Abstract
Itch/pruritus is the most common side effect associated with spinal administration of morphine given to humans for analgesia. The aim of this study was to investigate the effectiveness of κ-opioid receptor (KOR) agonists with diverse chemical structures as antipruritics and to elucidate the receptor mechanism underlying the antipruritic effect in monkeys. In particular, previously proposed non-KOR-1 agonists, including nalfurafine [TRK-820, 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[N-methyl-trans-3-(3-furyl)acrylamido]morphinan], bremazocine [(±)-6-ethyl-1,2,3,4,5,6-hexahydro-3-[(1-hydroxycyclopropy)-methyl]-11,11-dimethyl-2,6-methano-3-benzazocin-8-ol], and GR 89696 [4-[(3,4-dichlorophenyl)acetyl]-3-(1-pyrrolidinylmethyl)-1-piperazinecarboxylic acid methyl ester] were studied in various behavioral assays for measuring itch/scratching, analgesia, and respiratory depression. Systemic administration of nalfurafine (0.1–1 μg/kg), bremazocine (0.1–1 μg/kg), or GR 89696 (0.01–0.1 μg/kg) dose-dependently attenuated intrathecal morphine (0.03 mg)-induced scratching responses without affecting morphine antinociception. The combination of intrathecal morphine with these KOR agonists did not cause sedation. In addition, pretreatment with effective antiscratching doses of nalfurafine, bremazocine, or GR 89696 did not antagonize systemic morphine-induced antinociception and respiratory depression. The dose-addition analysis revealed that there is no subadditivity for nalfurafine in combination with morphine in the antinociceptive effect. Furthermore, the KOR antagonist study revealed that antiscratching effects of both nalfurafine and a prototypical KOR-1 agonist, U-50488H [trans-(±)-3,4-dichloro-N-methyl-N-(2-[1-pyrrolidinyl]-cyclohexyl)-benzeneacetamide], could be blocked completely by a selective KOR antagonist, nor-binaltorphimine (3 mg/kg). These findings suggest that the agonist action on KOR mainly contributes to the effectiveness of these atypical KOR agonists as antipruritics, and there is no evidence for KOR subtypes or μ-opioid antagonist action underlying the effects of these KOR agonists. This mechanism-based study further supports the clinical potential of KOR agonists as antipruritics under the context of spinal opioid analgesia.
Spinal administration of μ-opioid receptor agonists is an important method for pain management. In particular, it is a widely used therapy for obstetric analgesia (Cousins and Mather, 1984; DeBalli and Breen, 2003). However, itch/pruritus is the most common side effect of spinal opioid administration, and it reduces the value of spinal opioids for pain relief (Cousins and Mather, 1984; Ganesh and Maxwell, 2007). Previous studies have demonstrated that the same μ-opioid receptors mediate both analgesic and itch/scratching responses in primates (Ko and Naughton, 2000; Ko et al., 2004). Therefore, opioid receptor antagonists such as naloxone are not ideal antipruritics to be used under this context because such compounds can reverse opioid analgesia concurrently (Rawal et al., 1986; Cohen et al., 1992; Wang et al., 1998). It is important to identify specific pharmacological agents that can inhibit spinal opioid-induced itch without attenuating analgesia.
The κ-opioid receptor (KOR) seems to be a promising target because several studies suggest that KOR agonists are potentially useful as antipruritics. For example, scratching was a prominent withdrawal sign in monkeys treated chronically with and withdrawn from a selective KOR agonist, U-50488H (Gmerek et al., 1987). Many withdrawal symptoms from opioids appear to be opposite to the acute effects of agonist administration (Heishman et al., 1989; Kishioka et al., 2000). Excessive scratching activity observed during KOR withdrawal indicates that acute administration of KOR agonists may have antipruritic effects. Animal studies seem to support this notion because systemic administration of KOR agonists inhibited scratching evoked by pruritogenic agents without interfering with locomotor activity in rodents (Cowan and Gmerek, 1986; Togashi et al., 2002). In addition, KOR agonists can prevent or reverse intrathecal morphine-induced itch/scratching responses without interfering with intrathecal morphine analgesia in monkeys (Ko et al., 2003a). More importantly, animal studies have led to a successful clinical trial of a novel KOR agonist, nalfurafine (TRK-820), in hemodialysis patients suffering from uremic pruritus, supporting the therapeutic potential of KOR agonists as antipruritics (Wikström et al., 2005).
It is interesting that KOR antagonist studies have indicated that KOR-mediated antinociceptive effects may occur through two KOR subtypes. This distinction between KOR-1 and non-KOR-1 agonists is derived primarily from the differential susceptibility of KOR agonists to a KOR antagonist, nor-binaltorphimine (nor-BNI), and other opioid receptor antagonists, such as naltrexone in primates (Butelman et al., 1993; Ko et al., 1998). Prototypical KOR-1 agonists such as U-50488H are more sensitive to the antagonist effects of nor-BNI and naltrexone (Butelman et al., 1993; Ko et al., 1998). In contrast, other KOR agonists, including nalfurafine, bremazocine, and GR 89696, are less sensitive to the antagonist effects of nor-BNI and naltrexone (Butelman et al., 1993, 2001; Ko et al., 1998; Endoh et al., 2001). Although there is only one KOR cloned, and it appears to resemble the KOR-1 (Simonin et al., 1995), additional studies are needed to understand the relevant functions of KOR across different behavioral assays. In particular, non-KOR-1 agonists have atypical KOR pharmacological properties, including low to moderate affinity for μ-opioid receptors and extremely high potency in vivo (Toll et al., 1998; Butelman et al., 2001; Endoh et al., 2001). It is pivotal to investigate the effectiveness of these atypical KOR agonists as antipruritics and to elucidate the receptor mechanisms underlying the antipruritic actions of these KOR agonists.
Intrathecal administration of a single dose of morphine produces both itch/scratching and antinociception simultaneously in monkeys (Ko and Naughton, 2000; Ko et al., 2003a). This finding parallels closely with the behavioral effects of spinal administration of morphine in humans (Bailey et al., 1993; Palmer et al., 1999). It is valuable to use this experimental model of itch to characterize effects of novel compounds in monkeys for translational research. Therefore, the aim of this study was to investigate the effectiveness of atypical KOR agonists with diverse chemical structures as antipruritics against intrathecal morphine-induced itch and analgesia. Using different pharmacological approaches, studies were conducted to elucidate the receptor mechanisms underlying the antipruritic actions of these KOR agonists under this context.
Materials and Methods
Subjects
Eighteen adult intact male and female rhesus monkeys (Macaca mulatta) with body weights ranging between 6.5 and 12.1 kg were used. The monkeys were housed individually with free access to water and were fed approximately 25 to 30 biscuits (Purina Monkey Chow; Ralston Purina, St. Louis, MO) and fresh fruit daily. No monkey had exposure to any opioid receptor agonist or antagonist 1 month before the present study. The monkeys were housed in facilities accredited by the American Association for the Accreditation of Laboratory Animal Care. The studies were conducted in accordance with the University Committee on the Use and Care of Animals in the University of Michigan (Ann Arbor, MI) and the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the United States National Institutes of Health (Institute of Laboratory Animal Resources, 1996).
Procedures
Scratching Responses. Scratching responses, inferred to be a response to an itch sensation (Ko and Naughton, 2000; Ko et al., 2004), were recorded on videotapes while the monkeys were in their home cages. Each recording session was conducted for 15 min/test session. A scratch was defined as one short-duration (<1 s) episode of scraping contact of the forepaw or hind paw on the skin surface of other body parts. Scratches occurred repetitively at the same location. Scratching responses were scored by trained individuals who were blinded to experimental conditions. In addition, sedation was evaluated by cumulative time for eye closure or lying down at the bottom of the cage.
Antinociception. The warm water (50°C) tail-withdrawal assay was used to evaluate thermal antinociceptive effects of the test compound (Ko et al., 1998). In brief, monkeys were seated in primate restraint chairs, and the lower parts of their shaved tails (approximately 15 cm) were immersed in a thermal flask containing water maintained at 42, 46, or 50°C. Tail-withdrawal latencies were measured using a computerized timer by an experimenter who was blinded to experimental conditions. In each test session, monkeys were evaluated once with three temperatures given in a random order. If the monkeys did not remove their tails within 20 s (cutoff), the flask was removed, and a maximum time of 20 s was recorded. Test sessions began with determining a control value at each temperature. Subsequent tail-withdrawal latencies were determined at multiple time points after the drug administration.
Respiratory Function. The monkey was seated in a primate restraint chair, enclosed within a sound-attenuating chamber. A rectangular helmet (13.5 × 17.0 × 13.5 cm) was placed over the head of the monkey and sealed around its neck by two closely fitting latex shields. Gas (either air or a mixture of 5% CO2 in air) flowed into the helmet and was pumped out at a rate of 8 l/min. The breathing of the monkeys produced changes in pressure inside the helmet that were measured with a pressure transducer connected to a polygraph (Grass model 7; Grass Instruments, Quincy, MA), and the data were recorded on a polygraph trace and in a microprocessor (IBM personal computer; IBM, White Plains, NY) via an analog-to-digital converter. The apparatus was calibrated routinely with known quantities of air. The polygraph integrator was connected to a computer, which analyzes the data collected over a 3-min period. The rate of breathing (f, respiratory frequency) was determined directly. The minute volume (VE) was determined from the integration of the plethysmograph system. When the test compound was given in a cumulative dosing procedure, the test session contained six consecutive cycles. Each cycle was 30 min, which included the first 23-min exposure to air alone and the remaining 7-min exposure to 5% CO2 mixed in air. Responses in the first two cycles were averaged as a control value. The test compound was administered intramuscularly in the beginning of each cycle for the remaining dosing injection cycles (i.e., from 3rd–6th cycle).
Experimental Designs. The first part of the study was to determine the intervention effectiveness of KOR agonists with diverse chemical structures (Fig. 1), nalfurafine, bremazocine, and GR 89696, as antipruritics in monkeys (n = 6). In particular, the dose-response studies were conducted to investigate whether these KOR agonists could block scratching responses subsequent to intrathecal administration of morphine. Nalfurafine (0.1–1 μg/kg), bremazocine (0.1–1 μg/kg), or GR 89696 (0.01–0.1 μg/kg) was administered intramuscularly 45 min after 0.03 mg of intrathecal morphine. This dose of intrathecal morphine was selected based on previous studies showing that it produced maximal scratching responses and antinociception, and it could be used to detect whether the test compound could attenuate scratching without interfering intrathecal morphine-induced antinociception (Ko and Naughton, 2000; Lee et al., 2007). In a separate experiment, the tail-withdrawal latencies were measured at the same time points (i.e., measurement per half hour) in the same monkeys that were given effective antiscratching doses of KOR agonists 45 min after intrathecal morphine. The interinjection interval between intrathecal morphine was 10 days.
Fig. 1.
Structures of κ-opioid receptor agonists, bremazocine, nalfurafine (TRK-820), and GR 89696.
The second part of the study was to determine the receptor mechanism underlying the antiscratching effects of these KOR agonists by using different pharmacological approaches. The dose-response curves of subcutaneous morphine-induced antinociception were studied in one group of monkeys (n = 6) by using a cumulative dosing procedure (0.1–3 mg/kg) with a 30-min interinjection interval. An effective antiscratching dose of nalfurafine (1 μg/kg), bremazocine (1 μg/kg), or GR 89696 (0.1 μg/kg) was administered subcutaneously 15 min before redetermination of the morphine dose-response curve for antinociception. Similarly, the dose-response curves of intramuscular morphine-induced respiratory depression were studied in another group of monkeys (n = 6). The same dose of nalfurafine, bremazocine, or GR 89696 was administered intramuscularly 30 min before redetermination of the morphine dose-response curve for respiratory depression. These experiments were conducted once per week between test sessions. Both endpoints for morphine-induced antinociception and respiratory depression were used to detect the potential μ-opioid receptor antagonist effects of these KOR agonists.
Additional antagonist studies were conducted to elucidate whether potential μ-opioid receptor antagonist effects could mediate the antiscratching effects of nalfurafine. The dose-addition analysis was used to evaluate the drug interaction between nalfurafine and morphine, and it could be used to differentiate the additive effects from the subadditive effects (Tallarida, 2000). Initially, dose-response curves for subcutaneous nalfurafine- and morphine-induced antinociception were determined. Depending on the potency of nalfurafine, three mixtures of nalfurafine in combination with morphine were redetermined for their dose-response curves for antinociception. Furthermore, a selective KOR antagonist, nor-BNI, was used to determine the involvement of KOR in both nalfurafine- and U-50488H-induced antiscratching effects. After administration of nor-BNI (3 mg/kg i.m.), effects of nalfurafine (1 μg/kg) and U-50488H (100 μg/kg) on intrathecal morphine (0.03 mg)-induced scratching were redetermined at 1 and 10 days, respectively. The dose and pretreatment time for nor-BNI were chosen based on previous studies showing that peak KOR antagonist effects of nor-BNI were observed within the first 2 weeks after systemic administration in monkeys (Butelman et al., 1993; Ko et al., 2003a).
Data Analysis. Mean values (mean ± S.E.M.) were calculated from individual values for all behavioral endpoints. Comparisons were made for the same monkeys across all test sessions in the same experiment. Data were analyzed by a two-way analysis of variance followed by the Newman-Keuls test for multiple (post hoc) comparisons. The criterion for significance was set at p < 0.05. For the dose-addition analysis, individual tail-withdrawal latencies in 50°C water were first converted to percentage of maximum possible effect, [(test latency - control latency)/(cutoff latency - control latency)] × 100. ED50 values were then calculated by least-squares regression with the portion of the dose-response curves spanning the 50% of maximal possible effect, and 95% confidence limits were also determined (p < 0.05). Finally, statistical evaluation of drug interactions between nalfurafine and morphine was conducted by comparing the experimentally determined ED50 values for each mixture (Zmix) with predicted additive ED50 values (Zadd) as described by Tallarida (2000). In brief, Zmix values were determined empirically as described above. Zadd values were calculated individually for each monkey by using the equation, Zadd = fA + (1 - f)B, where A is the ED50 for nalfurafine alone, B is the ED50 for morphine alone, and f = 0.25, 0.5, 0.75 represents the fractional multiplier for three mixtures of nalfurafine in combination with morphine.
Drugs. GR 89696 fumarate salt and U-50488H methanesulfonate salt (Sigma-Aldrich, St. Louis, MO), bremazocine hydrochloride (RBI/Sigma, Natick, MA), nalfurafine hydrochloride (provided by Dr. S. M. Husbands, University of Bath, Bath, UK), nor-BNI dihydrochloride (Tocris Bioscience, Ellisville, MO), and morphine sulfate (Mallinckrodt, Hazelwood, MO) were dissolved in sterile water. Doses are presented in the compound forms listed above. For systemic administration, all compounds were administered at a volume of 0.1 ml/kg. For intrathecal administration, morphine was administered at a total volume of 1 ml. The detailed description for intrathecal drug delivery can be referred to Ko et al. (2003a).
Results
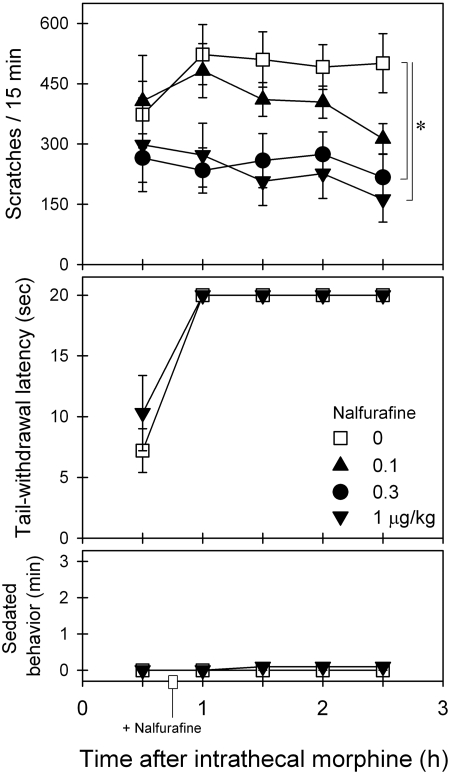
Figure 2 illustrates the effects of intramuscular nalfurafine on intrathecal morphine-induced scratching and antinociception. Nalfurafine intervention dose-dependently attenuated intrathecal morphine-induced scratching [F(3,15) = 7.0; p < 0.05]. Post hoc comparisons indicated that nalfurafine from 0.3 to 1 μg/kg significantly attenuated scratching between time points 1 and 2.5 h. Nalfurafine did not alter intrathecal morphine-induced antinociception [F(1,5) = 2.1; p > 0.05]. In addition, nalfurafine did not increase the sedation score [F(3,15) = 0.9; p > 0.05] under these conditions. Nalfurafine alone at the highest dose 1 μg/kg tested herein did not significantly increase scratching responses compared with the vehicle condition (data not shown).
Fig. 2.
Effects of nalfurafine intervention on intrathecal morphine-induced itch/scratching and antinociception against 50°C water. Nalfurafine (micrograms per kilogram intramuscularly) was given 45 min after intrathecal administration of 0.03 mg of morphine. Each value represents mean ± S.E.M. (n = 6). Symbols represent different dosing conditions for the same monkeys. *, significant difference from the vehicle condition between time points 1 and 2.5 h (p < 0.05).
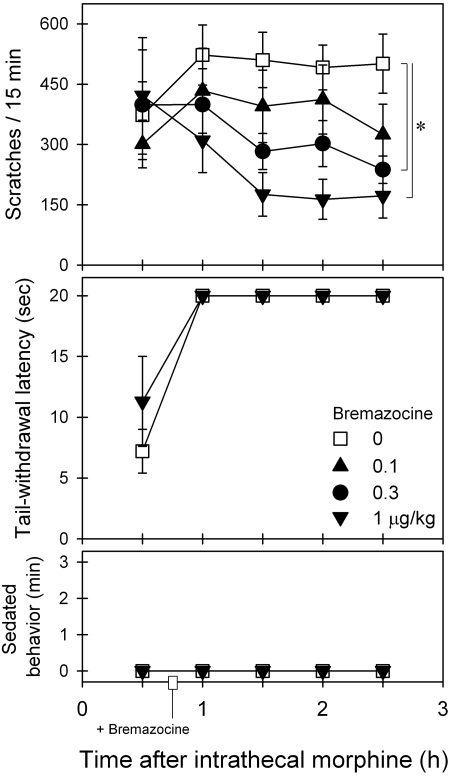
Figure 3 illustrates the effects of intramuscular bremazocine on intrathecal morphine-induced scratching and antinociception. Bremazocine intervention dose-dependently attenuated intrathecal morphine-induced scratching [F(3,15) = 3.1; p < 0.05]. Post hoc comparisons indicated that bremazocine from 0.3 to 1 μg/kg significantly attenuated scratching between time points 1.5 and 2.5 h. Bremazocine did not alter intrathecal morphine-induced antinociception [F(1,5) = 2.5; p > 0.05]. In addition, bremazocine did not increase the sedation score [F(3,15) = 1.5; p > 0.05] under these conditions.
Fig. 3.
Effects of bremazocine intervention on intrathecal morphine-induced itch/scratching and antinociception against 50°C water. Bremazocine (micrograms per kilogram intramuscularly) was given 45 min after intrathecal administration of 0.03 mg of morphine. Each value represents mean ± S.E.M. (n = 6). Symbols represent different dosing conditions for the same monkeys. *, significant difference from the vehicle condition between time points 1.5 and 2.5 h (p < 0.05).
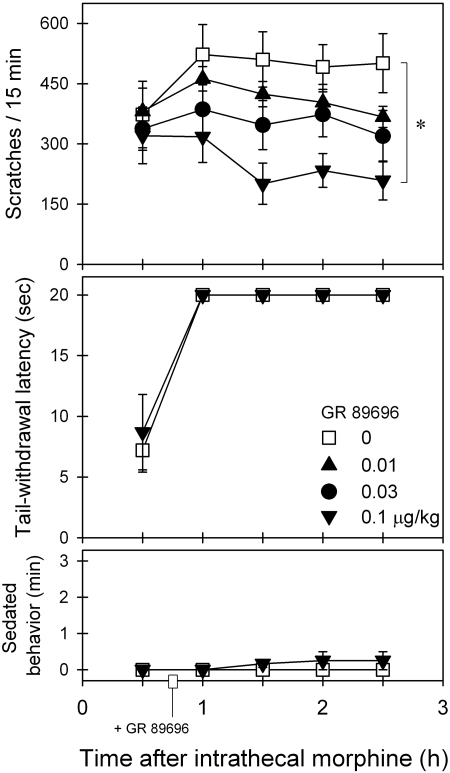
Figure 4 illustrates the effects of intramuscular GR 89696 on intrathecal morphine-induced scratching and antinociception. GR 89696 intervention dose-dependently attenuated intrathecal morphine-induced scratching [F(3,15) = 6.8; p < 0.05]. Post hoc comparisons indicated that 0.1 μg/kg GR 89696 significantly attenuated scratching between time points 1.5 and 2.5 h. GR 89696 did not alter intrathecal morphine-induced antinociception [F(1,5) = 0.5; p > 0.05]. In addition, GR 89696 did not increase the sedation score [F(3,15) = 1.4; p > 0.05] under these conditions.
Fig. 4.
Effects of GR 89696 intervention on intrathecal morphine-induced itch/scratching and antinociception against 50°C water. GR 89696 (micrograms per kilogram intramuscularly) was given 45 min after intrathecal administration of 0.03 mg of morphine. Each value represents mean ± S.E.M. (n = 6). Symbols represent different dosing conditions for the same monkeys. *, significant difference from the vehicle condition between time points 1 and 2.5 h (p < 0.05).
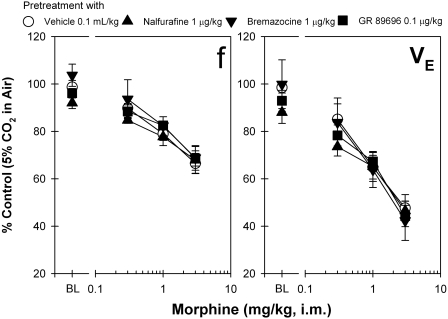
The dose-response curves of morphine-induced antinociception in the presence of different KOR agonists (i.e., 0.1 ml/kg vehicle, 1 μg/kg nalfurafine, 1 μg/kg bremazocine, and 0.1 μg/kg GR 89696) were further compared. Mean ED50 values for morphine-induced antinociception were similar among different dosing conditions, ranging between 0.5 and 0.7 mg/kg, indicating that these dosing conditions of KOR agonists did not change morphine-induced antinociception (data not shown). In addition, Fig. 5 shows that systemic morphine-induced respiratory depression was not significantly changed by pretreatment with different KOR agonists quantified by both parameters f and VE in monkeys breathing 5% CO2 in air. The dose response of these changes is the same for monkeys breathing air (data not shown). It is worth noting that these doses of KOR agonists alone did not suppress the respiratory function of monkeys because there were no changes in the baseline value.
Fig. 5.
Effects of pretreatment with κ-opioid receptor agonists on the dose-response curve of systemic morphine-induced respiratory depression. A single dose of the compound (micrograms per kilogram intramuscularly) was given 30 min before administration of the first dose of morphine (milligrams per kilogram intramuscularly). BL represents the effects of κ-opioid receptor agonists alone on the respiratory function of the subjects (i.e., the control value). Each value represents mean ± S.E.M. (n = 6).
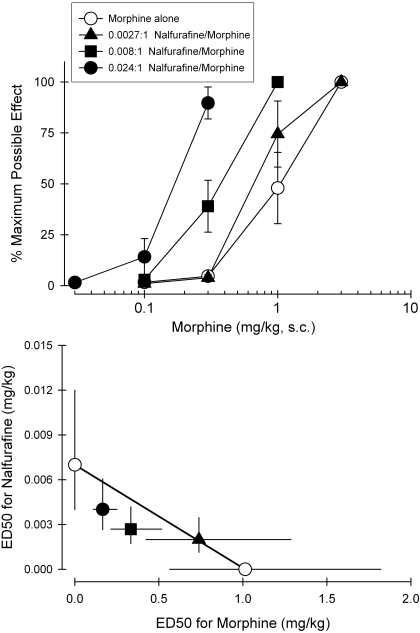
Figure 6, top, illustrates the antinociceptive effects of morphine alone or in combination with nalfurafine. All nalfurafine + morphine mixtures produced dose-dependent antinociceptive effects. Addition of nalfurafine produced left-ward shifts in the dose-response curve of morphine-induced antinociception. The magnitude of these left shifts depended on the proportion of nalfurafine in the mixture. Figure 6, bottom, displays an isobologram for the mixture of nalfurafine + morphine. The predicted additive values (Zadd) and experimentally determined ED50 values (Zmix) of mixtures of nalfurafine in combination with morphine are shown in Table 1. Statistical analysis confirmed that the Zmix values are not significantly different from the Zadd values for each mixture, indicating that additive effects (i.e., no subadditivity or superadditivity) were observed for nalfurafine in combination with morphine in the antinociceptive effect in monkeys.
Fig. 6.
Effects of nalfurafine in combined with morphine on antinociception against 50°C water. Top, dose-response curves for systemic morphine alone (milligrams per kilogram subcutaneously) and in mixtures with systemic nalfurafine (milligrams per kilogram subcutaneously). Bottom, isobologram for the mixture of nalfurafine- and morphine-induced antinociception. Each value represents mean ± S.E.M. (n = 6).
TABLE 1.
Predicted additive ED50 values (Zadd) and experimentally determined ED50 values (Zmix) of mixtures of nalfurafine administered in combination with morphine in the antinociceptive assay in monkeys
| Zadd (95% CL) | Zmix (95% CL) | |
|---|---|---|
| Nalfurafine + morphine | ||
| 0.0027:1, Nalfurafine/morphine | 0.76 (0.43–1.37) | 0.74 (0.43–1.29) |
| 0.008:1, Nalfurafine/morphine | 0.51 (0.29–0.92) | 0.34 (0.22–0.52) |
| 0.024:1, Nalfurafine/morphine | 0.26 (0.15–0.46) | 0.17 (0.11–0.26) |
CL, confidence limit
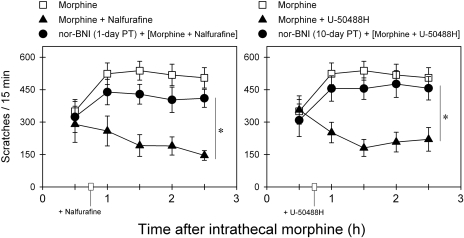
Figure 7 shows the KOR antagonist effects of nor-BNI on the inhibitory actions of nalfurafine and U-50488H against intrathecal morphine-induced scratching responses. One-day pretreatment with 3 mg/kg nor-BNI significantly blocked the ability of 1 μg/kg nalfurafine to attenuate intrathecal morphine (0.03 mg)-induced scratching [F(2,10) = 22.8; p < 0.05]. In addition, 10-day pretreatment with the same dose of nor-BNI also significantly blocked the ability of 100 μg/kg U-50488H to attenuate intrathecal morphine-induced scratching [F(2,10) = 16.2; p < 0.05].
Fig. 7.
Antagonist effects of nor-BNI on the actions of nalfurafine and U-50488H against intrathecal morphine-evoked scratching responses. Nalfurafine (1 μg/kg i.m.) or U-50488H (100 μg/kg i.m.) was given 45 min after intrathecal administration of 0.03 mg of morphine. Filled circles, effects of nor-BNI (3 mg/kg i.m.) 1- and 10-day pretreatment (PT) on the action of nalfurafine and U-50488H, respectively. Each value represents mean ± S.E.M. (n = 6). *, significant difference between conditions with or without nor-BNI pretreatment (p < 0.05).
Discussion
The first part of the study showed that systemic administration of atypical KOR agonists, nalfurafine (TRK-820), bremazocine, and GR 89696, potently attenuated intrathecal morphine-induced itch/scratching responses in a dose-dependent manner. It is important that intervention with these KOR agonists maintained intrathecal morphine-induced antinociception without producing sedation, supporting the notion of using KOR agonists for treating pruritus associated with spinal opioid analgesia in primates. It is worth noting that these KOR agonists used in the present study are extremely potent (i.e., in the unit of micrograms per kilogram), and these doses alone do not produce significant antinociceptive effects in monkeys (Butelman et al., 1993, 2001; Ko et al., 1998; Endoh et al., 2001). It may indicate that there are more KORs expressed in sensory neurons modulating itch sensation compared with those in nociceptors and/or that activation of KOR is more efficient in producing antipruritic actions than antinociceptive action. Nevertheless, it should be noted that these nonantinociceptive, nonsedative doses of KOR agonists did not completely suppress intrathecal morphine-elicited scratching. It is possible that distributions of morphine administered intrathecally and KOR agonists administered intramuscularly are not equivalent, and distinct sites of actions for both intrathecal morphine and intramuscular KOR agonists contribute to remaining scratching observed under this context. It will be interesting to examine whether intrathecal administration of morphine and KOR agonists together can produce maximal suppression of scratching responses in future studies.
The second part of the study demonstrated that antiscratching doses of these KOR agonists did not produce μ-opioid receptor antagonist effects. In particular, pretreatment with effective antiscratching doses of nalfurafine, bremazocine, or GR 89696 did not antagonize systemic morphine-induced antinociception and respiratory depression. Although these KOR agonists have moderate binding affinities for the μ-opioid receptor, they have approximately 100-fold lower potency in stimulating μ-opioid receptors compared with KOR measured by a functional guanosine 5′-3-O-(thio)-triphosphate binding assay (Emmerson et al., 1994; Toll et al., 1998; Butelman et al., 2001; Wang et al., 2005). These findings may suggest that potential μ-opioid antagonist effects of these KOR agonists can only be manifested at much higher doses in the guanosine 5′-3-O-(thio)triphosphate binding assay, and they cannot be detected in vivo in monkeys because of untoward effects elicited by high doses of KOR agonists (Butelman et al., 2001; Mizoguchi et al., 2003).
Because nalfurafine has been studied in the clinical trial (Wikström et al., 2005), additional pharmacological approaches were used to elucidate the receptor mechanisms underlying this compound's antiscratching effects. The dose-addition analysis and its isobologram indicate that there were additive effects for nalfurafine in combination with morphine in producing antinociceptive effects. This finding strongly supports the notion that μ-opioid antagonist effects of atypical KOR agonists identified from in vitro studies may not contribute to their behavioral effects observed herein (Butelman et al., 2001; Mizoguchi et al., 2003; Wang et al., 2005). More importantly, the KOR antagonist study revealed that antiscratching effects of both nalfurafine and U-50488H could be blocked completely by nor-BNI pretreatment. For comparison, a recent study has demonstrated that nor-BNI can only partially block antiscratching effects of butorphanol because this drug has dual actions (i.e., partial agonist action at both KOR and μ-opioid receptors) as an antipruritic (Lee et al., 2007). If nalfurafine's μ-opioid antagonist or partial agonist effect significantly contributes to its antiscratching effects, it is expected that pretreatment with nor-BNI could only partially reverse nalfurafine's antiscratching effects. Lack of partial blockade by nor-BNI against nalfurafine's actions may suggest that the agonist action at KOR mainly contribute to the effectiveness of nalfurafine as an antipruritic under these conditions.
It is important to note that systemic 3 mg/kg nor-BNI has been used previously to distinguish the antinociceptive effects of KOR-1 agonists versus non-KOR-1 agonists in monkeys (Butelman et al., 1993, 2001). Lack of relative nor-BNI selectivity for nalfurafine- versus U-50488H-induced antiscratching effects seems to suggest there is no evidence for KOR subtypes underlying KOR agonist-induced antipruritic effects. It is worth noting that KOR-1 and non-KOR-1 agonists may have differential sensitivity to nor-BNI's antagonist effects depending on functional assays. For example, intracisternal administration of 0.3 mg of nor-BNI produced a significant blockade of U-50488H-induced antinociception for 5 weeks, but it failed to block antinociceptive effects of bremazocine in the same monkeys (Ko et al., 1999). In contrast, intracisternal nor-BNI at the same dose equally blocked both U-50488H- and bremazocine-induced diuresis for 20 weeks (Ko et al., 2003b). These findings may indicate that the KOR population required for producing antinociception is greater than that for diuretic and antiscratching effects in primates. Although functional KOR subtypes have been proposed based on different pharmacological studies (Butelman et al., 1993, 1998; Ko et al., 1998), both KOR-1 and non-KOR-1 agonists are equally active in a variety of behavioral assays in monkeys (France et al., 1994; Butelman et al., 2001; Ko et al., 2003b).
Previous studies have found differences in KOR-regulated desensitization and phosphorylation between human KOR and rat KOR (Li et al., 2002; Liu-Chen, 2004). The major difference of KOR in vivo between rodents and primates is that the KOR antagonist, nor-BNI, elicits scratching responses in mice (Kamei and Nagase, 2001), but it does not do so in monkeys (Ko et al., 2003a). nor-BNI only acts as a neutral KOR antagonist to block the behavioral effects of KOR agonists in primates (Butelman et al., 1993; Ko et al., 1999, 2003a). Nevertheless, both types of KOR agonists, such as nalfurafine and U-50488H, have been shown to have a broader application as antipruritics by using diverse pruritogenic agents across both rodents and primates (Togashi et al., 2002; Ko et al., 2003a; Wakasa et al., 2004; Wang et al., 2005; Inan and Cowan, 2006). An early study has shown that subcutaneous administration of nalfurafine could effectively suppress intracisternal morphine-induced scratching in mice (Umeuchi et al., 2003). However, as previously demonstrated, intrathecal morphine simultaneously produces both scratching and antinociception in monkeys, but not in rodents (Ko and Naughton, 2000; Ko et al., 2003a; Lee et al., 2003). The present study of KOR agonists as antipruritics in primates further extends the therapeutic application of KOR agonists under the context of spinal opioid analgesia (Ko et al., 2003a; Lee et al., 2007; the present study). More importantly, a recent study demonstrated that there are two separate populations of spinothalamic tract neurons responding to histamine versus nonhistaminergic pruritogenic agent, cowhage, in monkeys (Davidson et al., 2007). It will be important to conduct more translational research in primates to compare the effectiveness of KOR agonists against scratching responses evoked by different pruritogenic agents.
In summary, this study is the first to provide direct evidence and translational value that previously proposed non-KOR-1 agonists, nalfurafine, bremazocine, and GR 89696, are effective and very potent in blocking intrathecal morphine-induced itch/scratching responses without interfering with intrathecal morphine-induced analgesia in primates. The agonist action of KOR mainly contributes to the effectiveness of nalfurafine as an antipruritic, and there is no evidence in vivo for KOR subtypes or μ-opioid antagonist action underlying the antipruritic effects of nalfurafine. These findings further support the therapeutic potential of KOR agonists, regardless of their KOR-1 selectivity, as antipruritics in the context of spinal opioid analgesia.
Acknowledgments
We thank Eric Hu for assistance of statistical analysis of dose-addition studies and Justin Knorr and Timothy Zdrodowski for technical assistance of data collection.
This study was supported by United States Public Health Service [Grant DA-013685]; and by Taiwan National Science Council [Grants NSC-96-2413-H-004-019, NSC-97-2628-H-004-089-MY2].
Article, publication date, and citation information can be found at http://jpet.aspetjournals.org.
doi:10.1124/jpet.108.143925.
ABBREVIATIONS: KOR, κ-opioid receptor; U-50488H, trans-(±)-3,4-dichloro-N-methyl-N-(2-[1-pyrrolidinyl]-cyclohexyl)-benzeneacetamide; nalfurafine (TRK-820), 17-cyclopropylmethyl-3,14β-dihydroxy-4,5α-epoxy-6β-[N-methyl-trans-3-(3-furyl)acrylamido]morphinan; nor-BNI, nor-binaltorphimine; bremazocine, (±)-6-ethyl-1,2,3,4,5,6-hexahydro-3-[(1-hydroxycyclopropy)methyl]-11,11-dimethyl-2,6-methano-3-benzazocin-8-ol; GR 89696, 4-[(3,4-dichlorophenyl)acetyl]-3-(1-pyrrolidinylmethyl)-1-piperazinecarboxylic acid methyl ester.
References
- Bailey PL, Rhondeau S, Schafer PG, Lu JK, Timmins BS, Foster W, Pace NL, and Stanley TH (1993) Dose-response pharmacology of intrathecal morphine in human volunteers. Anesthesiology 79 49-59. [DOI] [PubMed] [Google Scholar]
- Butelman ER, Ko MC, Sobczyk-Kojiro K, Mosberg HI, Van Bemmel B, Zernig G, and Woods JH (1998) Kappa-opioid receptor binding populations in rhesus monkey brain: relationship to an assay of thermal antinociception. J Pharmacol Exp Ther 285 595-601. [PubMed] [Google Scholar]
- Butelman ER, Ko MC, Traynor JR, Vivian JA, Kreek MJ, and Woods JH (2001) GR89,696: a potent κ-opioid agonist with subtype selectivity in rhesus monkeys. J Pharmacol Exp Ther 298 1049-1059. [PubMed] [Google Scholar]
- Butelman ER, Negus SS, Ai Y, de Costa BR, and Woods JH (1993) Kappa opioid antagonist effects of systemically administered nor-binaltorphimine in a thermal antinociception assay in rhesus monkeys. J Pharmacol Exp Ther 267 1269-1276. [PubMed] [Google Scholar]
- Cohen SE, Ratner EF, Kreitzman TR, Archer JH, and Mignano LR (1992) Nalbuphine is better than naloxone for treatment of side effects after epidural morphine. Anesth Analg 75 747-752. [PubMed] [Google Scholar]
- Cousins MJ and Mather LE (1984) Intrathecal and epidural administration of opioids. Anesthesiology 61 276-310. [PubMed] [Google Scholar]
- Cowan A and Gmerek DE (1986) In vivo studies on kappa opioid receptors. Trends Pharmacol Sci 7 69-72. [Google Scholar]
- Davidson S, Zhang X, Yoon CH, Khasabov SG, Simone DA, and Giesler GJ Jr (2007) The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci 27: 10007-10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBalli P and Breen TW (2003) Intrathecal opioids for combined spinal-epidural analgesia during labour. CNS Drugs 17 889-904. [DOI] [PubMed] [Google Scholar]
- Emmerson PJ, Liu MR, Woods JH, and Medzihradsky F (1994) Binding affinity and selectivity of opioids at mu, delta and kappa receptors in monkey brain membranes. J Pharmacol Exp Ther 271 1630-1637. [PubMed] [Google Scholar]
- Endoh T, Tajima A, Izumimoto N, Suzuki T, Saitoh A, Suzuki T, Narita M, Kamei J, Tseng LF, Mizoguchi H, et al. (2001) TRK-820, a selective κ-opioid agonist, produces potent antinociception in cynomolgus monkeys. Jpn J Pharmacol 85 282-290. [DOI] [PubMed] [Google Scholar]
- France CP, Medzihradsky F, and Woods JH (1994) Comparison of kappa opioids in rhesus monkeys: behavioral effects and receptor binding affinities. J Pharmacol Exp Ther 268 47-58. [PubMed] [Google Scholar]
- Ganesh A and Maxwell LG (2007) Pathophysiology and management of opioid-induced pruritus. Drugs 67 2323-2333. [DOI] [PubMed] [Google Scholar]
- Gmerek DE, Dykstra LA, and Woods JH (1987) Kappa opioids in rhesus monkeys: III. Dependence associated with chronic administration. J Pharmacol Exp Ther 242 428-436. [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Bigelow GE, and Liebson IA (1989) Acute opioid physical dependence in postaddict humans: naloxone dose effects after brief morphine exposure. J Pharmacol Exp Ther 248 127-134. [PubMed] [Google Scholar]
- Inan S and Cowan A (2006) Nalfurafine, a kappa opioid receptor agonist, inhibits scratching behavior secondary to cholestasis induced by chronic ethynylestradiol injections in rats. Pharmacol Biochem Behav 85 39-43. [DOI] [PubMed] [Google Scholar]
- Institute of Laboratory Animal Resources (1996) Guide for the Care and Use of Laboratory Animals, 7th ed, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council and National Academy Press, Washington, DC.
- Kamei J and Nagase H (2001) Norbinaltorphimine, a selective kappa-opioid receptor antagonist, induces an itch-associated response in mice. Eur J Pharmacol 418 141-145. [DOI] [PubMed] [Google Scholar]
- Kishioka S, Paronis CA, and Woods JH (2000) Acute dependence on, but not tolerance to, heroin and morphine as measured by respiratory effects in rhesus monkeys. Eur J Pharmacol 398 121-130. [DOI] [PubMed] [Google Scholar]
- Ko MC, Butelman ER, Traynor JR, and Woods JH (1998) Differentiation of kappa opioid agonist-induced antinociception by naltrexone apparent pA2 analysis in rhesus monkeys. J Pharmacol Exp Ther 285 518-526. [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Johnson MD, Butelman ER, Willmont KJ, Mosberg HI, and Woods JH (1999) Intracisternal nor-binaltorphimine distinguishes central and peripheral κ-opioid antinociception in rhesus monkeys. J Pharmacol Exp Ther 291 1113-1120. [PMC free article] [PubMed] [Google Scholar]
- Ko MCH and Naughton NN (2000) An experimental itch model in monkeys: characterization of intrathecal morphine-induced scratching and antinociception. Anesthesiology 92 795-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Lee H, Song MS, Sobczyk-Kojiro K, Mosberg HI, Kishioka S, Woods JH, and Naughton NN (2003a) Activation of κ-opioid receptors inhibits pruritus evoked by subcutaneous or intrathecal administration of morphine in monkeys. J Pharmacol Exp Ther 305 173-179. [DOI] [PubMed] [Google Scholar]
- Ko MC, Willmont KJ, Lee H, Flory GS, and Woods JH (2003b) Ultra-long antagonism of kappa opioid agonist-induced diuresis by intracisternal nor-binaltorphimine in monkeys. Brain Res 982 38-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko MC, Song MS, Edwards T, Lee H, and Naughton NN (2004) The role of central μ opioid receptors in opioid-induced itch in primates. J Pharmacol Exp Ther 310 169-176. [DOI] [PubMed] [Google Scholar]
- Lee H, Naughton NN, Woods JH, and Ko MCH (2003) Characterization of scratching responses in rats following centrally administered morphine or bombesin. Behav Pharmacol 14 501-508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Naughton NN, Woods JH, and Ko MC (2007) Effects of butorphanol on morphine-induced itch and analgesia in primates. Anesthesiology 107 478-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Li JG, Chen C, Zhang F, and Liu-Chen LY (2002) Molecular basis of differences in (-)(trans)-3,4-dichloro-N-methyl-N-[2-(1-pyrrolidiny)-cyclohexyl]benzeneacetamide-induced desensitization and phosphorylation between human and rat kappa-opioid receptors expressed in chinese hamster ovary cells. Mol Pharmacol 61 73-84. [DOI] [PubMed] [Google Scholar]
- Liu-Chen LY (2004) Agonist-induced regulation and trafficking of kappa opioid receptors. Life Sci 75 511-536. [DOI] [PubMed] [Google Scholar]
- Mizoguchi H, Hung KC, Leitermann R, Narita M, Nagase H, Suzuki T, and Tseng LF (2003) Blockade of μ-opioid receptor-mediated G-protein activation and antinociception by TRK-820 in mice. Eur J Pharmacol 461 35-39. [DOI] [PubMed] [Google Scholar]
- Palmer CM, Emerson S, Volgoropolous D, and Alves D (1999) Dose-response relationship of intrathecal morphine for postcesarean analgesia. Anesthesiology 90 437-444. [DOI] [PubMed] [Google Scholar]
- Rawal N, Schött U, Dahlström B, Inturrisi CE, Tandon B, Sjöstrand U, and Wennhager M (1986) Influence of naloxone infusion on analgesia and respiratory depression following epidural morphine. Anesthesiology 64 194-201. [DOI] [PubMed] [Google Scholar]
- Simonin F, Gavériaux-Ruff C, Befort K, Matthes H, Lannes B, Micheletti G, Mattéi MG, Charron G, Bloch B, and Kieffer B (1995) κ-Opioid receptor in humans: cDNA and genomic cloning, chromosomal assignment, functional expression, pharmacology, and expression pattern in the central nervous system. Proc Natl Acad Sci U S A 92 7006-7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallarida RJ (2000) Drug Synergism and Dose-Effect Data Analysis, CRC Press, Boca Raton, FL.
- Togashi Y, Umeuchi H, Okano K, Ando N, Yoshizawa Y, Honda T, Kawamura K, Endoh T, Utsumi J, Kamei J, et al. (2002) Antipruritic activity of the κ-opioid receptor agonist, TRK-820. Eur J Pharmacol 435 259-264. [DOI] [PubMed] [Google Scholar]
- Toll L, Berzetei-Gurske IP, Polgar WE, Brandt SR, Adapa ID, Rodriguez L, Schwartz RW, Haggart D, O'Brien A, White A, et al. (1998) Standard binding and functional assays related to medications development division testing for potential cocaine and opiate narcotic treatment medications. NIDA Res Monogr 178 440-466. [PubMed] [Google Scholar]
- Umeuchi H, Togashi Y, Honda T, Nakao K, Okano K, Tanaka T, and Nagase H (2003) Involvement of central μ-opioid system in the scratching behavior in mice, and the suppression of it by the activation of κ-opioid system. Eur J Pharmacol 477 29-35. [DOI] [PubMed] [Google Scholar]
- Wakasa Y, Fujiwara A, Umeuchi H, Endoh T, Okano K, Tanaka T, and Nagase H (2004) Inhibitory effects of TRK-820 on systemic skin scratching induced by morphine in rhesus monkeys. Life Sci 75 2947-2957. [DOI] [PubMed] [Google Scholar]
- Wang JJ, Ho ST, and Tzeng JI (1998) Comparison of intravenous nalbuphine infusion versus naloxone in the prevention of epidural morphine-related side effects. Reg Anesth Pain Med 23 479-484. [DOI] [PubMed] [Google Scholar]
- Wang Y, Tang K, Inan S, Siebert D, Holzgrabe U, Lee DY, Huang P, Li JG, Cowan A, and Liu-Chen LY (2005) Comparison of pharmacologic activities of three distinct κ ligands (salvinorin A, TRK-820 and 3FLB) on κ opioid receptors in vitro and their antipruritic and antinociceptive activities in vivo. J Pharmacol Exp Ther 312 220-230. [DOI] [PubMed] [Google Scholar]
- Wikström B, Gellert R, Ladefoged SD, Danda Y, Akai M, Ide K, Ogasawara M, Kawashima Y, Ueno K, Mori A, et al. (2005) Kappa-opioid system in uremic pruritus: multicenter, randomized, double-blinded, placebo-controlled clinical studies. J Am Soc Nephrol 16 3742-3747. [DOI] [PubMed] [Google Scholar]