Abstract
Hereditary stomatocytosis describes a wide spectrum of autosomal dominantly inherited hemolytic disorders in which the basal red cell membrane cation permeability is increased. In this perspective article, Drs. Flatt and Bruce summarize our current knowledge in the field. See related article on page 1049.
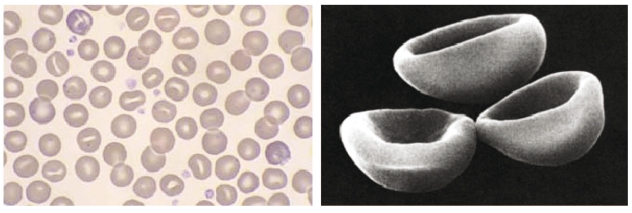
In healthy erythrocytes, cell volume is regulated by the transport of cations (Na+ and K+), which are pumped across the plasma membrane to maintain the osmotic potential and keep the cell correctly hydrated. Hereditary stomatocytosis (HSt) describes a wide spectrum of autosomal dominantly inherited hemolytic disorders in which the basal red cell membrane cation permeability is increased. This leak can be quantified by measuring the membrane permeability to cations when the Na+K+-ATPase and Na+K+2Cl− co-transporter are specifically inhibited by ouabain and bumetanide, respectively. Physiologically, these two transporters are most important in maintaining the ionic homeostasis in red cells.1 The cation leak results in deregulation of cellular volume, which can lead to morphological abnormality. This is illustrated by a typical HSt peripheral blood smear, which contains distinctive, cup-shaped stomatocytes (Figure 1). The clinical presentation of HSt varies widely, but common features are hemolytic anemia and reticulocytosis.2
Figure 1.
Hereditary stomatocytosis red cell slide. Stomatocytes are bowl-shaped red cells showing a slit-like area of central pallor. Reproduced with permission from Rozenberg G. Microscopic Haematology: A Practical Guide for the Laboratory. Second Edition 2003 pp 42. Palgrave Macmillan, Sydney, Australia.
One of the difficulties in diagnosing and classifying new cases of HSt is that the clinical presentation may easily be mistaken for the more common condition hereditary spherocytosis (HS), especially when there are few stomatocytes present in the blood film. HSt should be considered when the clinical severity of a presumed HS is greater than can be accounted for by reduction in band 3 (spectrin, ankyrin or protein 4.2) by SDS-PAGE analysis.
The hereditary stomatocytoses are classified according to phenotype; they are divided into overhydrated and dehydrated types, and can then be further sub-divided according to the temperature dependence of the cation leak.1,3 The genetic bases for these conditions have long remained unknown. Recently, investigations have uncovered the gene defects behind some of the variants, which offer new insights into the mechanisms underlying the disease.2
It was discovered that mutations in SLC4A1, which encodes the erythrocyte anion exchanger protein 1 (AE1, band 3), are responsible for some types of HSt.4 Band 3 is the most abundant erythrocyte membrane protein and plays a central role in red cell structure and function. Through its large N-terminal domain, band 3 binds ankyrin (along with other cytosolic proteins) and forms a link between the red cell membrane and the flexible spectrin cytoskeleton. The transmembrane domain of band 3 is responsible for its anion exchange activity, transporting bicarbonate ions (produced from the carbon dioxide released from respiring tissues) out of the red cell in exchange for chloride ions, which allows the whole blood volume to be used for carriage of carbon dioxide.
Mutations in band 3 are responsible for a number of conditions. These include HS, in which vertical interactions between band 3 and the red cell cytoskeleton are lost, distal renal tubular acidosis, in which the kidney isoform of band 3 (kAE1) is defective, and Southeast Asian ovalocytosis (SAO). SAO could be classed as a cryohydrocytosis form of HSt (see below), as the erythrocytes display a cold-induced increase in K+ and Na+ permeability, and peripheral blood smears contain stomatocytes as well as ovalocytes.5 The defect in SLC4A1 results in a nine amino acid deletion (400–408),6 which causes the band 3 protein to misfold; however, SAO band 3 is still successfully trafficked to the plasma membrane where it can conduct cations.
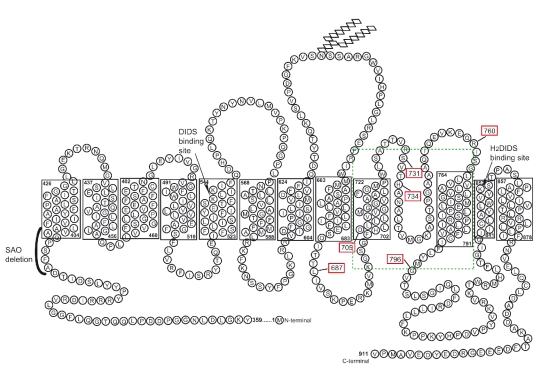
Band 3 mutations associated with HSt often produce an increased cation leak at low temperature (cryohydrocytosis; CHC). Patients with these variants show mild to moderate hemolytic anemia. Again, it is thought that the mutations responsible (Ser731Pro and His734Gln) affect the normal folding of band 3 and convert the protein into a cation conductor.4 Another mutation in band 3 has been identified as responsible for HSt Blackburn (Leu687Pro), in which affected erythrocytes share features of CHC and familial pseudohyperkalemia (see below), but the cation leak is not increased at low temperatures.4 Other band 3 mutants, originally classified as HS, have been reclassified as hereditary spherocytosis with a low temperature leak (HS-LTL) (Asp705Tyr and Arg760Gln) due to their increased cation leak at low temperatures.4 The known HSt point mutations in band 3 are illustrated in Figure 2, and occur in a restricted region of the trans-membrane domain of the protein. In the current issue of the journal, Iolascon et al.7 report a new mutation in band 3, associated with hereditary stomatocytosis (Gly796Arg). The proband also displays dyserythropoiesis that is phenotypically similar to congenital diserythropoietic anemia (CDA) type 1. Interestingly, there is another report of stomatocytosis with CDA,8 although the relationship between band 3 and CDA is not clear.
Figure 2.
Diagram of the membrane domain of band 3 showing the HSt mutations. Reprinted from Bruce LJ. Hereditary stomatocytosis and cation leaky red cells -recent developments. Blood Cells Mol Dis 2009;42:216-2, with permission from Elsevier.
Other HSt conditions are known to be caused by defects in proteins other than band 3. In a recently published paper it is reported that point mutations in Rh-associated glycoprotein (RhAG) result in overhydrated stomatocytosis (OHSt), in which the red cells are very cation-leaky at physiological temperature.9 RhAG is a member of the band 3 macrocomplex and there is evidence that in normal cells it may function as an ammonium transporter, and/or gas channel.10,11 The mutations identified (Phe65Ser, Ile61Arg) occur in the predicted transmembrane span 2 of the protein and are thought to widen the pore, allowing passage of cations.9 This condition is unusual, in that it is characterized by a large reduction or even absence of the membrane lipid raft protein, stomatin. The precise function of stomatin and its role in OHSt has remained a mystery. No mutations have been found in the stomatin gene in OHSt patients and production of the protein is normal,12 suggesting that the loss of stomatin is a secondary change. Recently, evidence has emerged that in normal cells stomatin interacts with and modulates the activity of the glucose transporter (glut1) by switching its preferred substrate from glucose to dehydroascorbic acid, an oxidized form of vitamin C.13 It has been suggested that by reducing the amount of stomatin in the membrane, OHSt cells maximize their capacity for glucose uptake. Increased glucose uptake would aid the metabolically-stressed OHSt cells to meet their energy demands.9
The most common type of HSt is dehydrated hereditary stomatocytosis (DHSt or xerocytosis); its exact genetic basis remains unknown, but linkage analysis in certain affected families has suggested that the condition segregates with chromosome 16q23–q24.14 The cells display a mild cation leak, with a tendency to leak K+ in particular, resulting in cellular dehydration. The manifestation of the condition may be quite mild, and diagnosis can be difficult. Hematologic parameters vary, including the quantity of stomatocytes - and the condition can present together with other pathologies, such as perinatal edema.15
Similar to DHSt, familial pseudohyperkalemia (FP) cells leak K+. However, this leak is not significant at physiological temperature, with no hemolytic anemia as observed in other HSt. The presence of stomatocytes is generally uncommon but has been reported in some cases.1 FP red cells display the leak when allowed to stand at room temperature. Although the gene defect(s) responsible for the condition have not yet been identified, it is interesting that some cases map to chromosome 16q23–q24 (the same region as DHSt),16 while others map to chromosome 2q35–q36, which may suggest the involvement of a heterodimer.17
Certain other conditions result in increased erythrocyte cation permeability, including sickle cell disease and the invasion of erythrocytes by the malaria parasite, Plasmodium falciparum.18 The transporter of the cation leak in these conditions is not known; however, it is tempting to speculate that these leaks may also be conducted by disrupted or misfolded red cell proteins, as is the case for HSt. HSt represents a distinct group of disorders caused by mutations that convert band 3 or RhAG into cation conductors in the red cell membrane; the molecular bases of DHSt and FP remain elusive but may yet be found to result from mutations causing mis-folding in other multispanning membrane proteins. The latest report from Iolascon et al. identifies a new band 3 mutation resulting in HSt, accompanied by dyserythropoietic features, and raises the idea that tyrosine phosphorylation and associated signaling is altered in these cells. Further investigations of HSt at the molecular level should aid our understanding of the processes underlying the range of pathologies observed in this diverse group of conditions.
Footnotes
Joanna Flatt is a PhD student at the Bristol Institute for Transfusion Science, University of Bristol. Dr. Bruce is a research scientist/project leader at the Bristol Institute for Transfusion Sciences. The experimental work described in this perspective article was supported by the UK National Health Service R & D Directorate.
References
- 1.Stewart GW. Hemolytic disease due to membrane ion channel disorders. Curr Opin Hematol. 2004;11:244–50. doi: 10.1097/01.moh.0000132240.20671.33. [DOI] [PubMed] [Google Scholar]
- 2.Bruce LJ. Hereditary stomatocytosis and cation leaky red cells-recent developments. Blood Cells Mol Dis. 2009;42:216–22. doi: 10.1016/j.bcmd.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 3.Delaunay J. The hereditary stomatocytoses: genetic disorders of the red cell membrane permeability to monovalent cations. Semin Hematol. 2004;41:165–72. doi: 10.1053/j.seminhematol.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Bruce LJ, Robinson HC, Guizouarn H, Borgese F, Harrison P, King MJ, et al. Monovalent cation leaks in human red cells caused by single amino-acid substitutions in the transport domain of the band 3 chloride-bicarbonate exchanger, AE1. Nat Genet. 2005;37:1258–63. doi: 10.1038/ng1656. [DOI] [PubMed] [Google Scholar]
- 5.Bruce LJ, Ring SM, Ridgwell K, Reardon DM, Seymour CA, Van Dort HM, et al. South-east Asian ovalocytic (SAO) erythrocytes have a cold sensitive cation leak: implications for in vitro studies on stored SAO red cells. Biochim Biophys Acta. 1999;1416:258–70. doi: 10.1016/s0005-2736(98)00231-4. [DOI] [PubMed] [Google Scholar]
- 6.Tanner MJ, Bruce L, Martin PG, Rearden DM, Jones GL. Melanesian hereditary ovalocytes have a deletion in red cell band 3. Blood. 1991;78:2785–6. [PubMed] [Google Scholar]
- 7.Iolascon A, De Falco L, Borgese F, Esposito MR, Avvisati RA, Izzo P, et al. A novel erythroid anion exchange variant (Gly796Arg) of hereditary stomatocytosis associated with dyserythropoiesis. Haematologica. 2009;94:1049–59. doi: 10.3324/haematol.2008.002873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Olivieri O, Girelli D, Vettore L, Balercia G, Corrocher R. A case of congenital dyserythropoietic anaemia with stomatocytosis, reduced bands 7 and 8 and normal cation content. Br J Haematol. 1992;80:258–60. doi: 10.1111/j.1365-2141.1992.tb08912.x. [DOI] [PubMed] [Google Scholar]
- 9.Bruce LJ, Guizouarn H, Burton NM, Gabillat N, Poole J, Flatt JF, et al. The monovalent cation leak in over-hydrated stomatocytic red blood cells results from amino acid substitutions in the Rh associated glycoprotein (RhAG) Blood. 2009;113:1350–7. doi: 10.1182/blood-2008-07-171140. [DOI] [PubMed] [Google Scholar]
- 10.Marini AM, Matassi G, Raynal V, André B, Cartron JP, Chérif-Zahar B. The human Rhesus-associated RhAG protein and a kidney homologue promote ammonium transport in yeast. Nat Genet. 2000;26:341–4. doi: 10.1038/81656. [DOI] [PubMed] [Google Scholar]
- 11.Endeward V, Cartron JP, Ripoche P, Gros G. RhAG protein of the Rhesus complex is a CO2 channel in the human red cell membrane. FASEB J. 2008;22:64–73. doi: 10.1096/fj.07-9097com. [DOI] [PubMed] [Google Scholar]
- 12.Fricke B, Argent AC, Chetty MC, Pizzey AR, Turner EJ, Ho MM, et al. The “stomatin” gene and protein in overhydrated hereditary stomatocytosis. Blood. 2003;102:2268–77. doi: 10.1182/blood-2002-06-1705. [DOI] [PubMed] [Google Scholar]
- 13.Montel-Hagen A, Kinet S, Manel N, Prohaska R, Battini JL, Delaunay J, et al. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell. 2008;132:1039–48. doi: 10.1016/j.cell.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 14.Carella M, Stewart G, Ajetunmobi JF, Perrotta S, Grootenboer S, Tchernia G, et al. Genomewide search for dehydrated hereditary stomatocytosis (hereditary xerocytosis): mapping of locus to chromosome 16 (16q23-qter) Am J Hum Genet. 1998;63:810–6. doi: 10.1086/302024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grootenboer S, Schischmanoff PO, Cynober T, Rodrigue JC, Delaunay J, Tchernia G, Dommergues JP. A genetic syndrome associating dehydrated hereditary stomatocytosis, pseudohyperkalaemia and perinatal oedema. Br J Haematol. 1998;103:383–6. doi: 10.1046/j.1365-2141.1998.00992.x. [DOI] [PubMed] [Google Scholar]
- 16.Iolascon A, Stewart GW, Ajetunmobi JF, Perrotta S, Delaunay J, Carella M, et al. Familial pseudohyperkalemia maps to the same locus as dehydrated hereditary stomatocytosis (hereditary xerocytosis) Blood. 1999;93:3120–3. [PubMed] [Google Scholar]
- 17.Carella M, d’Adamo AP, Grootenboer-Mignot S, Vantyghem MC, Esposito L, D’Eustacchio A, et al. A second locus mapping to 2q35–36 for familial pseudohyperkalaemia. Eur J Hum Genet. 2004;12:1073–6. doi: 10.1038/sj.ejhg.5201280. [DOI] [PubMed] [Google Scholar]
- 18.Ellory JC, Guizouarn H, Borgese F, Bruce LJ, Wilkins RJ, Stewart GW. Abnormal permeability pathways in human red blood cells. Blood Cells Mol Dis. 2007;39:1–6. doi: 10.1016/j.bcmd.2007.02.011. [DOI] [PubMed] [Google Scholar]