Abstract
We recently reported a computational method (CHAMP) to design sequence-specific peptides that bind to the membrane-embedded portions of transmembrane proteins. We successfully applied this method to design membrane-spanning peptides targeting the transmembrane domains of the αIIb-subunit of integrin αIIbβ3. Previously, we demonstrated that these CHAMP peptides bind specifically with reasonable affinity to isolated transmembrane helices of the targeted transmembrane region. These peptides also induced integrin αIIbβ3 activation due to disruption of the helix-helix interactions between the transmembrane domains of the αIIb and β3 subunits. In this paper, we show the direct interaction of the designed anti-αIIb CHAMP peptide with isolated full-length integrin αIIbβ3 in detergent micelles. Further, the behavior of the designed peptides in phospholipid bilayers is essentially identical to their behavior in detergent micelles. In particular, the peptides assume a membrane-spanning α-helical conformation that does not disrupt bilayer integrity. The activity and selectivity of the CHAMP peptides was further explored in platelets, comfirming that anti-αIIb activates wild type αIIbβ3 in whole cells as a result of its disruption of the protein-protein interactions between the α- and β-subunits at the transmembrane regions. These results demonstrate that CHAMP is a successful chemical biology approach that can provide specific tools to probe the transmembrane domains of proteins.
Keywords: membrane protein, protein-protein interactions, transmembrane domain, computational design, integrin
Membrane proteins account for approximately 30% of the entire human proteome; however, studies of their transmembrane (TM) domains have lagged behind due to limitation of their availability, the complexity of the model systems used to study membrane proteins (e.g. micelles, phospholipids vesicles, and bicelles), and in particular, the lack of exogenous probes with high affinity and specificity (1). Conventional antibody-based probing techniques are only useful for water-soluble regions of proteins. Currently, there is no widely used chemical biology method to specifically target the TM domains of proteins using exogenous agents.
Computational protein engineering has made major strides (2). There are now a variety of methods to design proteins that recognize water-soluble regions of target proteins (3–6), but few companion methods for targeting TM regions have been successful. Recently, we reported a general strategy for the computational design of TM domain-targeted peptides, designated the CHAMP (computed helical anti-membrane protein) method (7). We illustrated the utility of the method by designing peptides that specifically recognize the TM helix of the α-subunit of the platelet integrin αIIbβ3. The TM helices of the α and β subunits of integrin αIIbβ3 are thought to associate heteromerically in unstimulated platelets and to dissociate following platelet stimulation (8–10). We showed that anti-αIIb, a peptide designed to target the αIIb TM helix, activated αIIbβ3 by disrupting the heteromeric αIIb/β3 TM helix-helix interaction of the resting integrin (Figure 1). These results illustrate the potential of CHAMP method for generating high affinity molecules that bind to and modulate functions of membrane proteins.
FIGURE 1.

Schematic diagram of integrin αIIbβ3 regulation. Because the αIIb and β3 subunit TM domains interact when integrins are inactive, any process that destabilizes this interaction would be expected to allow dissociation of the TM domains with concomitant integrin activation. In platelets, this occurs when platelets are stimulated by agonists such as adenosine diphosphate (ADP), inducing binding of the talin phosphotyrosine-binding domain to integrin β3-subunit cytoplasmic domains (11). CHAMP peptides activate platelets by blocking the interactions between the TM helices of the α- and β-subunits of integrin αIIbβ3. Extracellular ligands for integrin αIIbβ3 (e.g. fibrinogen) are shown in black tubes. Question marks indicate unclear interactions.
In this paper, we validate the specificity of the anti-αIIb CHAMP peptide, using biophysical methods to confirm that it recognizes full-length integrin αIIbβ3 in vitro and in living cells. We found that anti-αIIb selectively binds to the isolated full-length integrin αIIbβ3 in detergent micelles and phospholipid bilayers, assumes a membrane-spanning α-helical conformation that does not disrupt the bilayer integrity, and activates αIIbβ3 by disrupting the association of αIIb and β3 transmembrane helices. These results support the notion that CHAMP is a general method for design membrane-spanning peptides targeting protein TM domains.
EXPREIMENTAL PROCEDURES
General Peptide Synthesis
Peptides were synthesized using an Applied Biosystems 430A peptide synthesizer at 0.25 mmole scales. These peptides were synthesized on a Rink Amide AM resin (200–400 mesh) (Nova Biochem) with a substitution level of 0.71 mmole/g. Activation of the free amino acids was achieved with 2-(1H-7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyl uronium hexafluorophosphate methanaminium (HATU, 0.40 M solution in DMF). The reaction solvent contains 25% DMSO and 75% NMP (HPLC grade, Aldrich). Side chain deprotection and simultaneous cleavage from the resin was performed using a mixture of trifluoroacetic acid (TFA)/thioanisole/1,2-ethanedithiol/anisole (90:5:3:3 v/v) at room temperature, under N2 flow for 2 hours. The crude peptides collected from precipitation with cold diethyl ether (Aldrich) were dissolved in a mixture of 2-propanol:acetonitrile: water (6:3:1) and then lyophilized overnight. The peptides were then purified on a preparative reverse phase HPLC system (Varian ProStar 210) with a C-4 preparative column (Vydac) using a linear gradient of buffer A (0.1% TFA in Millipore water) and buffer B (6:3:1 2-propanol: acetonitrile: water) containing 0.1% TFA. Elution of the purified peptides occurred at approximately 75% of buffer B. The identities of the purified peptides were confirmed by MALDI-TOF mass spectroscopy on a Voyager Biospectrometry Workstation (PerSeptive Biosystems), and their purity was assessed using HP1100 analytical HPLC system (Hewlett Packard) with an analytical C-4 column (Vydac) and a linear A/B gradient.
The coumarin label of anti-αIIb and anti-αIIbmut was attached using standard method (12). Two additional glycine residues were coupled to the amino terminal of the peptide-resin using standard manual peptide synthesis conditions. The Fmoc protection group was removed with 20% piperidine in DMF. Resin was rinsed with DMF four times then swelled with dichloromethane and drained. 7-Hydroxycoumarin-3-carboxylic acid (Anaspec) was dissolved in a mixture of pyridine/DMF/DCM (12:7:5) to prepare 0.1 M solution. The resulting solution was added to the resin. The suspension mixture was stirred under room temperature in dark until the ninhydrin test indicates that the reaction is completed.
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
Electrophoresis was carried out using precast SDS polyacrylamide gels (12% NuPAGE 10-well Bis-Tris gels, Invitrogen). The peptide samples were prepared in buffer (10 mM HEPES (pH=7.5), 60 mM N-octyl-β-D- glucopyranoside, 0.5 mM CaCl2, 0.02% NaN3) and left incubated overnight. Before electrophoresis, each sample was incubated at 90°C for 7 min. Electrophoresis was carried out at room temperature with NuPAGE MES SDS running buffer (Invitrogen) at 125 mV for 55 minutes. The resulting gel was stained using NOVEX stain kit (Invitrogen).
Fluorescence Anisotropy Assay
The full-length integrin αIIbβ3 protein in buffer (7.9 mg/ml, 10 mM HEPES (pH=7.5), 60 mM N-octyl-β-D-glucopyranoside, 0.5 mM CaCl2, 0.02% NaN3) is prepared using the previously reported method (13). Fluorescence polarization experiments were conducted on an ATF105 spectrofluorometer (Aviv Instrument, Inc) using a 0.3 cm path length cuvette. Spectra were measured at 25 °C using 1.0 nm slit widths. Excitation at 408 nm was used for the coumarin-labeled peptide. Anisotropy measurements were recorded upon titration of the integrin αIIbβ3 protein at varying concentrations into a solution of 64 nM of the anti-αIIb CHAMP peptide. Data analysis was carried out using previously described method (14).
Attenuated Total Reflection Infrared (ATR-IR) Spectroscopy
ATR-IR spectroscopy was performed as previously described (15). Briefly, the purified anti-αIIb peptide was solubilized in a 1:1 (v:v) mixture of 2-propanol:H2O containing 0.1% HCl, frozen, and lyophilized. This process was repeated for a total of 3 rounds to remove any TFA salts from the purification process. The anti-αIIb peptide was then solubilized in a 1:1 (v:v) mixture of 2-propanol:H2O, mixed with an appropriate volume of lipid dissolved in CHCl3, and dried under a stream of N2. The lipid composition was a mixture of 1- palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC) and 1-palmitoyl-2-oleoyl-sn-glycero-3- [phospho-rac-(1-glycerol)] (POPG) (7:3 mol:mol). The final peptide:lipid ratio in the sample was 1:50. The dried peptide-lipid film was subjected to high vacuum for 2–3 hours to remove any traces of solvent. The film was reconstituted in 5 mM HEPES buffered D2O at ~pH 7.1 by vigorous vortexing. The peptide-lipid suspension was then extruded 17 times using an Avestin liposofast mini extruder (Avestin Inc.) equipped with 2 stacked polycarbonate membranes with average pore diameter of 200 nm. The peptide-containing vesicles were deposited on the ATR crystal, gently spread with a Teflon bar to form a film, and dried under a gentle stream of N2. Infrared spectra were recorded on a Nicolet 4700 infrared spectrophotometer (Thermo-Electron Corp.) equipped with a DTGS detector, a ZnSe wire-grid polarizer, and a home built flow chamber to allow a stream of N2 bubbled through D2O to flow over the sample during collection. The internal reflection element was a zinc-selenide ATR crystal (80 × 20 × 3 mm) with an angle of 45° yielding 25 internal reflections. A total of 512 scans at polarizations of 0° and 90° were collected for each sample. Spectra were recorded at 2 cm−1 resolution and analyzed using the OMNIC software package for peak deconvolution and area analysis. Helix orientation angle was calculated from the spectra as previously described (16) with the exception that the value used in this study for the crystal refractive index was 2.42 (ZnSe).
Dye Release Assay
Lipids were purchased from Avanti Polar Lipids Inc. (Alabaster, Al) and used without further purification. Formation of large unilamellar vesicles with entrapped Tb(DPA)3 was performed as previously described with several modifications (17). POPC and POPG dissolved in chloroform were mixed (7:3 mol:mol) with a trace amount of L-3-phosphatidylcholine-1,2-di[14C]oleoyl (Amersham Biosciences). The lipid mixture was initially dried under a stream of N2, then further dessicated under vacuum for at least 3 hours to remove any residual chloroform. The dried lipid film was then stored under a head of N2 at −20°C until used. To form liposomes, the lipid film was rehydrated in HBS buffer (10mM HEPES, 100mM NaCl, pH 7.1) containing 5mM TbCl3 (Molecular Probes, a division of Invitrogen, Carlsbad, CA) and 15mM 2,6-pyridinedicarboxylic acid (DPA, Sigma-Aldrich, neutralized to pH 7.0) to obtain a final lipid concentration of 20mM (typically a final volume of 0.5 ml was used). The sample was vigorously vortexed for at least 3 minutes to ensure complete resuspension of the lipid film. The sample was then subjected to 7 rounds of freezing in a dry-ice/acetone bath and thawed at 37°C, to increase trapping yield. The sample was then passed 21 times through a Liposofast extruder (Avestin Inc., Ottowa, Canada) with two stacked polycarbonate membranes, each with a pore size of 200nm. Liposomes were then separated from untrapped molecules by gel filtration on a Sepharose CL-2B column (Sigma, 1.0 cm i.d. × 22cm). Lipid concentration was calculated by measuring radioactivity in liposome containing fractions after the column with a comparison to the radioactivity in an aliquot of liposomes that was taken before the gel filtration step. Liposomes were stored at 4°C and used for up to 1 week after preparation. Each liposome preparation was checked for nonspecific leakage before use by monitoring fluorescence in the absence of any externally added analytes.
An aliquot of Tb(DPA)3-containing liposomes were added to HBS supplemented with 10mM EDTA for a final concentration of 20 μM total lipid in 1700 μl volume. Samples were allowed to equilibrate for approximately 5 minutes at which point initial intensity readings were taken (F0). Peptides (or controls) were then added to the sample from a concentrated stock in DMSO to obtain the desired peptide:lipid ratio and allowed to equilibrate for 10 minutes with constant stirring at which point emission intensity was again recorded (F). Leakage was normalized to the fluorescence intensity value for complete vesicle disruption induced by addition of detergent (FM).
Hemolysis Assay
The hemolytic effects of the anti-αIIb and anti-αIIbmut peptides were tested using previously described method (18). Suspension of human erythrocytes (RBC, 1 %) with peptides of different concentrations were incubated in 150 mM sodium chloride and 10 mM Tris buffer (pH= 7.0), in the presence or absence of 1 mg/ml bovine serum albumin (BSA). The samples were prepared by combining 400 mL of the RBC suspension and peptide stock solutions (10 mM in DMSO). After incubation at 37°C for 1 h, the samples were centrifuged at 14 000 rpm for 5 min, and the OD400 of the supernatant was measured.
Transmission Electron Microscopy (TEM)
Electron microscopy of purified integrin αIIbβ3 heterodimers was performed as previously described (19). Rotary-shadowed samples were prepared using a modification of standard procedures (20) by spraying a dilute solution of molecules in a volatile buffer (0.05 M ammonium formate) and glycerol (30–50%) onto freshly-cleaved mica and shadowing with tungsten in a vacuum evaporator (Denton Vacuum Co., Cherry Hill, NJ). All specimens were examined in a FEI/Philips 400 electron microscope (Philips Electronic Instruments Co., Mahwah, NJ), operating at 80 kV and at a magnification of 60,000 ×.
Fluorescence-Activated Cell Sorting (FACS)
Freshly isolated platelets were added to 200 mg/ml FITC-conjugated fibrinogen, then incubated with 20 μM ADP or 2 μM the anti-αIIb CHAMP peptides in presence or absence of 5 mM EDTA for 3 minutes at room temperature. After incubation, the platelets were fixed with 0.37% formalin in PBS buffer for 10 minutes, then washed and examined by FACS analysis as previously reported (21).
RESULTS AND DISCUSSION
Anti-αIIb was prepared using Fmoc solid-state peptide synthesis (SSPS) with HATU as the activating agent to overcome the difficulty of synthesizing membrane-embed protein sequences (22). Double coupling conditions were used at the β-branched amino acid residues. High purity was confirmed by chromatography. A control peptide, anti-αIIbmut, in which the three Gly residues in the “Gly zipper” motif (GXXXGXXXG) were mutated to Leu (23), was also synthesized, as was a peptide, designated αIIb-TM, spanning residues Trp968 to Lys989 of αIIb (Figure 2A). For binding measurements using fluorescence spectroscopy, we prepared 7-hydroxycoumarin-3-carboxamide-labeled anti-αIIb (coum- anti-αIIb) and anti-αIIbmut (coum-anti-αIIbmut) via a linker to the N-termini of the peptides.
FIGURE 2.
(A) Sequences of the anti-αIIb CHAMP peptide, the control peptide anti-αIIbmut, and the targeted αIIb-TM helix; (B) SDS-PAGE of anti-αIIb (1), coumarin-labeled anti-αIIb (2), and anti-αIIbmut (3). (C) Computational model of anti-aIIb bound to the αIIb TM domain. The model predicts that anti-αIIb (red stick) recognizes the “hot spot” on the αIIb-TM binding surface (light blue) with spatial complementarity at the helix-crossing site.
To confirm that the designed CHAMP peptides, as well as its target αIIb-TM, take up structured conformations in artificial membrane systems (detergent micelles) and undergo oligomerization as previously reported in cellular membranes (7, 24), we used SDS-PAGE to study the oligomerization state of the free and tagged anti-αIIb peptides as well as anti-αIIbmut. SDS has previously been shown to provide a micellar environment that mimicks phospholipid bilayers (25). We found that both anti-αIIb (M.W.=3.7 kD) and coumarin-labelled anti-αIIb (M.W.=4.0 kD) migrated as dimers (Figure 2B), consistent with previous experiment using the TOXCAT system that measures transmembrane domain interactions in bacterial membranes (7). By contrast, anti-αIIbmut migrated as a monomer, confirmed that the Gly zipper motif is critical for the inter-helical recognition. Taken together, these results indicate that detergent micelles are a valid model system to study CHAMP peptide/target interactions. They also demonstrate that CHAMP peptides have high propensity to homo-dimerize, even in the presence of excessive competing detergent molecules.
Previously, we found that anti-αIIb associates with a short peptide (M.W.= ca. 3 kD) corresponding to the TM region of the αIIb-subunit of integrin αIIbβ3 in micelles and phospholipid vesicles (7). However, αIIbβ3 is a complex macromolecule (MW= ca. 230 kD) containing two non-identical transmembrane domains. To demonstrate that anti-αIIb also binds to the αllb transmembrane domain in full-length αIIbβ3, we used fluorescence anisotropy titrations of anti-αIIb to full-length αIIbβ3 in N-octyl-β-D-glucopyranoside micelles. The fluorescence anisotropy of a coumarin-labeled peptide in solution correlates with its tumbling and rotational rates (14). However, when bound to the much larger protein, its tumbling is constrained and polarization increases. The advantages of this assay are that it does not require immobilization of either the receptor or the ligand, requires small amounts of peptide and protein as fluorescein has high quantum yield, and can be readily developed into a high-throughput format. We added increasing amounts of coumarin-labeled anti-αIIb or anti-αIIbmut to a constant concentration of αIIbβ3 solution and monitored the resulting polarization increase. Plotting the anisotropy of coum-anti-αIIb as a function of the concentration of αIIbβ3 revealed a binding isotherm with an apparent Kdiss of 1.3±0.2 ×10−5 in mole fraction units, indicating that the peptide also binds tightly to the intact integrin (Figure 3). The control peptide, coum-anti-αIIbmut, displayed an ≈ 100-fold lower affinity for αIIbβ3. We also found that coum-anti-αIIb specifically recognized the TM domain of intergrin αIIb, but not that of intergrin αv, demonstrating that these CHAMP peptides can differentiate closely relevant homologous integral membrane targets (7).
FIGURE 3.

Fluorescence polarization titration shows that full-length integrin αIIbβ3 selectively associates with anti-αIIb over anti-αIIbmut.
Integrins are inactive when their TM domain-containing stalks are in proximity and active when the stalks separate (19, 26). Consistent with our previous report, transmission electron microscopy (TEM) of purified rotary shadowed αIIbβ3 in buffer containing N-octyl-β-D-glucopyranoside and 1 mM CaCl2 revealed that the majority of inactive αIIbβ3 molecules had a closed configuration with their stalks touching at the their tips (19). By contrast, when anti-αIIb was present, most of the αIIbβ3 molecules had an open configuration with separated stalks. Statistical analyses indicated that the presence of 5.0 μM anti-αIIb induced the majority of integrin αIIbβ3 molecules to convert to its active form (Figure 4). By contrast, anti-αIIbmut induced negligible activation under the same concentrations. These observations are consistent with the notion that anti-αIIb activates αIIbβ3 by disrupting the TM heterodimer maintaining the integrin in its inactive state.
FIGURE 4.
Transmission electron microscopy of purified αIIbβ3 in “closed”, (A, CaCl2-containing buffer alone) and “open” (B, in CaCl2-containing buffer + 3 μM anti-αIIb) forms. Individual αIIbβ3 molecules were visualized using TEM after rotary shadowing with tungsten (magnification 170,000×; magnification bar = 30 nm). Integrin molecules are found either in the inactive or active state with their transmembrane stalks close and open, respectively. (C) Statistical analyses of the effects of αIIb–TM, anti-αIIb, and anti-αIIbmut in activating integrin αIIbβ3.
To demonstrate that anti-αIIb takes up a membrane-spanning orientation in phospholipid bilayers, we used phospholipid vesicles as the artificial membrane system and polarized attenuated total reflection infrared (ATR-IR) spectroscopy to confirm that anti-αIIb spans the membrane. ATR-IR spectroscopy exploits the fact that in an ordered sample, a given bond will absorb infrared radiation differentially depending on the polarization of the light and the angle at which the bond is oriented relative to the polarized light. If the secondary structure of a peptide is known, the dichroic ratio (RATR) of the amide-I absorbance when the incident light is polarized at 0° to the amide-I absorbance when the light is polarized at 90° can be used to calculate the angle between the helical axis of the peptide and the bilayer normal. We observed the position of the amide-I vibration at 1656 cm−1, indicating that anti-αIIb adopted primarily an α-helical conformation in the POPC/POPG bilayers, consistent with the previously published CD data (Fig. 5). The RATR of 4.7 corresponds to an angle ~20°, indicating that the peptide was inserted nearly perpendicular to the plane of the bilayer. The ~20° angle of insertion is not surprising considering the 33 amino acid length of the peptide, which, if completely helical, would form an α-helix of ~49Å. This is somewhat longer than the hydrophobic thickness of the POPC/POPG bilayer core and could result in peptide tilting to compensate for the hydrophobic mismatch. The representative spectra of the methylene stretching region of the polarized ATR-IR spectra of POPC/POPG bilayers in the absence and in the presence of anti-αIIb are shown in Figure 5C. The dichroic ratios (RATR) values calculated from this region of the spectra are indicative of bilayer ordering, as the major signal arises from the symmetric (~2850cm−1) and asymmetric (~2920cm−1) stretching of the -CH2- groups in the lipid acyl chains (27, 28). The comparison between the PCPG/PCPG bilayers with and without anti-αIIb showed only a small difference in the dichroic ratios, indicating that the peptide is not significantly disrupting the internal organization of the bilayer or the ability of the bilayers to stack into multilayers on the ZnSe crystal (27).
FIGURE 5.
ATR-IR (A) Schematic representation of ATR-IR experiments. Helix angle with respect to the bilayer normal is calculated using the ratio of the AmideI absorbance band using 0° and 90° polarized incident radiation, RATR; (B) ATR-IR spectra collected at 0° (//) and 90° (⊥) polarized incident radiation. (C) The methylene stretching region of the polarized ATR-IR spectra of POPC/POPG bilayers in the absence (green) and in the presence (red) of anti-αIIb. The average RATR for pure POPC/POPG lipid bilayers was ~1.45 while the RATR for bilayers containing anti-αIIb was ~1.33 (RATR values are averages of 2–3 independent samples).
Because amphiphilic peptides can cause cell lysis (29), we examined the ability of anti-αIIb to permeabilize phospholipid membranes. Anti-αIIb did not induce significant leakage of a fluorescent metal complex, Tb(DPA)3, from lipid vesicles at lipid/peptide ratio up to 10:1 suggesting that the anti-αIIb peptide does not significantly perturb the cell membrane (Fig. 6). Further toxicity tests for these compounds were conducted using a lactate dehydrogenase (LDH) release assay. Platelets release LDH when their plasma membrane is perturbed. In the presence of 10 μM of anti-αIIb, the level of LDH released by platelets is not significantly higher than the negative control. Moreover, anti--αIIb did not lyse human erythrocyte membranes at the concentrations up to 10 μM.
FIGURE 6.
Toxicity assays. (A) Dye release induced by anti-αIIb. POPC:POPG (7:3) vesicles containing trapped Tb and DPA were exposed to anti-αIIb at various peptide/lipid ratios or to the known pore-forming peptide melittin. Peptides were incubated with the loaded vesicles for 10 minutes before fluorescence was measured. Release was quantified using intensity before addition as a baseline (F0) and fluorescence after the vesicles had been completely disrupted with detergent as a final maximum fluorescence (FM). (B) Hemolysis induced by the CHAMP peptides. Human erythrocyte hemolysis induced by increasing concentrations of anti-αIIb (circle), αIIb-TM (square), and anti-αIIbmut (diamonds) in 10 mM Tris buffer (pH= 7.0), 1mg/mL BSA.
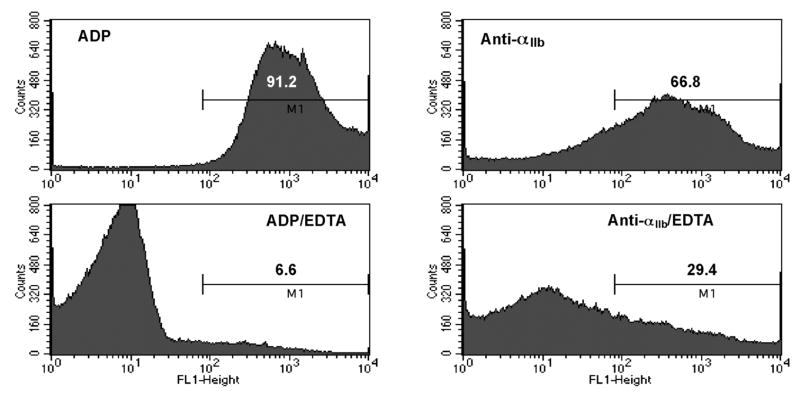
Agonist-stimulated platelets undergo rapid αIIbβ3-dependent aggregation in response to agonists such as ADP when the plasma protein fibrinogen binds to the activated conformation of αIIbβ3 (30). To directly demonstrate whether anti-αIIb induces the active conformation of αIIbβ3 in intact platelets, we used fluorescence-activated cell sorting (FACS) to measure FITC-labeled fibrinogen binding to platelets. The difference in agonist-stimulated fibrinogen binding in presence and absence of the calcium chelator EDTA indicated the amount of fibrinogen specifically bound to αIIbβ3. As shown in Figure 7, 2.0 μM anti-αIIb induced ≈ 50% as much specific FITC-fibrinogen binding to platelets as did platelet stimulation by 20 μM ADP. By contrast, anti-αIIbmut showed little ability to induce fibrinogen binding to platelets (data not shown). Thus, these data indicate that although not as potent a stimulus at ADP, anti-αIIb does directly enable αIIbβ3 to bind fibrinogen.
FIGURE 7.
Measurement of anti-αIIb-induced binding of FITC-fibrinogen to gel-filtered human platelets by flow cytometry. Fibrinogen binding stimulated by (Top Left) 20 μM ADP, (Top Right) 2 μM anti- αIIb; (Bottom Left) 20 μM ADP in the presence of 5 mM EDTA, and (Bottom Right) 2 μM anti-αIIb in the presence of 5 mM EDTA. The numbers above M1 represent the percent of analyzed platelets present in the gate.
In summary, these results validate the CHAMP method as a successful general chemical biology approach to provide molecular probes with high affinity and specificity for membrane-embedded segments of proteins. In particular, the CHAMP peptide, anti-αIIb, provides a specific tool for addressing the role of TM domain association in regulating the function of the integrin αIIbβ3. By blocking the site on the αIIb TM helix that engages the β3 helix, anti-αIIb activates the integrin, providing strong support for the hypothesis that separation of the helices is required for activation (10). CHAMP peptides provide a route to molecules that bind TM regions of their targets, expanding the range of conventional antibody-based methods that can only be applied to water-soluble regions of proteins. Given the current interests involving studying the lateral TM helix associations in membrane protein folding, assembly, and signal transduction (31), CHAMP peptides may provide much-needed reagents for probing these processes. Last, although hurdles (such as poor solubility in aqueous solution) associated with the physical properties of the peptides need to be overcome, it is encouraging that engineered peptides from TM helices have shown promises in animal models (32, 33). The CHAMP peptides can serve as lead sequences for the development of more drug-like, small-molecule peptidomimetic inhibitors of membrane protein-protein interactions, which might ultimately find applications as clinical diagnostics or therapeutics.
Acknowledgments
This work was supported by NIH grants GM60610, GM54616, HL40387, and HL81012. H.Y. thanks financial supports from the University of Colorado, the Association for Research of Childhood Cancer, and the Sidney Kimmel Foundation for Cancer Research (SKF-08-101).
ABBREVIATIONS
- ADP
adenosine diphosphate
- ATR-IR
attenuated total reflection infrared
- BSA
bovine serum albumin
- CHAMP
computed helical anti-membranef protein
- DMF
Dimethylformamide
- DMSO
dimethyl sulfoxide
- HATU
2-(1H-7-azabenzotriazol-1-yl)-1,1,3,3-tetramethyl uronium hexafluorophosphate methanaminium
- EDTA
ethylenediaminetetraacetic acid
- DPA
dipicolinic acid
- FACS
fluorescence activated cell sorting
- FITC
fluorescein isothiocyanate
- LDH
lactate dehydrogenase
- NMP
N-Methyl-Pyrrolidine
- POPC
1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine
- POPG
1-palmitoyl-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)]
- RBC
human erythrocytes
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TEM
transmission electron microscopy
- TFA
trifluoroacetic acid
- TFE
2,2,2-trifluoroethanol
- TM
transmembrane
References
- 1.Yin H. Exogenous Agents that Target Transmembrane Domains of Proteins. Angew Chem Int Ed. 2008;47:2744–2752. doi: 10.1002/anie.200704780. [DOI] [PubMed] [Google Scholar]
- 2.Kang SG, Saven JG. Computational protein design: structure, function and combinatorial diversity. Curr Opin Chem Biol. 2007;11:329–334. doi: 10.1016/j.cbpa.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Kortemme T, Baker D. Computational design of protein-protein interactions. Curr Opin Chem Biol. 2004;8:91–97. doi: 10.1016/j.cbpa.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Shifman JM, Mayo SL. Exploring the origins of binding specificity through the computational redesign of calmodulin. Proc Natl Acad Sci U S A. 2003;100:13274–13279. doi: 10.1073/pnas.2234277100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reina J, Lacroix E, Hobson SD, Fernandez-Ballester G, Rybin V, Schwab MS, Serrano L, Gonzalez C. Computer-aided design of a PDZ domain to recognize new target sequences. Nat Struct Biol. 2002;9:621–627. doi: 10.1038/nsb815. [DOI] [PubMed] [Google Scholar]
- 6.Ogihara NL, Ghirlanda G, Bryson JW, Gingery M, DeGrado WF, Eisenberg D. Design of three-dimensional domain-swapped dimers and fibrous oligomers. Proc Natl Acad Sci U S A. 2001;98:1404–1409. doi: 10.1073/pnas.98.4.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yin H, Slusky JS, Berger BW, Walters RS, Vilaire G, Litvinov RI, Lear JD, Caputo GA, Bennett JS, DeGrado WF. Computational design of peptides that target transmembrane helices. Science. 2007;315:1817–1822. doi: 10.1126/science.1136782. [DOI] [PubMed] [Google Scholar]
- 8.Partridge AW, Liu SC, Kim S, Bowie JU, Ginsberg MH. Transmembrane domain helix packing stabilizes integrin αIIbβ3 in the low affinity state. J Biol Chem. 2005;280:7294–7300. doi: 10.1074/jbc.M412701200. [DOI] [PubMed] [Google Scholar]
- 9.Luo BH, Springer TA, Takagi J. A specific interface between integrin transmembrane helices and affinity for ligand. Plos Biol. 2004;2:776–786. doi: 10.1371/journal.pbio.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W, Metcalf DG, Gorelik R, Li RH, Mitra N, Nanda V, Law PB, Lear JD, DeGrado WF, Bennett JS. A push-pull mechanism for regulating integrin function. Proc Natl Acad Sci U S A. 2005;102:1424–1429. doi: 10.1073/pnas.0409334102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID. Structural basis of integrin activation by talin. Cell. 2007;128:171–182. doi: 10.1016/j.cell.2006.10.048. [DOI] [PubMed] [Google Scholar]
- 12.http://www.anaspec.com.
- 13.Weisel JW, Nagaswami C, Vilaire G, Bennett JS. Examination of the platelet membrane glycoprotein IIb-IIIa complex and its interaction with fibrinogen and other ligands by electron microscopy. J Biol Chem. 1992;267:16637–16643. [PubMed] [Google Scholar]
- 14.Yin H, Lee GI, Park HS, Payne GA, Rodriguez JM, Sebti SM, Hamilton AD. Terphenyl-based helical mimetics that disrupt the p53/HDM2 interaction. Angew Chem Int Ed. 2005;44:2704–2707. doi: 10.1002/anie.200462316. [DOI] [PubMed] [Google Scholar]
- 15.Caputo GA, London E. Cumulative effects of amino acid substitutions and hydrophobic mismatch upon the transmembrane stability and conformation of hydrophobic alpha-helices. Biochemistry. 2003;42:3275–3285. doi: 10.1021/bi026697d. [DOI] [PubMed] [Google Scholar]
- 16.Arkin IT, MacKenzie KR, Brunger AT. Site-directed dichroism as a method for obtaining rotational and orientational constraints for oriented polymers. J Am Chem Soc. 1997;119:8973–8980. [Google Scholar]
- 17.Heuck AP, Tweten RK, Johnson AE. Assembly and topography of the prepore complex in cholesterol-dependent cytolysins. J Biol Chem. 2003;278:31218–31225. doi: 10.1074/jbc.M303151200. [DOI] [PubMed] [Google Scholar]
- 18.Liu D, DeGrado WF. De novo design, synthesis, and characterization of antimicrobial beta-peptides. J Am Chem Soc. 2001;123:7553–7559. doi: 10.1021/ja0107475. [DOI] [PubMed] [Google Scholar]
- 19.Litvinov RI, Nagaswami C, Vilaire G, Shuman H, Bennett JS, Weisel JW. Functional and structural correlations of individual αIIbβ3 molecules. Blood. 2004;104:3979–3985. doi: 10.1182/blood-2004-04-1411. [DOI] [PubMed] [Google Scholar]
- 20.Weisel JW, Stauffacher CV, Bullitt E, Cohen C. A model for fibrinogen: domains and sequence. Science. 1985;230:1388–1391. doi: 10.1126/science.4071058. [DOI] [PubMed] [Google Scholar]
- 21.Basani RB, D’Andrea G, Mitra N, Vilaire G, Richberg M, Kowalska MA, Bennett JS, Poncz M. RGD-containing peptides inhibit fibrinogen binding to platelet αIIbβ3 by inducing an allosteric change in the amino-terminal portion of αIIb. J Biol Chem. 2001;276:13975–13981. doi: 10.1074/jbc.M011511200. [DOI] [PubMed] [Google Scholar]
- 22.Fisher LE, Engelman DM. High-yield synthesis and purification of an α-helical transmembrane domain. Anal Biochem. 2001;293:102–108. doi: 10.1006/abio.2001.5122. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Jeon TJ, Oberai A, Yang D, Schmidt JJ, Bowie JU. Transmembrane glycine zippers: physiological and pathological roles in membrane proteins. Proc Natl Acad Sci U S A. 2005;102:14278–14283. doi: 10.1073/pnas.0501234102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yin H, Litvinov RI, Vilaire G, Zhu H, Li W, Caputo GA, Moore DT, Lear JD, Weisel JW, Degrado WF, Bennett JS. Activation of platelet αIIbβ3 by an exogenous peptide corresponding to the transmembrane domain of αIIb. J Biol Chem. 2006;281:36732–36741. doi: 10.1074/jbc.M605877200. [DOI] [PubMed] [Google Scholar]
- 25.DeGrado WF, Gratkowski H, Lear JD. How do helix-helix interactions help determine the folds of membrane proteins? Perspectives from the study of homo-oligomeric helical bundles. Protein Sci. 2003;12:647–665. doi: 10.1110/ps.0236503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim M, Carman CV, Springer TA. Bidirectional transmembrane signaling by cytoplasmic domain separation in integrins. Science. 2003;301:1720–1725. doi: 10.1126/science.1084174. [DOI] [PubMed] [Google Scholar]
- 27.Torrecillas A, Martínez-Senac MM, Goormaghtigh E, de Godos A, Corbalán-García S, Gómez-Fernández JC. Modulation of the membrane orientation and secondary structure of the C-terminal domains of Bak and Bcl-2 by lipids. Biochemistry. 2005;44:10796–10809. doi: 10.1021/bi0503192. [DOI] [PubMed] [Google Scholar]
- 28.Goormaghtigh E, Raussens V, Ruysschaert JM. Attenuated total reflection infrared spectroscopy of proteins and lipids in biological membranes. Biochim Biophys Acta. 1999;1422:105–185. doi: 10.1016/s0304-4157(99)00004-0. [DOI] [PubMed] [Google Scholar]
- 29.Bechinger B. Structure and functions of channel-forming peptides: Magainins, cecropins, melittin and alamethicin. J Membr Biol. 1997;156:197–211. doi: 10.1007/s002329900201. [DOI] [PubMed] [Google Scholar]
- 30.Bennett JS. Novel platelet inhibitors. Annu Rev Med. 2001;52:161–184. doi: 10.1146/annurev.med.52.1.161. [DOI] [PubMed] [Google Scholar]
- 31.Senes A, Engel DE, DeGrado WF. Folding of helical membrane proteins: the role of polar, GXXXG-like and proline motifs. Curr Opin Struct Biol. 2004;14:465–479. doi: 10.1016/j.sbi.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Manolios N, Collier S, Taylor J, Pollard J, Harrison LC, Bender V. T-cell antigen receptor transmembrane peptides modulate T-cell function and T cell-mediated disease. Nat Med. 1997;3:84–88. doi: 10.1038/nm0197-84. [DOI] [PubMed] [Google Scholar]
- 33.Gerber D, Quintana FJ, Bloch I, Cohen IR, Shai Y. D-enantiomer peptide of the TCR alpha transmembrane domain inhibits T-cell activation in vitro and in vivo. FASEB J. 2005;19:1190–1192. doi: 10.1096/fj.04-3498fje. [DOI] [PubMed] [Google Scholar]