Abstract
In this perspective article, Dr. Cazzola examines standard and novel tools for the diagnosis of myelodysplastic syndrome. Flow cytometry immunophenotyping may provide complementary information in the diagnostic approach to a patient with this condition. See related articles on pages 1066, 1124 and 1160.
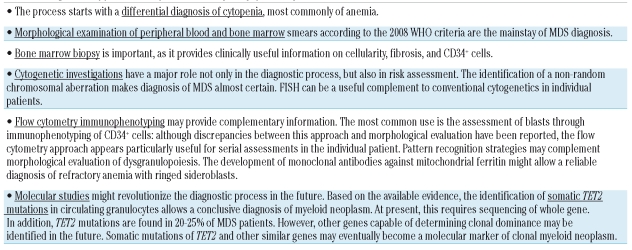
The typical patient with myelodysplastic syndrome (MDS) presents with a normocytic or slightly macrocytic anemia that is refractory to treatment with folates and vitamin B12.1 Many patients also have abnormal white blood cell and platelet counts, typically neutropenia and/or thrombocytopenia.
A rational approach to the diagnosis of myelodysplastic syndrome should be initially based on the exclusion of more common anemic disorders (Table 1). When anemia is frankly macrocytic, differential diagnosis should primarily include megaloblastic anemia. The real challenge is represented by the patient with normocytic anemia, whose differential diagnosis should not only consider renal failure and chronic disease, but also less common conditions such as celiac disease.2 Microcytic anemia is very rarely found in myelodysplastic syndromes and may be caused by somatic deletions of globin genes.
Table 1.
Diagnostic approach to a patient with myelodysplastic syndrome: standard and novel tools.
Examination of peripheral blood smear is mandatory in the initial work-up of any hematologic disorder, particularly of a myelodysplastic syndrome. This examination often reveals such morphological abnormalities as hypogranulated neutrophils with hyposegmented nuclei (pseudo-Pelger-Huët anomaly) and large platelets. Bone marrow aspiration is required for the assessment of dyserythropoiesis, dysgranulopoiesis, and dysmegakaryocytopoiesis, and for the enumeration of ringed sideroblasts and blast cells.3
While bone marrow biopsy may be avoided in elderly patients who are in any case only going to receive supportive care, it should be performed in the remaining patients due to its diagnostic and prognostic utility.4 In fact, bone marrow biopsy may provide information about marrow cellularity, fibrosis and CD34+ cell clusters. Hypoplastic MDS needs to be distinguished from both aplastic anemias and hypocellular acute myeloid leukemia.5 Bone marrow fibrosis identifies a distinct subgroup of MDS with multilineage dysplasia, high transfusion requirement, and poor prognosis, while the presence of CD34+ cell clusters is an independent risk factor for progression to acute leukemia.4
Cytogenetic abnormalities have a major role in the diagnosis of myelodysplastic syndrome and in risk assessment.6,7 Fluorescence in situ hybridization (FISH) should complement conventional cytogenetics in particular cases. Specifically, FISH may improve the detection of deletion 5q31-q32 in patients with MDS without cytogenetic evidence of del(5q).8,9
The pathological hallmark of myelodysplastic syndrome is marrow dysplasia, which represents the basis of the World Health Organization (WHO) classification of these disorders.10 This classification has been recently revised,6,11 and provides clinicians with a very useful tool for defining the different subtypes of myelodysplastic syndrome. The combination of overt marrow dysplasia and clonal cytogenetic abnormality allows a conclusive diagnosis of MDS, but this is found in only a portion of MDS patients. In many instances, cytogenetics is not informative, and the diagnosis of MDS is based entirely and exclusively on morphological criteria. Diagnosis of MDS may be particularly difficult in patients with a normal karyotype or non-informative cytogenetics who do not have robust morphological markers, such as ringed sideroblasts or excess of blasts.
Flow cytometry immunophenotyping is a reliable method for quantitative and qualitative evaluation of hematopoietic cells, and not surprisingly has been evaluated as a potential diagnostic tool for myelodysplastic syndromes.12–17 Despite many efforts, no one single simple immunophenotypic parameter has been proved to be diagnostic of MDS. Three articles in this issue of the journal provide additional observations in this field.
Ogata and co-workers18 designed a flow cytometry protocol applicable in many laboratories, and verified its diagnostic utility in patients with low-risk myelodysplastic syndromes. The cardinal parameters were blasts, B-cell progenitors, myeloblast CD45 expression, and channel number of side scatter where the maximum number of granulocytes occurs. This protocol was able to discriminate between low-grade MDS without conventional markers (cytogenetic abnormalities, ringed sideroblasts) and nonclonal cytopenias with good specificity.
Goardon and coworkers19 investigated whether reduced mean fluorescence intensity (MFI) of CD38 expression on CD34+ cells could be used as a surrogate marker for abnormalities in the MDS CD34+ compartment, and whether this may provide a single simple useful flow cytometric measurement diagnostic of MDS. They found that the examined immunophenotypic parameter diagnosed low-risk MDS with 95% sensitivity and 92% specificity, and concluded that it may be of value in the routine clinical diagnosis of MDS, especially in cases with a low blast count and normal karyotype.
The report by van de Loosdrecht et al.20 describes the results of the first European LeukemiaNet (ELN) working conference on flow cytometry immunophenotyping in MDS. This article is a very comprehensive analysis of this topic, and provides detailed information on what is currently known in the field. The ENL group agreed that flow cytometry reports should always be descriptive in nature, with a statement that findings could be consistent with MDS. However, the group concluded that despite strong evidence for an impact of flow cytometry immunophenotyping in MDS, prospective validation of markers and immunophenotypic patterns are required against control patient groups, as well as further standardization in multi-center studies.
Standardization of flow cytometry in MDS may improve not only diagnosis of MDS, but also its prognostication.21,22 However, it is not likely to revolutionize the approach to the MDS patient. This is more likely to happen with molecular markers such as mutant genes, as was the case with the myeloproliferative neoplasms. Indeed, recent papers report somatic mutations of TET2 in about 20–25% of patients with MDS.23,24 These mutations would cause clonal dominance of mutated stem cells, and predispose to the acquisition of additional mutations that determine the clinical phenotype. A new molecular era in the diagnosis of MDS might be starting.
Footnotes
Dr. Cazzola is a Professor of Hematology at the University of Pavia, Pavia, Italy. The author reported no potential conflict of interest.
References
- 1.Cazzola M, Malcovati L. Myelodysplastic syndromes--coping with ineffective hematopoiesis. N Engl J Med. 2005;352:536–8. doi: 10.1056/NEJMp048266. [DOI] [PubMed] [Google Scholar]
- 2.Bergamaschi G, Markopoulos K, Albertini R, Di Sabatino A, Biagi F, Ciccocioppo R, et al. Anemia of chronic disease and defective erythropoietin production in patients with celiac disease. Haematologica. 2008;93:1785–91. doi: 10.3324/haematol.13255. [DOI] [PubMed] [Google Scholar]
- 3.Mufti GJ, Bennett JM, Goasguen J, Bain BJ, Baumann I, Brunning R, et al. Diagnosis and classification of myelodysplastic syndrome: International Working Group on Morphology of myelodysplastic syndrome (IWGM-MDS) consensus proposals for the definition and enumeration of myeloblasts and ring sideroblasts. Haematologica. 2008;93:1712–7. doi: 10.3324/haematol.13405. [DOI] [PubMed] [Google Scholar]
- 4.Della Porta MG, Malcovati L, Boveri E, Travaglino E, Pietra D, Pascutto C, et al. Clinical relevance of bone marrow fibrosis and CD34-positive cell clusters in primary myelodysplastic syndromes. J Clin Oncol. 2009;27:754–62. doi: 10.1200/JCO.2008.18.2246. [DOI] [PubMed] [Google Scholar]
- 5.Bennett JM, Orazi A. Diagnostic criteria to distinguish hypocellular acute myeloid leukemia from hypocellular myelodysplastic syndromes and aplastic anemia: recommendations for a standardized approach. Haematologica. 2009;94:264–8. doi: 10.3324/haematol.13755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunning RDAO, Germing U, Le Beau MM, Porwit A, Bauman I, et al. Myelodysplastic syndromes/Neoplasms, overview. In: Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al., editors. WHO classification of tumours of haematopoietic and lymphoid tissues. Lyon: IARC; 2008. pp. 88–93. [Google Scholar]
- 7.Haase D, Germing U, Schanz J, Pfeilstocker M, Nosslinger T, Hildebrandt B, et al. New insights into the prognostic impact of the karyotype in MDS and correlation with subtypes: evidence from a core dataset of 2124 patients. Blood. 2007;110:4385–95. doi: 10.1182/blood-2007-03-082404. [DOI] [PubMed] [Google Scholar]
- 8.Mallo M, Arenillas L, Espinet B, Salido M, Hernandez JM, Lumbreras E, et al. Fluorescence in situ hybridization improves the detection of 5q31 deletion in myelodysplastic syndromes without cytogenetic evidence of 5q. Haematologica. 2008;93:1001–8. doi: 10.3324/haematol.13012. [DOI] [PubMed] [Google Scholar]
- 9.Cazzola M. Myelodysplastic syndrome with isolated 5q deletion (5q- syndrome). A clonal stem cell disorder characterized by defective ribosome biogenesis. Haematologica. 2008;93:967–72. doi: 10.3324/haematol.13377. [DOI] [PubMed] [Google Scholar]
- 10.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 11.Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the WHO classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood. 2009 Apr 8; doi: 10.1182/blood-2009-03-209262. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 12.Stetler-Stevenson M, Arthur DC, Jabbour N, Xie XY, Molldrem J, Barrett AJ, et al. Diagnostic utility of flow cytometric immunophenotyping in myelodysplastic syndrome. Blood. 2001;98:979–87. doi: 10.1182/blood.v98.4.979. [DOI] [PubMed] [Google Scholar]
- 13.Maynadie M, Picard F, Husson B, Chatelain B, Cornet Y, Le Roux G, et al. Immunophenotypic clustering of myelodysplastic syndromes. Blood. 2002;100:2349–56. doi: 10.1182/blood-2002-01-0230. [DOI] [PubMed] [Google Scholar]
- 14.Ogata K, Nakamura K, Yokose N, Tamura H, Tachibana M, Taniguchi O, et al. Clinical significance of phenotypic features of blasts in patients with myelodysplastic syndrome. Blood. 2002;100:3887–96. doi: 10.1182/blood-2002-01-0222. [DOI] [PubMed] [Google Scholar]
- 15.Malcovati L, Della Porta MG, Lunghi M, Pascutto C, Vanelli L, Travaglino E, et al. Flow cytometry evaluation of erythroid and myeloid dysplasia in patients with myelodysplastic syndrome. Leukemia. 2005;19:776–83. doi: 10.1038/sj.leu.2403680. [DOI] [PubMed] [Google Scholar]
- 16.Della Porta MG, Malcovati L, Invernizzi R, Travaglino E, Pascutto C, Maffioli M, et al. Flow cytometry evaluation of erythroid dysplasia in patients with myelodysplastic syndrome. Leukemia. 2006;20:549–55. doi: 10.1038/sj.leu.2404142. [DOI] [PubMed] [Google Scholar]
- 17.Matarraz S, Lopez A, Barrena S, Fernandez C, Jensen E, Flores J, et al. The immunophenotype of different immature, myeloid and B-cell lineage-committed CD34+ hematopoietic cells allows discrimination between normal/reactive and myelodysplastic syndrome precursors. Leukemia. 2008;22:1175–83. doi: 10.1038/leu.2008.49. [DOI] [PubMed] [Google Scholar]
- 18.Ogata K, Della Porta MG, Malcovati L, Picone C, Yokose N, Matsuda A, et al. Diagnostic utility of flow cytometry in low-grade myelodysplastic syndromes: a prospective validation study. Haematologica. 2009;94:1066–74. doi: 10.3324/haematol.2009.008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goardon N, Nikolousis E, Sternberg A, Chu W-K, Craddock C, Richardson P, et al. Reduced CD38 expression on CD34+ cells as a diagnsotic test in myelodysplastic syndromes. Haematologica. 2009;94:1160–3. doi: 10.3324/haematol.2008.004085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Loosdrecht AA, Alhan C, Béné MC, Della Porta MG, Dräger AM, Feuillard J, et al. Standardization of flow cytometry in myelodysplastic syndromes: report from the first European LeukemiaNet working conference on FCM in MDS. Haematologica. 2009;94:1124–34. doi: 10.3324/haematol.2009.005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van de Loosdrecht AA, Westers TM, Westra AH, Drager AM, van der Velden VH, Ossenkoppele GJ. Identification of distinct prognostic subgroups in low- and intermediate-1-risk myelodysplastic syndromes by flow cytometry. Blood. 2008;111:1067–77. doi: 10.1182/blood-2007-07-098764. [DOI] [PubMed] [Google Scholar]
- 22.Scott BL, Wells DA, Loken MR, Myerson D, Leisenring WM, Deeg HJ. Validation of a flow cytometric scoring system as a prognostic indicator for posttransplantation outcome in patients with myelodysplastic syndrome. Blood. 2008;112:2681–6. doi: 10.1182/blood-2008-05-153700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Delhommeau F, Dupont S, Della Valle V, James C, Trannoy S, Masse A, et al. Mutation in TET2 in myeloid cancers. N Engl J Med. 2009;360:2289–301. doi: 10.1056/NEJMoa0810069. [DOI] [PubMed] [Google Scholar]
- 24.Langemeijer SM, Kuiper RP, Berends M, Knops R, Aslanyan MG, Massop M, et al. Acquired mutations in TET2 are common in myelodysplastic syndromes. Nat Genet. 2009;41:838–42. doi: 10.1038/ng.391. [DOI] [PubMed] [Google Scholar]