Chronic autoimmune or pathogen-induced immune reactions resulting in lymphoid neogenesis are associated with development of malignant lymphomas, mostly extranodal marginal zone B-cell lymphomas. This review article examines the role of chronic inflammatory responses and the molecular mechanisms involved in the development and progression of extranodal marginal zone B-cell lymphomas.
Keywords: B-cell non-Hodgkin’s lymphoma, extranodal marginal zone B-cell lymphoma, immunoglobulin, B-cell antigen receptor, inflammation, lymphoid tissue neogenesis
Abstract
Chronic autoimmune or pathogen-induced immune reactions resulting in lymphoid neogenesis are associated with development of malignant lymphomas, mostly extranodal marginal zone B-cell lymphomas (MZBCLs). In this review we address (i) chemokines and adhesion molecules involved in lymphoid neogenesis; (ii) the autoimmune diseases and pathogens which are associated with development of B-cell lymphomas; (iii) the molecular mechanisms involved in the initiation and progression of MZBCL; and (iv) ‘potential’ mouse models for MZBCL.
Lymphoid tissue neogenesis and ectopic germinal center formation
Inflammation is a local response to cellular injury and is initiated by macrophages and local epithelial and/or stromal cells that sense microorganisms and cell damage by pattern recognition receptors, i.e. the Toll-like receptors (TLRs), soluble intra-cellular NOD-like receptors and RIG-like helicases.1 The triggered cells respond by secretion of a plethora of inflammatory mediators such as histamine, prostaglandins, leukotrienes, platelet-activating factors and typical pro-inflammatory chemokines and cytokines like IL-1β, IL-6, IL-8 (CXCL8) and TNF.2 These mediators, and in particular TNF, lead to endothelial activation and vasodilatation followed by a local efflux of circulating leukocytes. The first leukocytes arriving on site are granulocytes which combat the microbial invaders, while monocytes/macrophages clean up dead cells, including apoptotic granulocytes and destroyed tissue.3 In parallel, dendritic cells (DCs) take up and process antigens (Ag) from the intruder, mature and migrate to a local lymph node to set off an adaptive immune response.
Chronic inflammatory conditions, due to improper eradication of pathogens, auto-immune processes or chronic allograft rejections, are associated with the genesis of organized lymphoid tissue. In recent years, a number of key molecular determinants operating during the generation of tertiary lymphoid tissue, have been identified. In the complex sequence of events, TNF is again one of the key molecules as it induces the production of CCL19 and CCL21 (SLC), which are important for the attraction of B- and T-lymphocytes.
The infiltrating lymphocytes switch on expression of membrane-bound lymphotoxin α1β2 (mLTα1β2) when activated e.g. by Ag.4 High levels of mLTα1β2 lead to lymphotoxin receptor (LTβ-R) ligation on stromal cells and/or macrophages and induce CXCL13 (BLC) production.5 The local production of CXCL13 mediates homing of B cells and induces the arrived B cells to further upregulate mLTα1β2 and probably also TNF. The enhanced interaction of CXCL13-producing stromal cells with the TNF- and mLTα1β2- producing B cells promotes differentiation of resident stromal cells into follicular dendritic cells (FDCs) which start expressing characteristic molecules to trap immune complexes, i.e. the complement receptors CD21 and CD35 and the FcγR-IIB.6–8 Subsequent production of CXCL13 by FDCs establishes a positive feedback loop essential for ectopic lymphoid tissue development, similar to embryonic lympho-organogenesis and normal follicle formation (Figure 1).9–12 The importance of LT and TNF in this process has been demonstrated by transgenic expression of TNF, LTα and LTα/β in the pancreas and the kidneys, leading to formation of organized lymphoid tissue including FDC-containing follicles.10,11 Transgenic expression of CCL21 alone, resulted in extensive lymphoid tissue development in the pancreas.12 However, ectopic expression of CXCL12 (SDF), CCL19 or CXCL13 leads to attraction of lymphocytes, some compartmentalization but not to the genesis of FDC-containing follicles.12
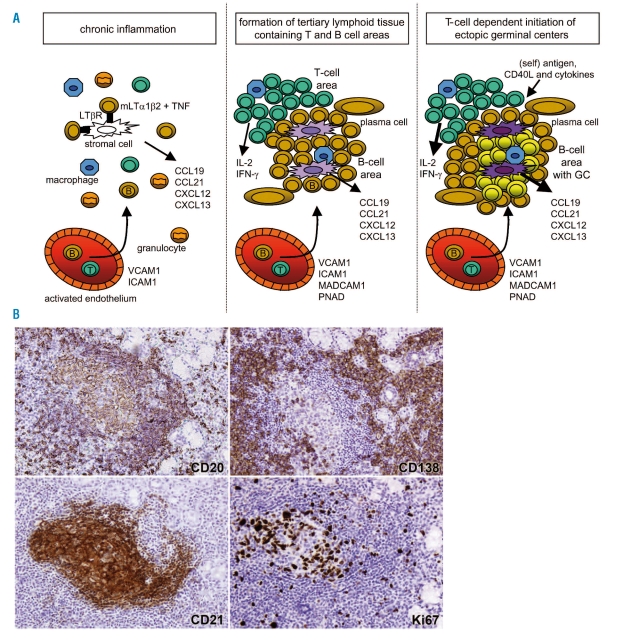
Figure 1.
Lymphoid tissue neogenesis and ectopic germinal center formation. Upper panels: (A) chronic inflammation is characterized by high levels of TNF, inducing stromal cells to produce CCL19 and CCL21 which attract B- and T- lymphocytes. (B) the interplay between CXCL13-producing stromal cells and increasing numbers of mLTα1β2 and TNF-expressing B lymphocytes, leads to the development of follicular dendritic cells (FDCs) and subsequent formation of lymphoid follicles. (c) T cells provide specific help to antigen activated B cells via costimulatory cytokines and membrane receptors. Lower panels: immunohistochemical stainings on a well-organized lymphoid infiltrate in a minor salivary gland of a Sjögren’s syndrome patient. Highlighted is a B-cell follicle including a germinal center using Abs specific for B cells CD20, plasma cells CD138, predominantly follicular dendritic cells CD21 and the proliferation marker Ki67.
Depending on the type of pathogen, i.e. differences in the Ag presentation mode and the combination of costimulatory molecules and cytokine signals, Ag presenting cells (APCs) guide T-cell differentiation into the direction of T-helper type 1 (TH1) or T-helper type 2 (TH2) cells. A TH1-polarized response depends on IL-12 and results in IFN-γ producing T cells which also produce IL-2 and TNF and in their turn stimulate macrophages, natural killer (NK) cells and CD8+ cytotoxic T cells. This type of reaction mainly generates cellular responses against intracellular pathogens like viruses but also evokes T-cell help leading to production of, for example, opsonizing IgG2 antibodies (Ab).13 A TH2 response is characterized by secretion of IL-4, IL-5 and IL-13, and yields humoral immunity, in particular the generation of plasma cells that secrete high-affinity Abs, mainly of IgG1, IgG4, IgA and IgE classes.13,14
In the ectopic follicles, germinal center (GC) reactions may occur (Figure 1). There are no reasons to assume that the biological processes operating in ectopic GCs differ from those in GCs of secondary lymphoid tissues. In secondary lymphoid tissues, GC reactions are initiated after activation of B cells and T cells by native and processed antigenic determinants, respectively. The B cells bind and internalize antigenic proteins with their membrane-bound immunoglobulins (mIg or B-cell Ag receptors, BCR) and, after intracellular processing, express Ag-derived peptides in MHC-II molecules at their surface. CD4 T cells, that are activated through cognate interactions with these peptide-MHC complexes, recompense the B cells by providing costimulatory signals (T-cell help) through CD40, CD80, CD86 and cytokine receptors.15,16 When properly stimulated at the follicular boundaries, the B cells directly differentiate into short-lived Ab-forming plasma cells or migrate back into the follicle to undergo a phase of brisk proliferation thereby creating the GC dark zone.17,18 The rapidly proliferating B cells, termed centroblasts, express high levels of the DNA mutator activation-induced-cytidine-deaminase (AID) and accumulate nucleotide substitutions in their Ig variable (IgV) genes, a process designated as somatic hypermutation (SHM).19,20 B cells in the GC are prone to undergo apoptosis except for those which, based on favorable point mutations, obtain higher affinities of their BCRs for the Ag. These high-affinity B cells are selected in the GC light zone based on successful competition for survival signals elicited by native Ag that is exposed at the surface of FDC, and by CD40L from GC T cells.21 In addition, the Ag-selected B cells may undergo class switch recombination (CSR) a process during which the switch (S) region sequence upstream of Cμ-Cδ is recombined with any of the other S region sequences located 5’ of each of the constant region genes Cγ3, Cγ1, Cα1, Cγ2, Cγ4, Cɛ and Cα2, thus leading to isotype switching from IgM/IgD to either IgG, IgA or IgE.22 The Ag-selected B cells, either class-switched or not class-switched, will finally differentiate into memory B cells or Ab-producing plasma cells.18
Marginal zone B cells
In humans and rodents, distinct populations of recirculating peripheral B cells are being distinguished, i.e. naïve (B2) or follicular (FO) B cells, naïve CD5+ B cells, marginal zone (MZ) B cells and class-switched memory B cells (Figure 2). Initial studies in mice and humans indicated that CD5-expressing naïve B cells frequently display poly-/self-reactivity.23–25 However, more recent work in humans demonstrated a similar frequency of poly-/self-reactivity between CD5− and CD5+ naïve B cells.26,27 MZ B cells particularly respond to T-cell independent type 2 (TI-2) Ags, like large polysaccharides of bacterial cell walls and polymeric bacterial flagellin, which by repetitive antigenic epitopes, are able to crosslink BCRs. Naïve B2 cells are involved in T-cell dependent (TD) GC reactions, generating plasma cells, secreting high affinity Igs, and CD27+ memory B cells. Recently, we obtained evidence in primary human lymph nodes that isotype-switched memory B cells can re-engage in GC reactions.28
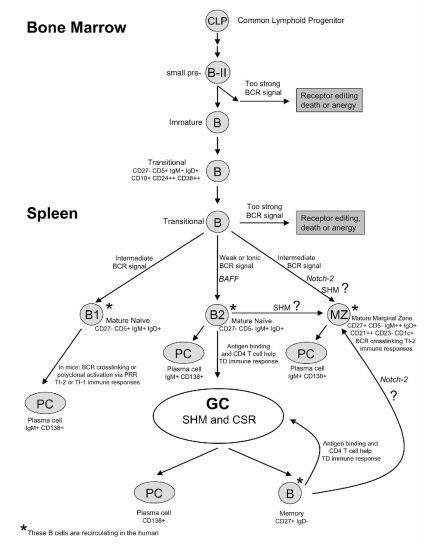
Figure 2.
B-cell development in man. Transitional B cells in the spleen potentially mature into three B cell subsets: (i) CD5+ mature naïve B cells; (ii) conventional mature naïve B2 or FO B cells; and (iii) marginal zone (MZ) B cells which contain mutated IgV genes possibly aquired in a T-cell independent manner. After antigen recognition, mature naïve B2 cells engage in T-cell dependent germinal center (GC) reactions in which SHM and CSR occur, generating high-affinity class-switched memory B cells and plasma cells (PC).
The marginal zone (MZ) was originally defined as an anatomical compartment within the spleen located around primary or secondary follicles and containing B cells with distinct phenotypic and functional characteristics. The MZ of the spleen is believed to be positioned in such a way that it primarily encounters blood borne pathogens. Later, primary mucosa-associated lymphoid tissues (MALT) of e.g. Waldeyer's ring, Peyer’s patches and appendix, locations known for a significant influx of Ags, were also found to contain a marginal zone. MZ B cells in mice and rats express essentially unmutated IgV genes and are supposed to be non-recirculating.29 On the other hand, human MZ B cells of both spleen and Peyer’s patches do harbor mutated IgV genes and recirculate (Figure 2).30–32 Human splenic MZ B cells are IgMhi IgDlo and co-express the B-cell markers CD20, CD22 and CD79a/b, the memory B-cell marker CD27, the complement receptors CD18/CD11b, CD21 and CD35, and the anti-apoptotic molecule BCL-2. MZ B cells are negative for CD5 and CD23 as well as for the GC markers CD10 and BCL-6. Human MZ B cells, both in tissues and in the circulation, express high levels of CD1c. Expression arrays of splenic and recirculating IgM+ IgD+ CD27+ B cells revealed similar profiles, including high expression of CD31, CD44 and IL-6, thus confirming the non-resident nature of MZ B cells in the humans.32
Where and when human MZ B cells obtain their somatic IgV mutations is still under debate. There is evidence that this occurs outside of GCs as part of an innate diversification program, like in sheep and birds.32 This is supported by the fact that mutated IgM+ IgD+ CD27+ MZ B cells are also found in CD40L-deficient, hyper-IgM patients lacking GCs and that the Ig repertoire of MZ B cells is as diverse as naïve B cells, thus not resembling the highly selected Ig repertoire of class-switched IgM− IgD−CD27+ B cells.33,34 Moreover, AID expression was observed in splenic MZ B cells of children under the age of two years, but not in older individuals.34 Recent data by Scheeren et al.35 indicated that MZ B cells, with mutated IgV genes, are already present in human fetuses in which no active immune responses are thought to happen. AID expression was found in fetal liver and mesenteric lymph nodes but not in the fetal spleen. Repopulation experiments with human hematopoietic stem cells in Rag2 −/− γc −/− mice showed that IgV-mutated MZ B cells develop in a T-cell independent manner.35 Others think that MZ B cells mutate their IgV genes within GCs and argued that some residual GCs may be present in CD40L-deficient patients.36 GC formation without T-cell help has been described in mice, albeit these GCs were smaller, short-lived and SHM frequencies were low. Moreover, CD40L-deficient patients have significantly lower numbers of circulating MZ B cells, being ~20–25% as compared to healthy individuals, indicative for at least a partial defect in MZ B-cell development.36 According to this scenario, MZ B cells would thus not belong to a distinct developmental lineage, but originate from conventional naïve B2 or follicular (FO) B cells. As currently there are no clues as to the heterogeneity of the MZ B-cell population, the possibility of multiple developmental routes producing hypermutated B cells with an MZ-like phenotype is not excluded (Figure 2).
It has been demonstrated that about 4% of MZ B cells are responsive to bacterial polysaccharides. Still, a large fraction of MZ B cells may thus have other specificities.37 In one donor, previously vaccinated with Streptococcus pneumoniae polysaccharide Ag, 2 of the 27 Abs (7%) generated out of the MZ B-cell fraction, specifically reacted with this bacterial Ag.37 Capolunghi et al.38 showed, by polyclonal activation of naïve and MZ B cells with CpG DNA, anti-S. pneumoniae (PnPS serotype 14) production exclusively by MZ B cells. In children below the age of two years, no or limited responses are detected against these TI-2 Ags.32 After the age of two, the percentage of IgM+ IgD+ CD27+ MZ B cells in the blood increases, which coincides with the appearance of the anatomical MZ structure in the spleen and with increased humoral responses to TI-2 Ags, such as pneumococcal polysaccharides.32,39
Human IgM+ CD27+ MZ B cells, when compared to IgM+ CD27− naïve B cells, appear to be selected against poly- and self-reactive BCRs. This selection is associated with a decrease in the average length of the IgVH complementarity determining regions 3 (IgVH-CDR3), which is largely due to deletion of B cells expressing the JH6 gene.37 Indeed, long IgVH-CDR3s have been associated with self- and poly-reactivity.40 Upon reversion of somatic IgV mutations to their corresponding germline IgV sequences, these Abs did not regain poly- and/or self-reactivity. This indicates that naïve B cells with poly-/self-reacting BCRs are already efficiently excluded from the MZ B-cell pool before the onset of SHM.37 Also in the rat, selection of naïve B cells into the MZ B-cell compartment is accompanied by a decrease in IgVH-CDR3 lengths.29 This selection is most likely driven by self antigens as it is also observed in germ-free rats.41 Notably, no preferential selection of short CDR3s is observed in conventional class-switched IgG+ CD27+ memory B cells. Surprisingly, it has been described that ~50% of IgG+ CD27+ B cells show poly-/self-reactivity which is generally lost when reverting IgV SHM. Thus, the poly-/self-reactivity of IgG+ memory B cells is due to the accumulation of IgV SHM and not due to intrinsic properties of the CDR3s.42
Auto-immune inflammatory conditions associated with B-cell lymphomagenesis
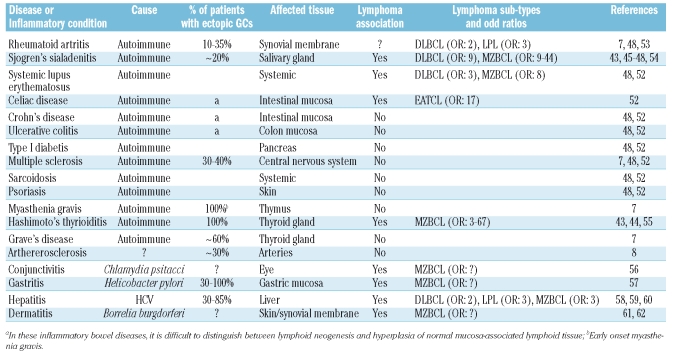
A number of chronic autoimmune conditions, organ-specific as well as systemic, are associated with an increased incidence of non-Hodgkin’s lymphomas (NHL). Among these, Hashimoto’s thyroiditis (HT) and Sjögren’s syndrome (SS) are the best examples with increased relative risks of 3–6743,44 and 9–44,43,45–48 respectively to develop extranodal marginal zone B-cell lymphomas (MZBCLs). Moreover, for SS patients a 9-fold increased risk of obtaining a diffuse large B-cell lymphoma (DLBCL) has been reported. These lymphomas may develop either de novo or by transformation out of a prior low-grade MZBCL.48–51 Systemic lupus erythematosus patients were reported to have 8-fold and 3-fold higher incidences of, respectively, MZBCL and DLBCL as well.48,52 Rheumatoid arthritis (RA) appears to be weakly associated with non-RA treatment related, development of DLBCL and lympho-plasmacytic lymphoma, with reported odd ratios of 1.8 and 2.5, respectively.52,53 A recent meta-analysis, however, did not reveal an overall statistically significant association between RA and NHL.48 Celiac disease is strongly associated with the occurrence of enteropathy-type T-cell lymphoma.52 It is not understood why some autoimmune diseases do and many others, like e.g. Crohn’s disease, ulcerative colitis, type I diabetes, multiple sclerosis, pernicious anemia and sarcoidosis, do not entail increased risks of developing NHLs (Table 1).48,52–62
Table 1.
Chronic inflammatory conditions and lymphoma association.
Sjögren’s syndrome
Sjögren’s syndrome (SS) is a systemic autoimmune disease characterized by complaints of dry mouth (xerostomia) and eyes (keratoconjunctivitis sicca). Biopsies of (minor) salivary and lacrymal glands typically show mixed infiltrates consisting of CD4 T cells, some CD8 T cells, macrophages, myeloid and plasmacytoid dendritic cells (DC), B cells (~20%) and plasma cells. In about 20% of the patients the infiltrates contain GCs (Figure 1).54 Compatibly, lymphocyte attracting chemokines such as CXCL12 and CCL21 which mainly attract T cells and CXCL13 which attracts B cells are abundantly expressed in SS.54,63–66 High expression levels of CXCL13 and CCL21 within inflamed tissues correlated with the extent of inflammatory aggregates, the degree of T-cell/B-cell compartmentalization, the number of peripheral lymph node addressin (PNAd)-positive high endothelial venules (HEVs) and the presence of FDC-network containing GCs.66 Salivary gland epithelial cells of both normal and SS patients produce CCL28, which mediates the homing of CCR10-expressing IgA plasmablasts.67 Plasmacytoid DCs are present in SS salivary glands which secrete high amounts of type I IFNs (IFN-α and β) and IL-6, supporting plasma cell differentiation.68 Furthermore, IFN-α induces the B cell and plasma cell survival cytokine BAFF, which was indeed found to be highly expressed in SS patients.68,69 CD4+ T cells from SS salivary glands express ~40-fold higher mRNA levels of IL-2, IFN-γ and IL-10, as compared to peripheral CD4+ T cells from SS patients or from healthy controls. In agreement with this, the IL-4 and IL-5 mRNA levels of the SS CD4+ T cells were low.70 Thus, in general, the proinflammatory T-helper cell 1 (TH1)-type cytokines IL-2 and IFN-γ are abundant in Sjögren’s sialadenitis.70 Accordingly, the IFN-γ induced inflammatory chemokines CXCL9 (MIG) and CXCL10 (IP-10) are highly expressed in SS salivary gland epithelial cells but not in normal salivary glands.64
A variety of nuclear auto-Ags are humoral immune targets in SS patients. Anti-nuclear Abs, among which are the anti-SSA/Ro and anti-SSB/La Abs, are detectable in 70–85% of the patients. SSA/Ro52, SSA/Ro60 and SSB/La Ags together form a complex with a small cytoplasmic uridine-rich Y RNA.71 Five anti-SSA/Ro human monoclonal Abs of SS patients have been produced, i.e. 2 anti-Ro52 IgM Abs derived from peripheral blood B cells and 3 anti-Ro60 IgG Abs obtained from B cells of affected salivary glands. The IgM anti-SSA/Ro52 Abs were regarded unmutated containing 0 and 3 somatic mutations in their IgVH genes, while the IgG anti-SSA/Ro60 Abs were heavily mutated, containing >20 somatic mutations.72,73 By immunohistochemistry using biotinylated SSA/Ro52, SSA/Ro60 and SSB/La, evidence was obtained that the anti-SSA/Ro and anti-SSB/La Abs are produced by local plasma cells, most likely generated within the ectopic GCs.54 Serum anti-SSA/SSB Ab levels correlated with the presence of ectopic GCs.74 By immunohistochemistry, evidence for AID expression was provided75 and by tissue microdissection formal proof was obtained of the occurrence of clonal B-cell expansion and SHM in the ectopic GCs76 (also RJ Bende et al., unpublished data, 2009). Ig repertoire analysis on cells isolated from crude tissues of parotid and minor salivary glands of SS patients, revealed that most (~80%) of the infiltrating B cells harbored mutated IgVH genes and thus are GC, marginal zone or memory B cells.77,78 The combined data strongly suggest local generation and affinity maturation of the auto-Abs.
Hashimoto’s thyroiditis
Hashimoto’s thyroiditis (HT) and Grave’s disease represent extremes of a clinical spectrum of typical organ-specific autoimmune diseases, histologically characterized by chronic lymphocytic infiltration. In Grave's disease, inflammation is generally mild and accompanied by the production of thyrotropin receptor stimulating Abs, resulting in hyperthyroidism. In HT, the infiltrates are more severe and progressive, ultimately causing destruction of the thyroid parenchyma and hypothyroidism. It has been shown that in all HT patients the lymphocytic infiltrate is well-organized containing GCs.55 T-cell attracting chemokines CXCL12 and CCL21 are produced by the follicular epithelium surrounding HEVs.79 Ectopic expression of CCL21 in the thyroid of mice leads to the development of lymphoid tissue containing FDCs, resembling HT.80 In this model, also the IFN-γ inducible inflammatory chemokines CXCL9, 10 and 11 were expressed.80,81 Cultures of CD4 and CD8 T cell clones retrieved from human HT patient thyroids yielded high levels of TNF and IFN-γ, whereas hardly any clones produced IL-4.82 Quantitative RT-PCR on patient thyroids revealed high levels of IFN-γ and IL-2, confirming that the TH1 cytokines are highly expressed in HT.83
Auto-Abs produced in HT are specific for thyroglobulin (Tg), thyroid peroxidase (TPO) and the thyroid stimulating hormone receptor (TSH-R). The Abs against Tg and TPO are not detected in all patients. Auto-Abs specific for the TSH-R are capable of blocking the activation of this receptor and thus can contribute to the impairment of the thyroid function.84 Numerous anti-Tg and anti-TPO human monoclonal Abs have been reported, isolated from peripheral blood B cells, thyroid tissue and cervical lymph node tissue. All these human monoclonal Abs were heavily mutated containing >10 mutations per IgVH gene.84 A correlation has been found between the levels of CCL21, CCL22 and CXCL13 in the inflamed thyroid and the titers of thyroid-specific auto Abs.79 Biotin-labeled Tg and TPO were shown to bind immunohistochemically to ectopic GCs in HT. The combined data support local generation and affinity maturation of anti-thyroideal Abs.55
Infections indirectly provoking B-cell lymphomagenesis
Helicobacter pylori-infection related gastric MZBCL is the most commonly mentioned example of bacterium-driven tumorigenesis but in fact is the only undisputed example.57 Cutaneous MZBCL has been linked to chronic Borrelia burgdorferi dermatitis (Lyme’s disease) in a minority of European patients, but not in cases from Asia or the United States.61,85–87 Recently, an association of Chlamydia psittaci and ocular adnexal MZBCL was found by PCR in studies from Italy, South Korea, Germany and Austria.56,88–90 Immunohistochemistry, laser assisted microdissection PCR and electron microscopy further provided evidence that C. psittaci was present in monocytes/macrophages within the MZBCL.91 Moreover, C. psittaci was cultured in vitro from conjuctival swabs and/or PBMCs from 25% of ocular adnexal MZBCL patients.92 However, the association between C. psittaci and ocular adnexal MZBCL could not be confirmed in studies from The Netherlands, Japan and The United States (Florida), suggesting geographical differences regarding this link.56,93–95 Hepatitis C virus (HCV) infection has been inferred in the development of malignant B-cell proliferations, in particular splenic and extranodal MZBCLs and DLBCLs.96,97 In a large intercontinental study, the relative risks for HCV patients to develop MZBCL, DLBCL or lymphoplasmacytic lymphoma were calculated as 2.5, 2.2 and 2.6, respectively (Table 1).58
H. pylori is a gram-negative bacterium able to persist during lifetime in the gastric mucosa and is present in ~50% of the world population. H. pylori binds tightly to epithelial cells via multiple bacterial surface components. H. pylori, like most intracellular bacteria, evoke TH1 immune responses characterized by high IFN-γ levels.98–103 IFN-γ targets genes with microbicidal properties such as enzymes that generate NO and O2 radicals. To circumvent the negative effects of these radicals H. pylori produces radical-scavenging enzymes.104 In addition, H. pylori secretes urease to neutralize the local low pH. Most strains of H. pylori possess the cag pathogenicity island, including the CagA gene. The CagA protein leads to a massive influx of neutrophils by inducing high production levels of the chemotactic factor CXCL8 (IL-8) by epithelial cells. Later, during the chronic phase also T cells, B cells, plasma cells and macrophages are recruited and secondary mucosa-associated lymphoid tissue (MALT) is formed within the gastric mucosa.105,106 By in situ hybridization and immunohistochemistry, CXCL13 was found to be expressed in the ectopic primary follicles and mainly in the mantle zones of ectopic GCs.106 H. pylori induces a strong Ab response which does not lead to eradication, but instead may contribute to the tissue damage. Two human anti-H. pylori single-chain Ig variable fragment (Ig-Fv) isolated from peripheral blood B cells of a H. pylori-infected patient have been reported, each displaying mutated IgVH genes (>7 mutations).107 Lymphoid aggregates with GCs have also been observed in B. burgdorferi-induced skin and synovial lesions, and in the liver of ~60% of the patients suffering from chronic HCV infections.59,60,108 In the ectopic GCs of B. burgdorferi-induced synovitis, B-cell expansion and IgVH diversification have been demonstrated.109
Extranodal marginal zone B-cell lymphoma
Extranodal marginal zone B-cell lymphoma (MZBCL) of mucosa-associated lymphoid tissue, also designated as MALT lymphoma, appears as heterogeneous infiltrates containing small centrocytic and monocytoid B cells, plasma cells and in some scattered immunoblasts and centroblasts. The growth characteristics of MZBCLs resemble those of the normal MZ of, for example, Peyer’s patches. MZBCLs typically expand around ectopic GCs and are able to invade the epithelium to form lympho-epithelial lesions. Colonization of ectopic GCs by tumor B cells contribute to a pseudo-follicular growth pattern. Also immunophenotypically, MZBCL cells are reminiscent of normal MZ B cells of the spleen and Peyer’s patches. They express the pan-B cell markers CD20, CD22 and CD79a/b, the memory B-cell marker CD27, the complement receptors CD18/CD11b, CD21 and CD35, the anti-apoptotic molecule BCL-2, and CD1c/CD1d. MZBCLs do not express CD5 and CD23, nor the GC molecules CD10 and BCL-6.110 There are as yet no markers by which MZBCLs can be unequivocally identified. MZBCLs have a low tendency to disseminate systemically, a feature which explains why the majority of these malignancies can be controlled by local treatments alone. About 30% of MZBCLs disseminate which, due to expression of the mucosal homing integrin α4β7, most often involves other mucosal sites or regional lymph nodes.111,112 Interestingly, in both lymph nodes and spleen, MZBCL cells also tend to expand peri-follicularly, in accordance with their MZ B-cell properties.113
As outlined, the chronic inflammatory conditions enabling MZBCL development are generally of TH1 type, characterized by high IFN-γ levels. IFN-γ receptor binding leads, via STAT1 activation, to induction of genes encoding microbicidal proteins and to induction of the transcription factor t-Bet which in its turn induces, among other genes, expression of the chemokine receptor CXCR3.98,114,115 CXCR3 is the specific receptor for the IFN-γ induced chemokines CXCL9 (MIG), CXCL10 (IP-10) and CXCL11 (ITAC). Indeed, extranodal and splenic marginal zone B-cell lymphomas almost invariably express CXCR3 and t-Bet.116–119 High expression of CXCL9 has been demonstrated in histiocytes, fibroblasts and endothelial cells of thyroid and gastric MZBCLs.120 We recently reported that within the group of MZBCLs, most cutaneous MZBCLs are distinct as they arise in a TH2 background and, in accordance with this immunological context, lack CXCR3 and t-Bet and carry isotype-switched Igs. Interestingly, a few cases of cutaneous MZBCL did express IgM and CXCR3 like other MZBCLs, and were thus most likely established in a typical TH1 inflammatory environment. Notably, the few B. burgdorferi-infection associated MZBCLs we have studied also co-expressed IgM and CXCR3.121
Genetic alterations in low- and high- grade extranodal marginal zone B-cell lymphomas
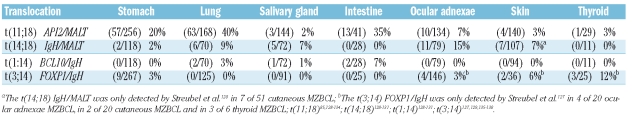
Recurrent chromosomal translocations identified in MZBCLs are t(11;18) (API-2/MALT1), t(14;18) (IgH/MALT1), t(1;14) (BCL-10/IgH) and t(3;14) (FOX-P1/IgH) (Table 2).122–138 Except for the t(11;18), these translocations involve IgH loci, like most translocations in other mature B-cell lymphomas.139 The t(11;18) is extraordinary since it does not involve the Ig locus and encodes a fusion protein which is constituted by the amino-terminal portion of API-2 and the carboxyl-terminal of MALT-1. The overexpression of either BCL-10 or MALT-1, but also the API2-MALT1 chimeric protein, cause constitutive activation of the canonical NF-κB signaling pathway.140,141 The t(14;18) is found in 5–15% of pulmonary, salivary gland and ocular adnexae MZBCLs. About 5% of intestinal and pulmonary MZBCLs harbor the t(1;14) (Table 2).128–131,142,143 More recently, three novel IgH translocations in non-gastric MZBCL involving ODZ2, CNN3 and JMJD2C have been described, of which ODZ2 and JMJD2C were found recurrently.144
Table 2.
Chromosomal translocations of MZBCL.
The t(11;18) is present in ~40% of pulmonary- and in ~20% of gastric- MZBCLs and is virtually absent in MZBCLs of the salivary gland (Table 2).86,128–134 Gastric MZBCLs harboring the t(11;18) were found to be associated with CagA-positive strains of H. pylori. CagA induces activation of neutrophils releasing reactive oxygen species. It has been hypothesized that these are the genotoxic conditions which are instrumental in generating the t(11;18).132 The assembled literature indicates that t(11;18)-carrying MZBCLs generally possess a limited degree of additional chromosomal imbalances, are non-responsive to H. pylori eradication therapy and are not prone to transform into high-grade DLBCLs.50,51,145–147 T(11;18)-negative gastric MZBCLs with a high degree of genomic imbalances were also associated with H. pylori independency.148 Trisomies of chromosome 3, 12 and 18 are observed in t(11;18)-negative gastric (20%), pulmonary (40%), ocular adnexae (40%) and salivary gland (60%) MZBCLs.128,149 Interestingly, in MZBCLs, concurrent gains at 8q24, 9q34, 11q11-13 and 18q21 are frequent.149,150 The gains of these loci appear to target genes whose products stimulate the NF-κB pathway (i.e. TRAF2 and CARD9 at 9q34, RELA at 11q11-13 and MALT-1 at 18q21) and the cell cycle (Cyclin D1 at 11q12-13) (Figure 3).150 Gain of 6p and loss of 6q23 was specifically found in ocular adnexal MZBCL in ~25% of the cases.149,151 High resolution tile-path array CGH indicated that 6p gains were centered at the TNF locus at 6p21.33 with NF-κB inhibitor-like 1, TNF, LTα and LTβ as putative target genes.149 The loss of 6q23, consistently deleted the TNF-induced protein 3 also known as A20 at 6q23.3.149,151 FISH assays further confirmed the occurrence of A20 deletions in MZBCLs of the ocular adnexa (19%), salivary gland (8%) and thyroid (11%) but not in MZBCLs of lung, stomach, skin and small intestine. A20 is a potent inhibitor of NF-κB signaling which is required for termination of TNF- and TLR- induced NF-κB activation. A recent study showed that both MALT1 and API2-MALT1 can inactivate the A20 inhibitor by proteolysis, which further implicates A20 in the pathogenesis of MZBCL.149
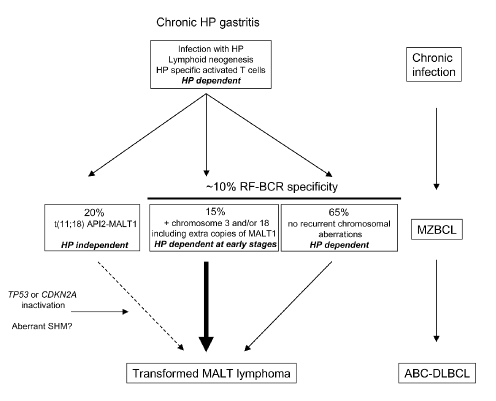
Figure 3.
Scenarios of multistep development of gastric MZBCL. Chronic H. pylori (HP) infection induces lymphoid tissue neogenesis. As a result of both direct and indirect stimulation infiltrating B cells will undergo active proliferation. Direct antigenic stimulation can be accomplished by auto-Ags like IgG-containing immune complexes, by bacterial or other, unknown, Ags. Indirect stimulation is provided by H. pylori-specific T cells. Due to the acquisition of genetic damage, B cells may obtain growth advantage. Gastric MZBCL with t(11;18) grow autonomously, do not respond to H. pylori eradication, but rarely progress to DLBCL. Gastric MZBCL with trisomy 3 and/or 18 and/or having extra copies of the MALT gene have a more aggressive clinical behavior. Following inactivation of the tumor suppressor genes TP53 or CDKN2A or due to mutation in oncogenes, possibly by aberrant SHM, MZBCL may transform to DLBCL.
The t(3;14)(FOX-P1/IgH), deregulating expression of the forkhead box P1 (FOX-P1) transcription factor, was initially reported by an Austrian study in as much as 4 out of 20 (20%) ocular adnexae MZBCLs and in 3 out of 6 (50%) thyroid MZBCLs.127 However, in more recent studies by North American and German groups, this translocation was not detected in series of 133 and 122 MZBCLs, respectively.129,135 Also others did not detect the t(3;14) in series of 126 ocular adnexae and 19 thyroid MZBCLs.129,135–137 On the other hand, Goatly et al.136 reported the t(3;14) in 8 out of 188 (4%) gastric MZBCLs (Table 2). Strong nuclear FOX-P1 expression has been found, irrespective of the t(3;14) or FOX-P1 copy number changes, in ~30% of MZBCLs.136,138 Sagaert et al.138 recently described 5 MALT lymphomas with strong nuclear FOX-P1 expression, one with the t(3;14) and 4 having trisomy 3 and 18, which all transformed into an aggressive, ABC-type DLBCL.138 Finally, there is little dispute that t(3;14) is prevalent in a subset of DLBCLs, with extranodal presentation and having the activated B cell-like (ABC) expression profile.135,137,152
Although the precise frequencies of transition of the various low-grade MZBCLs into DLBCLs are not clear in literature, ample evidence exists that MZBCLs can transform particularly into ABC-type DLBCLs rather than into GC-type DLBCLs: (i) trisomy 3 has been observed as a characteristic alteration in both MZBCLs and ABC-type DLBCLs;49,51,153 (ii) MZBCLs with high nuclear FOX-P1 were documented to progress into ABC-type DLBCLs;138 (iii) the majority of genomic alterations in t(11;18)-negative MZBCLs are also found in ABC-type DLBCLs;49,153 (iv) both MZBCLs and ABC-type DLBCLs are characterized by constitutive NF-κB signaling;154,155 (v) DLBCLs which still contain a low-grade lymphoma component are mostly of the ABC type;156 (vi) primary gastrointestinal DLBCLs show a similar expression profile as gastrointestinal MALT lymphoma;157 and (vii) the majority of rheumatoid arthritis-associated DLBCLs are of the ABC type.158
The molecular mechanisms underlying MZBCL progression are as yet ill-defined. A number of genetic alterations has been associated with histological transformation such as allelic loss and mutation of TP53 and hypermethylation or deletion of CDKN2A (p16-INK4A, ARF).159–161 Furthermore, several chromosomal gains and losses are associated with transformation.50 Since most MZBCLs express mutated IgV genes with intra-clonal sequence variation, proving previous and suggesting continued exposure to the SHM machinery, a role for the DNA mutator AID in MZBCL transformation cannot be excluded. However, immunohistochemical expression analyses showed that AID is detectable only in a minority of the cases.75,156 Accordingly, several investigators demonstrated variable, but generally low AID mRNA expression levels in MALT lymphomas.156,162,163 On the other hand, in ~50% of DLBCL, several protooncogenes, including PIM1, PAX5, RhoH/TTF and cMYC are targeted by aberrant SHM. Sequence analysis of MALT lymphomas revealed that 75% of low-grade MZBCLs and 100% of low-grade MZBCLs with a DLBCL component contained mutations in one or more of these oncogenes. In the latter group, higher frequencies of aberrant SHM were found as compared to pure low-grade MZBCLs, supporting the concept of AID-mediated lymphoma progression.156
BCR specificity of marginal zone B-cell lymphomas
The general idea is that MZBCLs still depend on environmental stimuli and on antigen-receptor ligands. IgVH and IgVL gene sequence analyses have revealed that in spite of high mutation loads the overall structure of the Ig is being preserved.119 Apparently, selective forces prevent the outgrowth of BCR- MZBCL mutants.119 Nearly 80% of early stage H. pylori-associated gastric MZBCLs, but also a proportion of cutaneous and ocular adnexal MZBCLs is curable by bacterial eradication alone.62,164,165 Similarly, IFNα-2b treatment can cause regression of HCV-associated MZBCL.166,167
In vitro culture experiments with gastric MZBCL cells have revealed that the tumor B cells do not respond to H. pylori directly, but instead depend for their survival on stimuli provided by intra-tumoral, H. pylori-specific T cells.168 We have recently produced soluble recombinant Abs derived from gastric and other MZBCLs, and indeed did not observe any reactivity with H. pylori bacteria. 119 Alternatively, it appeared that ~10% of gastric, and as much as ~40% of salivary gland MZBCLs expressed V1-69/JH4- and V3-7/JH3- encoded BCRs with strong IgVH-CDR3 amino acid sequence homology to canonical rheumatoid factors (RF).119,169,170 Among an extensive panel of B-NHLs, this RF homology was unique for MZBCL.119 Indeed 7 out of 10 recombinant MZBCL-derived Abs showed strong in vitro binding activity to immobilized human IgG.119 MZBCLs with high affinity IgG-specific BCRs may thus be continuously stimulated by Ab-Ag immune complexes, like IgG-opsonized H. pylori in chronic gastritis or IgG-chromatin and/or IgG-SSA/SSB-RNA in Sjögrens sialadenitis. The IgG-reactive BCRs may also capture and internalize Ab-Ag complexes and activate TLR9 and/or TLR7 by autologous or bacterial CpG DNA or by autoantigen-associated RNA, consequently potentiating the NF-κB pathway (Figure 4). Synergistic effects of BCR and TLR9 or TLR7 engagement have originally been shown in the mouse by T-cell independent activation of IgG-reactive B cells, using IgG-chromatin or IgG-RNA complexes.171,172
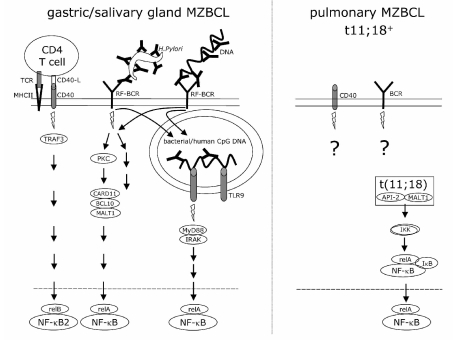
Figure 4.
MZBCL proliferation depends on constitutive NF-κB signaling provoked either by combined CD40/BCR/TLR9 signaling or by the API2-MALT1 fusion protein. Gastric- and salivary gland MZBCL, lacking t(11;18), depend on CD40L and other T-help factors, together with the (RF-specific) BCR and/or TLR7/9 NF-κB signals. MZBCL with constitutive NF-κB signaling due to t(11;18), do not depend on T-helper factors, BCR nor TLR signaling.
Intriguingly, none of 8 previously published,119 nor 12 newly analyzed MZBCLs that harbored the t(11;18), express BCRs with RF homology or reactivity170 (also H. Inagaki, written communication, Department of Pathology, University Graduate School of Medical Sciences, Nagoya, Japan, April 4, 2008). Moreover, the frequency of RF-BCRs, being ~40% of salivary gland, ~10% of gastric and <1% of pulmonary MZBCLs, inversely correlates with the t(11;18) frequencies found in these entities (Table 2).119,128,132,133 This tentative inverse relation suggests that t(11;18)+ MZBCLs do not depend on BCR (and perhaps neither on CD40 and TLR7/9) signals for their expansion since constitutive NF-κB activation is already guaranteed due to the expressed fusion protein (Figures 3 and 4). The facts that: (i) t(11;18)+ gastric MZBCLs are resistant to H. pylori eradication therapy; and that (ii) within the overall group of MZBCLs, t(11;18) and trisomy 3 harboring cases were from patients without underlying autoimmune diseases, support this hypothesis.145,147,173 Of note, the finding that t(11;18)+ MZBCLs lack RF-BCRs, indicates that this genetic aberration occurs independently of the selection process favoring this specificity.
Mouse models of marginal zone B-cell lymphoma
As yet a limited number of mouse models have been generated aimed at MZBCL development. In general, four categories of potential 'MZBCL' models can be distinguished: (i) mice with transgenic expression of genes involved in lympho-organogenesis; (ii) mice chronically challenged with Helicobacter species; (iii) mice carrying MZBCL-specific gene alterations; and (iv) mice with chronic or uncontrolled T-cell mediated B-cell activation.
As delineated in the first chapter, transgenic expression of the key molecules LT and TNF results in augmented lymphoid tissue neogenesis.10,11 Similarly, transgenic expression of B- and T-lymphocyte-attracting chemokines initiates formation of ectopic lymphoid tissues, presumably also via the LT/TNF axis.12 Although it could be argued that in these transgenic animals the continued ectopic lymphoproliferation would ultimately lead to cellular transformation, development of MZBCLs has not been described. This may be due to the fact that in these mice chronic antigenic stimulation has not been assayed.
In mice infected with Helicobacter species, the occurrence of organized lymphoid tissues in and beyond the gastric mucosa has been described.174–177 Oral infection of A/J mice with Helicobacter sp leads to development of hepatic inflammatory lesions containing HEVs, the production of CCL21 and CXCL13 and influx of B and T cells.177 Infection of BALB/c mice with Helicobacter felis resulted in a massive influx of B cells and lympho-organogenesis in the stomach.174,175 It was reported that after 23 months of infection, 25-75% of these mice developed low- or intermediate-grade MZBCLs. In these mice, regression of the infiltrates after anti-bacterial therapy was also demonstrated.174,175 Other investigators have infected BALB/c mice with different 'H. heilmannii' isolates originating from human and animal hosts. MZBCLs developed in ~25% of the infected mice. The lymphoma prevalence was dependent on the origin of the infecting isolates and the duration of infection.176 Finally, infection of C57BL/6 mice with Candidatus H. heilmannii resulted in the development of gastric MALT lymphoma in 100% of the mice after six months.178 It is noted that in all these infection protocols, the diagnosis of MZBCLs was based on morphological grounds alone, i.e. the presence of centrocyte-like cells and lympho-epithelial lesions, but not on the assessment of monoclonality by Ig gene rearrangement assays nor on any other molecular genetic analyses.174,175
Transgenic FVB mice expressing the API2-MALT1 fusion gene, driven by the SRα promoter under control of the EμIg heavy chain enhancer, specifically triggered the expansion of splenic MZ B cells. However, the expression of the API2-MALT1 fusion protein alone was not sufficient for the development of lymphomas over a period of 50 weeks.179 Immunization of these mice with complete Freund's adjuvants induced the loss of splenic compartmentalization and a poly- or oligoclonal lymphoid hyperplasia which gradually disappeared after cessation of the antigenic stimulation.180 P100−/− mice with signal-independent activation of the non-canonical NF-κB pathway had markedly elevated MZ B-cell numbers and a disturbed spleen microarchitecture.181 BAFF overexpression, activating the non-canonical NF-κB pathway in BAFF-Tg and BAFF-Tg/TNF −/− mice, resulted in increased survival and accumulation of transitional T2 and MZ B cells.182,183 Interestingly, the BAFF-Tg/TNF −/− mice showed a high incidence of B-cell infiltrates with histological features of extranodal MZBCL, but again not substantiated by molecular analyses.183 Mice with constitutively active IKK2, enhancing the canonical NF-κB pathway, showed a mild B-cell hyperplasia due rather to prolonged survival than to proliferation. Cell proliferation was dramatically enhanced when the IKK2 B cells were stimulated via the BCR or TLR4/9.184 Mice with B cell-specific expression of a chimeric CD40/latent membrane protein 1 (LMP1) protein showed an increased number of FO- and MZ- B cells in secondary lymphoid organs. The constitutive CD40-like signaling via the cytoplasmic LMP-1 tail in the B cells induced activation of the non-canonial NF-κB pathway, but also of the MAP kinases, Jnk and ERK.185 Interestingly, in mice of >12 months, oligo- and mono-clonal B-cell lymphomas developed at a high incidence. These B-cell lymphomas did not resemble human MZBCLs as they did not express CD21.
Some mouse models nicely underscore the role of chronic T-cell help in the development of B-cell lymphomas. For example, in Igλ transgenic mice with B cells presenting Igλ idiotype (Id)-derived peptides on MHC class II, the transferral of Id-specific CD4 T cells resulted in B-cell lymphoma development after approximately 40 weeks. The lymphomas resembled MZBCLs as they expressed CD21, CD35, CD1d and IgM. Moreover, the lymphomas were shown to be mono-/biclonal, harbored some somatic IgVH mutations and had major cytogenetic aberrations.186 Mice deficient for the autoimmune regulator (Aire) gene showed a high frequency of MZBCLs after 15–24 months. The B-cell lymphoproliferations were shown to be oligoclonal in the spleens of 4 out of 9 mice and displayed a MZ B-cell phenotype with low IgD and high CD1d.187 Interestingly, Aire is a recently discovered transcription factor that is expressed in thymic medullary epithelial cells and plays a key role in central tolerance induction.188 Thus, in these mice MZBCL development is most likely related to chronic help from autoreactive T cells.
In conclusion, the assembled literature points towards a key role of constitutive NF-κB signaling in MZBCL development. This requirement is fulfilled by the combination of persistent BCR triggering, chronic T-cell help and TLR stimulation elicited by chronic infection or autoimmunity. In the ectopically formed lymphoid tissue, these physiological stimuli can be overruled by genetic alterations which guarantee constitutive NF-κB signaling, thus making the cells less dependent on the environmental stimuli.
Acknowledgments
we thank Robbert Hoogeboom and Rogier M. Reijmers for critical reading of the manuscript.
Footnotes
Authorship and Disclosures
RJB designed, discussed, wrote and performed original work, FVM wrote, discussed and performed original work and CJVN wrote, approved and discussed original work.
The authors reported no potential conflicts of interest.
References
- 1.Meylan E, Tschopp J, Karin M. Intracellular pattern recognition receptors in the host response. Nature. 2006;442:39–44. doi: 10.1038/nature04946. [DOI] [PubMed] [Google Scholar]
- 2.Kumar V, Cotran RS, Robbins SL. Basic pathology. 7th ed. Philadelphia: Saunders; 2003. [Google Scholar]
- 3.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6:1191–7. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 4.Ware CF. Network communications: lymphotoxins, LIGHT, and TNF. Annu Rev Immunol. 2005;23:787–819. doi: 10.1146/annurev.immunol.23.021704.115719. [DOI] [PubMed] [Google Scholar]
- 5.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–8. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 6.Victoratos P, Lagnel J, Tzima S, Alimzhanov MB, Rajewsky K, Pasparakis M, Kollias G. FDC-specific functions of p55TNFR and IKK2 in the development of FDC networks and of antibody responses. Immunity. 2006;24:65–77. doi: 10.1016/j.immuni.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 7.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–17. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 8.Houtkamp MA, de Boer OJ, van der Loos CM, van der Wal AC, Becker AE. Adventitial infiltrates associated with advanced atherosclerotic plaques: structural organization suggests generation of local humoral immune responses. J Pathol. 2001;193:263–9. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH774>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 9.Ansel KM, Ngo VN, Hyman PL, Luther SA, Förster R, Sedgwick JD, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–14. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 10.Kratz A, Campos-Neto A, Hanson MS, Ruddle NH. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J Exp Med. 1996;183:1461–72. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LT alpha beta directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med. 2003;197:1153–63. doi: 10.1084/jem.20021761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169:424–33. doi: 10.4049/jimmunol.169.1.424. [DOI] [PubMed] [Google Scholar]
- 13.Kitani A, Strober W. Regulation of C gamma subclass germ-line transcripts in human peripheral blood B cells. J Immunol. 1993;151:3478–88. [PubMed] [Google Scholar]
- 14.Punnonen J, Aversa G, Cocks BG, McKenzie AN, Menon S, Zurawski G, et al. Interleukin 13 induces interleukin 4-independent IgG4 and IgE synthesis and CD23 expression by human B cells. Proc Natl Acad Sci USA. 1993;90:3730–4. doi: 10.1073/pnas.90.8.3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han S, Hathcock K, Zheng B, Kepler TB, Hodes R, Kelsoe G. Cellular interaction in germinal centers. Roles of CD40 ligand and B7-2 in established germinal centers. J Immunol. 1995;155:556–67. [PubMed] [Google Scholar]
- 16.Callard RE, Armitage RJ, Fanslow WC, Spriggs MK. CD40 ligand and i ts role in X-linked hyper-IgM syndrome. Immunol Today. 1993;14:559–64. doi: 10.1016/0167-5699(93)90188-Q. [DOI] [PubMed] [Google Scholar]
- 17.Kroese FG, Timens W, Nieuwenhuis P. Germinal center reaction and B lymphocytes: morphology and function. Curr Top Pathol. 1990;84 (Pt 1):103–48. doi: 10.1007/978-3-642-75519-4_5. [DOI] [PubMed] [Google Scholar]
- 18.MacLennan ICM. Germinal centers. Annu Rev Immunol. 1994;12:117–39. doi: 10.1146/annurev.iy.12.040194.001001. [DOI] [PubMed] [Google Scholar]
- 19.Berek C, Berger A, Apel M. Maturation of the immune response in germinal centers. Cell. 1991;67:1121–9. doi: 10.1016/0092-8674(91)90289-b. [DOI] [PubMed] [Google Scholar]
- 20.Jacob J, Kelsoe G, Rajewsky K, Weiss U. Intraclonal generation of antibody mutants in germinal centres. Nature. 1991;354:389–92. doi: 10.1038/354389a0. [DOI] [PubMed] [Google Scholar]
- 21.Lindhout E, Koopman G, Pals ST, de Groot C. Triple check for antigen specificity of B cells during germinal centre reactions. Immunol Today. 1997;18:573–7. doi: 10.1016/s0167-5699(97)01160-2. [DOI] [PubMed] [Google Scholar]
- 22.Liu YJ, Malisan F, de Bouteiller O, Guret C, Lebecque S, Banchereau J, et al. Within germinal centers, isotype switching of immunoglobulin genes occurs after the onset of somatic mutation. Immunity. 1996;4:241–50. doi: 10.1016/s1074-7613(00)80432-x. [DOI] [PubMed] [Google Scholar]
- 23.Schettino EW, Chai SK, Kasaian MT, Schroeder HW, Jr, Casali P. VHDJH gene sequences and antigen reactivity of monoclonal antibodies produced by human B-1 cells, evidence for somatic selection. J Immunol. 1997;158:2477–89. [PMC free article] [PubMed] [Google Scholar]
- 24.Hayakawa K, Hardy RR, Parks DR, Herzenberg LA. The "Ly-1 B" cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983;157:202–18. doi: 10.1084/jem.157.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casali P, Burastero SE, Nakamura M, Inghirami G, Notkins AL. Human lymphocytes making rheumatoid factor and antibody to ssDNA belong to Leu-1+ B-cell subset. Science. 1987;236:77–81. doi: 10.1126/science.3105056. [DOI] [PubMed] [Google Scholar]
- 26.Chen ZJ, Wheeler CJ, Shi W, Wu AJ, Yarboro CH, Gallagher M, Notkins AL. Polyreactive antigen-binding B cells are the predominant cell type in the newborn B cell repertoire. Eur J Immunol. 1998;28:989–94. doi: 10.1002/(SICI)1521-4141(199803)28:03<989::AID-IMMU989>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 27.Hervé M, Xu K, Ng YS, Wardemann H, Albesiano E, Messmer BT, et al. Unmutated and mutated chronic lymphocytic leukemias derive from self-reactive B cell precursors despite expressing different antibody reactivity. J Clin Invest. 2005;115:1636–43. doi: 10.1172/JCI24387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bende RJ, van Maldegem F, Triesscheijn M, Wormhoudt TA, Guijt R, van Noesel CJ. Germinal centers in human lymph nodes contain reactivated memory B cells. J Exp Med. 2007;204:2655–65. doi: 10.1084/jem.20071006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dammers PM, Visser A, Popa ER, Nieuwenhuis P, Kroese FG. Most marginal zone B cells in rat express germline encoded Ig VH genes and are ligand selected. J Immunol. 2000;165:6156–69. doi: 10.4049/jimmunol.165.11.6156. [DOI] [PubMed] [Google Scholar]
- 30.Dunn-Walters DK, Isaacson PG, Spencer J. Analysis of mutations in immunoglobulin heavy chain variable region genes of microdissected marginal zone (MGZ) B cells suggests that the MGZ of human spleen is a reservoir of memory B cells. J Exp Med. 1995;182:559–66. doi: 10.1084/jem.182.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn-Walters DK, Isaacson PG, Spencer J. Sequence analysis of rearranged IgVH genes from microdissected human Peyer’s patch marginal zone B cells. Immunology. 1996;88:618–24. [PMC free article] [PubMed] [Google Scholar]
- 32.Weller S, Braun MC, Tan BK, Rosenwald A, Cordier C, Conley ME, et al. Human blood IgM "memory" B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood. 2004;104:3647–54. doi: 10.1182/blood-2004-01-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weller S, Faili A, Garcia C, Braun MC, Le Deist F F, de Saint Basile G G, et al. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci USA. 2001;98:1166–70. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weller S, Mamani-Matsuda M, Picard C, Cordier C, Lecoeuche D, Gauthier F, et al. Somatic diversification in the absence of antigen-driven responses is the hallmark of the IgM+ IgD+ CD27+ B cell repertoire in infants. J Exp Med. 2008;205:1331–42. doi: 10.1084/jem.20071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scheeren FA, Nagasawa M, Weijer K, Cupedo T, Kirberg J, Legrand N, Spits H. T cell-independent development and induction of somatic hypermutation in human IgM+ IgD+ CD27+ B cells. J Exp Med. 2008;205:2033–42. doi: 10.1084/jem.20070447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tangye SG, Good KL. Human IgM+CD27+ B cells: memory B cells or "memory" B cells? J Immunol. 2007;179:13–9. doi: 10.4049/jimmunol.179.1.13. [DOI] [PubMed] [Google Scholar]
- 37.Tsuiji M, Yurasov S, Velinzon K, Thomas S, Nussenzweig MC, Wardemann H. A checkpoint for autoreactivity in human IgM+ memory B cell development. J Exp Med. 2006;203:393–400. doi: 10.1084/jem.20052033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Capolunghi F, Cascioli S, Giorda E, Rosado MM, Plebani A, Auriti C, et al. CpG drives human transitional B cells to terminal differentiation and production of natural antibodies. J Immunol. 2008;180:800–8. doi: 10.4049/jimmunol.180.2.800. [DOI] [PubMed] [Google Scholar]
- 39.Zandvoort A, Lodewijk ME, de Boer NK, Dammers PM, Kroese FG, Timens W. CD27 expression in the human splenic marginal zone: the infant marginal zone is populated by naive B cells. Tissue Antigens. 2001;58:234–42. doi: 10.1034/j.1399-0039.2001.580403.x. [DOI] [PubMed] [Google Scholar]
- 40.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–7. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 41.Dammers PM, Kroese FG. Recruitment and selection of marginal zone B cells is independent of exogenous antigens. Eur J Immunol. 2005;35:2089–99. doi: 10.1002/eji.200526118. [DOI] [PubMed] [Google Scholar]
- 42.Tiller T, Tsuiji M, Yurasov S, Velinzon K, Nussenzweig MC, Wardemann H. Autoreactivity in human IgG+ memory B cells. Immunity. 2007;26:205–13. doi: 10.1016/j.immuni.2007.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mellemkjaer L, Pfeiffer RM, Engels EA, Gridley G, Wheeler W, Hemminki K, et al. Autoimmune disease in individuals and close family members and susceptibility to non-Hodgkin's lymphoma. Arthritis Rheum. 2008;58:657–66. doi: 10.1002/art.23267. [DOI] [PubMed] [Google Scholar]
- 44.Holm LE, Blomgren H, Lowhagen T. Cancer risks in patients with chronic lymphocytic thyroiditis. N Engl J Med. 1985;312:601–4. doi: 10.1056/NEJM198503073121001. [DOI] [PubMed] [Google Scholar]
- 45.Kassan SS, Thomas TL, Moutsopoulos HM, Hoover R, Kimberly RP, Budman DR, et al. Increased risk of lymphoma in sicca syndrome. Ann Intern Med. 1978;89:888–92. doi: 10.7326/0003-4819-89-6-888. [DOI] [PubMed] [Google Scholar]
- 46.Leandro MJ, Isenberg DA. Rheumatic diseases and malignancy--is there an association? Scand J Rheumatol. 2001;30:185–8. doi: 10.1080/030097401316909486. [DOI] [PubMed] [Google Scholar]
- 47.Pertovaara M, Pukkala E, Laippala P, Miettinen A, Pasternack A. A longitudinal cohort study of Finnish patients with primary Sjogren's syndrome: clinical, immunological, and epidemiological aspects. Ann Rheum Dis. 2001;60:467–72. doi: 10.1136/ard.60.5.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ekström Smedby K, Vajdic CM, Falster M, Engels EA, Martínez-Maza O, Turner J, et al. Auto-immune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029–38. doi: 10.1182/blood-2007-10-119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barth TF, Bentz M, Leithäuser F, Stilgenbauer S, Siebert R, Schlotter M, et al. Pathogenic complexity of gastric B-cell lymphoma. Blood. 2002;100:1095–6. doi: 10.1182/blood-2002-02-0423. [DOI] [PubMed] [Google Scholar]
- 50.Starostik P, Patzner J, Greiner A, Schwarz S, Kalla J, Ott G, Müller-Hermelink HK. Gastric marginal zone B-cell lymphomas of MALT type develop along 2 distinct pathogenetic pathways. Blood. 2002;99:3–9. doi: 10.1182/blood.v99.1.3. [DOI] [PubMed] [Google Scholar]
- 51.Schreuder MI, Hoeve MA, Hebeda KM, Verdijk MA, Ligtenberg MJ, Bot FJ, et al. Mutual exclusion of t(11;18)(q21;q21) and numerical chromosomal aberrations in the development of different types of primary gastric lymphomas. Br J Haematol. 2003;123:590–9. doi: 10.1046/j.1365-2141.2003.04630.x. [DOI] [PubMed] [Google Scholar]
- 52.Smedby KE, Hjalgrim H, Askling J, Chang ET, Gregersen H, Porwit-MacDonald A, et al. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98:51–60. doi: 10.1093/jnci/djj004. [DOI] [PubMed] [Google Scholar]
- 53.Baecklund E, Iliadou A, Askling J, Ekbom A, Backlin C, Granath F, et al. Association of chronic inflammation, not its treatment, with increased lymphoma risk in rheumatoid arthritis. Arthritis Rheum. 2006;54:692–701. doi: 10.1002/art.21675. [DOI] [PubMed] [Google Scholar]
- 54.Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmström P, Wahren-Herlenius M, Jonsson R. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjogren's syndrome. Arthritis Rheum. 2003;48:3187–201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 55.Armengol MP, Juan M, Lucas-Martín A, Fernández-Figueras MT, Jaraquemada D, Gallart T, Pujol-Borrell R. Thyroid autoimmune disease: demonstration of thyroid antigen-specific B cells and recombination-activating gene expression in chemokine-containing active intrathyroidal germinal centers. Am J Pathol. 2001;159:861–73. doi: 10.1016/S0002-9440(10)61762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zucca E, Bertoni F. Chlamydia or not Chlamydia, that is the question: which is the microorganism associated with MALT lymphomas of the ocular adnexa? J Natl Cancer Inst. 2006;98:1348–9. doi: 10.1093/jnci/djj406. [DOI] [PubMed] [Google Scholar]
- 57.Wotherspoon AC, Ortiz-Hidalgo C, Falzon MR, Isaacson PG. Helicobacter pylori-associated gastritis and primary B-cell gastric lymphoma. Lancet. 1991;338:1175–6. doi: 10.1016/0140-6736(91)92035-z. [DOI] [PubMed] [Google Scholar]
- 58.de Sanjose S, Benavente Y, Vajdic CM, Engels EA, Morton LM, Bracci PM, et al. Hepatitis C and non-Hodgkin lymphoma among 4784 cases and 6269 controls from the International Lymphoma Epidemiology Consortium. Clin Gastroenterol Hepatol. 2008;6:451–8. doi: 10.1016/j.cgh.2008.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mosnier JF, Degott C, Marcellin P, Hénin D, Erlinger S, Benhamou JP. The intraportal lymphoid nodule and its environment in chronic active hepatitis C: an immunohistochemical study. Hepatology. 1993;17:366–71. [PubMed] [Google Scholar]
- 60.Freni MA, Artuso D, Gerken G, Spanti C, Marafioti T, Alessi N, et al. Focal lymphocytic aggregates in chronic hepatitis C: occurrence, immunohistochemical characterization, and relation to markers of autoimmunity. Hepatology. 1995;22:389–94. [PubMed] [Google Scholar]
- 61.Garbe C, Stein H, Dienemann D, Orfanos CE. Borrelia burgdorferi-associated cutaneous B cell lymphoma: clinical and immunohistologic characterization of four cases. J Am Acad Dermatol. 1991;24:584–90. doi: 10.1016/0190-9622(91)70088-j. [DOI] [PubMed] [Google Scholar]
- 62.Cerroni L, Signoretti S, Höfler G, Annessi G, Pütz B, Lackinger E, et al. Primary cutaneous marginal zone B-cell lymphoma: a recently described entity of low-grade malignant cutaneous B-cell lymphoma. Am J Surg Pathol. 1997;21:1307–15. doi: 10.1097/00000478-199711000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Amft N, Curnow SJ, Scheel-Toellner D, Devadas A, Oates J, Crocker J, et al. Ectopic expression of the B cell-attracting chemokine BCA-1 (CXCL13) on endothelial cells and within lymphoid follicles contributes to the establishment of germinal center-like structures in Sjogren's syndrome. Arthritis Rheum. 2001;44:2633–41. doi: 10.1002/1529-0131(200111)44:11<2633::aid-art443>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 64.Ogawa N, Ping L, Zhenjun L, Takada Y, Sugai S. Involvement of the interferon-gamma-induced T cell-attracting chemokines, interferon-gamma-inducible 10-kd protein (CXCL10) and monokine induced by interferon-gamma (CXCL9), in the salivary gland lesions of patients with Sjogren's syndrome. Arthritis Rheum. 2002;46:2730–41. doi: 10.1002/art.10577. [DOI] [PubMed] [Google Scholar]
- 65.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–63. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barone F, Bombardieri M, Manzo A, Blades MC, Morgan PR, Challacombe SJ, et al. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjogren’s syndrome. Arthritis Rheum. 2005;52:1773–84. doi: 10.1002/art.21062. [DOI] [PubMed] [Google Scholar]
- 67.Kunkel EJ, Kim CH, Lazarus NH, Vierra MA, Soler D, Bowman EP, Butcher EC. CCR10 expression is a common feature of circulating and mucosal epithelial tissue IgA Ab-secreting cells. J Clin Invest. 2003;111:1001–10. doi: 10.1172/JCI17244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gottenberg JE, Cagnard N, Lucchesi C, Letourneur F, Mistou S, Lazure T, et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjogren's syndrome. Proc Natl Acad Sci USA. 2006;103:2770–5. doi: 10.1073/pnas.0510837103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, et al. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren's syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fox RI, Kang HI, Ando D, Abrams J, Pisa E. Cytokine mRNA expression in salivary gland biopsies of Sjogren's syndrome. J Immunol. 1994;152:5532–9. [PubMed] [Google Scholar]
- 71.Slobbe RL, Pluk W, van Venrooij WJ, Pruijn GJ. Ro ribonucleoprotein assembly in vitro. Identification of RNA-protein and protein-protein interactions. J Mol Biol. 1992;227:361–6. doi: 10.1016/0022-2836(92)90890-v. [DOI] [PubMed] [Google Scholar]
- 72.Elagib KE, Tengnér P, Levi M, Jonsson R, Thompson KM, Natvig JB, Wahren-Herlenius M. Immunoglobulin variable genes and epitope recognition of human monoclonal anti-Ro 52-kd in primary Sjogren's syndrome. Arthritis Rheum. 1999;42:2471–81. doi: 10.1002/1529-0131(199911)42:11<2471::AID-ANR26>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 73.Suzuki H, Takemura H, Suzuki M, Sekine Y, Kashiwagi H. Molecular cloning of anti-SS-A/Ro 60-kDa peptide Fab fragments from infiltrating salivary gland lymphocytes of a patient with Sjogren's syndrome. Biochem Biophys Res Commun. 1997;232:101–6. doi: 10.1006/bbrc.1997.6233. [DOI] [PubMed] [Google Scholar]
- 74.Salomonsson S, Wahren-Herlenius M. Local production of Ro/SSA and La/SSB autoantibodies in the target organ coincides with high levels of circulating antibodies in sera of patients with Sjogren's syndrome. Scand J Rheumatol. 2003;32:79–82. doi: 10.1080/03009740310000076. [DOI] [PubMed] [Google Scholar]
- 75.Bombardieri M, Barone F, Humby F, Kelly S, McGurk M, Morgan P, et al. Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjogren's syndrome. J Immunol. 2007;179:4929–38. doi: 10.4049/jimmunol.179.7.4929. [DOI] [PubMed] [Google Scholar]
- 76.Stott DI, Hiepe F, Hummel M, Steinhauser G, Berek C. Antigen-driven clonal proliferation of B cells within the target tissue of an autoimmune disease. The salivary glands of patients with Sjogren's syndrome. J Clin Invest. 1998;102:938–46. doi: 10.1172/JCI3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gellrich S, Rutz S, Borkowski A, Golembowski S, Gromnica-Ihle E, Sterry W, Jahn S. Analysis of V(H)-D-J(H) gene transcripts in B cells infiltrating the salivary glands and lymph node tissues of patients with Sjogren's syndrome. Arthritis Rheum. 1999;42:240–7. doi: 10.1002/1529-0131(199902)42:2<240::AID-ANR5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 78.Hansen A, Jacobi A, Pruss A, Kaufmann O, Scholze J, Lipsky PE, Dörner T. Comparison of immunoglobulin heavy chain rearrangements between peripheral and glandular B cells in a patient with primary Sjogren’s syndrome. Scand J Immunol. 2003;57:470–9. doi: 10.1046/j.1365-3083.2003.01226.x. [DOI] [PubMed] [Google Scholar]
- 79.Armengol MP, Cardoso-Schmidt CB, Fernández M, Ferrer X, Pujol-Borrell R, Juan M. Chemokines determine local lymphoneogenesis and a reduction of circulating CXCR4+ T and CCR7 B and T lymphocytes in thyroid autoimmune diseases. J Immunol. 2003;170:6320–8. doi: 10.4049/jimmunol.170.12.6320. [DOI] [PubMed] [Google Scholar]
- 80.Marinkovic T, Garin A, Yokota Y, Fu YX, Ruddle NH, Furtado GC, Lira SA. Interaction of mature CD3+ CD4+ T cells with dendritic cells triggers the development of tertiary lymphoid structures in the thyroid. J Clin Invest. 2006;116:2622–32. doi: 10.1172/JCI28993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Garcia-Lopez MA, Sancho D, Sanchez-Madrid F, Marazuela M. Thyrocytes from autoimmune thyroid disorders produce the chemokines IP-10 and Mig and attract CXCR3+ lymphocytes. J Clin Endocrinol Metab. 2001;86:5008–16. doi: 10.1210/jcem.86.10.7953. [DOI] [PubMed] [Google Scholar]
- 82.Del Prete GF, Tiri A, De Carli M, Mariotti S, Pinchera A, Chretien I, et al. High potential to tumor necrosis factor alpha (TNF-alpha) production of thyroid infiltrating T lymphocytes in Hashimoto's thyroiditis: a peculiar feature of destructive thyroid autoimmunity. Autoimmunity. 1989;4:267–76. doi: 10.3109/08916938909014703. [DOI] [PubMed] [Google Scholar]
- 83.Heuer M, Aust G, Ode-Hakim S, Scherbaum WA. Different cytokine mRNA profiles in Graves' disease, Hashimoto's thyroiditis, and nonautoimmune thyroid disorders determined by quantitative reverse transcriptase polymerase chain reaction (RT-PCR) Thyroid. 1996;6:97–106. doi: 10.1089/thy.1996.6.97. [DOI] [PubMed] [Google Scholar]
- 84.McIntosh R, Watson P, Weetman A. Somatic hypermutation in autoimmune thyroid disease. Immunol Rev. 1998;162:219–31. doi: 10.1111/j.1600-065x.1998.tb01444.x. [DOI] [PubMed] [Google Scholar]
- 85.Cerroni L, Zochling N, Putz B, Kerl H. Infection by Borrelia burgdorferi and cutaneous B-cell lymphoma. J Cutan Pathol. 1997;24:457–61. doi: 10.1111/j.1600-0560.1997.tb01318.x. [DOI] [PubMed] [Google Scholar]
- 86.Li C, Inagaki H, Kuo TT, Hu S, Okabe M, Eimoto T. Primary cutaneous marginal zone B-cell lymphoma: a molecular and clinicopathologic study of 24 asian cases. Am J Surg Pathol. 2003;27:1061–9. doi: 10.1097/00000478-200308000-00003. [DOI] [PubMed] [Google Scholar]
- 87.Wood GS, Kamath NV, Guitart J, Heald P, Kohler S, Smoller BR, Cerroni L. Absence of Borrelia burgdorferi DNA in cutaneous B-cell lymphomas from the United States. J Cutan Pathol. 2001;28:502–7. doi: 10.1034/j.1600-0560.2001.281002.x. [DOI] [PubMed] [Google Scholar]
- 88.Ferreri AJ, Guidoboni M, Ponzoni M, De Conciliis C, Dell'Oro S, Fleischhauer K, et al. Evidence for an association between Chlamydia psittaci and ocular adnexal lymphomas. J Natl Cancer Inst. 2004;96:586–94. doi: 10.1093/jnci/djh102. [DOI] [PubMed] [Google Scholar]
- 89.Chanudet E, Zhou Y, Bacon CM, Wotherspoon AC, Müller-Hermelink HK, Adam P, et al. Chlamydia psittaci is variably associated with ocular adnexal MALT lymphoma in different geographical regions. J Pathol. 2006;209:344–51. doi: 10.1002/path.1984. [DOI] [PubMed] [Google Scholar]
- 90.Aigelsreiter A, Leitner E, Deutsch AJ, Kessler HH, Stelzl E, Beham-Schmid C, et al. Chlamydia psittaci in MALT lymphomas of ocular adnexals: the Austrian experience. Leuk Res. 2008;32:1292–4. doi: 10.1016/j.leukres.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 91.Ponzoni M, Ferreri AJ, Guidoboni M, Lettini AA, Cangi MG, Pasini E, et al. Chlamydia infection and lymphomas: association beyond ocular adnexal lymphomas highlighted by multiple detection methods. Clin Cancer Res. 2008;14:5794–800. doi: 10.1158/1078-0432.CCR-08-0676. [DOI] [PubMed] [Google Scholar]
- 92.Ferreri AJ, Dolcetti R, Dognini GP, Malabarba L, Vicari N, Pasini E, et al. Chlamydophila psittaci is viable and infectious in the conjunctiva and peripheral blood of patients with ocular adnexal lymphoma: results of a single-center prospective case-control study. Int J Cancer. 2008;123:1089–93. doi: 10.1002/ijc.23596. [DOI] [PubMed] [Google Scholar]
- 93.Mulder MM, Heddema ER, Pannekoek Y, Faridpooya K, Oud ME, Schilder-Tol E, et al. No evidence for an association of ocular adnexal lymphoma with Chlamydia psittaci in a cohort of patients from the Netherlands. Leuk Res. 2006;30:1305–7. doi: 10.1016/j.leukres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 94.Daibata M, Nemoto Y, Togitani K, Fukushima A, Ueno H, Ouchi K, et al. Absence of Chlamydia psittaci in ocular adnexal lymphoma from Japanese patients. Br J Haematol. 2006;132:651–2. doi: 10.1111/j.1365-2141.2005.05943.x. [DOI] [PubMed] [Google Scholar]
- 95.Rosado MF, Byrne GE, Jr, Ding F, Fields KA, Ruiz P, Dubovy SR, et al. Ocular adnexal lymphoma: a clinicopathologic study of a large cohort of patients with no evidence for an association with Chlamydia psittaci. Blood. 2006;107:467–72. doi: 10.1182/blood-2005-06-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.De Vita S, Sacco C, Sansonno D, Gloghini A, Dammacco F, Crovatto M, et al. Characterization of overt B-cell lymphomas in patients with hepatitis C virus infection. Blood. 1997;90:776–82. [PubMed] [Google Scholar]
- 97.Silvestri F, Pipan C, Barillari G, Zaja F, Fanin R, Infanti L, et al. Prevalence of hepatitis C virus infection in patients with lymphoproliferative disorders. Blood. 1996;87:4296–301. [PubMed] [Google Scholar]
- 98.Decker T, Muller M, Stockinger S. The yin and yang of type I interferon activity in bacterial infection. Nat Rev Immunol. 2005;5:675–87. doi: 10.1038/nri1684. [DOI] [PubMed] [Google Scholar]
- 99.D'Elios MM, Manghetti M, De Carli M, Costa F, Baldari CT, Burroni D, et al. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–7. [PubMed] [Google Scholar]
- 100.Karttunen R, Karttunen T, Ekre HP, MacDonald TT. Interferon gamma and interleukin 4 secreting cells in the gastric antrum in Helicobacter pylori positive and negative gastritis. Gut. 1995;36:341–5. doi: 10.1136/gut.36.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sommer F, Faller G, Konturek P, Kirchner T, Hahn EG, Zeus J, et al. Antrum- and corpus mucosa-infiltrating CD4(+) lymphocytes in Helicobacter pylori gastritis display a Th1 phenotype. Infect Immun. 1998;66:5543–6. doi: 10.1128/iai.66.11.5543-5546.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dame TM, Orenzoff BL, Palmer LE, Furie MB. IFN-gamma alters the response of Borrelia burgdorferi-activated endothelium to favor chronic inflammation. J Immunol. 2007;178:1172–9. doi: 10.4049/jimmunol.178.2.1172. [DOI] [PubMed] [Google Scholar]
- 103.Del Río L, Buendía AJ, Sánchez J, Garcés B, Caro MR, Gallego MC, et al. Chlamydophila abortus (Chlamydia psittaci serotype 1) clearance is associated with the early recruitment of neutrophils and CD8(+)T cells in a mouse model. J Comp Pathol. 2000;123:171–81. doi: 10.1053/jcpa.2000.0411. [DOI] [PubMed] [Google Scholar]
- 104.Baldari CT, Lanzavecchia A, Telford JL. Immune subversion by Helicobacter pylori. Trends Immunol. 2005;26:199–207. doi: 10.1016/j.it.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 105.Genta RM, Hamner HW, Graham DY. Gastric lymphoid follicles in Helicobacter pylori infection: frequency, distribution, and response to triple therapy. Hum Pathol. 1993;24:577–83. doi: 10.1016/0046-8177(93)90235-9. [DOI] [PubMed] [Google Scholar]
- 106.Mazzucchelli L, Blaser A, Kappeler A, Schärli P, Laissue JA, Baggiolini M, Uguccioni M. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J Clin Invest. 1999;104:R49–R54. doi: 10.1172/JCI7830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Reiche N, Jung A, Brabletz T, Vater T, Kirchner T, Faller G. Generation and characterization of human monoclonal scFv antibodies against Helicobacter pylori antigens. Infect Immun. 2002;70:4158–64. doi: 10.1128/IAI.70.8.4158-4164.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Steere AC, Duray PH, Butcher EC. Spirochetal antigens and lymphoid cell surface markers in Lyme synovitis. Comparison with rheumatoid synovium and tonsillar lymphoid tissue. Arthritis Rheum. 1988;31:487–95. doi: 10.1002/art.1780310405. [DOI] [PubMed] [Google Scholar]
- 109.Ghosh S, Steere AC, Stollar BD, Huber BT. In situ diversification of the antibody repertoire in chronic Lyme arthritis synovium. J Immunol. 2005;174:2860–9. doi: 10.4049/jimmunol.174.5.2860. [DOI] [PubMed] [Google Scholar]
- 110.Dierlamm J, Pittaluga S, Wlodarska I, Stul M, Thomas J, Boogaerts M, et al. Marginal zone B-cell lymphomas of different sites share similar cytogenetic and morphologic features. Blood. 1996;87:299–307. [PubMed] [Google Scholar]
- 111.Ferreri AJ, Zucca E. Marginal-zone lymphoma. Crit Rev Oncol Hematol. 2007;63:245–56. doi: 10.1016/j.critrevonc.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 112.Drillenburg P, van der Voort R, Koopman G, Dragosics B, van Krieken JH, Kluin P, et al. Preferential expression of the mucosal homing receptor integrin alpha4 beta7 in gastrointestinal non-Hodgkin’s lymphomas. Am J Pathol. 1996;150:919–27. [PMC free article] [PubMed] [Google Scholar]
- 113.Du MQ, Peng HZ, Dogan A, Diss TC, Liu H, Pan LX, et al. Preferential dissemination of B-cell gastric mucosa-associated lymphoid tissue (MALT) lymphoma to the splenic marginal zone. Blood. 1997;90:4071–7. [PubMed] [Google Scholar]
- 114.Beima KM, Miazgowicz MM, Lewis MD, Yan PS, Huang TH, Weinmann AS. T-bet binding to newly identified target gene promoters is cell type-independent but results in variable context-dependent functional effects. J Biol Chem. 2006;281:11992–12000. doi: 10.1074/jbc.M513613200. [DOI] [PubMed] [Google Scholar]
- 115.Muehlinghaus G, Cigliano L, Huehn S, Peddinghaus A, Leyendeckers H, Hauser AE, et al. Regulation of CXCR3 and CXCR4 expression during terminal differentiation of memory B cells into plasma cells. Blood. 2005;105:3965–71. doi: 10.1182/blood-2004-08-2992. [DOI] [PubMed] [Google Scholar]
- 116.Jones D, Benjamin RJ, Shahsafaei A, Dorfman DM. The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood. 2000;95:627–32. [PubMed] [Google Scholar]
- 117.Dorfman DM, Hwang ES, Shahsafaei A, Glimcher LH. T-bet, a T-cell-associated transcription factor, is expressed in a subset of B-cell lymphoproliferative disorders. Am J Clin Pathol. 2004;122:292–7. doi: 10.1309/AQQ2-DVM7-5DVY-0PWP. [DOI] [PubMed] [Google Scholar]
- 118.Bende RJ, Smit LA, Bossenbroek JG, Aarts WM, Spaargaren M, de Leval L, et al. Primary follicular lymphoma of the small intestine: alpha4beta7 expression and immunoglobulin configuration suggest an origin from local antigen-experienced B cells. Am J Pathol. 2003;162:105–13. doi: 10.1016/s0002-9440(10)63802-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bende RJ, Aarts WM, de Jong D, Pals ST, van Noesel CJ. Among B-cell non-Hodgkin's lymphomas, MALT lymphomas express a unique antibody repertoire with frequent rheumatoid factor reactivity. J Exp Med. 2005;201:1229–41. doi: 10.1084/jem.20050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ohshima K, Suefuji H, Karube K, Hamasaki M, Hatano B, Tutiya T, et al. Expression of chemokine receptor CXCR3 and its ligand, mig, in gastric and thyroid marginal zone lymphomas. Possible migration and autocrine mechanism. Leuk Lymphoma. 2003;44:329–36. doi: 10.1080/1042819031000060546. [DOI] [PubMed] [Google Scholar]
- 121.van Maldegem F, van Dijk R, Wormhoudt TA, Kluin PM, Willemze R, Cerroni L, et al. The majority of cutaneous marginal zone B cell lymphomas express class switched immunoglobulins and develop in a T helper type 2 inflammatory environment. Blood. 2008;112:3355–61. doi: 10.1182/blood-2008-01-132415. [DOI] [PubMed] [Google Scholar]
- 122.Auer IA, Gascoyne RD, Connors JM, Cotter FE, Greiner TC, Sanger WG, Horsman DE. t(11;18) (q21;q21) is the most common translocation in MALT lymphomas. Ann Oncol. 1997;8:979–85. doi: 10.1023/a:1008202303666. [DOI] [PubMed] [Google Scholar]
- 123.Ott G, Katzenberger T, Greiner A, Kalla J, Rosenwald A, Heinrich U, et al. The t(11;18)(q21;q21) chromosome translocation is a frequent and specific aberration in low-grade but not high-grade malignant non-Hodgkin's lymphomas of the mucosa-associated lymphoid tissue (MALT-) type. Cancer Res. 1997;57:3944–8. [PubMed] [Google Scholar]
- 124.Wotherspoon AC, Pan LX, Diss TC, Isaacson PG. Cytogenetic study of B-cell lymphoma of mucosa-associated lymphoid tissue. Cancer Genet Cytogenet. 1992;58:35–8. doi: 10.1016/0165-4608(92)90130-z. [DOI] [PubMed] [Google Scholar]
- 125.Streubel B, Lamprecht A, Dierlamm J, Cerroni L, Stolte M, Ott G, et al. T(14;18)(q32;q21) involving IGH and MALT1 is a frequent chromosomal aberration in MALT lymphoma. Blood. 2003;101:2335–9. doi: 10.1182/blood-2002-09-2963. [DOI] [PubMed] [Google Scholar]
- 126.Sanchez-Izquierdo D, Buchonnet G, Siebert R, Gascoyne RD, Climent J, Karran L, et al. MALT1 is deregulated by both chromosomal translocation and amplification in B-cell non-Hodgkin lymphoma. Blood. 2003;101:4539–46. doi: 10.1182/blood-2002-10-3236. [DOI] [PubMed] [Google Scholar]
- 127.Streubel B, Vinatzer U, Lamprecht A, Raderer M, Chott A. t(3;14) (p14.1;q32) involving IGH and FOXP1 is a novel recurrent chromosomal aberration in MALT lymphoma. Leukemia. 2005;19:652–8. doi: 10.1038/sj.leu.2403644. [DOI] [PubMed] [Google Scholar]
- 128.Streubel B, Simonitsch-Klupp I, Müllauer L, Lamprecht A, Huber D, Siebert R, et al. Variable frequencies of MALT lymphoma-associated genetic aberrations in MALT lymphomas of different sites. Leukemia. 2004;18:1722–6. doi: 10.1038/sj.leu.2403501. [DOI] [PubMed] [Google Scholar]
- 129.Remstein ED, Dogan A, Einerson RR, Paternoster SF, Fink SR, Law M, et al. The incidence and anatomic site specificity of chromosomal translocations in primary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) in North America. Am J Surg Pathol. 2006;30:1546–53. doi: 10.1097/01.pas.0000213275.60962.2a. [DOI] [PubMed] [Google Scholar]
- 130.Sagaert X, Laurent M, Baens M, Wlodarska I, Wolf-Peeters C. MALT1 and BCL10 aberrations in MALT lymphomas and their effect on the expression of BCL10 in the tumour cells. Mod Pathol. 2006;19:225–32. doi: 10.1038/modpathol.3800523. [DOI] [PubMed] [Google Scholar]
- 131.Schreuder MI, Hoefnagel JJ, Jansen PM, van Krieken JH, Willemze R, Hebeda KM. FISH analysis of MALT lymphoma-specific translocations and aneuploidy in primary cutaneous marginal zone lymphoma. J Pathol. 2005;205:302–10. doi: 10.1002/path.1711. [DOI] [PubMed] [Google Scholar]
- 132.Ye H, Liu H, Attygalle A, Wotherspoon AC, Nicholson AG, Charlotte F, et al. Variable frequencies of t(11;18)(q21;q21) in MALT lymphomas of different sites: significant association with CagA strains of H pylori in gastric MALT lymphoma. Blood. 2003;102:1012–8. doi: 10.1182/blood-2002-11-3502. [DOI] [PubMed] [Google Scholar]
- 133.Okabe M, Inagaki H, Ohshima K, Yoshino T, Li C, Eimoto T, et al. API2-MALT1 fusion defines a distinctive clinicopathologic subtype in pulmonary extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Am J Pathol. 2003;162:1113–22. doi: 10.1016/S0002-9440(10)63908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gallardo F, Bellosillo B, Espinet B, Pujol RM, Estrach T, Servitje O, et al. Aberrant nuclear BCL10 expression and lack of t(11;18)(q21;q21) in primary cutaneous marginal zone B-cell lymphoma. Hum Pathol. 2006;37:867–73. doi: 10.1016/j.humpath.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 135.Haralambieva E, Adam P, Ventura R, et al. Genetic rearrangement of FOXP1 is predominantly detected in a subset of diffuse large B-cell lymphomas with extranodal presentation. Leukemia. 2006;20:1300–3. doi: 10.1038/sj.leu.2404244. [DOI] [PubMed] [Google Scholar]
- 136.Goatly A, Bacon CM, Nakamura S, Ye H, Kim I, Brown PJ, et al. FOXP1 abnormalities in lymphoma: translocation breakpoint mapping reveals insights into deregulated transcriptional control. Mod Pathol. 2008;21:902–11. doi: 10.1038/modpathol.2008.74. [DOI] [PubMed] [Google Scholar]
- 137.Wlodarska I, Veyt E, De Paepe P, Vandenberghe P, Nooijen P, Theate I, et al. FOXP1, a gene highly expressed in a subset of diffuse large B-cell lymphoma, is recurrently targeted by genomic aberrations. Leukemia. 2005;19:1299–1305. doi: 10.1038/sj.leu.2403813. [DOI] [PubMed] [Google Scholar]
- 138.Sagaert X, de Paepe P, Libbrecht L, Vanhentenrijk V, Verhoef G, Thomas J, et al. Forkhead box protein P1 expression in mucosa-associated lymphoid tissue lymphomas predicts poor prognosis and transformation to diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:2490–7. doi: 10.1200/JCO.2006.05.6150. [DOI] [PubMed] [Google Scholar]
- 139.Kuppers R, Dalla-Favera R. Mechanisms of chromosomal translocations in B cell lymphomas. Oncogene. 2001;20:5580–94. doi: 10.1038/sj.onc.1204640. [DOI] [PubMed] [Google Scholar]
- 140.Lucas PC, Yonezumi M, Inohara N, McAllister-Lucas LM, Abazeed ME, Chen FF, et al. Bcl10 and MALT1, independent targets of chromosomal translocation in malt lymphoma, cooperate in a novel NF-kappa B signaling pathway. J Biol Chem. 2001;276:19012–9. doi: 10.1074/jbc.M009984200. [DOI] [PubMed] [Google Scholar]
- 141.Ho L, Davis RE, Conne B, Chappuis R, Berczy M, Mhawech P, et al. MALT1 and the API2-MALT1 fusion act between CD40 and IKK and confer NF-kappa B-dependent proliferative advantage and resistance against FAS-induced cell death in B cells. Blood. 2005;105:2891–9. doi: 10.1182/blood-2004-06-2297. [DOI] [PubMed] [Google Scholar]
- 142.Wongchaowart NT, Kim B, Hsi ED, Swerdlow SH, Tubbs RR, Cook JR. t(14;18)(q32;q21) involving IGH and MALT1 is uncommon in cutaneous MALT lymphomas and primary cutaneous diffuse large B-cell lymphomas. J Cutan Pathol. 2006;33:286–92. doi: 10.1111/j.0303-6987.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- 143.Espinet B, Gallardo F, Pujol RM, Estrach T, Servitje O, Solé F. Absence of MALT1 translocations in primary cutaneous marginal zone B-cell lymphoma. Haematologica. 2004;89:ELT14. [PubMed] [Google Scholar]
- 144.Vinatzer U, Gollinger M, Müllauer L, Raderer M, Chott A, Streubel B. Mucosa-associated lymphoid tissue lymphoma: novel translocations including rearrangements of ODZ2, JMJD2C, and CNN3. Clin Cancer Res. 2008;14:6426–31. doi: 10.1158/1078-0432.CCR-08-0702. [DOI] [PubMed] [Google Scholar]
- 145.Sugiyama T, Asaka M, Nakamura T, Nakamura S, Yonezumi S, Seto M. API2-MALT1 chimeric transcript is a predictive marker for the responsiveness of H. pylori eradication treatment in low-grade gastric MALT lymphoma. Gastroenterology. 2001;120:1884–5. doi: 10.1053/gast.2001.25305. [DOI] [PubMed] [Google Scholar]
- 146.Liu H, Ruskon-Fourmestraux A, Lavergne-Slove A, Ye H, Molina T, Bouhnik Y, et al. Resistance of t(11;18) positive gastric mucosa-associated lymphoid tissue lymphoma to Helicobacter pylori eradication therapy. Lancet. 2001;357:39–40. doi: 10.1016/S0140-6736(00)03571-6. [DOI] [PubMed] [Google Scholar]
- 147.Liu H, Ye H, Ruskone-Fourmestraux A, De Jong D, Pileri S, Thiede C, et al. T(11;18) is a marker for all stage gastric MALT lymphomas that will not respond to H. pylori eradication. Gastroenterology. 2002;122:1286–94. doi: 10.1053/gast.2002.33047. [DOI] [PubMed] [Google Scholar]
- 148.Fukuhara N, Nakamura T, Nakagawa M, Tagawa H, Takeuchi I, Yatabe Y, et al. Chromosomal imbalances are associated with outcome of Helicobacter pylori eradication in t(11;18)(q21;q21) negative gastric mucosa-associated lymphoid tissue lymphomas. Genes Chromosomes Cancer. 2007;46:784–90. doi: 10.1002/gcc.20464. [DOI] [PubMed] [Google Scholar]
- 149.Chanudet E, Ye H, Ferry J, Bacon CM, Adam P, Müller-Hermelink HK, et al. A20 deletion is associated with copy number gain at the TNFA/B/C locus and occurs preferentially in translocation-negative MALT lymphoma of the ocular adnexa and salivary glands. J Pathol. 2009;217:420–30. doi: 10.1002/path.2466. [DOI] [PubMed] [Google Scholar]
- 150.Zhou Y, Ye H, Martin-Subero JI, Gesk S, Hamoudi R, Lu YJ, et al. The pattern of genomic gains in salivary gland MALT lymphomas. Haematologica. 2007;92:921–7. doi: 10.3324/haematol.10191. [DOI] [PubMed] [Google Scholar]
- 151.Honma K, Tsuzuki S, Nakagawa M, Karnan S, Aizawa Y, Kim WS, et al. TNFAIP3 is the target gene of chromosome band 6q23.3-q24.1 loss in ocular adnexal marginal zone B cell lymphoma. Genes Chromosomes Cancer. 2008;47:1–7. doi: 10.1002/gcc.20499. [DOI] [PubMed] [Google Scholar]
- 152.Fenton JA, Schuuring E, Barrans SL, Banham AH, Rollinson SJ, Morgan GJ, et al. t(3;14)(p14;q32) results in aberrant expression of FOXP1 in a case of diffuse large B-cell lymphoma. Genes Chromosomes Cancer. 2006;45:164–8. doi: 10.1002/gcc.20278. [DOI] [PubMed] [Google Scholar]
- 153.Bea S, Zettl A, Wright G, Salaverria I, Jehn P, Moreno V, et al. Diffuse large B-cell lymphoma subgroups have distinct genetic profiles that influence tumor biology and improve gene-expression-based survival prediction. Blood. 2005;106:3183–90. doi: 10.1182/blood-2005-04-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Jost PJ, Ruland J. Aberrant NF-kappaB signaling in lymphoma: mechanisms, consequences, and therapeutic implications. Blood. 2007;109:2700–7. doi: 10.1182/blood-2006-07-025809. [DOI] [PubMed] [Google Scholar]
- 155.Davis RE, Brown KD, Siebenlist U, Staudt LM. Constitutive nuclear factor kappaB activity is required for survival of activated B cell-like diffuse large B cell lymphoma cells. J Exp Med. 2001;194:1861–74. doi: 10.1084/jem.194.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Deutsch AJ, Aigelsreiter A, Staber PB, Beham A, Linkesch W, Guelly C, et al. MALT lymphoma and extranodal diffuse large B-cell lymphoma are targeted by aberrant somatic hypermutation. Blood. 2007;109:3500–4. doi: 10.1182/blood-2006-06-030494. [DOI] [PubMed] [Google Scholar]
- 157.Barth TF, Barth CA, Kestler HA, Michl P, Weniger MA, Buchholz M, et al. Transcriptional profiling suggests that secondary and primary large B-cell lymphomas of the gastrointestinal (GI) tract are blastic variants of GI marginal zone lymphoma. J Pathol. 2007;211:305–13. doi: 10.1002/path.2096. [DOI] [PubMed] [Google Scholar]
- 158.Baecklund E, Backlin C, Iliadou A, Granath F, Ekbom A, Amini RM, et al. Characteristics of diffuse large B cell lymphomas in rheumatoid arthritis. Arthritis Rheum. 2006;54:3774–81. doi: 10.1002/art.22277. [DOI] [PubMed] [Google Scholar]
- 159.Du M, Peng H, Singh N, Isaacson PG, Pan L. The accumulation of p53 abnormalities is associated with progression of mucosa-associated lymphoid tissue lymphoma. Blood. 1995;86:4587–93. [PubMed] [Google Scholar]
- 160.Neumeister P, Hoefler G, Beham-Schmid C, Schmidt H, Apfelbeck U, Schaider H, et al. Deletion analysis of the p16 tumor suppressor gene in gastrointestinal mucosa-associated lymphoid tissue lymphomas. Gastroenterology. 1997;112:1871–5. doi: 10.1053/gast.1997.v112.pm9178679. [DOI] [PubMed] [Google Scholar]
- 161.Martinez-Delgado B, Fernandez-Piqueras J, Garcia MJ, Arranz E, Gallego J, et al. Hypermethylation of a 5' CpG island of p16 is a frequent event in non-Hodgkin's lym-phoma. Leukemia. 1997;11:425–8. doi: 10.1038/sj.leu.2400579. [DOI] [PubMed] [Google Scholar]
- 162.Greeve J, Philipsen A, Krause K, Klapper W, Heidorn K, Castle BE, et al. Expression of activation-induced cytidine deaminase in human B-cell non-Hodgkin lymphomas. Blood. 2003;101:3574–80. doi: 10.1182/blood-2002-08-2424. [DOI] [PubMed] [Google Scholar]
- 163.Smit LA, Bende RJ, Aten J, Guikema JE, Aarts WM, van Noesel CJ. Expression of activation-induced cytidine deaminase is confined to B-cell non-Hodgkin's lymphomas of germinal-center phenotype. Cancer Res. 2003;63:3894–8. [PubMed] [Google Scholar]
- 164.Wotherspoon AC. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]
- 165.Ferreri AJ, Ponzoni M, Guidoboni M, Resti AG, Politi LS, Cortelazzo S, et al. Bacteria-eradicating therapy with doxycycline in ocular adnexal MALT lymphoma: a multicenter prospective trial. J Natl Cancer Inst. 2006;98:1375–82. doi: 10.1093/jnci/djj373. [DOI] [PubMed] [Google Scholar]
- 166.Hermine O, Lefrère F, Bronowicki JP, Mariette X, Jondeau K, Eclache-Saudreau V, et al. Regression of splenic lymphoma with villous lymphocytes after treatment of hepatitis C virus infection. N Engl J Med. 2002;347:89–94. doi: 10.1056/NEJMoa013376. [DOI] [PubMed] [Google Scholar]
- 167.Casato M, Mecucci C, Agnello V, Fiorilli M, Knight GB, Matteucci C, et al. Regression of lymphoproliferative disorder after treatment for hepatitis C virus infection in a patient with partial trisomy 3, Bcl-2 overexpression, and type II cryoglobulinemia. Blood. 2002;99:2259–61. doi: 10.1182/blood.v99.6.2259. [DOI] [PubMed] [Google Scholar]
- 168.Hussell T, Isaacson PG, Crabtree JE, Spencer J. Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J Pathol. 1996;178:122–7. doi: 10.1002/(SICI)1096-9896(199602)178:2<122::AID-PATH486>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 169.Miklos JA, Swerdlow SH, Bahler DW. Salivary gland mucosa-associated lymphoid tissue lymphoma immunoglobulin VH genes show frequent use of V1–69 with distinctive CDR3 features. Blood. 2000;95:3878–84. [PubMed] [Google Scholar]
- 170.Sakuma H, Nakamura T, Uemura N, Chiba T, Sugiyama T, Asaka M, et al. Immunoglobulin VH gene analysis in gastric MALT lymphomas. Mod Pathol. 2007;20:460–6. doi: 10.1038/modpathol.3800758. [DOI] [PubMed] [Google Scholar]
- 171.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–7. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 172.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, et al. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–7. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wöhrer S, Troch M, Streubel B, Zwerina J, Skrabs C, Formanek M, et al. MALT lymphoma in patients with autoimmune diseases: a comparative analysis of characteristics and clinical course. Leukemia. 2007;21:1812–8. doi: 10.1038/sj.leu.2404782. [DOI] [PubMed] [Google Scholar]
- 174.Enno A, O'rourke J, Braye S, Howlett R, Lee A. Antigen-dependent progression of mucosa-associated lymphoid tissue (MALT)-type lymphoma in the stomach. Effects of antimicrobial therapy on gastric MALT lymphoma in mice. Am J Pathol. 1998;152:1625–32. [PMC free article] [PubMed] [Google Scholar]
- 175.Mueller A, O'rourke J, Chu P, Chu A, Dixon MF, Bouley DM, et al. The role of antigenic drive and tumor-infiltrating accessory cells in the pathogenesis of helicobacter-induced mucosa-associated lymphoid tissue lymphoma. Am J Pathol. 2005;167:797–812. doi: 10.1016/S0002-9440(10)62052-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.O'Rourke JL, Dixon MF, Jack A, Enno A, Lee A. Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma in an animal model of 'Helicobacter heilmannii' infection. J Pathol. 2004;203:896–903. doi: 10.1002/path.1593. [DOI] [PubMed] [Google Scholar]
- 177.Shomer NH, Fox JG, Juedes AE, Ruddle NH. Helicobacter-induced chronic active lymphoid aggregates have characteristics of tertiary lymphoid tissue. Infect Immun. 2003;71:3572–7. doi: 10.1128/IAI.71.6.3572-3577.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nakamura M, Murayama SY, Serizawa H, Sekiya Y, Eguchi M, Takahashi S, et al. "Candidatus Helicobacter heilmannii" from a cynomolgus monkey induces gastric mucosa-associated lymphoid tissue lymphomas in C57BL/6 mice. Infect Immun. 2007;75:1214–22. doi: 10.1128/IAI.01459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Baens M, Fevery S, Sagaert X, Noels H, Hagens S, Broeckx V, et al. Selective expansion of marginal zone B cells in Emicro-API2-MALT1 mice is linked to enhanced IkappaB kinase gamma polyubiquitination. Cancer Res. 2006;66:5270–7. doi: 10.1158/0008-5472.CAN-05-4590. [DOI] [PubMed] [Google Scholar]
- 180.Sagaert X, Theys T, Wolf-Peeters C, Marynen P, Baens M. Splenic marginal zone lymphoma-like features in API2-MALT1 transgenic mice that are exposed to antigenic stimulation. Haematologica. 2006;91:1693–6. [PubMed] [Google Scholar]
- 181.Guo F, Weih D, Meier E, Weih F. Constitutive alternative NF-kappaB signaling promotes marginal zone B-cell development but disrupts the marginal sinus and induces HEV-like structures in the spleen. Blood. 2007;110:2381–9. doi: 10.1182/blood-2007-02-075143. [DOI] [PubMed] [Google Scholar]
- 182.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, et al. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–66. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Batten M, Fletcher C, Ng LG, Groom J, Wheway J, Laâbi Y, et al. TNF deficiency fails to protect BAFF transgenic mice against autoimmunity and reveals a predisposition to B cell lymphoma. J Immunol. 2004;172:812–22. doi: 10.4049/jimmunol.172.2.812. [DOI] [PubMed] [Google Scholar]
- 184.Sasaki Y, Schmidt-Supprian M, Derudder E, Rajewsky K. Role of NFkappaB signaling in normal and malignant B cell development. Adv Exp Med Biol. 2007;596:149–54. doi: 10.1007/0-387-46530-8_13. [DOI] [PubMed] [Google Scholar]
- 185.Hömig-Hölzel C, Hojer C, Rastelli J, Casola S, Strobl LJ, Müller W, et al. Constitutive CD40 signaling in B cells selectively activates the non-canonical NF-kappaB pathway and promotes lymphomagenesis. J Exp Med. 2008;205:1317–29. doi: 10.1084/jem.20080238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zangani MM, Frøyland M, Qiu GY, Meza-Zepeda LA, Kutok JL, et al. Lymphomas can develop from B cells chronically helped by idiotype-specific T cells. J Exp Med. 2007;204:1181–91. doi: 10.1084/jem.20061220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hässler S, Ramsey C, Karlsson MC, Larsson D, Herrmann B, Rozell B, et al. Aire-deficient mice develop hematopoetic irregularities and marginal zone B-cell lymphoma. Blood. 2006;108:1941–8. doi: 10.1182/blood-2006-04-019679. [DOI] [PubMed] [Google Scholar]
- 188.Offerhaus GJ, Schipper ME, Lazenby AJ, Montgomery E, Morsink FH, Bende RJ, et al. Graft-versus-host-like disease complicating thymoma: lack of AIRE expression as a cause of non-hereditary autoimmunity? Immunol Lett. 2007;114:31–7. doi: 10.1016/j.imlet.2007.08.010. [DOI] [PubMed] [Google Scholar]