Respiratory virus infections are important causes of morbidity and mortality after hematopoietic cell transplantation. Their clinical course can be severe with progression to lower respiratory tract infection, co-infection with serious pulmonary co-pathogens, and high mortality. The findings of this retrospective cohort study indicate that viral lower respiratory tract infection during the first 100 days after hematopoietic cell transplantation was less common among patients undergoing non-myeloablative conditioning regimens than in those receiving myeloablative conditioning, despite a similar overall rate of acquisition.
Keywords: respiratory virus, stem cell transplantation
Abstract
Background
Respiratory virus infections are important causes of morbidity and mortality after hematopoietic cell transplantation. Their clinical course can be severe with progression to lower respiratory tract infection, co-infection with serious pulmonary co-pathogens, and high mortality. Non-myeloablative conditioning regimens achieve engraftment without eradication of host hematopoietic cells, which potentially allows for protection against infections commonly seen in hematopoietic cell transplantation patients treated with standard intensity conditioning regimens.
Design and Methods
We performed a retrospective cohort study to measure the incidence and severity of parainfluenza types 1–4, influenza (A and B), respiratory syncitial virus and human rhinovirus disease in myeloablative versus non-myeloablative versus autologous hematopoietic cell transplantation patients.
Results
The incidences of all respiratory virus infections were similar in the non-myeloablative and myeloablative cohorts but less in the autologous cohort (33/420 [7.9%], 150/1593 [9.4%], and 37/751 [4.9%], respectively, p<0.0001). However, respiratory virus lower tract infections were significantly less common during the first 100 days after transplantation in non-myeloablative patients compared to myeloablative and autologous patients (1/420 [0.2%], 34/1593 [2.1%] and 16/751 [2.1%], respectively, p=0.005. Respiratory virus lower tract infection had high co-infection and attributable mortality rates.
Conclusions
Respiratory virus lower tract infection during the first 100 days after hematopoietic cell transplantation was less common in persons receiving non-myeloablative conditioning regimens compared to myeloablative conditioning, despite a similar overall rate of acquisition.
Introduction
Community-acquired respiratory infections (parainfluenza 1–4, influenza A and B, respiratory syncitial virus, and rhinovirus) are important causes of morbidity and mortality after hematopoietic cell transplantation.1–5 Though the incidence of these infections in hematopoietic cell transplantation patients usually parallels that in the community, their course can be more severe with progression to lower respiratory tract infection, co-infection with serious pulmonary co-pathogens such as invasive molds, and late airflow decline.3,6–8
Allogeneic hematopoietic cell transplantation with non-myeloablative or reduced-intensity conditioning regimens is an increasingly common modality in patients who because of age or co-morbidity are not eligible for conventional transplantation.9–11 Non-myeloablative conditioning regimens achieve engraftment with less toxicity and host hematopoietic cells are not immediately eradicated. This potentially allows for a period of protection against infections commonly seen in hematopoietic cell transplantation patients treated with standard intensity conditioning; patients who are placed on non-myeloablative protocols are more frequently followed as outpatients than patients undergoing myeloablative transplantation. However, graft-versus-host disease and its therapies continue to be significant risk factors for invasive cytomegalovirus and fungal infection in patients undergoing non-myeloablative transplantation, particularly in the late period after conditioning.12,13
It is unknown whether non-myeloablative conditioning has an effect on acquisition of respiratory virus infection, likelihood of respiratory virus associated lower tract infection, risk of progression of disease from upper to lower tract infection, or mortality rate associated with respiratory virus infection. We performed a retrospective cohort study to measure the incidence and severity of these infections in myeloablative versus non-myeloablative hematopoietic cell transplantation patients.
Design and Methods
Patient population
From December 1997 through March 2005, 2,453 patients underwent myeloablative hematopoietic cell transplantation at the FHCRC (1,620 allogeneic, 767 autologous/syngeneic, and 66 tandem: i.e. autologous followed by planned non-myeloablative hematopoietic cell transplantation six months later). This cohort included 1,111 women and 1,342 men with a mean age of 40.5 years (range 0.5–73.8). Indications for transplantation included acute leukemia (30%), chronic leukemia (18%), lymphoma (15%), myelodysplastic syndrome (14%), myeloma (10%), solid tumor (6%), aplastic anemia (2%) and other causes (4%).
Four hundred and forty-eight patients underwent non-myeloablative hematopoietic cell transplantation, including the 66 tandem transplants described above. Tandem patients were analyzed in the non-myeloablative cohort. This cohort included 172 women and 276 men with a mean age of 49.5 years (range 0.5–74.5). Indications for transplantation included acute leukemia (26%), chronic leukemia (12%), lymphoma (26%), myelodysplastic syndrome (8%), myeloma (19%), solid tumors (4%), and other causes (4%).
For myeloablative patients, conditioning consisted of cyclophosphamide plus either 12–16-Gy total body irradiation or busulfan targeted to 800–900 ng/mL. Post-transplant immunosuppression usually consisted of cyclosporine plus methotrexate. Non-myeloablative patients were conditioned with 2-Gy total body irradiation alone, or preceded by fludarabine (90 mg/m2/dose). Post-transplant immunosuppression consisted of cyclosporine or tacrolimus, and mycophenolate mofetil. Autologous hematopoietic cell transplantation recipients were typically conditioned with cyclophosphamide (100 mg/kg), etoposide (60 mg/kg) and fractionated 12-Gy total body irradiation.14–16
Hematopoietic cell transplantation patients were treated as inpatients from the start of conditioning until recovery from neutropenia. Thereafter, they were followed twice weekly in the outpatient department until 100 days after transplantation, when they were returned to the care of their referring physician. Autologous hematopoietic cell transplantation patients returned to their referring physician 60 days after transplantation.
We reviewed virology records and identified all transplantation recipients with isolation of parainfluenza 1–4, influenza A–B, respiratory syncitial virus or human rhino virus from respiratory secretions, demonstration of viral antigen by direct fluorescent antibody, positive conventional tube culture, or positive shell vial centrifugation cultures (respiratory syncitial virus only); infections before conditioning were excluded. We extracted data from a prospectively entered database. Data from the first 100 days after hematopoietic cell transplantation were from FHCRC databases. Long-term follow-up data were gathered from FHCRC databases and from follow-up medical records and structured correspondence letters from physicians outside of the FHCRC.
Virology and microbiology procedures
A nasopharyngeal-throat wash or swab for viral direct fluorescent antibody staining and viral culture was standard practice for all patients with upper respiratory infection symptoms throughout the study period. Direct fluorescent antibody and cultures were performed on bronchoalveolar lavage, lung biopsy, and autopsy specimens. Culture, hemadsorption and direct fluorescent antibody techniques are described elsewhere.7
All bronchoalveolar, biopsy, and autopsy specimens were submitted for routine bacterial, fungal, and acid-fast bacilli cultures, Aspergillus Galactomannan EIA and direct fluorescent antibody staining, and Legionella species culture. Cytospins were performed for cytomegalovirus and herpes simplex virus. Shell vial cultures were performed for cytomegalovirus and respiratory syncitial virus. Tube cultures were inoculated into four cell lines that support the growth of herpes viruses and respiratory viruses.7
Definitions
Parainfluenza, influenza, respiratory syncitial virus and rhinovirus upper tract infections were defined as isolation of virus from nasopharyngeal-throat wash by culture or evidence of viral specific antigen by direct fluorescent antibody in conjunction with consistent symptoms, without the presence of a new infiltrate on chest radiography. Parainfluenza, influenza, respiratory syncitial virus and rhinovirus lower tract infections were defined as isolation of virus by culture, evidence of viral specific antigen by direct fluorescent antibody, or positive respiratory syncitial virus shell vial centrifugation culture, from bronchoalveolar lavage or lung biopsy specimens in association with symptoms and a new radiographic infiltrate. Lower tract infections included cases where virus was first detected in the upper respiratory tract and then detected on lavage or lung biopsy. An upper tract infection was considered to have progressed to the lower tract if the virus was detected in the lower tract more than 48 h after the upper tract.
The presence of a co-pathogen was defined as isolation of a respiratory virus plus a pathogenic bacterium, mold or opportunistic virus from the same lavage or lung biopsy specimen, or from lavage or lung biopsy specimen obtained within a week of respiratory virus lower tract infection diagnosis. Attributable mortality was defined as progressive respiratory failure in the presence of respiratory virus lower tract infection and the absence of other conditions thought by the oncologist or pathologist to have contributed to the patient’s death.
Management
Once positive by nasopharyngeal-throat wash or bronchoalveolar lavage for a respiratory virus, patients were isolated to prevent transmission to staff and other patients. Patients underwent repeat nasopharyngeal-throat wash on the day following identification of infection and weekly to document clearance.
Treatment with aerosolized ribavirin for respiratory syncitial virus and parainfluenza lower tract infection was performed at the discretion of the treating physician. The dose and duration, 2 g (60 mg/mL) administered 3 times daily over two hours via nebulizer for 7–10 days, was per FHCRC protocol.17 Respiratory syncitial virus lower tract infection patients received respiratory syncitial virus specific monoclonal antibody at the discretion of the attending physician. A subset of parainfluenza lower tract infection patients also received 3–5 doses of intravenous immune globulin (0.5 g/kg) given every other day; others had their immunosuppression decreased. Oseltamivir became available for influenza in 1999 and was used thereafter. Patients were treated with appropriate antibiotics or antifungal agents directed at co-pathogens.
Statistical analysis
We measured the incidence of upper and lower tract infection in allogeneic myeloablative versus allogeneic non-myeloablative versus autologous hematopoietic cell transplantation patients during the first 100 days after transplant. We assumed that all patients contributed 100 days to the analysis because 100-day survival after myeloablative and non-myeloablative transplantation exceeds 90% and is equal in both groups.18–21 p-values were derived from a one-way Fisher’s exact test.
For determination of risk factors for acquisition of parainfluenza lower tract infection, or for progression from parainfluenza upper to lower tract infection, a logistic regression model was fit with respiratory virus lower tract infection as outcome and day zero as date of transplant. p values from regression models were obtained using the Wald test. No adjustments were made for multiple comparisons.
Results
Respiratory virus infection incidence
From December 1997 through March 2005, 8.0% (233/2901) of hematopoietic cell transplantation patients developed respiratory virus infections within 100 days of transplantation. Median times [range] to infection with parainfluenza, influenza, respiratory syncitial virus and rhinovirus infection were 46 [1–100], 55 [2–98], 60 [5–94] and 40 [6–99] days, respectively.
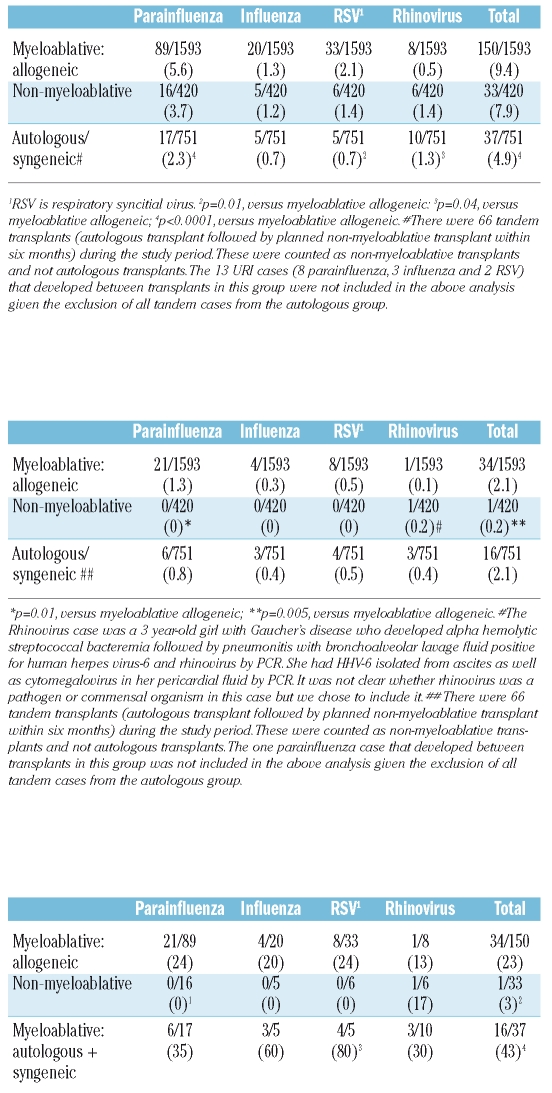
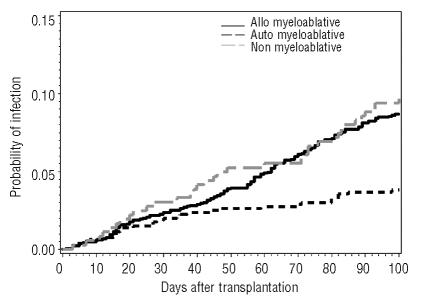
The incidence of respiratory virus infections was slightly lower for hematopoietic cell transplantation patients following non-myeloablative conditioning compared with myeloablative conditioning though this did not reach statistical significance. The incidence of respiratory virus infection was higher in allogeneic hematopoietic cell transplantation patients than autologous patients (Table 1A). The cumulative incidence in the autologous cohort tracked that of the allogeneic myeloablative cohort until day 50 (Figure 1). We expected this trend because data for autologous transplantation patients are less reliable beyond 60 days after transplantation because these patients are typically returned to their outside providers. Parainfluenza was the most common infection followed by respiratory syncitial virus, influenza and rhinovirus (Table 1A).
Table 1A.
Incidence of any respiratory virus infection (upper and lower tract infection) in hematopoietic stem cell transplant patients (per 100-person days).
Figure 1.
100-day cumulative incidence of respiratory virus infection following myeloablative, non-myeloablative and autologous/syngeneic hematopoietic stem cell transplantation.*
Influenza infection occurred predominately during the winter; RSV infection typically occurred during late winter; parainfluenza infection occurred year round. We detected no difference in seasonal patterns between non-myeloablative and myeloablative cohorts (data not shown).
Lower respiratory tract infection incidence
Of the respiratory tract disease cases, 22% (52/233) were lower tract infection. Only one case of lower tract infection was in a patient who underwent non-myeloablative conditioning. The causative agent in this case was rhinovirus whose role as an uncommon but potentially lethal lung pathogen in hematopoietic cell transplantation patients has become clear.22
The incidence of respiratory virus lower tract infection was lower in patients who underwent non-myeloablative conditioning compared to patients who underwent myeloablative conditioning followed by either an allogeneic or autologous transplantation (Table 1B). The percentages of viral respiratory tract infections resulting in lower tract infection were significantly lower in patients in the non-myeloablative cohort compared to the allogeneic myeloablative cohorts. The percentage of cases of viral respiratory tract infection resulting in lower tract infection was significantly higher in the autologous cohort compared to the allogeneic myeloablative cohort, probably due to the artificially low cumulative incidence of total respiratory infection in autologous patients (Table 1C). Most of the difference in lower tract infection between non-myeloablative and myeloablative cohorts was due to difference in incidence of parainfluenza infection.
Table 1B.
Incidence of respiratory virus lower tract infection in hematopoietic stem cell transplant patients (per 100-person days).
Table 1C.
Percentage of total respiratory virus cases in the first 100 days after hematopoietic stem cell transplant that represent lower tract infection (%).
p=0.02, versus myeloablative allogeneic;
p=0.005, versus myeloablative allogeneic;
p=0.03, versus myeloablative allogeneic;
p=0.01, versus myeloablative allogeneic.
Clinical characteristics of patients with parainfluenza virus upper and lower tract infection
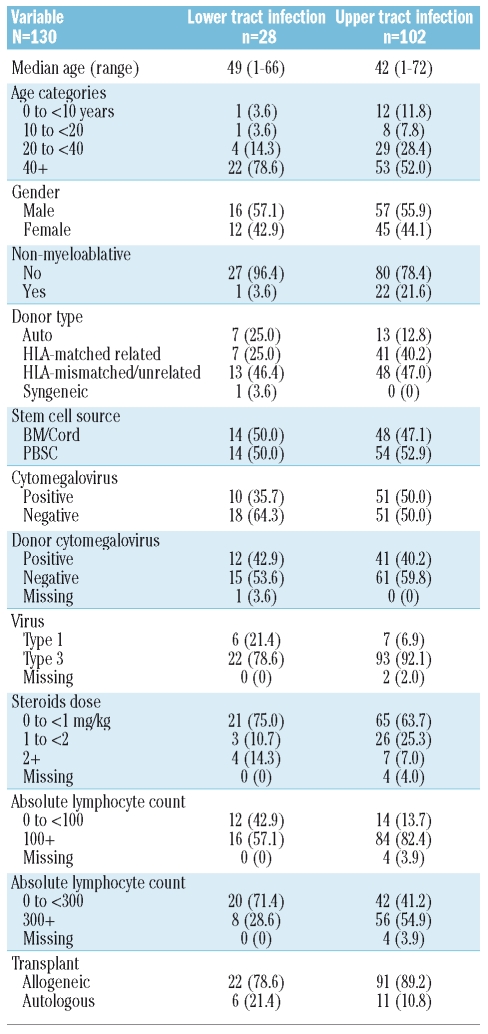
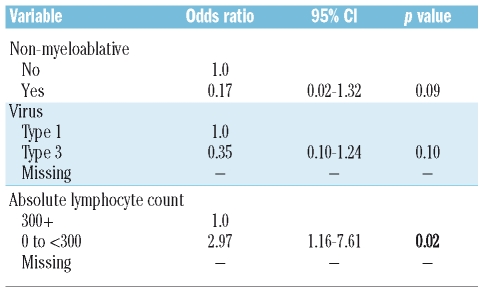
Clinical characteristics were available for 28 patients with parainfluenza lower tract infection and 102 patients with upper tract infection (Table 2). In univariate logistic regression analysis, type-1 parainfluenza (p=0.03) and low absolute lymphocyte count (300 cells/mm3) on the first day of infection (p<0.01) correlated with higher likelihood of lower tract infection. Non-myeloablative conditioning was associated with a non-significant lower likelihood of lower tract infection (p=0.06). In multivariate analysis, absolute lymphocyte count less than 300 cells/mm3 correlated with increased risk for lower tract infection (Table 3).
Table 2.
Characteristics of stem cell transplant recipients who developed parainfluenza virus lower tract infection or upper tract infection.
Table 3.
Factors associated with the development of parainfluenza virus lower tract infection among hematopoietic cell transplant recipients. Multivariate logistic regression results for lower tract versus upper tract parainfluenza infection.
Clinical characteristics of patients who progressed from parainfluenza upper to lower tract infection
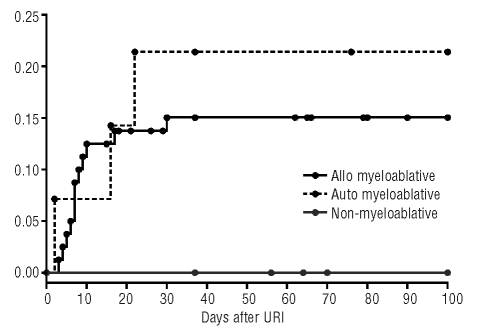
For all respiratory viruses, likelihood of progression from upper to lower tract infection (lower respiratory tract infection more than 48 h after diagnosis of upper tract infection) was 3% (1/33) in non-myeloablative patients compared to 14% (19/135) in allogeneic myeloablative patients (p=0.06) and 19% (5/26) in autologous patients. For parainfluenza virus, the likelihood of progression from upper to lower tract infection was 0% (0/16) in non-myeloablative patients compared to 15% (12/80) in allogeneic myeloablative patients (p=0.10) and 21% (3/14) in autologous patients (Figure 2).
Figure 2.
100-day progression from parainfluenza virus upper respiratory tract infection to lower respiratory tract infection.
According to univariate logistic regression analysis, absolute lymphocyte count < 300/mm3 on first day of infection (hazard ratio = 5.33 [1.42, 20.10], p=0.01), but not type of conditioning or corticosteroid dose correlated with progression to lower tract infection; parainfluenza virus type 3 infection was less likely to progress to lower tract infection than parainfluenza virus type 1 infection (hazard ratio=0.21 [0.05–0.82], p=0.03).
Morbidity and mortality of respiratory virus lower respiratory tract infections
Respiratory virus lower tract infections were notable for high rates of co-infection, mechanical ventilation, and attributable mortality. Forty-one percent (11/27), 100% (7/7) and 25% (3/12) of parainfluenza, influenza and respiratory syncitial virus infections were notable for isolation of another virulent pathogen. Pathogens included Aspergillus species (n=11), cytomegalovirus (n=10), Pseudomonas species (n=3), Legionella species (n=2), Stenotrophomonas (n=1), Mirabella catarrhalis (n=1) and Klebsiella pneumoniae (n=1). Cytomegalovirus was isolated in 5/7 (71%) of influenza lower tract infections compared to 3/28 (11%) parainfluenza lower tract infections. Aspergillus species were isolated in 3/7 (43%) of influenza lower tract infections compared to 6/28 (21%) parainfluenza infections.
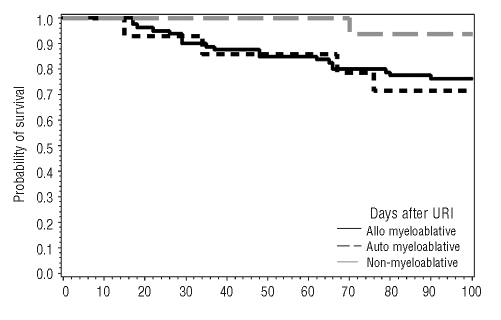
Rates of mechanical ventilation, and lower respiratory infection-attributable mortality were 29% (8/28) and 46% (13/28) for parainfluenza, 66% (4/6) and 100% (7/7) for influenza, and 18% (2/11) and 0% (0/12) for respiratory syncitial virus infection. Fewer patients who developed viral upper respiratory tract infection after non-myeloablative conditioning died within 100 days of infection than patients who developed upper respiratory tract infection after myeloablative conditioning (Figure 3).
Figure 3.
Time to death for allogeneic myeloablative, autologous myeloablative, and non-myeloablative hematopoietic stem cell patients following diagnosis of upper respiratory virus infection.
All (9/9) respiratory syncitial virus lower tract infections and 46% (12/26) of parainfluenza infections were treated with inhaled ribavirin. Seventeen of 26 (65%) parainfluenza patients received intravenous immunoglobulin therapy. Three of five (60%) of influenza cases received oseltamivir while 4/5 (80%) received amantadine or rimantadine therapy. Three of 9 (33%) respiratory syncitial virus infected patients received intravenous immunoglobulin or respiratory syncitial virus-directed immunoglobulin. Eleven percent (14/129), 0% (0/129), 89% (115/129) and 0.8% (1/129) of serotyped parainfluenza cases were types-1, -2, -3 and -4, respectively. Seventy-five percent (46/61) and 25% (15/61) of typed influenza cases were influenza A and influenza B, respectively. There were not enough data to compare clinical outcomes between viral subtypes.
Respiratory virus infection incidence more than 100 days after hematopoietic cell transplantation
For the total duration of follow-up, 12.5% (363/2,901) of patients had respiratory virus disease and cumulative incidences of total respiratory virus infection more than 100 days after hematopoietic cell transplantation were not significantly different for patients following non-myeloablative 24/448 (5.4%) and myeloablative plus autologous transplantation 106/2453 (4.3%). Cumulative incidences of total lower tract infection occurring more than 100 days after transplantation also did not differ between the two groups [5/448 (1.1%) vs. 30/2453 (1.2%)]. Cumulative incidences of parainfluenza lower tract infection in patients undergoing non-myeloablative conditioning 1/448 (0.22%) were similar compared to 8/2,453 (0.33%) in patients undergoing autologous or myeloablative conditioning. Due to the small number of cases of lower tract infection and the inconsistent work-up of upper respiratory symptoms, we were not able to perform a risk factor analysis for respiratory virus lower tract infection after day 100, nor were we able to separate myeloablative conditioning from autologous conditioning in the analysis.
Discussion
Our results indicate that during the first 100 days after hematopoietic cell transplantation, allogeneic non-myeloablative and myeloablative transplantation patients had similar rates of respiratory virus infection. Patients undergoing autologous hematopoietic cell transplantation had a smaller incidence of respiratory virus infection. However, cumulative incidences of respiratory virus infection during the first 50 days in allogeneic myeloablative and autologous cohorts were nearly identical: the reason for the lower cumulative incidence of infection in autologous transplantation at 100 days is likely truncated follow-up in our system after transplant, rather than a biological effect. Typical seasonality for all three viruses was demonstrated, though the pattern of seasonal infection was no different between myeloablative and non-myeloablative cohorts.
Despite comparable numbers of infections, the incidence of lower tract infection during the first 100 days after hematopoietic cell transplantation was lower in patients who underwent non-myeloablative conditioning, with a non-significant decrease in the incidence of parainfluenza lower tract infection. Only one patient who received non-myeloablative conditioning developed lower tract infection during the first 100 days after transplantation. The absence of parainfluenza, influenza and respiratory syncitial virus lower tract infections in non-myeloablative transplantation patients was consistent with our hypothesis that persistence of host T cells might result in protection against serious infections, though more evidence will be needed to definitively prove this point.23,24 Our data revealed equal incidences of respiratory virus infections and lower tract infections among non-myeloablative and myeloablative cohorts beyond 100 days after transplantation. This signified a limited duration of protective immunity in non-myeloablative cohorts The transition from a preponderance of early-onset to late-onset infections has been described for invasive fungi12 and cytomegalovirus.13
Parainfluenza infection accounted for the statistical difference between the two cohorts and was the most common virus isolated. High doses of corticosteroids for graft versus host disease and lymphopenia were risk factors for progression from parainfluenza upper to lower tract infection, perhaps due to a decrease in T-cell immunity.7 In our current analysis, corticosteroid dose did not correlate with progression to lower tract infection, possibly based on our policy to attempt to reduce steroid doses in patients infected with parainfluenza that was introduced following analysis of our earlier cohort.7 Another risk factor for lower tract infection was parainfluenza type-1 infection (rather than type 3). Though more clinical data are needed to confirm this finding, it was a novel observation and might suggest that factors related to pathogen, and not just host, predict severity of disease.
Respiratory virus pneumonia carried a high mortality rate and was associated with invasive molds, pathogenic bacteria and viruses seen in immunocompromised hosts. The particularly high rate of cytomegalovirus infection in cases of influenza was notable as were the generally poor outcomes in this group. Patients who developed respiratory syncitial virus infection were less likely to die from this infection than patients with parainfluenza or influenza, possibly due to early therapy with inhaled ribavirin and palivizumab,25 though this should be interpreted with caution based on the small number of lower tract infection patients in the cohort.
Strengths of our analysis include large sample size and standardized diagnostic approach for all hematopoietic cell transplantation patients with respiratory symptoms. Limitations included its retrospective nature and use of only conventional diagnostic tests that lack the sensitivity of polymerase chain reaction, as well as the lack of a quantitative assay as a measure of disease severity. The exclusion of other respiratory viruses that are detected with PCR (such as human metapneumovirus and coronavirus) is another weakness. Routine molecular testing for these viruses or the viruses included in our study, including quantitative measures, was not routinely performed in our center or elsewhere during the time period of this retrospective cohort. Data collected more than 100 days after allogeneic hematopoietic cell transplantation or 60 days after autologous transplantation should be interpreted with caution. We were reliant upon accurate reports from outside physicians: it was probable that low morbidity events were underrepresented.
In conclusion, respiratory virus lower tract infection during the first 100 days after hematopoietic cell transplantation was significantly less common in persons receiving non-myeloablative conditioning despite a similar overall rate of acquisition. Respiratory virus lower tract infection had high co-infection and mortality rates. Screening for respiratory viruses should continue in all hematopoietic cell transplantation patients, although for patients on non-myeloablative protocols, the likelihood of developing a respiratory virus lower tract infection was very low during the first 100 days after hematopoietic cell transplantation. The low risk of progression to lower tract infection among non-myeloablative transplantation recipients suggests the intriguing possibility of using this conditioning regimen in patients who develop respiratory virus disease before transplantation. In such cases, delay of transplantation until resolution of infections is currently recommended, especially for infections due to respiratory syncitial virus, parainfluenza, and influenza.26 However, this delay may increase the risk of progression of the underlying hematologic malignancy. Thus, non-myeloablative conditioning may provide a safer alternative if transplantation is urgent. An evaluation of this approach is needed.
Acknowledgments
we thank Peter Choe and Nido Nguyen for database services and laboratory staff at the University of Washington Clinical Virology Laboratory for specimen processing, testing and expertise. We thank the HCT teams at the FHCRC for their contributions, and all patients for participation in these studies. This research was presented in part at the Infectious Diseases Society of America meeting in 2006 in Toronto.
Footnotes
Authorship and Disclosures
JTS and MB participated in the conception, design, analysis and interpretation of data, drafting and critical revision of the article for important intellectual content; and on final approval of the version to be published; KK participated in the analysis and interpretation of data, the drafting of the article and on final approval of the version to be published; BS and RS participated in analysis and interpretation of data; revising the article critically for important intellectual content; and on final approval of the version to be published; LC participated in conception and design of the study, revising it critically for important intellectual content; and on final approval of the version to be published.
Sources of support: NIH CA 18029, CA 15704, CA 78902, CA 18029, HL 36444, and HL 081595. The authors reported no potential conflicts of interest.
Funding: this study was supported in part by NIH CA18029, CA15704, CA78902, CA18029, HL36444, and HL081595.
References
- 1.Ison MG, Hayden FG, Kaiser L, Corey L, Boeckh M. Rhinovirus infections in hematopoietic stem cell transplant recipients with pneumonia. Clin Infect Dis. 2003;36:1139–43. doi: 10.1086/374340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ljungman P. Respiratory virus infections in stem cell transplant patients: the European experience. Biol Blood Marrow Transplant. 2001;7 (Suppl):5S–7S. doi: 10.1053/bbmt.2001.v7.pm11777102. [DOI] [PubMed] [Google Scholar]
- 3.Nichols WG, Gooley T, Boeckh M. Community-acquired respiratory syncytial virus and parainfluenza virus infections after hematopoietic stem cell transplantation: the Fred Hutchinson Cancer Research Center experience. Biol Blood Marrow Transplant. 2001;7 (Suppl):11S–5S. doi: 10.1053/bbmt.2001.v7.pm11777098. [DOI] [PubMed] [Google Scholar]
- 4.Whimbey E, Englund JA, Couch RB. Community respiratory virus infections in immunocompromised patients with cancer. Am J Med. 1997;102:10–8. doi: 10.1016/S0002-9343(97)80004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whimbey E, Vartivarian SE, Champlin RE, Elting LS, Luna M, Bodey GP. Parainfluenza virus infection in adult bone marrow transplant recipients. Eur J Clin Microbiol Infect Dis. 1993;12:699–701. doi: 10.1007/BF02009383. [DOI] [PubMed] [Google Scholar]
- 6.Harrington RD, Hooton TM, Hackman RC, Storch GA, Osborne B, Gleaves CA, et al. An outbreak of respiratory syncytial virus in a bone marrow transplant center. J Infect Dis. 1992;165:987–93. doi: 10.1093/infdis/165.6.987. [DOI] [PubMed] [Google Scholar]
- 7.Nichols WG, Corey L, Gooley T, Davis C, Boeckh M. Parainfluenza virus infections after hematopoietic stem cell transplantation: risk factors, response to antiviral therapy, and effect on transplant outcome. Blood. 2001;98:573–8. doi: 10.1182/blood.v98.3.573. [DOI] [PubMed] [Google Scholar]
- 8.Erard V, Chien JW, Kim HW, Nichols WG, Flowers ME, Martin PJ, et al. Airflow decline after myeloablative allogeneic hematopoietic cell transplantation: the role of community respiratory viruses. J Infect Dis. 2006;193:1619–25. doi: 10.1086/504268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giralt S, Estey E, Albitar M, van Besien K, Rondon G, Anderlini P, et al. Engraftment of allogeneic hematopoietic progenitor cells with purine analog-containing chemotherapy: harnessing graft-versus-leukemia without myeloablative therapy. Blood. 1997;89:4531–6. [PubMed] [Google Scholar]
- 10.McSweeney PA, Niederwieser D, Shizuru JA, Sandmaier BM, Molina AJ, Maloney DG, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–400. doi: 10.1182/blood.v97.11.3390. [DOI] [PubMed] [Google Scholar]
- 11.Slavin S, Nagler A, Naparstek E, Kapelushnik Y, Aker M, Cividalli G, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91:756–63. [PubMed] [Google Scholar]
- 12.Fukuda T, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003;102:827–33. doi: 10.1182/blood-2003-02-0456. [DOI] [PubMed] [Google Scholar]
- 13.Junghanss C, Boeckh M, Carter RA, Sandmaier BM, Maris MB, Maloney DG, et al. Incidence and outcome of cytomegalovirus infections following nonmyeloablative compared with myeloablative allogeneic stem cell transplantation, a matched control study. Blood. 2002;99:1978–85. doi: 10.1182/blood.v99.6.1978. [DOI] [PubMed] [Google Scholar]
- 14.Buckner C, Clift R, Appelbaum F, Storb R, Fefer A, Petersen F, et al. Effects of treatment regimens on post marrow transplant relapse. Semin Hematol. 1991 Jul;28(Suppl 4):32–4. [PubMed] [Google Scholar]
- 15.Clift R, Buckner C, Thomas E, Bensinger W, Bowden R, Bryant E, et al. Marrow transplantation for chronic myeloid leukemia: a randomized study comparing cyclophosphamide and total body irradiation with busulfan and cyclophosphamide. Blood. 1994;84:2036–43. [PubMed] [Google Scholar]
- 16.Lynch M, Petersen F, Appelbaum F, Bensinger W, Clift R, Storb R, et al. Phase II study of busulfan, cyclophosphamide and fractionated total body irradiation as a preparatory regimen for allogeneic bone marrow transplantation in patients with advanced myeloid malignancies. Bone Marrow Transplant. 1995;15:59–64. [PubMed] [Google Scholar]
- 17.Boeckh M, Englund J, Li Y, Miller C, Cross A, Fernandez H, et al. Randomized controlled multicenter trial of aerosolized ribavirin for respiratory syncytial virus upper respiratory tract infection in hematopoietic cell transplant recipients. Clin Infect Dis. 2007;44:245–9. doi: 10.1086/509930. [DOI] [PubMed] [Google Scholar]
- 18.Sorror M, Storer B, Maloney D, Sandmaier B, Martin P, Storb R. Outcomes after allogeneic hematopoietic cell transplantation with non-myeloablative or myeloablative conditioning regimens for treatment of lymphoma and chronic lymphocytic leukemia. Blood. 2008;111:446–52. doi: 10.1182/blood-2007-07-098483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laport G, Sandmaier B, Storer B, Scott B, Stuart M, Lange T, et al. Reduced-intensity conditioning followed by allogeneic hematopoietic cell transplantation for adult patients with myelodysplastic syndrome and myeloproliferative disorders. Biol Blood Marrow Transplant. 2008;14:246–55. doi: 10.1016/j.bbmt.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rezvani A, Storer B, Maris M, Sorror M, Agura E, Maziarz R, et al. Nonmyeloablative allogeneic hematopoietic cell transplantation in relapsed, refractory, and transformed indolent non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26:211–7. doi: 10.1200/JCO.2007.11.5477. [DOI] [PubMed] [Google Scholar]
- 21.Georges G, Maris M, Maloney D, Sandmaier B, Sorror M, Shizuru J, et al. Nonmyeloablative unrelated donor hematopoietic cell transplantation to treat patients with poor-risk, relapsed, or refractory multiple myeloma. Biol Blood Marrow Transplant. 2007;13:423–32. doi: 10.1016/j.bbmt.2006.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutman JA, Peck AJ, Kuypers J, Boeckh M. Rhinovirus as a cause of fatal lower respiratory tract infection in adult stem cell transplantation patients: a report of two cases. Bone Marrow Transplant. 2007;40:809–11. doi: 10.1038/sj.bmt.1705827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maris M, Boeckh M, Storer B, Dawson M, White K, Keng M, et al. Immunologic recovery after hematopoietic cell transplantation with non-myeloablative conditioning. Exp Hematol. 2003;31:941–52. doi: 10.1016/s0301-472x(03)00201-7. [DOI] [PubMed] [Google Scholar]
- 24.Baron F, Storer B, Maris MB, Storek J, Piette F, Metcalf M, et al. Unrelated donor status and high donor age independently affect immunologic recovery after nonmyeloablative conditioning. Biol Blood Marrow Transplant. 2006;12:1176–87. doi: 10.1016/j.bbmt.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Boeckh M, Berrey M, Bowden R, Crawford S, Balsley J, Corey L. Phase 1 evaluation of the respiratory syncytial virus-specific monoclonal antibody palivizumab in recipients of hematopoietic stem cell transplants. J Infect Dis. 2001;184:350–4. doi: 10.1086/322043. [DOI] [PubMed] [Google Scholar]
- 26.Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients. MMWR Recomm Rep. 2000;49:1–125. [PubMed] [Google Scholar]