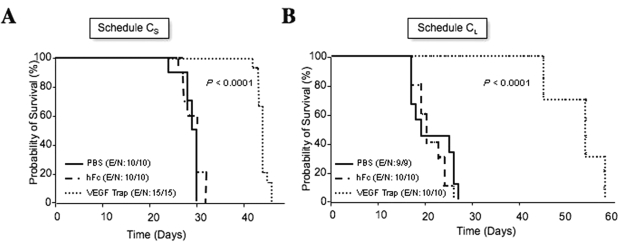
Fig. 3.
Effect of the anti-vascular endothelial growth factor (VEGF) agent VEGF Trap on advanced glioma disease: survival analyses of glioma-bearing animals that were treated with VEGF Trap starting on day 10 after cell implantation in either a 3-week (schedule CS) or 6-week (schedule CL) regimen, as pictured in Fig. 1. Kaplan-Meier survival curves begin on the day of U-87 MG intracranial implantation following the subcutaneous injection of VEGF Trap or control agent (vehicle or human Fc). The p-values (determined by log-rank test) show significant overall survival differences between VEGF Trap-treated and control-treated animals. Abbreviations: E, events; N, number of animals.
Antiglioma Effect of Prolonged VEGF Trap Treatment
We next explored the effect in vivo of more prolonged VEGF Trap treatment. In this experiment, animals bearing intracranial human gliomas were treated with VEGF Trap (25 mg/kg) twice weekly for 6 weeks starting on day 10 after cell implantation (Fig. 1, schedule CL). Control animals were treated with vehicle or hFc (25 mg/kg) twice weekly until they showed signs of disease, at which time they were euthanized according to institutional regulations. Animals treated with VEGF Trap for 6 weeks survived longer than did animals treated with hFc (median overall survival, 55 days and 21 days, respectively; Fig. 3B) (p < 0.0001). We also analyzed the difference in median survival times between the animals treated with VEGF Trap for 6 weeks and those treated for 3 weeks. Using the permutation test and after adjusting for overall survival on PBS-treated groups, we found the increase in survival obtained with the 6-week VEGF Trap treatment to be significantly greater than the increase in survival obtained with the 3-week treatment (p < 0.05). These data suggest that VEGF Trap is more effective in prolonging overall survival when administered in a prolonged treatment schedule.
Histological Examination of VEGF Trap-Treated Tumors
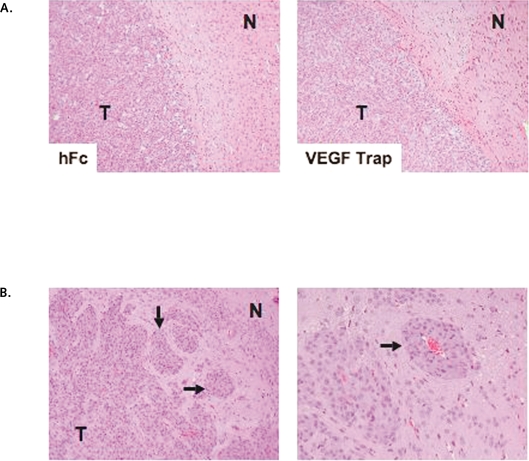
Microscopic analysis of histological sections from formalin-fixed, paraffin-embedded brains revealed that control- and VEGF Trap-treated animals eventually suffered from the lethal growth of their tumors. Because of previous studies describing that treatment with antiangiogenic agents may result in intracranial hemorrhages or enhance tumor invasion,2,17 we specifically examined the tumors for the presence of these adverse effects. Histological examination of the brains of the cohorts treated for 3 weeks did not reveal either phenomenon. Treated U-87 MG-derived tumors displayed a very well-defined border with the normal host parenchyma (Fig. 4A). However, examination of the brains of animals that received prolonged treatment (6 weeks) of VEGF Trap, which survived longer than those treated on a 3-week schedule, revealed the signs of mass effect and the presence of the so-called “secondary structures” or “satellitosis” consisting of aggregations of glioma cells in the perivascular regions, as well as the presence of glioma cells along the Virchow-Robin spaces (Fig. 4B). These data suggest that U-87 MG-derived xenografts acquired an invasive phenotype in response to anti-VEGF therapy. These results are in agreement with a similar pattern of growth of intracranial G55 xenografts in animals treated with an antibody against mouse VEGFR-2, DC101,17 or a neutralizing VEGF antibody.18 These results may be likewise in agreement with those from clinical trials in patients with cancer treated with VEGF inhibitors, in that they survived longer but eventually exhibited resistance to the treatment.19,20 Of importance, the model described here offers us the possibility of testing combined therapies designed to counteract the emergence of a resistant phenotype to anti-VEGF therapies.
Fig. 4.
Histological examination of brain sections from animals treated with the anti-vascular endothelial growth factor (VEGF) agent VEGF Trap. (A) Hematoxylineosin staining of mouse brains bearing U-87 MG xenografts treated with human Fc (hFc) or VEGF Trap according to schedule B. No signs of hemorrhagic areas or an enhanced invasive phenotype were observed after VEGF Trap treatment. N, normal tissue; T, tumor tissue. Original magnification, ×100. (B) Histological examination of brain sections from animals treated with VEGF Trap as described for schedule CL. Sections stained with hematoxylin and eosin show the presence of an invasive phenotype with satellitosis characterized by glioma clustering around vascular vessels and accumulation of invasive glioma cells far from the main tumor mass (arrows). Original magnification: left, ×100; right, ×200.
Taken together, our data show that treatment with VEGF Trap significantly prolonged the survival of glioma xenograft-bearing mice. Of great interest, initial/residual disease and disease burden were both similarly affected by the antiangiogenesis treatment. In addition, the prolonged use of VEGF Trap (over 6 weeks) improved outcomes significantly more than did treatment administered in a short schedule (over 3 weeks).
The traits for personalized medicine are emerging for the treatment of brain tumors, and they will need to take into consideration the highly heterogeneous nature of these tumors.1,21 However, the fact that all brain tumor subtypes rely on blood vessels for survival and growth indicates the broad applicability of this strategy. Thus, our report provides data that encourage the testing of VEGF Trap in patients with recurrent malignant gliomas, and in this regard, results from a multicenter study consisting of a phase II clinical trial of VEGF Trap in patients with recurrent gliomas will soon be available. Finally, we suggest that VEGF Trap should also be considered for the treatment of patients after extensive surgery, which we would regard as carrying minimal residual disease, in combination with therapies targeting the migratory and invasive properties of gliomas.
Acknowledgments
We greatly thank Drs. John S. Rudge and Risa Shapiro (Regeneron Pharmaceuticals, Inc.) for their useful comments and the ELISA studies, Betty Notzon (Department of Scientific Publications, M. D. Anderson) for editorial assistance, and Verlene Henry and Jennifer Edge for technical assistance (Brain Tumor Center, M. D. Anderson). This work was supported by National Cancer Institute grant CA-16672 (supporting the Research Histopathology and Research Animal Support core facilities at M. D. Anderson) and was partially sponsored by Regeneron Pharmaceuticals, Inc.
J.H. is currently at Novartis Institutes for BioMedical Research, Emeryville, CA, USA.
References
- 1.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pope WB, Lai A, Nghiemphu P, et al. MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology. 2006;66:1258–1260. doi: 10.1212/01.wnl.0000208958.29600.87. [DOI] [PubMed] [Google Scholar]
- 3.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13:1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 4.Jain RK, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8:610–622. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 5.Holash J, Davis S, Papadopoulos N, et al. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99:11393–11398. doi: 10.1073/pnas.172398299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Byrne AT, Ross L, Holash J, et al. Vascular endothelial growth factor-trap decreases tumor burden, inhibits ascites, and causes dramatic vascular remodeling in an ovarian cancer model. Clin Cancer Res. 2003;9:5721–5728. [PubMed] [Google Scholar]
- 7.Kim ES, Serur A, Huang J, et al. Potent VEGF blockade causes regression of coopted vessels in a model of neuroblastoma. Proc Natl Acad Sci U S A. 2002;99:11399–11404. doi: 10.1073/pnas.172398399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Riely GJ, Miller VA. Vascular endothelial growth factor trap in non small cell lung cancer. Clin Cancer Res. 2007;13:s4623–s4627. doi: 10.1158/1078-0432.CCR-07-0544. [DOI] [PubMed] [Google Scholar]
- 9.Verheul HM, Hammers H, van Erp K, et al. Vascular endothelial growth factor trap blocks tumor growth, metastasis formation, and vascular leakage in an orthotopic murine renal cell cancer model. Clin Cancer Res. 2007;13:4201–4208. doi: 10.1158/1078-0432.CCR-06-2553. [DOI] [PubMed] [Google Scholar]
- 10.Wachsberger PR, Burd R, Cardi C, et al. VEGF trap in combination with radiotherapy improves tumor control in u87 glioblastoma. Int J Radiat Oncol Biol Phys. 2007;67:1526–1537. doi: 10.1016/j.ijrobp.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Blouw B, Song H, Tihan T, et al. The hypoxic response of tumors is dependent on their microenvironment. Cancer Cell. 2003;4:133–146. doi: 10.1016/s1535-6108(03)00194-6. [DOI] [PubMed] [Google Scholar]
- 12.Roberts WG, Delaat J, Nagane M, et al. Host microvasculature influence on tumor vascular morphology and endothelial gene expression. Am J Pathol. 1998;153:1239–1248. doi: 10.1016/s0002-9440(10)65668-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee OH, Fueyo J, Xu J, et al. Sustained angiopoietin-2 expression disrupts vessel formation and inhibits glioma growth. Neoplasia. 2006;8:419–428. doi: 10.1593/neo.06109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 15.Holash J, Maisonpierre PC, Compton D, et al. Vessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGF. Science. 1999;284:1994–1998. doi: 10.1126/science.284.5422.1994. [DOI] [PubMed] [Google Scholar]
- 16.Rudge JS, Holash J, Hylton D, Russell M, et al. Inaugural article: VEGF Trap complex formation measures production rates of VEGF, providing a biomarker for predicting efficacious angiogenic blockade. Proc Natl Acad Sci U S A. 2007;104:18363–18370. doi: 10.1073/pnas.0708865104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61:6624–6628. [PubMed] [Google Scholar]
- 18.Rubenstein JL, Kim J, Ozawa T, et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2:306–314. doi: 10.1038/sj.neo.7900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerbel RS, Yu J, Tran J, et al. Possible mechanisms of acquired resistance to anti-angiogenic drugs: implications for the use of combination therapy approaches. Cancer Metastasis Rev. 2001;20:79–86. doi: 10.1023/a:1013172910858. [DOI] [PubMed] [Google Scholar]
- 20.Miller KD, Sweeney CJ, Sledge GW., Jr The snark is a boojum: the continuing problem of drug resistance in the antiangiogenic era. Ann Oncol. 2003;14:20–28. doi: 10.1093/annonc/mdg033. [DOI] [PubMed] [Google Scholar]
- 21.Kleihues P, Burger PC, Scheithauer BW. Histological typing of tumours of the central nervous system. 2nd ed. Berlin: Springer; 1993. pp. 1–105. [Google Scholar]