Abstract
Purpose
The goal of this study was to determine the impact of age, gender and race on the prevalence and severity of hearing loss in elder adults, aged 72–96 years, after accounting for income, education, smoking, and clinical and subclinical cardiovascular disease.
Methods
Air-conduction thresholds for standard and extended high-frequency puretones were obtained from a cohort of 548 (out of 717) elderly adults (ages 72–96 years) who were recruited during the Year 11 clinical visit (1999–2000) of the Cardiovascular Health Study (CHS) at the Pittsburgh, Pennsylvania site. Participant smoking, income, education, and cardiovascular disease histories were obtained from the CHS database and were included as factors.
Results
Hearing loss was more common and more severe for the participants in their 80s than those in their 70s, the men more than the women, and the White participants more than the Black participants. The inclusion of education, income, smoking and cardiovascular disease (clinical and subclinical) histories as factors did not substantively impact the overall results.
Conclusion
Although the data reported in this paper were cross-sectional and a cohort phenomenon might have been operational, they suggested that hearing loss is more substantive in the eighth than the seventh decade of life, and that race and gender influence this decline in audition. Given the high prevalence in the aging population and the differences across groups there is a clear need to understand the nature and causes of hearing loss across various groups in order to improve prevention and develop appropriate interventions.
Hearing loss is very common in the general population. Approximately 16% of adults in the United States report some difficulty with hearing, and after arthritis and hypertension, hearing impairment is the third most commonly reported chronic condition in persons over 65 (National Center for Health Statistics, 1982; Pleis & Coles, 2003). By age 70 years, approximately 30% of the population perceives themselves as being hearing impaired, and by 80 years, 50% report being hearing impaired (Desai, Pratt, Lentzner, & Robinson, 2001). There also is indication that the prevalence of hearing impairment in persons 45–69 years of age is increasing, especially among men (Wallhagen, Strawbridge, Cohen, & Kaplan, 1997). Men have consistently reported more hearing problems than women regardless of race. Furthermore, White adults have reported hearing impairment more than Black adults (Desai et al., 2001; Pleis & Lethbridge-Çejku, 2006).
The prevalence of self-reported hearing impairment not only varies by race but also by ethnicity. For example, the 1999 National Health Interview Survey (Pleis & Coles, 2003) found that adults from Asian and African decent were less likely to experience hearing problems (7.8% and 7.4% respectively) than Whites or Native Americans (17.2 and 20.1, respectively). Whereas 15% of non-Hispanic White adults reported some form of hearing difficulty, 6% of non-Hispanic Black adults and 6% of Hispanic White adults reported hearing problems. A similar pattern of results was reported in the 2005 National Health Interview Survey although the rates of reported hearing impairment were slightly higher for all groups (Pleis & Lethbridge-Çejku, 2006).
Hearing Loss, Age and Gender
Studies in which hearing was tested directly have shown similar age and gender effects although the prevalence of hearing loss is consistently greater than that found in self-report studies (Agrawal, Platz & Niparko, 2008; Brainerd & Frankel, 1985; Clark, Sowers, Wallace, & Anderson, 1991; Gomez, Hwang, Sobotova, Stark, & May, 2001). In a cross-sectional study of 1662 adults (mean age of 73 years) drawn from the Framingham Heart Study (primarily White cohort), hearing loss was more common with age, and men exhibited more severe hearing loss than women (Moscicki, Elkins, Baum, & McNamara, 1985). With hearing loss defined as puretone average (M for 500, 1000 and 2000 Hz) >25 dB HL in the better ear, 32% of the men and 26.7% of the women had a hearing loss.
A population-based study of 3556 adults from the predominately White agricultural community of Beaver Dam, Wisconsin, (The Epidemiology of Hearing Study) found similar patterns of hearing loss for age and gender but the prevalence of hearing loss was greater for all groups than was found in the Framingham study, which was likely due to differing definitions of hearing loss (Cruickshanks, Wiley, et al., 1998). With hearing loss defined as the occurrence of a puretone average threshold of 500, 1000, 2000 and 4000 Hz in the worse ear of > 25 dB HL, 21% of the adults aged 48–59 years, 44% of the adults aged 60–69, 66% of the adults aged 70–79, and 90% of the adults aged 80–92 had a hearing loss (Cruickshanks, Wiley, et al., 1998). Moreover, hearing loss was nearly four times more prevalent in men than women. Extended high-frequency (9000–20,000 Hz) hearing thresholds reported for 3396 participants from this same pool of participants indicated a similar pattern with greater threshold elevation with increased age, and males having significantly poorer thresholds than females. However, the threshold differences between the men and women were observed for 9000 to 14,000 Hz tones but not the highest of the extended high-frequency tones. The inability to obtain thresholds in the extended high-frequencies became more common with age but more so for men. Similar age and gender differences for the extended high-frequencies also were observed by Matthews, Lee, Mills, and Dubno (1997) in a smaller sample of adults between the ages of 60 and 79 years.
The typical hearing loss configuration in adults is a bilateral high-frequency sensorineural loss with normal or near-normal hearing in the low frequencies (Cruickshanks, Wiley, et al., 1998; Morrell, Gordon-Salant, Pearson, Brant, & Fozard, 1996; Moscicki et al., 1985). Men tend to exhibit a more constant rate of decline in the mid- and high-frequency range across the lifespan while women have smaller decreases in hearing initially with an accelerated declination with age (Morrell et al., 1996). The inter-participant range of thresholds also is greater for men than women and increases with age for both sexes. Correspondingly, it should be noted that a small subset of elderly adults does not acquire a clinically significant loss of hearing. For example, in a cross sectional analysis of the hearing data from the Baltimore Longitudinal Study of Aging, approximately 10% of the octogenarian men and 25% percent of the women had thresholds 25 dB HL or better at 500 through 4000 Hz (Morrell et al., 1996). Similar results were reported by Cruickshanks, Wiley, et al.
The explanation for the differences in hearing sensitivity between men and women largely has been attributed to differences in occupational and recreational preferences and associated levels of noise exposure. There also may be some intrinsic factors that could account for the differences. Although the structures of the auditory system appear similar for males and females at birth, minor differences in otoacoustic emissions and the auditory brainstem response have been observed suggesting that other intrinsic factors such as hormonal and metabolic differences may influence hearing across the lifespan (McFadden, 1993; McFadden & Loehlin, 1995). For example, Guimaraes, Zhu, Cannon, Kim and Frisina (2004) observed in an aging mouse model that the male mice exhibited decline in otoacoustic emissions in middle age, and that the decline continued into old age, while the female mice did not show substantive decline in otoacoustic emissions until after menopause when the mice were considered old. These results argue that hormones, like estrogen, may play an otoprotective role in the cochlea. There are a limited number of studies that support this contention although the cochlear mechanism is not clear. Reron, Reron, Modrzejew, Strek and Trajnar-Podlesney (2002) found that three months after hysterectomy with adrexia, women tended to show a slight elevation in hearing thresholds. Kilicdag et al. (2004) found that postmenopausal women receiving estrogen therapy may have a slowing of age-related hearing loss. Kim, Kang, Chae, and Kim (2002) also found a relationship between serum estradiol levels and hearing in postmenopausal women receiving hormone replacement therapy. They observed that lower levels of serum estradiol were associated with reduced hearing sensitivity. Other biochemical factors may play a role as well. Houston et al. (1999) observed increased hearing loss in healthy elderly women who had low serum vitamin B-12 and red cell folate levels. So too, environmental, social and healthcare issues may contribute to gender differences in hearing loss (Lee, Matthews, Mills, Dubno, & Adkins, 1998).
Hearing Loss and Race
As previously suggested, most of the studies looking at changes in hearing sensitivity with age have sampled primarily White populations, did not separate racial groups, or relied on self-report to infer changes in hearing status. However, in a recent study by from the 1999–2004 National Health and Nutrition Examination Survey hearing thresholds were obtained from 5742 participants, aged 20–69 years, with the data examined by age-group, gender, race (White, Black, Mexican American) and hearing loss configuration (Agrawal et al., 2008). The study showed differences in hearing loss prevalence by race and gender for all age groups. For the 20 to 29 year age-group, the prevalence of hearing loss was higher for the White and Mexican American men than the Black men (15%, 15% and 6.8% respectively), whereas the prevalence for the women was relatively low for all racial groups. Differences by gender and race were more pronounced for the participants aged 40 to 49 years and increased for the two older age groups (50–59 and 60–69 years). In general, the men had higher rates of hearing loss than women, and the White participants were more likely to have a hearing loss than the Mexican American and Black participants. In addition, other risk factors such as smoking, noise exposure, and cardiovascular risk increased the likelihood of hearing loss, especially in White men. The results from this study also suggested that these risk factors not only increased the likelihood hearing loss but also lowered the age of onset.
The assessment of hearing status across the major racial groups has been particularly limited in the elderly population. In a study of 100 Black adults aged 65 to 95; Marcus-Bernstein (1986) found that older Black adults exhibited high-frequency hearing loss similar in character to that observed with other older adult populations. More recently, Pugh and Crandell (2002) compared the puretone thresholds of Black and White adults aged 60 to 89 and found similar results for both groups. They observed significantly better puretone thresholds for 8000 Hz signals for their Black participants than their White participants, but no differences were found between the two groups at the other audiometric frequencies tested.
A report from the Health, Aging and Body Composition Study on the prevalence of hearing loss by gender and Black and White races included a large number of older male and female participants and did find differences by race and gender (Helzner et al., 2005). The study accounted for the group differences by health and lifestyle factors. The women and Black groups had a lower prevalence of hearing loss than the men and White groups respectively, and after multivariable adjustment this study found that older age, White race, diabetes mellitus, cerebrovascular disease, smoking, occupational noise exposure, and ear surgery were associated with hearing loss. Race and gender-specific factors relating to the prevalence of hearing loss included high blood pressure and occupational noise exposure for White men, smoking and poorer cognitive status for Black women, and low total hip bone mineral density for Black men.
There are a number of reasons why differences might be observed across racial groups such as cultural attitudes towards noise exposure, and environmental, dietary and healthcare disparities. Intrinsic factors also may play a role as suggested by the Helzner et al. (2005). However, despite some potential risk factors such as higher rates of diabetes mellitus, hypertension, high-density lipoprotein cholesterol, congestive heart failure, and stroke (Arnold et al., 2005) the Black adult population in the United States tends to demonstrate better hearing than the White population.
A commonly debated intrinsic factor is the role of melanin. Melanin containing melanocytes are distributed throughout much of the cochlea, and in humans and many animals the density of melanin within the cochlea corresponds to general pigmentation as reflected in eye and skin color (Barrenas & Hellstrom, 1996; Keithley, Ryan, & Feldman, 1993). Although not well specified, some studies have suggested that melanin is involved in the structural, metabolic and vascular health of the cochlea (Barrenas, 1997; Barrenas & Axelsson, 1992). Melanin also has been found to accentuate some antioxidant activities in the cochlea and as such, likely plays an otoprotective role (Wu et al., 2001). For example, melanin can reduce the formation of superoxide radicals and lipid peroxides (Scalia et al., 1990; Sichel, Coraro, Scalia, Di Bilio, & Bonomo, 1991) that are generated by aminoglycosides and associated with ototoxicity (Priuska & Schacht, 1995; Sha & Schacht, 2000). Yet, melanin also is associated with increased ototoxicity because many toxins and pharmacologic agents bind with melanin, resulting in an accumulation of the chemicals in melanin-rich tissues and potentiation of their actions (Barr-Hamilton, Matheson, & Keay, 1992; Heimen, Klis, de Groot, & Smoorenburg, 1999; Wrześniok, Buszman, Karna, Nawrat, & Pałka, 2002). Although not clear, melanin likely plays a complex role in the long term health of the cochlea.
Most particularly, it has been suggested that melanin is otoprotective against excessive noise exposure although the mechanism has not been well established and there has been considerable debate on the issue (Conlee, Abdul-Baqi, McCandless, & Creel, 1986; Gratton & Wright, 1992; Kawaguchi, 1992). Some animal models have demonstrated a relationship between pigmentation and noise resistance but there is substantive variability observed within and across studies, which appears to be due to genetic variation, how general pigmentation is classified, and its relationship to the melanocytes within the cochlea (Barrenas, 1997; Bartels, Ito, Trune, & Nuttall, 2001). Studies observing a relationship between pigmentation and noise resistance reported that acoustic trauma was associated with hyperpigmentation of the stria vascularis, and migration of melanin to the marginal and basil cells in the cochlea of chinchillas and guinea pigs (Gratton & Wright, 1992; Kawaguchi, 1992). These animal models also tended to demonstrate a positive association between melanin density in the stria vascularis and protection against noise induced hearing loss (Conlee et al., 1986).
A number of human studies have suggested that darker skin and iris pigmentation is associated with resistance to noise-induced hearing loss. Ishii and Talbott (1998) found that even after accounting for years of occupational noise exposure; race/ethnicity had a substantive influence on the magnitude of noise-induced hearing loss. In a study of 39,006 soldiers followed in the U.S. Army hearing conservation program, White soldiers exhibited less hearing sensitivity than Black soldiers, and soldiers of other races, even after accounting for noise exposure and age (Henselman et al., 1995). The Black soldiers also demonstrated the best retention of hearing sensitivity across years of military service. In general, the literature suggests that racial differences may reflect differences in hardiness or resistance to the permanent tissue damage (i.e., spiral ligament and stria vascularis) that commonly is associated with the aging cochlea (Lang, Schulte, & Schmiedt, 2003; Schuknecht & Gacek, 1993; Spicer, Gratton, & Schulte, 1997).
Extrinsic Factors
There are a number of life style and environmental factors that have been linked to hearing loss in adults, and likely interact with the influences of age, race and gender. A large number of studies have linked cigarette smoking to hearing loss, particularly high-frequency hearing loss, and have found it additive to the effects of noise exposure (Cruickshanks, Klein et al, 1998; Uchida, Nakashimat, Ando, Niino, & Shimokata, 2005). However, cigarette smoking is not uniform across groups. The 2006 National Health Interview Survey indicated that in the United States men (23.9%) smoke substantively more than women (18%) (Centers for Disease Control and Prevention, 2007). Among race and ethnicity groups American Indian/Alaska Natives have the highest prevalence of smoking (32.4%) followed by non-Hispanic Blacks (23.0%), non-Hispanic Whites (21.9%), Hispanics (15.2%) and Asians (10.4%). Historically the rates of cigarette smoking have varied across these groups, so the impact on hearing likely varies by group as a function of smoking rates over time.
Although the literature is limited in this area, potential gender and race differences in environmental, occupational and recreational noise exposure could result in differences across groups as they age (Agrawal et al., 2008). So too could other modifiable influences such as diet, exercise and healthcare, all of which strongly relate to income and education (Daniel, 2007; Willott, Hnath Chisolm, & Lister, 2001). Furthermore, family income has been found to be predictive of hearing loss in both children and adults. In a national survey, adults classified as poor or nearly poor were more likely to report having hearing difficulties than those classified as not poor, and hearing loss was reported least by adults who had family incomes of $75,000 or greater (Pleis & Lethbridge-Çejku, 2006). Income also might be predictive of hearing loss severity. In a study of hearing loss in Hispanic subgroups in the United States, Lee, Gómez-Marín and Lee (1996) found that both the presence and severity of hearing loss was related to income and access to health insurance. Similarly, a study in the United Kingdom monitored the hearing thresholds of 1958 persons from childhood to middle-age and found that social status (as defined by type of paternal and current employment) was related to hearing loss and hearing loss severity (Ecob et al., 2008). At middle age, hearing loss increased with decreased social class regardless of whether childhood or adulthood social class was considered, suggesting that a person’s susceptibility to hearing loss could be determined in early childhood. Poorer hearing thresholds also were associated with positive histories of occupational noise exposure, smoking and alcohol consumption, clearly suggesting that the acquisition of hearing loss with age is dependent on multiple factors.
Purpose
The purpose of this study was to describe the auditory sensitivity of a group of elderly adults enrolled in the Cardiovascular Health Study (CHS) and to determine whether their audiometric and extended high-frequency puretone thresholds differed by age, gender, and race. The impact of education, smoking, income category, and cardiovascular disease histories (clinical and subclinical) also were investigated as factors. Unlike many previous studies ear- and frequency-specific analyses were conducted in an effort to capture differences in audiometric configuration. In addition, the hearing status of the groups was compared by average thresholds and to previous population-based studies.
It was hypothesized that the participants would demonstrate primarily high-frequency hearing loss consistent with previous studies looking at the impact of aging on audition. It was believed that women would have better thresholds than the men, and Black participants would have better thresholds than White participants. It also was hypothesized that more years of education, higher income, and negative histories of smoking, and cardiovascular disease (clinical and subclinical) would be associated with greater preservation of hearing.
Methods
Participants
The participants in this study comprised a subset of adults from the CHS, which was a prospective population-based study of risk factors for and consequences of cardiovascular disease in older adults sponsored by the National Heart, Lung, and Blood Institute. The original cohort of 5201 participants was enrolled in 1989–1990 from Sacramento County, California; Washington County, Maryland; Forsyth County, North Carolina, and Allegheny County, Pennsylvania (Greater Pittsburgh Metropolitan Area). In 1992 and 1993 additional African American elders (n=685) were recruited to increase the racial diversity of the sample and to allow for the assessment of racial differences in cardiovascular disease. All potential participants were first identified with Health Care Financing Administration Medicare Enrollment Lists. These lists were randomized and stratified by age group (65–74, 75–84 and ≥ 85 years) and all households with age-eligible members were contacted. Age-eligible members indicating that they were planning to reside in their community for at least three years were invited to participate with the exception of persons who were wheelchair bound to their homes, unable to participate in the examinations at the clinical sites, or were undergoing active treatment for cancer. The recruitment rate was approximately 43% and the participants who agreed to participate tended to be better educated and healthier than those who declined participation. The elements and recruitment procedures of the CHS have been described previously (Fried et al., 1998; Kuller et al., 2006).
The participants of the current sub-study included 548 out of 717 elderly adults who were sufficiently ambulatory to be seen at the Pittsburgh, Pennsylvania clinical site of the CHS during Year 11 (1999–2000) and who had at least one ear canal that was clear upon otoscopic inspection at the time of testing. The cohort ranged in age from 72 to 96 and consisted of 227 men and 321 women. The racial composition was 122 Black (48 men; 74 women) and 426 White (179 men: 247 women) adults. Other characteristics of the participant groups are shown in Table 1.
Table 1.
Group Characteristics
| CHARACTERISTIC | GROUP | |||||||
|---|---|---|---|---|---|---|---|---|
| Females | Males | |||||||
| Black | White | Black | White | |||||
| <80 Years | ≥80 Years | <80 Years | ≥80 Years | <80 Years | ≥80 Years | <80 Years | ≥80 Years | |
| Number of Participants | 56 | 18 | 125 | 122 | 33 | 15 | 80 | 100 |
| Mean Age (1 SD) | 75.9 (2.22) | 83.2 (3.17) | 77.4 (1.52) | 83.4 (2.88) | 75.8 (2.66) | 83.5 (2.71) | 77.4 (1.65) | 83.9 (3.51) |
| Mean Years of Education (1 SD) | 12.6 (3.06) | 12.0 (2.74) | 14.1 (3.06) | 13.7 (2.79) | 12.0 (3.45) | 14.1 (3.60) | 15.3 (3.16) | 14.8 (3.31) |
| Income Category | ||||||||
| Under $5000 | 3.6% | 0.0% | 0.9% | 3.6% | 3.3% | 0.0% | 1.4% | 0.0% |
| $5000–$7,999 | 25.5% | 50.0% | 2.7% | 5.4% | 10.0% | 15.4% | 1.4% | 1.0% |
| $8,000–$11,999 | 16.4% | 12.5% | 9.1% | 9.8% | 26.7% | 7.7% | 1.4% | 4.1% |
| $12,000–$15,999 | 21.8% | 12.5% | 13.6% | 22.3% | 26.7% | 23.1% | 8.2% | 12.4% |
| $16,000–$15,000 | 14.5% | 18.8% | 13.6% | 16.1% | 16.7% | 7.7% | 19.2% | 14.4% |
| $25,000–$34,999 | 10.9% | 6.3% | 14.5% | 13.4% | 10.0% | 0.0% | 11.0% | 10.3% |
| $35,000–$49,999 | 1.8% | 0.0% | 10.9% | 8.0% | 0.0% | 15.4% | 15.1% | 15.5% |
| Over $50,000 | 5.5% | 0.0% | 34.5% | 21.4% | 6.7% | 30.8% | 42.5% | 42.3% |
| History of CVD1 | ||||||||
| No CVD | 40.1% | 11.1% | 48.0% | 34.9% | 33.3% | 25.0% | 39.2% | 25.5% |
| Subclinical CVD | 33.3% | 38.9% | 38.6% | 45.2% | 48.5% | 43.8% | 38.0% | 37.3% |
| Clinical CVD | 26.3% | 50.0% | 13.4% | 19.8% | 18.2% | 31.3% | 22.8% | 37.3% |
| History of Smoking | 56.1% | 50.0% | 59.8% | 53.2% | 75.8% | 50.0% | 58.2% | 63.7% |
| Self-reported Hearing Problem2 | 17.9% | 17.6% | 18.4% | 27.9% | 18.2% | 40.0% | 24.1% | 48.0% |
| Reported Using a Hearing Aid | 1.8% | 5.6% | 12.4% | 25.6% | 15% | 26.6% | 15.4% | 21.0% |
Note. Clinical and Subclinical Cardiovascular Disease (CVD) as defined by Arnold et al. (2005) and Kuller et al. (2006).
Self-reported a current hearing problem per Gates et al. (2003).
The <80 years group included the 72–79 years age range, while the ≥80 years group included the 80–96 years age range.
Procedures
Otoscopy was completed to ensure that the participants’ ear canals were clear of debris and not impacted with cerumen. Five participants had one ear that failed otoscopic inspection and one participant was excluded from the study because both ears failed inspection. For all of the ears that passed the otoscopic inspection, thresholds for puretone air-conducted signals were obtained individually using standard audiometric frequencies (250, 500, 1000250, 500, 2000, 4000 and 8000 Hz) and the ANSI manual puretone threshold procedure (ANSI, 1978). Threshold was considered the lowest hearing level at which responses occurred for at least half of a series of ascending trials with a minimum of two responses out of three presentations at a single level. Thresholds were similarly obtained with four extended high-frequency signals (10,000, 12,000, 14,000 and 16,000 Hz) in the better ear based on the standard audiometric test results. If the puretone thresholds at the standard audiometric frequencies were similar across ears, then the ear preferred by the participant was chosen for the extended high-frequency testing. Sensitivity to extended high-frequency signals was examined to allow more direct comparison to previous studies on aging, and because of the pronounced effects of aging at the basal end of the cochlea (Cruickshanks, Wiley, et al., 1998; Matthews et al., 1997). Extended high-frequency hearing loss also has been associated with noise exposure, ototoxic medications, and vascular disease (Bohme, 1987; Fausti, Erickson, Frey, Rappaport, & Schechter, 1981; Fausti et al., 1999). Moreover, the impact of noise exposure on extended high-frequency thresholds, and the rate at which these thresholds increase with age have been found to differ by gender in elder adults (Lee, Matthews, Dubno, & Mills, 2005). Although sensorineural hearing loss is most commonly associated with aging, as well as hearing differences betweens men and women, it should be emphasized that in the current study thresholds only were obtained with air-conducted puretone signals due to time limitations. Bone-conduction, admittance and otoacoustic emission testing were not completed, so the results represent the combined effects of any conductive and sensorineural components.
All of the testing was completed in a 3′ × 3′ single-walled telephone-booth-style sound booth, which met ANSI ambient noise standards for testing puretones under earphones (ANSI, 1991). The standard audiometric tones were presented with a portable audiometer (Maico, MA-19 and TDH-39 earphones with MX-41/AR cushions). The extended high-frequency tones were presented from a Monitor Instruments audiometer (model 20-P with HV/1A-Plus earphones). Both audiometers were calibrated to appropriate ANSI standards (ANSI, 1989, 1996). For descriptive purposes participants also were asked if they currently had a hearing problem, a question that has been found to be more sensitive to hearing loss in older adults than the Hearing Handicap Inventory for the Elderly – Screening questionnaire (Gates, Murphy, Rees, & Fraher, 2003; Weinstein, 1989). Nondahl et al. (1997) similarly found that a global question about the presence of a hearing loss was sensitive to hearing loss in older adults. The participants also reported whether they wore hearing aids and in what ear if they only wore one hearing aid.
Occupation, income, education, smoking and medication histories were obtained from the CHS database. These data were obtained from the participants through interview as part of their longitudinal participation in the CHS. Household annual income and education (as reflected in years of schooling) were queried at entry into the study. The participants had not been questioned about their history of noise exposure and the occupational information for the Black participants was limited due to later entry into the larger CHS. Baseline history of smoking (ever smoked), treatment for cancer, use of aspirin and aspirin-containing medications, and the use of loop diuretics were extracted from the database. Information about exposure to other potential ototoxic medications was not available. Composite predictors of cardiovascular disease (CVD) at entry into the study also were considered and included clinical CVD, subclinical CVD and no CVD. Clinical CVD included a history of myocardial infarction, angina pectoris, use of nitroglycerin, atrial fibrillation, pacemaker, coronary artery bypass or angioplasty, intermittent claudication, congestive heart failure, stroke, transient ischemic attack or carotid endarterectomy. Subclinical CVD was defined in the absence of clinical disease by any one of the following: (1) major abnormalities in electrocardiographic findings based on the Minnesota Code (Blackburn, Keys, Simonson, Rautharaju, & Punsar, 1960), (2) a ratio of ankle to arm systolic blood pressure of 0.9 or less, (3) positive finds on the Rose Questionnaire (Rose, McCartney, & Reid, 1977) without clinical history of angina or intermittent claudication, (4) stenosis of the internal carotid artery (based on ultrasonographic findings) greater than 25%, or intimal medial thickness of the internal or common carotid artery above the 80th percentile for the overall CHS distribution, (5) abnormalities in echocardiographic findings (6) abnormal wall motion, or (7) low ejection fraction. The two composite variables have been validated and reported for the entire CHS population in previous publications (Arnold et al., 2005; Kuller et al., 2006; Kuller et al., 1995). Participants were considered to have no CVD if they had negative histories of clinical and subclinical CVD.
Statistical Analysis
For descriptive analysis, the participants were grouped by age (72–79 years old vs. 80–96 years old), gender (male vs. female), and race (Black vs. White). The group formed by the 72–79 year old participants are here after referred to as the <80 years old group, while those in the 80–96 years age range are referred to as the ≥80 years old group. The number and percentage distributions of each categorical characteristic in groups were calculated.
Multiple linear regressions were applied to examine the linear relationships between hearing thresholds for the different standard audiometric frequencies and the selected participant characteristics. The dependent variables were the air-conduction puretone thresholds from the right and left ears obtained at the standard audiometric frequencies. Separate analyses were completed independently for each ear because previous studies had found ear differences in older adults, and because preliminary examination of the data suggested that the ears might show different patterns. The variables of age, race, gender, income, education, smoking, and CVD were selected to fit the model with the method of entry. Smoking was the only ototoxic agent entered because preliminary analyses showed no differences between the groups for medication recorded in the CHS database.
In addition, an analysis of variance was used to test for group differences in rates of hearing loss based on averaged hearing thresholds. It is common practice to characterize hearing status with the average of the thresholds obtained for the 500, 1000 and 2000 Hz test signals because these frequencies reflect hearing in the frequency range most critical for speech recognition. This average usually is referred to as the puretone average (PTA) or the speech frequency puretone average (Clark, 1984; Lichtenstein, Bess, & Logan, 1988). However, the PTA might be less sensitive to the effects of aging than averages that include higher frequency tones. So, along with the PTA, the four-frequency average used by Cruickshanks, Wiley, et al. (1998) (PTA-4; M of 500, 1000, 2000 and 4000 Hz) and a high-frequency average (HFA; M of 2000, 4000 and 8000 Hz) were calculated for each participant per ear and tested across groups. For descriptive purposes, Spearman correlations were between the threshold averages and self-reported hearing problems and using a hearing aid also were calculated.
Group differences for the extended high-frequency thresholds also were assessed with an analysis of variance. A Bonferonni correction for multiple comparisons was applied to further tests of the groups for both the threshold averages and extended high-frequency tests. The significance tests were two-sided at the 0.05 level.
Results
Puretone Thresholds for Standard Audiometric Frequencies
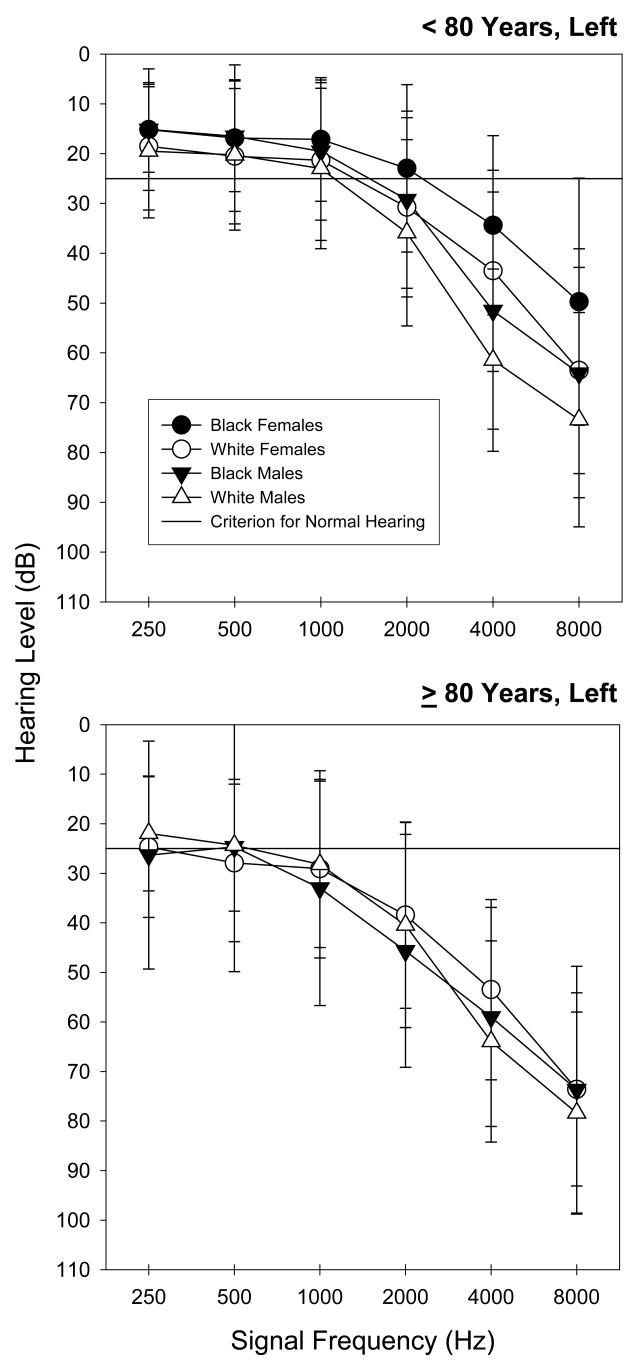
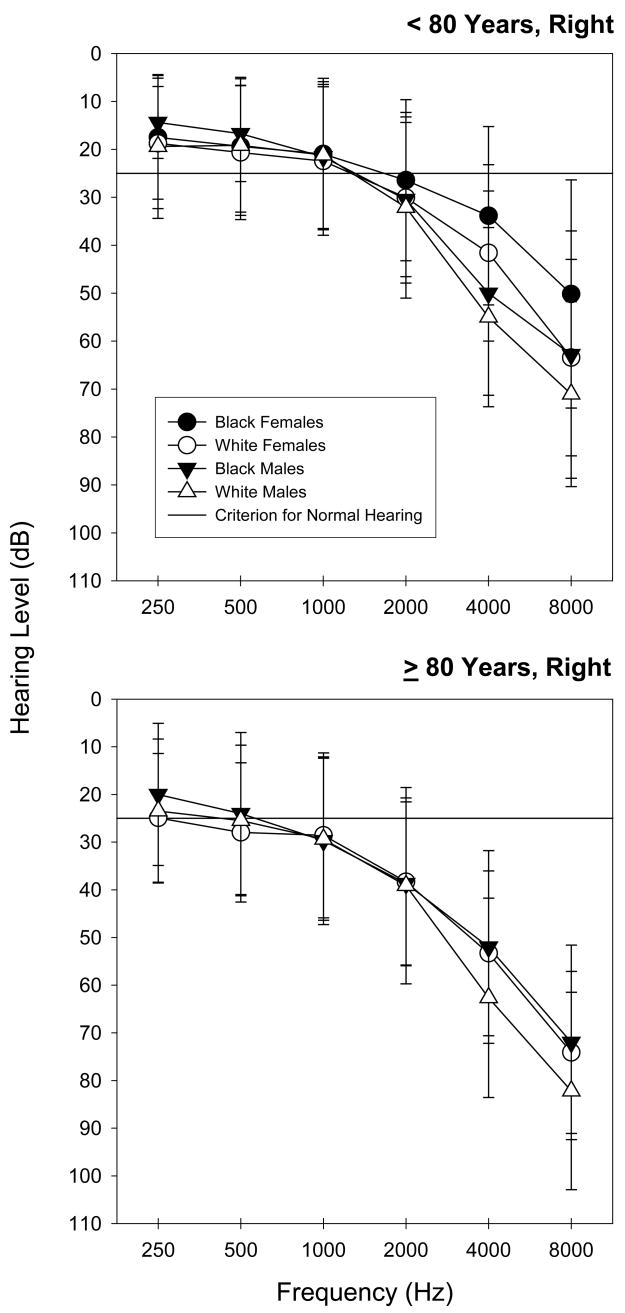
All but three right and two left ear canals were sufficiently clear for hearing testing and as indicated previously only one participant was excluded due to impacted cerumen in both ears. The mean hearing thresholds are illustrated in Figures 1 and 2. The data were grouped according to race and gender for each age-group. A predominately high-frequency audiometric configuration was observed with the thresholds becoming more elevated with age. The White male group had the most elevated thresholds, while the Black female group produced the least elevated thresholds.
Figure 1.
Mean left ear hearing thresholds for standard audiometric frequencies. The error bars reflect +/− 1 SD.
Figure 2.
Mean right ear hearing thresholds for standard audiometric frequencies. The error bars reflect +/− 1 SD.
The linear relationships between hearing loss (looking at each ear independently) under the different standard audiometric frequencies and the selected characteristic factors are illustrated in Tables 2 and 3. Age group was significantly associated with hearing loss for all signal frequencies in both ears (p<0.01). The effects of gender were evident for all three high frequencies (2000, 4000 and 8000 Hz) in left ear and for two high frequencies (4000 and 8000 Hz) in right ear (p<0.01). The variable of race also was significantly related with hearing loss for the frequencies (250, 500, 2000 and 8000 Hz) for the right ears and for all the standard audiometric frequencies for the left ears. Despite substantive income differences across groups, income was not associated with a significant effect on hearing. So too, smoking, education, and CVD histories were not significant factors at any test frequency for either ear.
Table 2.
Multiple linear analysis between air-conduction puretone thresholds for the right ear standard audiometric frequencies and participant characteristics factors
| COVARIATES | SIGNAL FREQUENCY |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz | 8000 Hz | |||||||
| B | Std. Error | B | Std. Error | B | Std. Error | B | Std. Error | B | Std. Error | B | Std. Error | |
| (Constant) | 32.599** | 3.869 | 32.288** | 4.109 | 32.018** | 4.572 | 38.589** | 5.148 | 56.949** | 5.326 | 72.240** | 5.693 |
| Age Group | 4.982** | 1.298 | 6.422** | 1.379 | 7.921** | 1.534 | 8.124** | 1.727 | 9.039** | 1.787 | 10.662** | 1.910 |
| Gender | −.047 | 1.301 | −1.150 | 1.382 | −.146 | 1.538 | 2.270 | 1.731 | 13.465** | 1.790 | 9.124** | 1.914 |
| Race | −5.344** | 1.662 | −4.970** | 1.766 | −2.674 | 1.965 | −4.226 | 2.212 | −9.417** | 2.288 | −10.901** | 2.446 |
| Years of Schoola | −.240 | .176 | .087 | .187 | −.205 | .208 | −.153 | .234 | −.208 | .242 | .012 | .258 |
| Ever Smokeda | .878 | 1.265 | 1.700 | 1.344 | .472 | 1.496 | .302 | 1.684 | .303 | 1.742 | 1.167 | 1.862 |
| Income Categorya | −.738 | .406 | −1.016 | .431 | −.691 | .480 | −.421 | .540 | −.399 | .559 | .137 | .597 |
| History of CVDa | −.198 | .825 | −.138 | .876 | −.418 | .975 | .056 | 1.097 | .148 | 1.135 | .257 | 1.214 |
Note. indicates that p <0.01.
indicates that p<0.05.
Years of School, Ever Smoked, Income Category, and History of CVD covariates were based on baseline data collected at participant entry into the CHS.
B is a non-standardized coefficient.
Table 3.
Multiple linear analysis between air-conduction puretone thresholds for the left ear standard audiometric frequencies and participant characteristics factors
| COVARIATES | SIGNAL FREQUENCY |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 250 Hz | 500 Hz | 1000 Hz | 2000 Hz | 4000 Hz | 8000 Hz | |||||||
| B | Std. Error | B | Std. Error | B | Std. Error | B | Std. Err | B | Std. Error | B | Std. Error | |
| (Constant) | 28.719** | 3.574 | 30.029** | 3.941 | 28.432** | 4.448 | 39.248** | 5.244 | 54.547** | 5.511 | 69.150** | 6.041 |
| Age Group | 4.992** | 1.194 | 5.913** | 1.316 | 7.172** | 1.485 | 7.211** | 1.748 | 6.244** | 1.840 | 7.833** | 2.018 |
| Gender | .414 | 1.194 | −1.074 | 1.317 | 1.449 | 1.486 | 4.457* | 1.749 | 14.950** | 1.842 | 8.395** | 2.019 |
| Race | −4.259** | 1.533 | −5.303** | 1.690 | −4.192* | 1.907 | −6.674** | 2.245 | −9.462** | 2.363 | −10.812** | 2.591 |
| Years of Schoola | −.286 | .161 | −.249 | .177 | −.212 | .200 | −.049 | .236 | −.199 | .248 | .003 | .272 |
| Ever Smokeda | −.362 | 1.160 | 1.506 | 1.279 | .371 | 1.444 | .199 | 1.699 | 1.253 | 1.789 | 2.811 | 1.961 |
| Income Categorya | −.268 | .376 | −.279 | .415 | −.169 | .468 | −.296 | .551 | .405 | .580 | .380 | .636 |
| History of CVDa | .500 | .759 | .828 | .837 | 1.122 | .944 | .364 | 1.111 | .020 | 1.170 | 2.261 | 1.283 |
Note. indicates that p <0.01.
indicates that p<0.05.
Years of School, Ever Smoked, Income Category, and History of CVD covariates were based on baseline data collected at participant entry into the CHS.
B is a non-standardized coefficient.
Hearing Loss and Average Thresholds
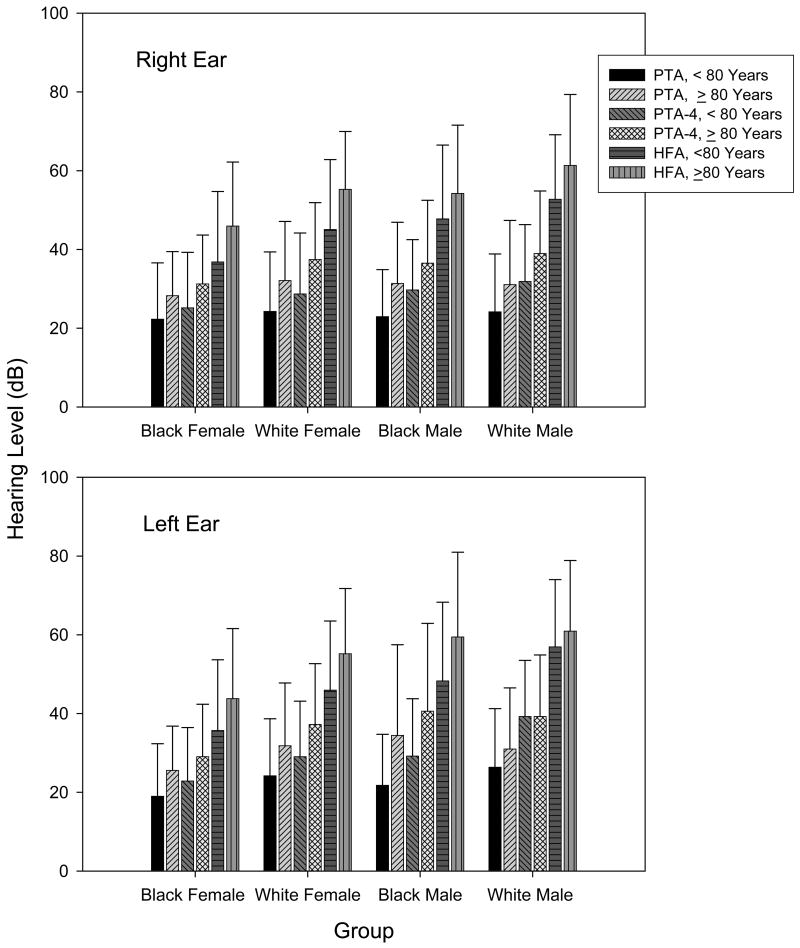
The three threshold averages for each group per ear are illustrated in Figure 3. Three-way ANOVAs (gender by race by age-group) for each ear indicated that PTA was different for age but not for race or gender [left ear, F(1, 540)=18.860, p= .006 to .000; right ear, F(1, 538)=4.427 to 17.752, p =.036 to .000]. However, age, race and gender differences were observed with the PTA-4 [left ear, F(1, 540)=7.622 to 14.356, p=.006 to .000;right ear, F(1, 536)=4.427 to 17.752, p=.036 to .000] and HFA [left ear, F(1, 540)=15.440 to 30.988, p=.000; right ear: F(1, 538)=14.448 to 19.463, p=.000] for both ears. No interactions were obtained.
Figure 3.
Mean puretone averages for each race and gender group per age-group. The error bars reflect +/− 1 SD.
With hearing loss defined as having any threshold greater than 25 dB HL, all of the participants who were 80 years or older had a hearing loss, while over 97% of the septuagenarians had a hearing loss. Those percentages dropped only 1 and 2% respectively if 8000 Hz was not considered. When using the more common definition of PTA ≥ 25 dB HL, 55% of the participants had a hearing loss when the worse ear was considered: 42% of the septuagenarians and 71% of the ≥ 80 years old group. When applying the definition of hearing loss used by Cruickshanks, Wiley, et al. (1998) (PTA-4 in the worse ear >25 dB HL) 64% of the < 80 years old group and 84% of the ≥ 80 years old group had a hearing loss. The prevalence of hearing loss segregated by age, gender and race is shown in Table 4. It should be highlighted that the relative pattern of hearing loss across the groups observed in the previous analyses was maintained except that the White men in the ≥ 80 years old group had a lower percentage of hearing loss than the Black men when using the PTA definition of hearing loss. Although, when the PTA-4 definition was used the older White men had a higher percentage of hearing loss than the older Black men.
Table 4.
Prevalence of hearing loss by hearing loss definition
| HEARING LOSS DEFINITION | GROUP | |||||||
|---|---|---|---|---|---|---|---|---|
| Females | Males | |||||||
| Black | White | Black | White | |||||
| <80 Years | ≥80 Years | <80 Years | ≥80 Years | <80 Years | ≥80 Years | <80 Years | ≥80 Years | |
| PTA >25 dB HL in the Worse Ear | 33.9% | 61.1% | 46.4% | 75.4% | 39.4% | 73.3% | 42.5% | 66.0% |
| PTA-4 >25 dB HL in the Worse Ear | 51.8% | 83.3% | 60.8% | 83.6% | 66.7% | 80.0% | 77.5% | 84.0% |
| Hearing Threshold>25 dB HL at any Audiometric Frequency | 92.9% | 100.0% | 97.6% | 100.0% | 97.0% | 100.0% | 98.8% | 100.0% |
Note. PTA is a three-frequency average of thresholds for the 500, 1000 and 2000 Hz signals.
The PTA-4 is a four-frequency average of thresholds for the 500, 1000, 2000 and 4000 Hz signals.
The <80 years group included the 72–79 years age range, while the ≥80 years group included the 80–96 years age range.
Subsequent binary Spearman correlations between the three different threshold averages and self-reported hearing problems were modest and similar across the three averages (r = .41 to .46, p<.01). The correlations between the threshold averages and the use of a hearing aid were similar (r = .40 to .51, p<.01), with the PTA and PTA-4 producing slightly higher correlations than the HFA. Controlling for age, gender or race did not affect the magnitude or the patterns of the correlations. These correlations are largely consistent with previous aging studies of hearing (Gates et al., 2003;Nondahl et al, 1997), and suggest that the participants in the current study were somewhat insensitive to their hearing losses or did not consider their losses to be problematic. The pattern of hearing aid use also was consistent with that previously reported by Popelka et al. (1998).
Extended High-Frequency Thresholds
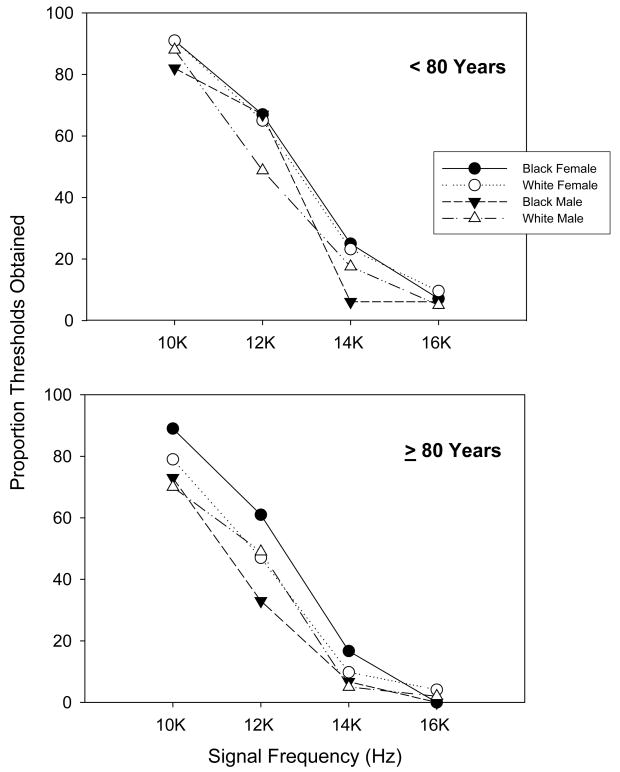
Few of the participants were able to hear the extended high-frequency tones, even when tested at the limits of the audiometer. Overall, 95% and 85% of the participants were unable to hear the 16,000 and 14,000 Hz signals, respectively. All of the groups had at least a 90% no-response rate for the 16,000 Hz signal (Figure 4). The female participants in the <80 year old group, particularly the Black females, were most likely to hear the 14,000 Hz signal (24%) while the ≥80 years old men were least likely to hear it (5%). Of the participants who could hear the 12,000 Hz signal (N=291), a univariate three-way ANOVA indicated a significant difference for race, F(1, 283)=5.026, p=.026, but not for age or gender, with the Black participants producing lower thresholds than the White participants. A total of 455 of the participants were able to produce thresholds for the 10,000 Hz signal and a univariate three-way ANOVA showed significant main effects for gender, F(1, 447)=5.382, p=.021, race, F(1, 447)=28.932, p=.000, and age, F(1, 447)=14.475, p=.000, with no interactions.
Figure 4.
Proportion of thresholds obtained for the extended high-frequency puretone signals per each group.
The general finding for the extended high-frequency tones was that the group differences observed for the higher standard audiometric frequencies extended into the 10,000 – 12,000 Hz range for which thresholds were measurable for most of the participants. Age, gender and race group differences were observed. The younger group had lower thresholds than the older group, the women had lower thresholds than the men, and the Black participants had lower thresholds than the White participants. Furthermore, the younger Black women produced the lowest mean thresholds while the older White men produced the highest (Table 5).
Table 5.
Average thresholds (dB SPL) for the 12,000 and 10,000 Hz test signals
| SIGNAL FREQUENCY | GROUP | |||||||
|---|---|---|---|---|---|---|---|---|
| Females | Males | |||||||
| Black | White | Black | White | |||||
| <80 Years | ≥80 Years | <80 Years | ≥80 Years | <80 Years | ≥80 Years | <80 Years | ≥80 Years | |
| 10,000 Hz N=455 | 77.65 (17.24) | 88.13 (8.54) | 91.10 (12.04) | 94.64 (9.13) | 85.00 (16.58) | 91.36 (13.98) | 93.21 (10.32) | 96.14 (11.01) |
| 12,000 Hz N=291 | 97.11 (9.98) | 99.55 (7.89) | 101.23 (8.57) | 103.60 (7.89) | 99.89 (11.12) | 101.00 (4.18) | 100.26 (8.35) | 104.08 (5.91) |
Note. The value in parentheses represents 1 SD. The thresholds were obtained from the better or preferred ear.
Thresholds for 14,000 and 16,000 Hz are not included because of high no-response rates.
The <80 years group included the 72–79 years age range, while the ≥80 years group included the 80–96 years age range.
Discussion
As indicated previously, the purpose of this study was to examine and describe hearing loss in a limited cohort of the CHS population. The impact of age, gender and race was examined, as were the influences of education, income, smoking, and CVD histories. The results of this study largely conformed to expectations in that increased age, White race, and being a man were associated with elevated puretone thresholds. Contrary to expectations; education, income, smoking and CVD histories did not appear to influence hearing thresholds in this group of elders, suggesting that characteristics intrinsic or strongly tied to the groups in this study disposed them or made them resistant to hearing loss.
Hearing loss was more common and more severe for the group in their eighth decade of life when compared to the group in their seventh decade regardless of gender and race. The loss of hearing sensitivity was found primarily for the higher frequency signals for all groups but involved more of the mid and low frequencies with the ≥80 participants than the <80 participants. The women were less likely to have a hearing loss and their hearing losses were less severe than those of the men, whereas the Black participants (particularly the female Black participants) were less likely to have a hearing loss and their hearing losses tended to be less severe than those of the White participants. These results are suggestive of a race effect but because the number of Black participants was substantively smaller than the White participants, these results should be viewed with caution. This sample-size disparity was most noticeable in the ≥80 age range, which potentially contributed to the differences between the racial groups and the lack of interactions. Given the issues with sample size and the cross sectional nature of this study, these results should be applied with care as a cohort effect might have been operational.
The lack of CVD influence on hearing was somewhat surprising given the rich capillary supply to the stria vascularis within the cochlea and its sensitivity to disruptions in arterial blood supply. It is possible that the lack of a CVD effect might relate to the composite nature of subclinical and clinical CVD as defined in this study. In previous studies not all characteristics consistent with or predictive of CVD have related to hearing loss, nor have they exerted uniform influences across groups. For example, Torre, Cruickshanks, Klein, Klein, and Nondahl (2005) assessed the cochlear function of 1501 adults from The Epidemiology of Hearing Loss Study using distortion product otoacoustic emission testing and found that their test results related to self-reported myocardial infarction in women but not in men. In addition, stroke, angina and hypertension were not related to cochlear dysfunction. In contrast, Gates et al. (1993) failed to find an influence of myocardial infarction on hearing in women although men with histories of coronary heart disease and heart attack were twice as likely to have low-frequency hearing losses as men with negative histories. In addition, they found that the risk of low-frequency hearing loss was doubled for women with histories of stroke, coronary heart disease, intermittent claudication, or overall CVD as compared to women with negative histories of CVD. The women with histories of stroke and overall CVD also were twice as likely to have high-frequency hearing losses as women without CVD. Moreover, there are a substantive number of studies that have not found any relationship between hearing loss and CVD or with CVD risk factors (Bunch, 1931; Karamitsos et al., 1996; Parving, Hein, Suadicani, Ostri, & Gyntelberg, 1993).
Environmental and Social Influences
In addition to hearing loss, the groups and subgroups in the current study were not uniform with regards to years of education and annual income. The Black women and the younger Black men tended to have fewer years of education and lower income levels than the White groups, and the older Black men. Of particular note is the extremely low income levels reported by the ≥80 year-old Black women, wherein 50% reported annual incomes of between $5,000 and $7,999. As previously mentioned, prior studies have related low income and educational levels to a higher prevalence of hearing loss and greater hearing loss severity (Agrawal et al., 2008; Ecob et al., 2008; Lee et al., 1996; Pleis & Lethbridge-Çejku, 2006; Schwarze, Notbohm, & Gärtner, 2005). The implication is that income and education influence access to healthcare and adequate nutrition, as well as increased exposure to adverse environmental and social conditions, which in turn impact hearing. However, a contradictory pattern was observed in the current study as the Black women had the least amount of hearing loss but were most economically and educationally disadvantaged. In contrast, the White men had the highest levels of education and income, but evidenced the highest rates of hearing loss and the most elevated hearing thresholds. This contradictory pattern is consistent with a race effect that is additive with the auditory benefits associated with being female, but it also could reflect a healthy survivor bias.
This account would assume that the Black female participants were healthier than the White male participants and/or the White male participants had income and education levels sufficient to preserve general health but insufficient to protect auditory function. General health status has been linked to the prevalence of reported hearing loss but this relationship is less evident when evaluated by race and gender. Health survey data from 1993 through 1997 compiled by the Centers for Disease Control and Prevention indicated that older adults in the United States who reported hearing loss were more likely to also report a higher number of unhealthy days and activity limitations than their peers who reported no hearing loss (Campbell, Crews, Moriarty, Zack, & Blackman, 1999). Yet, this surveillance study also found that Black and female respondents were less likely to report hearing loss than White and male respondents even though their reported health status was poorer. Income and education, which typically are lower in Black and female populations, might have played a role in these health status results in that annual income less than $15,000 and not graduating from high school also were associated with reduced health status. It should be highlighted, however, that accounting for income and educational differences between the groups in the current study did not change the outcome, nor did accounting for other potential health risk-factors such as smoking, and clinical and subclinical CVD.
An additional concern with the current study is that the history of occupational and recreational noise exposure was not accounted for in the analyses, and therefore, not eliminated as a contributor to the group differences. Very little historical data were available on the occupational, recreational and environmental noise exposure of the participants, especially the Black participants. The literature to date is limited with regard to racial and gender differences in occupational and recreational noise exposure patterns across the age-span or its relative impact on elderly adults who would have been working primarily in the l940–l980 time period. It should be noted, however, that Helzner et al. (2005) found that self-reported history of noise exposure only was predictive of hearing loss within their White male group and not their female or Black male groups. Additional studies are needed that follow various groups of men and women over their employment, recreational and environmental histories, and document noise exposure and changes in hearing sensitivity.
Thresholds for Extended High-frequency Signals
The extended high-frequency results were consistent with previous studies in older adults with regard to age and gender differences (Cruickshanks, Wiley, et al., 1998; Matthews et al., 1997). There were no group differences at the highest frequencies because few of the participants could hear the signals at the maximum output level of the audiometer. Although, thresholds could be obtained from most of the participants with the 10,000 and 12,000 Hz signals. Only race effects were observed for the 12,000 Hz signals but race, age and gender effects were observed for the 10,000 Hz signal with no interactions. The older group and the men producing more elevated thresholds, and the White participants produced greater threshold elevation than the Black participants, with the Black women producing the best thresholds of all of the groups. This pattern is consistent with that found for the standard audiometric frequencies.
It has been argued that extended high-frequency hearing is sensitive to cochlear insult (e.g., noise, ototoxic drugs, and vascular disease) and that extended high-frequency hearing loss might be useful in identifying ears that are vulnerable to insult and increased sensory hearing loss even into old age. The results of this study and that of Cruickshanks, Wiley, et al. (1998) and Matthews et al. (1997) showed that extended high-frequency thresholds cannot be obtained from many adults, and that the patterns found for the frequencies at which thresholds can be obtained are largely redundant with those observed when testing with the standard audiometric frequencies. Lee et al. (2005) found that the rate of threshold elevation for the extended high-frequency was not dependent on history of noise exposure in a group of older adults studied longitudinally. In addition, Schwarze et al. (2005) suggested that noise exposure accounts poorly for extended high-frequency hearing even in young and middle-aged adults. They found that the frequency range most commonly associated with noise-induced hearing loss (3000 to 6000 Hz, Cooper & Owens, 1976) was best explained by age, gender and smoking rather than noise exposure, despite a strong univariate relationship between hearing loss and noise exposure. Moreover, Schmuziger, Patscheke and Probst (2007) found that extended high-frequency thresholds are not associated with temporary threshold shift secondary to high-intensity music exposure. Overall, these findings suggest that extended high-frequency testing does not provide additional information in older adults. One clinical implication is that adding the frequencies above 8000 Hz may not be worth the time, expense and calibration difficulties associated with testing at these frequencies.
Prevalence of Hearing Loss
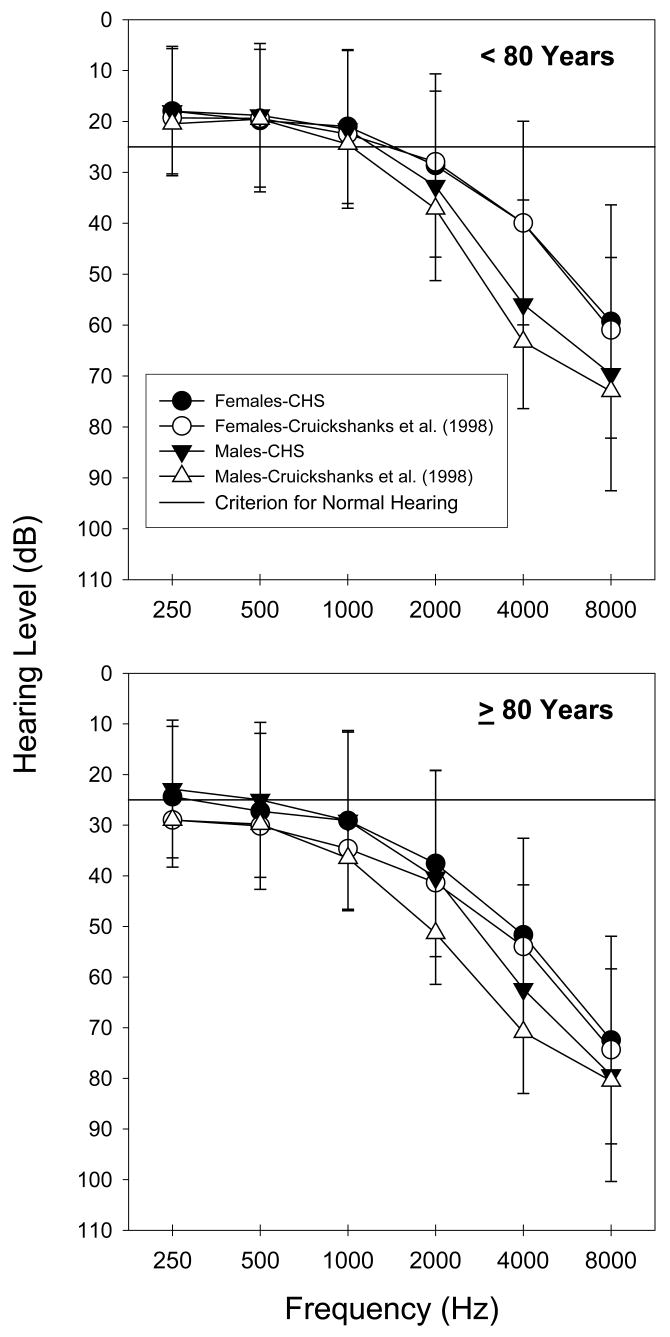
The prevalence of hearing loss found in this study was similar to that observed by Helzner et al.(2005) and Cruickshanks, Wiley, et al. (1998) when using their definitions of hearing loss, which were not only sensitive to age-related hearing loss, but also sensitive to gender and race differences. A minor difference across the studies is that the men in the current study had slightly lower rates of hearing loss than the men of corresponding age in the Cruickshanks, Wiley, et al. study and the White men in the Helzner et al. study. The lower rate of hearing loss in the men in the current study also corresponded with reduced hearing loss severity when compared to the men in the Cruickshanks, Wiley, et al. study. With ears combined, Figure 5 shows that, on average, the men from the Cruickshanks, Wiley, et al. study had higher thresholds than the men in the current study regardless of the age group. When the thresholds of the Black participants were removed in order to equalize the racial composition of the two studies, the threshold differences were reduced slightly for the men in the <80 group but not for the men in the ≥80 years group. This may reflect differences associated with dissimilar living and working environments (urban vs. rural) sampled in the two studies. The septuagenarian women were similar across the two studies even when the thresholds from the Black women were removed from the averages. Yet, the ≥80 years old women from the Cruickshanks, Wiley, et al. study demonstrated more threshold elevation in the lower and mid frequencies. This pattern has been associated presbyacusis in women (Jerger, Chmiel, Stach, & Spretnjak, 1993), as well as differences in cardiovascular health and disease profiles (Gates, Cobb, D’Agostino, & Wolf, 1993; Lee et al., 1998); although less pronounced in the current study.
Figure 5.
Mean air-conduction thresholds for the 70–79 and ≥80 years old participants from the Cruickshanks, Wiley, et al. (1998) and CHS. The thresholds represent both ears combined. The CHS data include thresholds from both White and Black participants. The error bars reflect +/− 1 SD for the CHS data.
The minor differences between the three studies could have resulted from a number of factors, such as the manner in which the participants were recruited from their prospective populations, as well as the potential health differences across the two studies. For example, the current study included only those CHS participants who could be tested at the Pittsburgh clinical site, while some of the participants in the Cruickshanks, Wiley, et al. (1998) study were tested at home. This allowed for inclusion of less ambulatory and potentially less healthy participants. The Helzner et al. (2005) study included participants from both Pittsburgh and Memphis, Tennessee and there was some suggestion of health differences existing across the two populations. These differences might have been consequent to distinct occupational, recreational, environmental and nutritional characteristics of the groups. The participants in the Helzner et al study who were recruited from Pittsburgh were enrolled after the current study was closed to participation, so there might have been some overlap of participants in the current study but the likelihood is low.
The results of the current study highlight the lack of uniformity in age-related hearing loss across gender and racial groups that should considered during a hearing assessment. It also argues for further research to determine the intrinsic and extrinsic factors that might make certain people resistant or disposed to hearing loss as they age such as estrogen levels, diet, exercise and preventative healthcare. With high prevalence of hearing loss in the aging populations there is a clear need to understand its etiologies, methods for prevention, and potential interventions as they have notable implications for social inclusion and quality of life.
Although the demographic, health and hearing loss differences across racial and gender groups suggest that effective rehabilitation also might differ across these groups, the group disparities highlight the need for individualized treatment approaches. Clinicians can not only assume that all elderly patients with hearing loss should be treated in the same manner, but clinicians cannot assume that all women, or all Black women with hearing loss will benefit from the same hearing aid algorithms, assistive devices, or type of counseling and instruction. As part of the intervention process, the specific problems and needs of each patient should be established. Optimal treatment approaches should be developed based on established evidence, and then refined to meet the individual problems and needs of the patient.
Acknowledgments
The research reported in this article was supported by contracts N01-HC-85079 through N01-HC-85086, N01-HC-35129, N01 HC-15103 and N01HC085082 from the National Heart, Lung, and Blood Institute. A full list of participating CHS investigators and institutions can be found at http://www.chs-nhlbi.org.
References
- Agrawal Y, Platz EA, Niparko JK. Prevalence of hearing loss and difference by demographic characteristics among US Adults. Archives of Internal Medicine. 2008;168:1522–1530. doi: 10.1001/archinte.168.14.1522. [DOI] [PubMed] [Google Scholar]
- American National Standards Institute (ANSI) Methods for manual pure-tone threshold audiometry, ANSI S3.21–1978. New York: ANSI; 1978. [Google Scholar]
- American National Standards Institute (ANSI) Maximum permissible ambient noise levels for audiometric test rooms, ANSI S3.1–1991. New York: ANSI; 1991. [Google Scholar]
- American National Standards Institute (ANSI) American national standard specification for audiometers. ANSI S3.6–1989. New York: ANSI; 1989. [Google Scholar]
- American National Standards Institute (ANSI) American national standard specification for audiometers. ANSI S3.6–1996. New York: ANSI; 1996. [Google Scholar]
- Arnold AM, Pasty BM, Kuller LH, Burke GL, Manolio TA, Fried LP, et al. Incidence of cardiovascular disease in older Americans: The Cardiovascular Health Study. Journal of the American Geriatrics Society. 2005;53:211–218. doi: 10.1111/j.1532-5415.2005.53105.x. [DOI] [PubMed] [Google Scholar]
- Barrenas ML. Hair cell loss from acoustic trauma in chloroquine-treated red, black and albino guinea pigs. Audiology. 1997;36:187–2001. [PubMed] [Google Scholar]
- Barrenas ML, Axelsson A. The development of melanin in the stria vascularis of the gerbil. Acta Otolaryngology. 1992;112:50–58. doi: 10.3109/00016489209100782. [DOI] [PubMed] [Google Scholar]
- Barrenas ML, Hellstrom PA. The effect of low level acoustic stimulation to noise in blue- and brown-eyed young human subjects. Ear and Hearing. 1996;17:58–63. doi: 10.1097/00003446-199602000-00008. [DOI] [PubMed] [Google Scholar]
- Barr-Hamilton RM, Matheson LM, Keay DG. Ototoxicity of cis-platinum and its relationship to eye colour. Journal of Laryngology and Otology. 1992;1005:7–11. doi: 10.1017/s0022215100114689. [DOI] [PubMed] [Google Scholar]
- Bartels S, Ito S, Trune DR, Nuttall AL. Noise-induced hearing loss: The effect of melanin in the stria vascularis. Hearing Research. 2001;154:116–123. doi: 10.1016/s0378-5955(01)00213-1. [DOI] [PubMed] [Google Scholar]
- Blackburn H, Keys A, Simonson E, Rautharaju P, Punsar S. The electrocardiogram in population studies: a classification system. Circulation. 1960;21:1160–1175. doi: 10.1161/01.cir.21.6.1160. [DOI] [PubMed] [Google Scholar]
- Bohme G. Hearing disorders in peripheral arterial vascular diseases. A contribution on hearing loss in the aged. Laryngologie, Rhinologie, Otologie. 1987;66:638–642. [PubMed] [Google Scholar]
- Brainerd SH, Frankel BG. The relationship between audiometric and self-report measures of hearing handicap. Ear and Hearing. 1985;6:89–92. doi: 10.1097/00003446-198503000-00005. [DOI] [PubMed] [Google Scholar]
- Bunch CC. Further observations on age variations in auditory acuity. Archives of Otolaryngology. 1993;13:170–180. [Google Scholar]
- Campbell VA, Crews JE, Moriarty DG, Zack MM, Blackman DK. Surveillance for sensory impairment, activity limitation, and health-related quality of life among older adults – United States, 1993–1997. MMWR Surveillance Summaries. 1999;48(SS08):131–156. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Cigarette smoking among adults – United States, 2006. Morbidity and Mortality Weekly Report. 2007;56:1157–1161. [PubMed] [Google Scholar]
- Clark JG. Uses and abuses of hearing loss classification. Asha. 1984;23:493–500. [PubMed] [Google Scholar]
- Clark K, Sowers MF, Wallace RB, Anderson C. The accuracy of self-reported hearing loss in women aged 60–85 years. American Journal of Epidemiology. 1991;134:704–708. doi: 10.1093/oxfordjournals.aje.a116147. [DOI] [PubMed] [Google Scholar]
- Conlee JW, Abdul-Baqi KJ, McCandless GA, Creel DJ. Differential susceptibility to noise-induced permanent threshold shift between albino and pigmented guinea pigs. Hearing Research. 1986;23:81–91. doi: 10.1016/0378-5955(86)90177-2. [DOI] [PubMed] [Google Scholar]
- Cooper JC, Owens JA. Audiologic profile of noise-induced hearing loss. Archives of Otolaryngology. 1976;102:148–150. doi: 10.1001/archotol.1976.00780080070007. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Klein R, Klein BEK, Wiley TL, Nondahl DM, Tweed TS. Cigarette smoking and hearing loss: The epidemiology of hearing loss study. Journal of the American Medical Association. 1998;280:1715–1719. doi: 10.1001/jama.279.21.1715. [DOI] [PubMed] [Google Scholar]
- Cruickshanks KJ, Wiley TL, Tweed T, Klein BEK, Klein R, Mares-Perlman JA, et al. Prevalence of hearing loss in older adults in Beaver Dam, Wisconsin. American Journal of Epidemiology. 1998;148:879–886. doi: 10.1093/oxfordjournals.aje.a009713. [DOI] [PubMed] [Google Scholar]
- Daniel E. Noise and hearing loss: A review. Journal of School Health. 2007;77:225–231. doi: 10.1111/j.1746-1561.2007.00197.x. [DOI] [PubMed] [Google Scholar]
- Desai M, Pratt L, Lentzner H, Robinson K. Aging Trends, No. 2. Hyattsville, MD: National Center of Health Statistics; 2001. Trends in vision and hearing among older Americans. [DOI] [PubMed] [Google Scholar]
- Ecob R, Sutton G, Rudnicka A, Smith P, Power C, Strachan D, et al. Is the relation of social class to change in hearing threshold levels from childhood to middle age explained by noise, smoking and drinking behavior? International Journal of Audiology. 2008;47:100–108. doi: 10.1080/14992020701647942. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Erickson DA, Frey RH, Rappaport BZ, Schechter MA. The effects of noise upon human hearing sensitivity from 8000 to 20 000 Hz. Journal of the Acoustical Society of America. 1981;69:1343–1347. doi: 10.1121/1.385805. [DOI] [PubMed] [Google Scholar]
- Fausti SA, Henry JA, Helt WJ, Phillips DS, Frey RH, Noffsinger D, et al. An individualized, sensitive frequency range for early detection of ototoxicity. Ear and Hearing. 1999;20:497–505. doi: 10.1097/00003446-199912000-00005. [DOI] [PubMed] [Google Scholar]
- Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, et al. The Cardiovascular Health Study: design and rationale. Annals of Epidemiology. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- Gates GA, Cobb JL, D’Agostino RB, Wolf PA. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Archives of Otolaryngology Head and Neck Surgery. 1993;119:156–161. doi: 10.1001/archotol.1993.01880140038006. [DOI] [PubMed] [Google Scholar]
- Gates GA, Murphy M, Rees TS, Fraher A. Screening for handicapping hearing loss in the elderly. The Journal of Family Practice. 2003;52:56–62. [PubMed] [Google Scholar]
- Gomez MI, Hwang S, Sobotova L, Stark AD, May JJ. A comparison of self-reported hearing loss and audiometry in a cohort of New York farmers. Journal of Speech, Language, and Hearing Research. 2001;44:1201–1208. doi: 10.1044/1092-4388(2001/093). [DOI] [PubMed] [Google Scholar]
- Gratton MA, Wright CG. Hyperpigmentation of chinchilla stria vascularis following acoustic trauma. Pigment Cell Research. 1992;5:30–37. doi: 10.1111/j.1600-0749.1992.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Guimaraes P, Zhu X, Cannon T, Kim S, Frisina RD. Gender differences in distortion product otoacoustic emissions as a function of age in CBA mice. Hearing Research. 2004;192:83–89. doi: 10.1016/j.heares.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Heimen PS, Klis SFL, de Groot JCMJ, Smoorenburg GF. Cisplatin ototoxicity and the possibly protective effect of α-melanocyte stimulating hormone. Hearing Research. 1999;128:27–39. doi: 10.1016/s0378-5955(98)00194-4. [DOI] [PubMed] [Google Scholar]
- Helzner EP, Cauley JA, Pratt SR, Wisniewski SR, Zmuda JM, Talbott EO, et al. Race and sex differences in age-related hearing loss: The Health, Aging and Body Composition Study. Journal of the American Geriatrics Society. 2005;53:2119–2147. doi: 10.1111/j.1532-5415.2005.00525.x. [DOI] [PubMed] [Google Scholar]
- Houston DK, Johnson MA, Nozza RJ, Gunter EW, Shea KJ, Cutler GM, et al. Age-related hearing loss, vitamin B-12, and folate in elderly woman. American Journal of Clinical Nutrition. 1999;69:564–571. doi: 10.1093/ajcn/69.3.564. [DOI] [PubMed] [Google Scholar]
- Ishii EK, Talbott EO. Race/ethnicity differences in the prevalence of noise-induced hearing loss in a group of metal fabricating workers. Journal of Occupational and Environmental Medicine. 1998;40:661–666. doi: 10.1097/00043764-199808000-00001. [DOI] [PubMed] [Google Scholar]
- Jerger J, Chmiel R, Stach B, Spretnjak M. Gender affects audiometric shape in presbyacusis. Journal of the American Academy of Audiology. 1993;4:42–49. [PubMed] [Google Scholar]
- Karamitsos DG, Kounis NG, Zavras GM, Kitrou MP, Goudevenos JA, Papdaki PJ, Koutsojannis CM. Brainstem auditory evoked potentials in patients with ischemic heart disease. Laryngoscope. 1996;106:54–57. doi: 10.1097/00005537-199601000-00011. [DOI] [PubMed] [Google Scholar]
- Kawaguchi K. Susceptibility of organ of Corti with or without melanin to acoustic overstimulation. Journal of the Oto-Rhino-Laryngological Society of Japan. 1992;95:556–66. doi: 10.3950/jibiinkoka.95.556. [DOI] [PubMed] [Google Scholar]
- Keithley EM, Ryan AF, Feldman ML. Cochlear degeneration in aged rats of four strains. Hearing Research. 1993;59:171–178. doi: 10.1016/0378-5955(92)90113-2. [DOI] [PubMed] [Google Scholar]
- Kilicdag EB, Yavuz H, Agis T, Tarim E, Erkan AN, Kazanci F. Effects of estrogen therapy on hearing in postmenopausal women. American Journal of Obstetrics and Gynecology. 2004;190:77–82. doi: 10.1016/j.ajog.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Kim SH, Kang BM, Chae HD, Kim CH. The association between serum estradiol level and hearing sensitivity in postmenopausal women. Obstetrics and Gynecology. 2002;99:726–730. doi: 10.1016/s0029-7844(02)01963-4. [DOI] [PubMed] [Google Scholar]
- Kuller LH, Arnold AM, Psaty BM, Robbins JA, O’Leary DH, Tracy RP, et al. 10-Year follow-up of subclinical cardiovascular disease and risk of coronary heart disease in the cardiovascular health study. Archives of Internal Medicine. 2006;166:71–78. doi: 10.1001/archinte.166.1.71. [DOI] [PubMed] [Google Scholar]
- Kuller LH, Shemanski L, Psaty BM, Borhani NO, Gardin J, Haan MN, et al. Subclinical disease as an independent risk factor for cardiovascular disease. Circulation. 1995;92:720–726. doi: 10.1161/01.cir.92.4.720. [DOI] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Schmiedt RA. Effects of chronic fuosemide treatment and age of cell division in the adult gerbil inner ear. Journal of the Association for Research in Otolaryngology. 2003;4:164–175. doi: 10.1007/s10162-002-2056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Gómez-Marín O, Lee HM. Sociodemographic correlates of hearing loss and hearing aid use in Hispanic adults. Epidemiology. 1996;7:443–446. doi: 10.1097/00001648-199607000-00019. [DOI] [PubMed] [Google Scholar]
- Lee FS, Matthews LJ, Dubno JR, Mills JH. Longitudinal study of pure-tone thresholds in older persons. Ear and Hearing. 2005;26:1–11. doi: 10.1097/00003446-200502000-00001. [DOI] [PubMed] [Google Scholar]
- Lee FS, Matthews LJ, Mills JH, Dubno JR, Adkins WW. Analysis of blood chemistry and hearing levels in a sample of older persons. Ear and Hearing. 1998;19:180–190. doi: 10.1097/00003446-199806000-00002. [DOI] [PubMed] [Google Scholar]
- Lichtenstein MJ, Bess FH, Logan SA. Diagnostic performance of the Hearing Handicap Inventory for the Elderly (Screening Version) against differing definitions of hearing loss. Ear and Hearing. 1988;9:208–211. doi: 10.1097/00003446-198808000-00006. [DOI] [PubMed] [Google Scholar]
- Marcus-Bernstein C. Audiologic and nonaudiologic correlates of hearing handicap in black elderly. Journal of Speech and Hearing Research. 1986;29:301–312. doi: 10.1044/jshr.2903.301. [DOI] [PubMed] [Google Scholar]
- Matthews LJ, Lee F, Mills JH, Dubno JR. Extended high-frequency thresholds in older adults. Journal of Speech, Language, and Hearing Research. 1997;40:208–214. doi: 10.1044/jslhr.4001.208. [DOI] [PubMed] [Google Scholar]
- McFadden D. A speculation about the parallel ear asymmetries and sex differences in hearing sensitivity and otoacoustic emissions. Hearing Research. 1993;68:143–151. doi: 10.1016/0378-5955(93)90118-k. [DOI] [PubMed] [Google Scholar]
- McFadden D, Loehlin JC. On the heritability of spontaneous otoacoustic emissions: A twin study. Hearing Research. 1995;85:181–198. doi: 10.1016/0378-5955(95)00045-6. [DOI] [PubMed] [Google Scholar]
- Morrell CH, Gordon-Salant S, Pearson JD, Brant LJ, Fozard JL. Age- and gender-specific reference ranges for hearing level and longitudinal changes in hearing level. Journal of the Acoustical Society of America. 1996;100:1949–1967. doi: 10.1121/1.417906. [DOI] [PubMed] [Google Scholar]
- Moscicki E, Elkins E, Baum H, McNamara P. Hearing loss in the elderly: an epidemiologic study of the Framingham Heart Study Cohort. Ear and Hearing. 1985;6:184–190. [PubMed] [Google Scholar]
- National Center for Health Statistics. Vital and Health Statistics (Series 10, No.140, DHS publications No. PHS 82–1568) Public Health Service; Washington, DC: US: 1982. Hearing ability of persons by sociodemographic and health characteristics: United States. [PubMed] [Google Scholar]
- Nondahl DM, Cruickshanks JJ, Wiley TL, Tweed TS, Klein R, Klein BEK. Accuracy of self-reported hearing loss. Audiology. 1998;37:295–301. doi: 10.3109/00206099809072983. [DOI] [PubMed] [Google Scholar]
- Parving A, Hein HO, Suadicani P, Ostri B, Gyntelberg F. Epidemology of hearing disorders. Some factors affecting hearing. The Copenhagen Male Study. Scandinavian Audiology. 1993;22:101–107. doi: 10.3109/01050399309046025. [DOI] [PubMed] [Google Scholar]
- Pleis JR, Coles R. Vital & Health Statistics - Series 10: Data from the National Health Survey. Vol. 212. U.S. Government Printing Office; 2003. Summary health statistics for U.S. adults: National Health Interview Survey, 1999; pp. 1–137. [PubMed] [Google Scholar]
- Pleis JR, Lethbridge-Çejku M. Vital Health Statistics – Series 10. Vol. 232. National Center for Health Statistics; 2006. Summary health statistics for U.S. adults: National Health Interview Survey, 2005; pp. 1–163. [PubMed] [Google Scholar]
- Popelka MM, Cruickshanks KJ, Wiley TL, Tweed TS, Klein BEK, Klein R. Low prevalence of hearing aid use among older adults with hearing loss: The Epidemiology of Hearing Loss Study. Journal of the American Geriatrics Society. 1998;46:1075–1078. doi: 10.1111/j.1532-5415.1998.tb06643.x. [DOI] [PubMed] [Google Scholar]
- Priuska EM, Schacht J. Formation of free radicals by gentamicin and iron and evidence for an iron/gentamicin complex. Biochemical Pharamacology. 1995;50:1749–1752. doi: 10.1016/0006-2952(95)02160-4. [DOI] [PubMed] [Google Scholar]
- Pugh KC, Crandell CC. Hearing loss, hearing handicap, and functional health status between African American and Caucasian American seniors. Journal of the American Academy of Audiology. 2002;13:493–502. [PubMed] [Google Scholar]
- Reron A, Reron E, Modrzejew M, Strek P, Trojnar-Podlesny M. The effect of hormonal factors on the hearing organ in women after surgical castration. Preliminary report. Neuroendocrinology Letters. 2002;23:455–458. [PubMed] [Google Scholar]
- Rose GA, McCartney P, Reid DD. Self-administration of a questionnaire on chest pain and intermittent claudication. British Journal of Preventive and Social Medicine. 1977;31:42–48. doi: 10.1136/jech.31.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalia M, Geremia E, Corsaro C, Santoro C, Baratta D, Sichel G. Lipid peroxidation in pigmented and unpigmented liver tissues: Protective role of melanin. Pigment Cell Research. 1990;3:115–119. doi: 10.1111/j.1600-0749.1990.tb00330.x. [DOI] [PubMed] [Google Scholar]
- Schmuziger N, Patscheke J, Probst R. An assessment of threshold shifts in nonprofessional pop/rock muscicians using conventional and extended high-frequency audiometry. Ear and Hearing. 2007;28:643–648. doi: 10.1097/AUD.0b013e31812f7144. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF, Gacek MR. Cochlear pathology in presbyacusis. Annals of Otology, Rhinology and Laryngology. 1993;102:1–16. doi: 10.1177/00034894931020S101. [DOI] [PubMed] [Google Scholar]
- Schwarze S, Notbohm G, Gärtner C. High-frequency audiometry and noise-induced hearing loss, a contribution to prevention by early diagnosis of a vulnerable hearing? Publication Series from the Federal Institute for Occupational Safety and Health, Research Report Fb 1063, 1–10. 2005 Retrieved August 1, 2007 from http://www.baua.de/nn_33078/sid_59C9F6A62FD7EBBB9F267E5A2735C148/nsc_true/en/Publications/Research-reports/2005/Fb1063,xv=lf.pdf.
- Sha SH, Schacht J. Antioxidants attenuate gentamicin-induced free radical formation in vitro and ototoxicity in vivo: D-methionine is a potential protectant. Hearing Research. 2000;142:34–40. doi: 10.1016/s0378-5955(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Sichel G, Corsaro C, Scalia M, Di Bilio AJ, Bonomo RP. In vitro scavenger activity of some flavonoids and melanins against O2-(.) Free Radical Biology and Medicine. 1991;11:1–8. doi: 10.1016/0891-5849(91)90181-2. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Gratton MA, Schulte BA. Expression patterns of ion transport enzymes in spiral ligament fibrocytes change in relation to strial atrophy in the aged gerbil cochlea. Hearing Research. 1997;111:93–102. doi: 10.1016/s0378-5955(97)00097-x. [DOI] [PubMed] [Google Scholar]
- Torre P, Cruickshanks KJ, Klein BEK, Klein R, Nondahl DM. The association between cardiovascular disease and cochlear function in older adults. Journal of Speech, Language, and Hearing Research. 2005;48:473–481. doi: 10.1044/1092-4388(2005/032). [DOI] [PubMed] [Google Scholar]
- Uchida Y, Nakashimat T, Ando F, Niino N, Shimokata H. Is there a relevant effect of noise and smoking on hearing? A population-based aging study. International Journal of Audiology. 2005;44:86–91. doi: 10.1080/14992020500031256. [DOI] [PubMed] [Google Scholar]
- Wallhagen M, Strawbridge W, Cohen R, Kaplan G. An increasing prevalence of hearing impairment and associated risk factors over three decades of the Alameda County Study. American Journal of Public Health. 1997;87:440–442. doi: 10.2105/ajph.87.3.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein BE. Geriatric hearing loss: myths, realities, resources for physicians. Geriatrics. 1989;44:42–48. 58–60. [PubMed] [Google Scholar]
- Willott JF, Hnath Chisolm T, Lister JJ. Modulation of presbycusis: Current status and future directions. Audiology & Neuro-Otology. 2001;6:231–239. doi: 10.1159/000046129. [DOI] [PubMed] [Google Scholar]
- Wrześniok D, Buszman E, Karna E, Nawrat P, Pałka J. Melanin potentiates gentamicin-induced inhibition of collagen biosynthesis in human skin fibroblasts. European Journal of Pharmacology. 2002;446:7–13. doi: 10.1016/s0014-2999(02)01793-4. [DOI] [PubMed] [Google Scholar]
- Wu WJ, Sha SH, McLaren JD, Kawamoto K, Raphael Y, Schacht J. Aminoglycoside ototoxicity in adult CBA, C57BL and BALB mice and the Sprague-Dawley rat. Hearing Research. 2001;158:165–178. doi: 10.1016/s0378-5955(01)00303-3. [DOI] [PubMed] [Google Scholar]