The diagnosis of myelodysplastic syndromes is not always straightforward when patients lack specific diagnostic markers, such as blast excess, karyotype abnormality, and ringed sideroblasts. This article proposes a flow cytometry protocol that can be used in the diagnostic work-up of low-grade myelodysplastic syndrome patients who lack specific diagnostic markers. See related perspective article on page 1041.
Keywords: myelodysplastic syndromes, flow cytometry, diagnosis
Abstract
Background
The diagnosis of myelodysplastic syndromes is not always straightforward when patients lack specific diagnostic markers, such as blast excess, karyotype abnormality, and ringed sideroblasts.
Design and Methods
We designed a flow cytometry protocol applicable in many laboratories and verified its diagnostic utility in patients without those diagnostic markers. The cardinal parameters, analyzable from one cell aliquot, were myeloblasts (%), B-cell progenitors (%), myeloblast CD45 expression, and channel number of side scatter where the maximum number of granulocytes occurs. The adjunctive parameters were CD11b, CD15, and CD56 expression (%) on myeloblasts. Marrow samples from 106 control patients with cytopenia and 134 low-grade myelodysplastic syndromes patients, including 81 lacking both ringed sideroblasts and cytogenetic aberrations, were prospectively analyzed in Japan and Italy.
Results
Data outside the predetermined reference range in 2 or more parameters (multiple abnormalities) were common in myelodysplastic syndromes patients. In those lacking ringed sideroblasts and cytogenetic aberrations, multiple abnormalities were observed in 8/26 Japanese (30.8%) and 37/55 Italians (67.3%) when the cardinal parameters alone were considered, and in 17/26 Japanese (65.4%) and 42/47 Italians (89.4%) when all parameters were taken into account. Multiple abnormalities were rare in controls. When data from all parameters were used, the diagnostic sensitivities were 65% and 89%, specificities were 98% and 90%, and likelihood ratios were 28.1 and 8.5 for the Japanese and Italian cohorts, respectively.
Conclusions
This protocol can be used in the diagnostic work-up of low-grade myelodysplastic syndromes patients who lack specific diagnostic markers, although further improvement in diagnostic power is desirable.
Introduction
Myelodysplastic syndromes (MDS) are clonal disorders of hematopoietic cells with variable clinical course and risk of evolution into acute myeloid leukemia (AML).1,2 MDS appear to be the most common myeloid malignancy and their incidence increases steeply with age.3 The diagnosis of MDS is based on a combination of clinical history, the morphological features of the peripheral blood (PB) and BM (e.g., percentages of blasts and dysplasia of cells), cytogenetic data, and ruling out other diseases.4,5 The diagnosis is straightforward if clearly objective abnormalities, i.e., increase in blasts and/or ringed sideroblasts (RS) and/or the presence of chromosomal aberration, are detected. In other words, a diagnostic challenge exists in low-grade MDS that lack conventional, specific diagnostic markers, RS and karyotypic aberration. The diagnosis of this category (called low-grade MDS without conventional markers in this paper) largely relies on the presence of dysplasia, and therefore experienced examiners (hematologists/hematopathologists) are required to make the diagnosis. On the other hand, the dysplastic features of myeloid cells do not in themselves establish a diagnosis. Conditions other than MDS can induce dysplastic myeloid cells (e.g., deficiencies of vitamin B12 and folate, viral infections, ethanol or lead), and thus such conditions should be ruled out by careful history taking and physical and laboratory examinations.
Flow cytometry (FCM) has been established as a useful, routine diagnostic tool for acute leukemia and Non-Hodgkin’s lymphoma but not for MDS. Recently, we and others have reported prototypes of diagnostic FCM for MDS.6–10 Based on those recent developments, in a report from a recent international working conference on MDS, FCM was proposed as one diagnostic test.11 To make diagnostic MDS FCM widely applicable, further studies on the parameters and analytical strategy usable in many laboratories are required.12
This study was conducted by two laboratories that have been working on diagnostic FCM for low-grade MDS.7,9,10,13 We investigated the diagnostic utility of our protocol, which was designed to be applicable in many laboratories and to minimize variations among them, for prospective application in low-grade MDS patients, in particular low-grade MDS without conventional markers, in the two laboratories.
Design and Methods
Patients
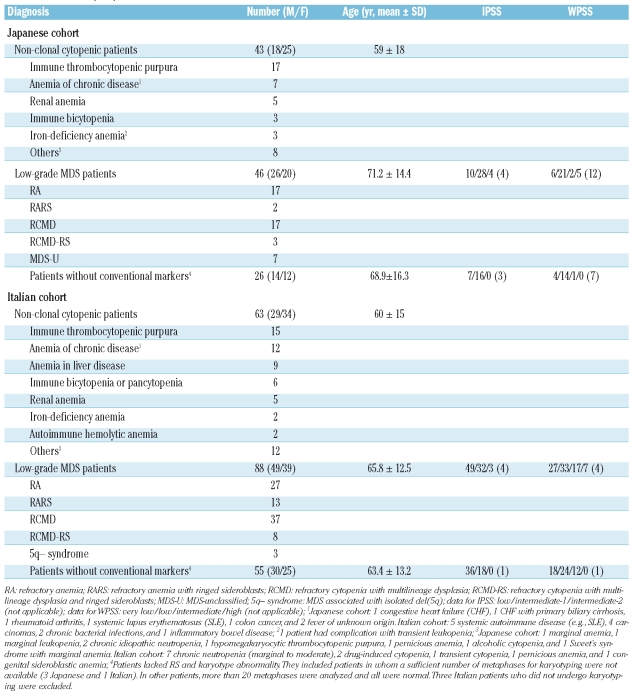
This study included four cohorts of patients. The first was a cohort reported in our previous study, in which FCM data on BM cells from 27 low-grade MDS patients lacking RS and 90 non-MDS patients including 70 non-clonal cytopenic patients were collected.10 The second cohort was made up of patients used for preliminary experiments, who were selected from patients who underwent BM aspiration in Nippon Medical School for diagnostic purposes. They consisted of 13 low-grade MDS patients lacking RS and 30 nonclonal cytopenic patients. Reference ranges (RRs) of parameters were determined using data from these cohorts before prospective FCM analysis (see Results). Patients in the prospective analysis were consecutive patients who underwent BM aspiration in our institutions in Japan (the third cohort) and Italy (the fourth cohort) and who were diagnosed with low-grade MDS or non-clonal cytopenia using conventional methods. These patients were either new patients with abnormal PB findings not ruling out low-grade MDS (e.g., cytopenia and/or macrocytosis without circulating blasts) for whom clinicians performed BM examination, or patients who had already been diagnosed with low-grade MDS and underwent BM examination to evaluate their disease status. Patients’ BM samples were subjected to the present FCM analysis as well as to conventional laboratory tests, which included cytological and pathological examinations, and cytogenetic analysis using the standard G-banding technique.14 Data on patients who underwent FCM analysis but had neither low-grade MDS nor non-clonal cytopenia, e.g., high-grade MDS, were excluded.
MDS was diagnosed according to the World Health Organization (WHO) criteria,15 as described preiously.16 In cases suspected to be low-grade MDS without conventional markers, observation was carried out for six months prior to making a diagnosis of MDS. Other diseases were also ruled out by repeated history taking and physical and laboratory examinations including follow-up PB data, for example, normalization of PB data after specific therapy for non-clonal cytopenia, and, if necessary, repeated BM examination. Karyotypes were interpreted using the standard criteria.17 The International Prognostic Score System (IPSS) and WHO classification-based prognostic scoring system (WPSS) were applied to MDS patients according to previous reports.18,19 Written, informed consent was obtained from all patients. This study protocol was approved by the Ethics Committee, Fondazione IRCCS Policlinico San Matteo, Pavia, Italy, and by the Institutional Review Board of Nippon Medical School, Tokyo, Japan.
Flow cytometry
BM cells of the patients were aspirated into a heparinized syringe, immediately diluted with RPMI 1640 medium containing 10% fetal calf serum, and stored at 4°C overnight. The next morning (19–24 h after cell aspiration), nucleated cells were counted and stained with antibodies.
Then, samples were treated with the standard ammonium chloride method to lyse erythrocytes and washed with phosphate-buffered saline. The rationale for sample handling was described in our previous report.10 Antibody staining was performed as follows: 100 μL of a cell aliquot containing 5–8×105 nucleated cells were placed into each tube and stained with three antibodies conjugated with fluorescein isothiocyanate (FITC), phycoerythrin (PE), or peridin chlorophyll (PerCP). Combinations of the three antibodies were CD10/CD34/ CD45 (FITC/PE/PerCP), CD15/CD34/CD45, CD34/ CD11b/CD45, and CD34/CD56/CD45. Antibodies were obtained from Becton Dickinson (BD, Franklin Lakes, NJ, USA) (CD45-PerCP, CD34-PE, CD11b-PE, and CD15-FITC) and Beckman Coulter (Fullerton, CA) (CD34-FITC, CD10-FITC, and CD56-PE). The optimal volume of each antibody reagent for the staining was determined beforehand. Fluorescence minus one (FMO) controls were used as described previously.13,20,21 Data were acquired using a FACSCalibur and FACSCanto flow cytometer (BD) in Tokyo and Pavia, respectively. At least 100,000 cell events were acquired.
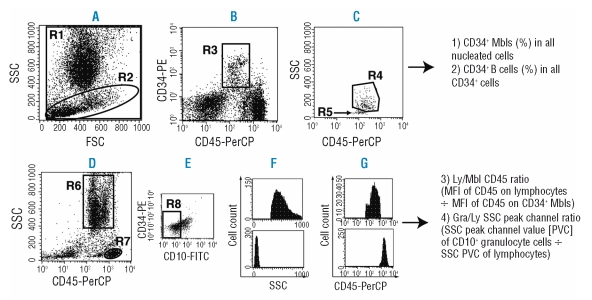
Data were analyzed with CellQuest software (BD) by investigators who were blinded to the patients’ clinical and laboratory data, including the diagnosis. First, four parameters were analyzed from a cell sample stained with the CD10/CD34/CD45 antibody combination. Analytical methods for three of these four parameters have been described previously.13 On the forward scatter (FSC)-versus-side scatter (SSC) display, we defined all nucleated cells (R1, Figure 1A) and cells with relatively low SSC were gated (R2) and then plotted on a CD45-versus-CD34 display (Figure 1B).13 Next, CD34+ cells with intermediate CD45 expression were gated (R3) and then plotted on a CD45-versus-SSC display (Figure 1C), in which CD34+ B-cell progenitors (stage I hematogones) formed an easily recognizable cluster (R5, arrow).10,22,23 Other CD34+ cells (R4) consist predominantly of myeloblasts, and thus these cells are called CD34+ myeloblasts in this paper. Meanwhile, all cells were plotted on a CD45-versus-SSC display (Figure 1D) and granulocytic cells other than myeloblasts (R6, called granulocytic cells in this paper) and lymphocytes (R7) were gated. For granulocytic cells, the CD10-negative cell fraction was gated (R8, Figure 1E). Then we examined the following parameters: (i) the percentage of CD34+ myeloblasts in all nucleated cells (called CD34+ myeloblasts [%] in this paper); (ii) the percentage of CD34+ B-cell progenitors in all CD34+ cells (called CD34+ B cells [%] in this paper); 3) Ly/Mbl CD45 ratio (mean fluorescence intensity [MFI] of CD45 on lymphocytes ÷ MFI of CD45 on CD34+ myeloblasts); and 4) Gra/Ly SSC peak channel ratio (SSC channel number where the maximum number of CD10- granulocytic cells occurs ÷ SSC channel number where the maximum number of lymphocytes occurs). From cell samples stained with CD15/CD34/CD45, CD34/CD11b/CD45, and CD34/ CD56/CD45 combinations, we analyzed the three other parameters. We gated CD34+ myeloblasts as described above and their CD15, CD11b, and CD56 expression was quantified by determining the percentages of antigen-positive cells in all CD34+ myeloblasts.
Figure 1.
Analysis of four cardinal parameters from a single cell aliquot stained with CD10-FITC, CD34-PE, and CD45-PerCP antibodies. (A) All nucleated cells (R1) and cells with relatively low SSC (R2). (B) Cells in R2 in panel A were displayed on a CD34-versus-CD45 plot. CD34+ cells with intermediate CD45 expression were gated (R3). (C) Cells in R3 in panel B were displayed on a CD45-versus-SSC plot. A cluster of CD34+ B-cell progenitors was identified in the lower left region of CD34+ cells (R5, arrow). The reliability of the R5 region was confirmed based on CD10 positivity. Cells in R4 were composed mainly of myeloblasts (Mbls) and thus simply called CD34+ Mbls in this paper. (D) Granulocytic cells other than Mbls (R6, called granulocytic cells in this paper) and lymphocytes (R7) were gated on a CD45-versus-SSC plot. (E) Cells in R6 in panel D were displayed, and the CD10− fraction was gated (R8). (F) SSC of CD10− granulocytic cells (upper panel) and lymphocytes (lower panel). SSC peak channel values (SSC channel number where the maximum number of cells occurs) of both fractions were computed using the software. (G) CD45 expression of CD34+ Mbls (upper panel) and lymphocytes (lower panel). Mean fluorescence intensity (MFI, GeoMean) of CD45 of both fractions was computed.
Determination of interlaboratory variability of flow data
Each of 10 BM samples obtained from the second patient cohort was divided into three aliquots and distributed to three laboratories in Tokyo. An FCM operator in each laboratory analyzed the above seven parameters according to the analysis manual written and distributed by Nippon Medical School. The manual contained all information described above, e.g, cell number, reagents for staining, and analytical methods.
Statistical analysis
Differences between two groups of data of continuous variables were analyzed using Student’s t-test. Differences in categorical variables were evaluated using the χ2 test. Interlaboratory variability of FCM data was determined based on the coefficient of variation (CV [%] = standard deviation [SD] ÷ mean x 100). To estimate the diagnostic power of the present FCM method, we calculated specificity, sensitivity, and the likelihood ratio according to the standard method,24 and 95% confidence intervals (CI) of specificity and sensitivity were based on the binomial distribution.25
Results
Determination of reference ranges and their application to the preliminary cohort
Using data from the first patient cohort, we analyzed four parameters, CD34+ myeloblasts (%), CD34+ B cells (%), Ly/Mbl CD45 ratio, and Gra/Ly SSC peak channel ratio, and compared the data between 27 low-grade MDS patients and 70 non-clonal cytopenic patients (Online Supplementary Figure S1, upper panel, most data on CD34+ myeloblasts, CD34+ B cells, and Ly/Mbl CD45 ratio were in our previous paper)13. Meanwhile, using BM samples from the second cohort, we determined CD15, CD11b, and CD56 expression on CD34+ myeloblasts (Online Supplementary Figure S1, lower panel) in addition to the above four parameters. The data suggest that these seven parameters might be useful in differentiating the two patient groups. Next, we examined the interlaboratory data variability of all seven parameters adopted (Online Supplementary Table S1). The data on CD34+ myeloblasts (%), CD34+ B cells (%), Ly/Mbl CD45 ratio, and Gra/Ly SSC peak channel ratio showed little interlaboratory variability, although data on CD11b, CD15, and CD56 expression (%) on CD34+ myeloblasts showed greater interlaboratory variability.
We then determined RRs for all parameters. For parameters showing little interlaboratory data variability, RRs were determined based on the receiver-operator characteristic curve using the data in the Online Supplementary Figure S1: CD34+ myeloblasts <2.4%, CD34+ B cells >5%, 4 <Ly/Mbl CD45 ratio <7.8, and Gra/Ly SSC peak ratio >6. For parameters showing substantial interlaboratory data variability, RRs were set to reduce/avoid events when non-clonal samples showed abnormal results (false-positive events), that is, CD11b expression on CD34+ myeloblasts <10%, CD15 expression on CD34+ myeloblasts <20%, and CD56 expression on CD34+ myeloblasts <10%. Using these RRs, the data from three laboratories shown in the Online Supplementary Table S1 did not show any false-positive results for all samples from non-clonal cytopenic patients (n=8).
Then, we applied FCM scoring for patients in the second cohort: one point was given for each parameter for which data were outside the RR. For example, if the data for two parameters were outside the RRs in an individual, the FCM score was 2. The results of scoring using four parameters (CD34+ myeloblasts [%], CD34+ B cells [%], Ly/Mbl CD45 ratio, and Gra/Ly SSC peak ratio) and all seven parameters are shown in Online Supplementary Table S2. An FCM score of 2 or more was not observed in any cytopenic control but observed in 46% and 69% of low-grade MDS patients in the scoring using four and seven parameters, respectively.
Application of diagnostic flow cytometry to the prospective cohorts
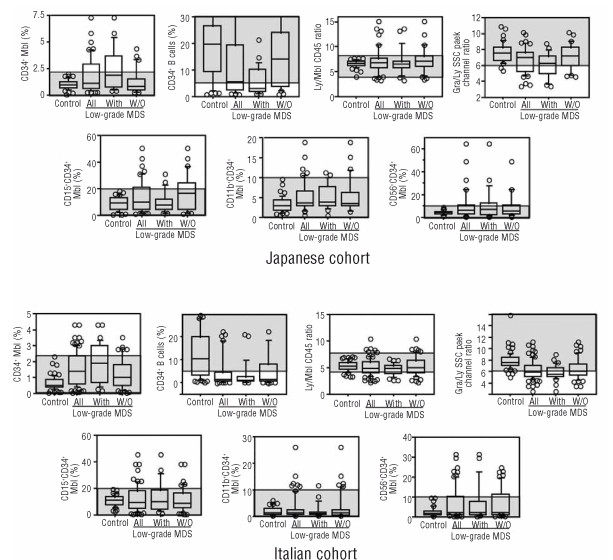
Using the same approach, we prospectively analyzed consecutive patients in Japan and Italy. Methodological details described in the Methods section and RRs were distributed to both laboratories beforehand. We defined CD34+ myeloblasts (%), CD34+ B cells (%), Ly/Mbl CD45 ratio, and Gra/Ly SSC peak ratio as cardinal parameters that must be analyzed for every sample, and defined CD11b, CD15, and CD56 expression on CD34+ myeloblasts as adjunctive parameters that should be analyzed in as many samples as possible. Patients analyzed are shown in Table 1. MDS subtypes in the Japanese cohort reflect the characteristics of Japanese MDS. That is, compared with MDS patients in Western countries, 5q- syndrome and MDS with RS are less frequent and hematopoietic cells are less dysplastic.26 Among 134 low-grade MDS patients, 81 (26 Japanese and 55 Italians) had low-grade MDS without conventional markers. All seven parameters were analyzed in all Japanese patients and in 38 non-clonal cytopenic patients and 66 low-grade MDS patients in the Italian cohort. All data from the Japanese and Italian cohorts are shown in Figure 2. The data from both cohorts showed the same characteristics. When looking at all low-grade MDS patients in these cohorts (the second boxplot from the left in each panel of Figure 2), the data on CD34+ myeloblasts (%) deviated upward and those on CD34+ B cells (%) and Gra/Ly SSC peak ratio deviated downward compared with those in non-clonal cytopenic patients (the extreme left plot in each panel of Figure 2). Meanwhile, the Ly/Mbl CD45 ratio deviated upward or downward depending on the individual patient in MDS. Furthermore, CD34+ myeloblasts from a considerable proportion of low-grade MDS patients expressed higher levels of CD15, CD11b, and CD56 compared with those from non-clonal cytopenic patients. The above characteristics also held true when low-grade MDS patients who had RS and/or karyotype abnormality (low-grade MDS with conventional markers) and low-grade MDS without conventional markers were analyzed separately (the two right plots in each panel). When data from these low-grade MDS groups were compared, low-grade MDS patients with conventional markers had more CD34+ myeloblasts (%) and less Gra/Ly SSC peak ratio compared with low-grade MDS patients without conventional markers in both cohorts (not shown in detail).
Table 1.
Patients in prospective cohorts.
Figure 2.
Analysis of seven flow cytometry parameters in the prospective cohorts. The horizontal lines in each boxplot represent the 90th, 75th, 50th, 25th, and 10th percentiles. Circles are outliers. Controls are nonclonal cytopenic patients. “All” indicates all low-grade myelodysplastic syndromes (MDS) patients, “With” indicates low-grade MDS with conventional markers, and “W/O” indicates low-grade MDS without conventional markers. Shaded areas are the predetermined reference ranges.
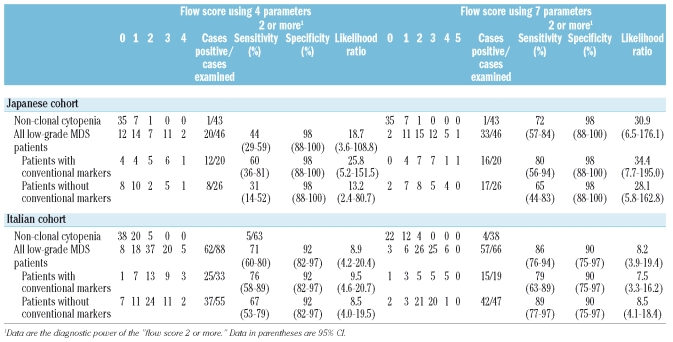
The results of FCM scoring using the predetermined RRs are shown in Table 2. When all low-grade MDS patients were scored using data from the four cardinal parameters, 20 of 46 (43.5%) Japanese and 62 of 88 (70.5%) Italian patients scored 2 or more, among whom 13 of 46 (28.3%) Japanese and 25 of 88 (28.4%) Italian patients scored 3 or more. However, among non-clonal cytopenic patients, most scored 0 or 1, only a few patients scored 2 (1/43 [2.3%] Japanese and 5/63 [7.9%] Italian patients), and none scored 3 or more. When data from all seven parameters were used to score all low-grade MDS patients, 33 of 46 (71.7%) Japanese and 57 of 66 (86.4%) Italian patients scored 2 or more, among whom 18 of 46 (39.1%) Japanese and 31 of 66 (47.0%) Italian patients scored 3 or more. In non-clonal cytopenic patients, most also scored 0 or 1, a few scored 2 (1/43 [2.3%] Japanese and 4/38 [10.5%] Italian patients), and none scored 3 or more. When low-grade MDS with conventional markers and low-grade MDS without conventional markers were scored separately, the results were similar between these two groups, except for the scoring using the four cardinal parameters in the Japanese cohort, among whom the number of patients who scored 2 or more was greater in low-grade MDS with conventional makers than in low-grade MDS without conventional markers (12/20 vs. 8/26, p=0.047).
Table 2.
Flow scores of patients in the prospective cohorts.
Based on the above data, the diagnostic power of the present FCM scoring, i.e., sensitivity, specificity, and more importantly the likelihood ratio, was calculated (Table 2). When patients lacking excess blasts, RS, and karyotype abnormality scored 2 or more in the present FCM method, the likelihood ratio of MDS (low-grade MDS without conventional markers) was 13.2 (four-parameter method, sensitivity for positivity 31%) and 28.1 (seven-parameter method, sensitivity 65%) in the Japanese cohort and 8.5 (in both four-parameter and seven-parameter methods with sensitivities 67% and 89%, respectively) in the Italian cohort.
When the FCM score 3 or more is defined as positive, the FCM scoring is 100% specific to MDS in our cohorts, and thus the likelihood ratio is infinity. However, the sensitivity of the test is reduced. In detecting low-grade MDS patients without conventional markers, the sensitivity was 23% and 35% (four- and seven-parameter methods, respectively) in the Japanese and 24% and 45% (four- and seven-parameter methods, respectively) in the Italian cohorts. Other combinations of parameters, e.g., CD34+ myeloblasts (%) and CD11b, CD15, and CD56 expression (%) on CD34+ myeloblasts, did not improve the diagnostic power.
Analysis of clinical parameters in association with flow cytometry scores in patients in the prospective cohorts
Age and gender do not affect low-grade MDS detection using the FCM score, because they did not differ between non-clonal cytopenic patients with an FCM score of 1 or 2 and those with an FCM score of 0 in either cohort, and did not differ between low-grade MDS patients with an FCM score of less than 2 and those with an FCM score of 2 or more. The analysis of clinical parameters in association with FCM scores in MDS patients is summarized in the Online Supplementary Table S3. In the Italian cohort, the presence of multilineage dysplasia was associated with a high prevalence of FCM scores of 2 or more, but this was not the case in the Japanese cohort. A common phenomenon in both cohorts was that MDS patients for whom a poor prognosis was indicated by the IPSS or WPSS were associated with a high prevalence of FCM scores of 2 or more. Karyotype categories and transfusion dependency, which are components in the IPSS and/or WPSS, were not associated with the FCM score. The presence or absence of chromosomal aberrations as well as the presence or absence of RS (> 15% of erythroblasts) was not associated with the FCM score in either cohort (p>0.12 in all analyses).
Discussion
To enable this FCM protocol to be applied in many laboratories with acceptable data variation, our method has the following characteristics: (i) three-color FCM was adopted rather than four or more-color FCM, because numerous laboratories use the former and data reproduction is probably easier with this; (ii) four cardinal parameters, which can be analyzed from a single cell aliquot and show little interlaboratory variability, were adopted.
The rationale for choosing our seven parameters was as follows: two major components in marrow CD34+ cells behave in an opposing fashion in MDS: CD34+ myeloblasts increase while CD34+ B cells decrease when compared with marrow samples from control individuals.10 Therefore, analyzing these two cell populations separately rather than analyzing all CD34+cells is more accurate and sensitive in detecting MDS-related changes. While other methods can be used to measure CD34+ B cells, such as examining CD34+CD19+ cells, we adopted the present method because data variability appears to be lower than with other methods.23
The Ly/Mbl CD45 ratio and the newly introduced Gra/Ly SSC peak channel ratio ensured the reproducibility of data by adjusting data on target cells with data on lymphocytes in the same sample. Although other groups reported that myeloblast CD45 expression decreases in a fraction of MDS patients,27,28 by analyzing the Ly/Mbl CD45 ratio, we showed that myeloblast CD45 expression can increase or decrease in MDS. It was reported that the SSC of granulocytic cells decreases in a variable proportion of MDS patients.6,27,29 In preliminary experiments, we found that data on the SSC peak channel were more useful in discriminating MDS from controls rather than other SSC-related data, such as mean SSC value. Also, we targeted CD10− granulocytic cells rather than all granulocytic cells in analyzing the Gra/Ly SSC peak channel ratio. There was no significant difference in the discriminating power between the Gra/Ly SSC peak channel ratios using CD10− granulocytic cells and using all granulocytic cells (data not shown). However, using the former might be beneficial to analyze samples contaminated significantly with PB, because most circulating neutrophils express CD10.30
In addition to the above cardinal parameters, we adopted three others, CD15, CD11b, and CD56 expression of CD34+ myeloblasts. MDS myeloblasts often show dysregulated expression of a variety of antigens.10,31 Among these antigens, we selected CD15, CD11b, and CD56 based on the frequency of dysregulation and feasibility of discrimination between antigen-positive and antigen-negative cells. Finally, it should be noted that none of our parameters, except for CD34+ myeloblasts (%), was affected by contamination with PB. Collecting data from a sufficient number of CD34+ cells provides necessary data on CD34-related parameters even if BM samples are diluted with PB. However, the dilution can influence all nucleated cells assessed by FCM and thus may cause falsely low CD34+ myeloblasts (%) data.
By applying these quantitative FCM parameters, we determined their RRs, which enabled us to judge the prospective FCM data objectively. We used non-clonal cytopenic patients as controls, rather than healthy individuals and patients with clonal diseases other than MDS, because non-clonal cytopenic patients are the main population that must be differentiated from MDS in clinical practice.4,5 At the same time, our MDS patients included low-grade subtypes alone. In particular, the prospective cohorts included 81 low-grade MDS patients without conventional markers. This is the largest study analyzing this MDS category using diagnostic FCM. We found that our scoring discriminated low-grade MDS patients from non-clonal cytopenic patients with likelihood ratios suitable for diagnosis. Likelihood ratios with pre- and post-test probabilities, which are capable of refining clinical diagnoses,24,32 may be underused in clinical practice.33 If patients with a 50% pre-test probability of having MDS are positive for a diagnostic test with a likelihood ratio of 8–10, the post-test probability of having MDS is greater than 90%.24 We would like to emphasize the importance of the diagnostic strategy using likelihood ratios, because FCM data in low-grade MDS are usually not absolutely specific, in contrast to other specific data in MDS, i.e., blast excess and cytogenetic aberrations.
There was a difference in the diagnostic power of the present FCM method between the Japanese and Italian cohorts. We think that although our parameters and their RRs have advantages for general use, fine-tuning of the RRs in each laboratory may further improve the diagnostic power. It should also be noted that FCM abnormalities may differ between ethnic groups as does cytomorphological dysplasia.26 It is also noted that, in addition to the scoring that we used in this paper, the degree of abnormality of flow data might help diagnose low-grade MDS. Data from six parameters other than CD34+ B cells (%) showed Gaussian distribution in patients with non-clonal cytopenia (Figure 2). Therefore, it is expected that if data on these parameters deviate more from the RRs, the diagnosis of low-grade MDS is more certain. Furthermore, only one-parameter data would be diagnostic if they deviated profoundly from RRs. This approach is worthy of examination in future prospective studies.
Finally, FCM scores were associated with IPSS and/or WPSS but not with the karyotype categories or the presence or absence of karyotype abnormality in our cohorts. This suggests the possibility that our FCM protocol detects MDS-related abnormality that cannot be detected by chromosomal analysis and is linked with prognosis, as suggested by other studies.31,34,35 Further study is required to verify whether the present FCM protocol has prognostic merit in MDS.
In conclusion, we showed a relatively simple, and thus applicable in many laboratories, FCM protocol for discriminating between low-grade MDS without conventional markers and non-clonal cytopenias. Further study is needed to improve the diagnostic power of this protocol.
Acknowledgments
we would like to thank all clinicians, technical staff, and patients of our institutions for their support of the work. We would further thank Kenji Takai, SRL, Inc. for his expertise on FCM and Mario Cazzola, University of Pavia Medical School and Fondazione IRCCS Policlinico San Matteo, Pavia for his thoughtful advice and support.
Footnotes
The online version of this article contains a supplementary appendix.
Authorship and Disclosures
KO conceived and designed the study, collected and analyzed data, and wrote the manuscript. MGDP designed the study, collected and analyzed data, and contributed to the writing of the manuscript. LM designed the study, collected and analyzed data, and edited the manuscript. CP, NY, AM, TY, HT, JT, and KD collected and analyzed data.
The authors reported no potential conflicts of interest.
Funding: the Pavia investigations were supported by grants from the Associazione Italiana per la Ricerca sul Cancro (AIRC) and Fondazione IRCCS Policlinico San Matteo to Mario Cazzola.
References
- 1.Mufti GJ. Pathobiology, classification, and diagnosis of myelodysplastic syndrome. Best Pract Res Clin Haematol. 2004;17:543–57. doi: 10.1016/j.beha.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 2.Cazzola M, Malcovati L. Myelodysplastic syndromes--coping with ineffective hematopoiesis. N Engl J Med. 2005;352:536–8. doi: 10.1056/NEJMp048266. [DOI] [PubMed] [Google Scholar]
- 3.Hamblin TJ. Epidemiology of the myelodysplastic syndromes. In: Bennett JM, editor. The Myelodysplastic Syndromes. New York: Marcel Dekker; 2002. pp. 15–27. [Google Scholar]
- 4.Heaney ML, Golde DW. Myelodysplasia. N Engl J Med. 1999;340:1649–60. doi: 10.1056/NEJM199905273402107. [DOI] [PubMed] [Google Scholar]
- 5.Kouides PA, Bennett JM. Understanding the myelodysplastic syndromes. Oncologist. 1997;2:389–401. [PubMed] [Google Scholar]
- 6.Stetler-Stevenson M, Arthur DC, Jabbour N, Xie XY, Molldrem J, Barrett AJ, et al. Diagnostic utility of flow cytometric immunophenotyping in myelodysplastic syndrome. Blood. 2001;98:979–87. doi: 10.1182/blood.v98.4.979. [DOI] [PubMed] [Google Scholar]
- 7.Malcovati L, Della Porta MG, Lunghi M, Pascutto C, Vanelli L, Travaglino E, et al. Flow cytometry evaluation of erythroid and myeloid dysplasia in patients with myelodysplastic syndrome. Leukemia. 2005;19:776–83. doi: 10.1038/sj.leu.2403680. [DOI] [PubMed] [Google Scholar]
- 8.Cherian S, Moore J, Bantly A, Vergilio JA, Klein P, Luger S, et al. Peripheral blood MDS score: a new flow cytometric tool for the diagnosis of myelodysplastic syndromes. Cytometry B Clin Cytom. 2005;64:9–17. doi: 10.1002/cyto.b.20041. [DOI] [PubMed] [Google Scholar]
- 9.Della Porta MG, Malcovati L, Invernizzi R, Travaglino E, Pascutto C, Maffioli M, et al. Flow cytometry evaluation of erythroid dysplasia in patients with myelodysplastic syndrome. Leukemia. 2006;20:549–55. doi: 10.1038/sj.leu.2404142. [DOI] [PubMed] [Google Scholar]
- 10.Ogata K, Kishikawa Y, Satoh C, Tamura H, Dan K, Hayashi A. Diagnostic application of flow cytometric characteristics of CD34+ cells in low-grade myelodysplastic syndromes. Blood. 2006;108:1037–44. doi: 10.1182/blood-2005-12-4916. [DOI] [PubMed] [Google Scholar]
- 11.Valent P, Horny HP, Bennett JM, Fonatsch C, Germing U, Greenberg P, et al. Definitions and standards in the diagnosis and treatment of the myelodysplastic syndromes: consensus statements and report from a working conference. Leuk Res. 2007;31:727–36. doi: 10.1016/j.leukres.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Loken MR, van de Loosdrecht A, Ogata K, Orfao A, Wells DA. Flow cytometry in myelodysplastic syndromes: report from a working conference. Leuk Res. 2008;32:5–17. doi: 10.1016/j.leukres.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 13.Satoh C, Dan K, Yamashita T, Jo R, Tamura H, Ogata K. Flow cytometric parameters with little interexaminer variability for diagnosing low-grade myelodysplastic syndromes. Leuk Res. 2008;32:699–707. doi: 10.1016/j.leukres.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 14.ELN Diagnostic Guidelines. Proposal for standardized diagnostic and prognostic procedures in the myelodysplastic syndromes, mixed myelodysplastic/myeloproliferative disorders, and acute myeloid leukemia. [Accessed December 24, 2008];LeukemiaNet WP8 MDS. 2007-09-26 http://www.leukemianet.org/content/leukemias/mds/standards_sop/
- 15.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 16.Malcovati L, Della Porta MG, Pascutto C, Invernizzi R, Boni M, Travaglino E, et al. Prognostic factors and life expectancy in myelodysplastic syndromes classified according to WHO criteria: a basis for clinical decision making. J Clin Oncol. 2005;23:7594–603. doi: 10.1200/JCO.2005.01.7038. [DOI] [PubMed] [Google Scholar]
- 17.Mitelman F ISCN. An International System for Human Cytogenetic Nomenclature. Basel: Karger; 1995. 1995. [Google Scholar]
- 18.Greenberg P, Cox C, LeBeau MM, Fenaux P, Morel P, Sanz G, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–88. [PubMed] [Google Scholar]
- 19.Malcovati L, Germing U, Kuendgen A, Della Porta MG, Pascutto C, Invernizzi R, et al. Time-dependent prognostic scoring system for predicting survival and leukemic evolution in myelodysplastic syndromes. J Clin Oncol. 2007;25:3503–10. doi: 10.1200/JCO.2006.08.5696. [DOI] [PubMed] [Google Scholar]
- 20.Baumgarth N, Roederer M. A practical approach to multicolor flow cytometry for immunophenotyping. J Immunol Methods. 2000;243:77–97. doi: 10.1016/s0022-1759(00)00229-5. [DOI] [PubMed] [Google Scholar]
- 21.Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry. 2001;45:194–205. doi: 10.1002/1097-0320(20011101)45:3<194::aid-cyto1163>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 22.Loken MR, Wells DA. Normal antigen expression in hematopoiesis Basis for interpreting leukemia phenotypes. In: Stewart CC, Nicholson JKA, editors. Immunophenotyping. New York, NY: Wiley-Liss; 2000. pp. 133–60. [Google Scholar]
- 23.Ogata K. Diagnostic flow cytometry for low-grade myelodysplastic syndromes. Hematol Oncol. 2008;26:193–8. doi: 10.1002/hon.857. [DOI] [PubMed] [Google Scholar]
- 24.Grimes DA, Schulz KF. Refining clinical diagnosis with likelihood ratios. Lancet. 2005;365:1500–5. doi: 10.1016/S0140-6736(05)66422-7. [DOI] [PubMed] [Google Scholar]
- 25.Leemis LM, Trivedi KS. A comparison of approximate interval estimators for the Bernoulli parameter. The American Statistician. 1996;50:63–8. [Google Scholar]
- 26.Matsuda A, Germing U, Jinnai I, Misumi M, Kuendgen A, Knipp S, et al. Difference in clinical features between Japanese and German patients with refractory anemia in myelodysplastic syndromes. Blood. 2005;106:2633–40. doi: 10.1182/blood-2005-01-0040. [DOI] [PubMed] [Google Scholar]
- 27.Wells DA, Benesch M, Loken MR, Vallejo C, Myerson D, Leisenring WM, et al. Myeloid and monocytic dyspoiesis as determined by flow cytometric scoring in myelodysplastic syndrome correlates with the IPSS and with outcome after hematopoietic stem cell transplantation. Blood. 2003;102:394–403. doi: 10.1182/blood-2002-09-2768. [DOI] [PubMed] [Google Scholar]
- 28.Pirruccello SJ, Young KH, Aoun P. Myeloblast phenotypic changes in myelodysplasia. CD34 and CD117 expression abnormalities are common. Am J Clin Pathol. 2006;125:884–94. doi: 10.1309/j3et7rxd1x4bkdlf. [DOI] [PubMed] [Google Scholar]
- 29.Kussick SJ, Fromm JR, Rossini A, Li Y, Chang A, Norwood TH, et al. Four-color flow cytometry shows strong concordance with bone marrow morphology and cytogenetics in the evaluation for myelodysplasia. Am J Clin Pathol. 2005;124:170–81. doi: 10.1309/6PBP-78G4-FBA1-FDG6. [DOI] [PubMed] [Google Scholar]
- 30.McCormack RT, Nelson RD, LeBien TW. Structure/function studies of the common acute lymphoblastic leukemia antigen (CALLA/CD10) expressed on human neutrophils. J Immunol. 1986;137:1075–82. [PubMed] [Google Scholar]
- 31.Ogata K, Nakamura K, Yokose N, Tamura H, Tachibana M, Taniguchi O, et al. Clinical significance of phenotypic features of blasts in patients with myelodysplastic syndrome. Blood. 2002;100:3887–96. doi: 10.1182/blood-2002-01-0222. [DOI] [PubMed] [Google Scholar]
- 32.Tosetto A, Castaman G, Rodeghiero F. Evidence-based diagnosis of type 1 von Willebrand disease: a Bayes theorem approach. Blood. 2008;111:3998–4003. doi: 10.1182/blood-2007-08-105940. [DOI] [PubMed] [Google Scholar]
- 33.Reid MC, Lane DA, Feinstein AR. Academic calculations versus clinical judgments: practicing physicians’ use of quantitative measures of test accuracy. Am J Med. 1998;104:374–80. doi: 10.1016/s0002-9343(98)00054-0. [DOI] [PubMed] [Google Scholar]
- 34.van de Loosdrecht AA, Westers TM, Westra AH, Drager AM, van der Velden VH, Ossenkoppele GJ. Identification of distinct prognostic subgroups in low- and intermediate-1-risk myelodysplastic syndromes by flow cytometry. Blood. 2008;111:1067–77. doi: 10.1182/blood-2007-07-098764. [DOI] [PubMed] [Google Scholar]
- 35.Matarraz S, Lopez A, Barrena S, Fernandez C, Jensen E, Flores J, et al. The immunophenotype of different immature, myeloid and B-cell lineage-committed CD34+ hematopoietic cells allows discrimination between normal/reactive and myelodysplastic syndrome precursors. Leukemia. 2008;22:1175–83. doi: 10.1038/leu.2008.49. [DOI] [PubMed] [Google Scholar]