Dicentric chromosomes are rare in acute lymphoblastic leukemia, dic(9;20) being a recurrent aberration. This study provides insight into the breakpoint complexity underlying dicentric chromosomal formation in acute lymphoblastic leukemia and highlights putative target gene loci.
Keywords: acute lymphoblastic leukemia, dic(9;20), fusion genes, genetic target
Abstract
The dic(9;20)(p11~13;q11) is a recurrent chromosomal abnormality in patients with acute lymphoblastic leukemia. Although it results in loss of material from 9p and 20q, the molecular targets on both chromosomes have not been fully elucidated. From an initial cohort of 58 with acute lymphoblastic leukemia patients with this translocation, breakpoint mapping with fluorescence in situ hybridization on 26 of them revealed breakpoint heterogeneity of both chromosomes. PAX5 has been proposed to be the target gene on 9p, while for 20q, FISH analysis implicated the involvement of the ASXL1 gene, either by a breakpoint within (n=4) or centromeric (deletion, n=12) of the gene. Molecular copy-number counting, long-distance inverse PCR and direct sequence analysis identified six dic(9;20) breakpoint sequences. In addition to the three previously reported: PAX5-ASXL1, PAX5-C20ORF112 and PAX5-KIF3B; we identified three new ones in this study: sequences 3’ of PAX5 disrupting ASXL1, and ZCCHC7 disrupted by sequences 3’ of FRG1B and LOC1499503. This study provides insight into the breakpoint complexity underlying dicentric chromosomal formation in acute lymphoblastic leukemia and highlights putative target gene loci.
Introduction
Chromosomes 7, 9, 12 and 20 are frequently involved in the formation of dicentric chromosomes in patients with B-cell precursor acute lymphoblastic leukemia (BCP ALL), constituting the dic(7;9)(p11;p11),1 dic(9;12)(p11~13;p13)2 and dic(9;20)(p11~13;q11).3 The dic(9;20) occurs in ~2% and ~0.5% of childhood and adult precursor-B ALL (BCP ALL), respectively.4 Although the cytogenetic and clinical associations of this translocation have been widely reported over the last decade, its molecular consequences remain unclear. FISH has shown that dic(9;20) contains the centromeres of both chromosomes 9 and 20, resulting in the loss of 9p and 20q material.3,5 Array-based comparative genomic hybridization (aCGH), with BAC clones at tiling path resolution, showed clustering of breakpoints within 9p13.2 (genomic position 37.1–38.7Mb) and 20q11.2 (29.2–30.8Mb).6 The 9p breakpoints precluded the involvement of PAX5 as a fusion gene. Notably, these data implicated the involvement of the hemopoietic cell kinase gene (HCK) at 20q11~12 in a single case.6
Our previous FISH mapping studies of dicentric chromosomal abnormalities showed partial or complete deletion of PAX5 on the short arm of chromosome 9, while molecular analysis identified five novel sequence partners of PAX5, three of which were found in patients with dic(9;20): ASXL1 (20q11.21), C20ORF112 (20q11.21) and KIF3B (20q11.21). With targeted expression analysis, this study highlighted disruption of PAX5, regardless of the heterogeneous breakpoints on 9p.7 The aim of this present study was to further unravel the breakpoint complexity in these patients and identify potential genetic targets on chromosome 20 in dic(9;20) patients.
Design and Methods
Samples were received from 58 patients with BCP ALL and dic(9;20)(p11~13;q11) entered onto a Medical Research Council (MRC/NCRI) United Kingdom Childhood or Adult ALL treatment trial. Local Ethical Committee approval was obtained by treating centers, and informed consent was given by parents and/or patients. Diagnostic cytogenetic and FISH data is shown in Table 1. The presence of dic(9;20) was confirmed according to previous studies.5 FISH mapping was performed on 26 cases with dic(9;20) in order to precisely define the breakpoints on 9p and 20q. FISH clones and their genomic positions are listed in Online Supplementary Table S1.
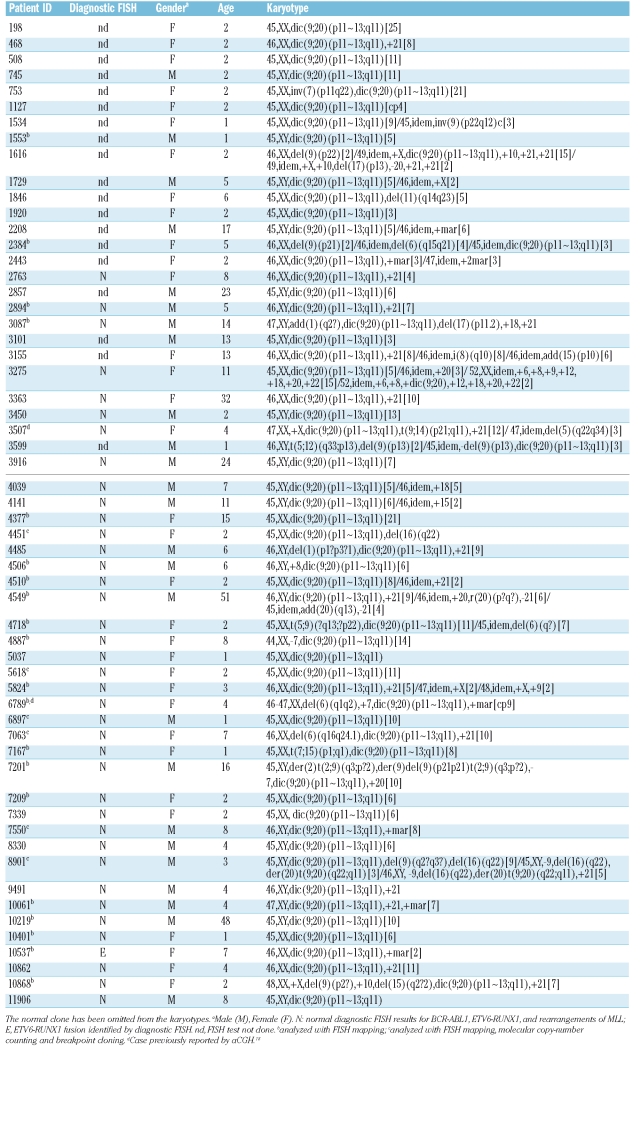
Table 1.
Clinical and cytogenetic data on patients with dic(9;20), with the molecular investigation performed on different cases.
Molecular copy number counting (MCC) was carried out on 2 cases as previously described.8 MCC allows the progressive delineation of unbalanced copy number breakpoints to within a few hundred base-pairs and facilitates rapid sequence analysis. With the use of subgenome quantities of DNA distributed into a 96-well plate, the frequency of PCR well positivity for any given primer pair is a direct reflection of the genomic copy number at that site. Based on the FISH data, genomic locations (markers) in PAX5, the PAX5 3’ downstream region and ZCCHC7 were chosen for MCC. The data were analyzed as previously described.9 Long distance inverse-PCR (LDI-PCR) was carried out on 3 cases, as previously described with modifications.7 The MCC primers, restriction enzymes and the sequences of LDI-PCR primers are shown in Online Supplementary Tables S2 and S3. Protein sequences were predicted using the online programme GENSCAN (http://genes.mit.edu/GENSCAN).
Results and Discussion
Since the first reports in 1995,3,10 several studies of dic(9;20) have characterized this abnormality at both the cytogenetic and molecular levels, revealing breakpoint heterogeneity on both chromosomes.5,6,11,12 In our series, dic(9;20) was the sole visible cytogenetic change in 21/58 patients. Confirming previous observations, we identified recurrent gain of chromosomes X (n=5) and 21 (n=21) (Table 1).11 Diagnostic FISH analysis performed in 39/58 (67.2%) cases did not confirm the previous association with the t(9;22)(q34;q11) (11), but did identify a single patient positive for the ETV6-RUNX1 fusion (10537; Table 1).
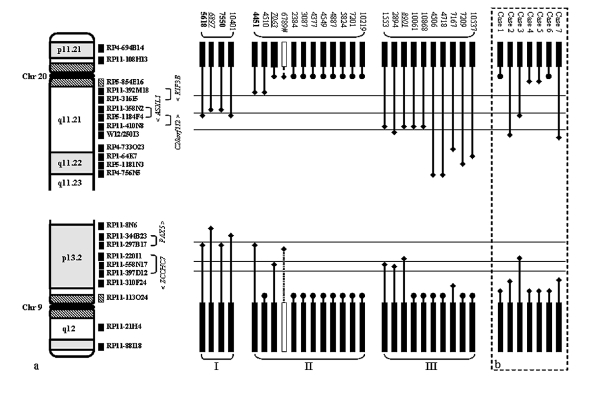
We have previously shown that breakpoints on dicentric chromosomes involving chromosome 9 target the PAX5 locus.7 However, the genetic targets on 20q in dic9;20 remain unclear. FISH mapping performed in this current study revealed considerable breakpoint heterogeneity on both 9p and 20q (Figure 1A). A total of 24/52 (46.2%) breakpoints were located within the centromeric regions of both chromosomes 9 and 20, either centromeric to RP11-113O24 (38.261–38.427Mb) on 9p or RP5-854E16 (29.267–29.338Mb) on 20q. These regions could not be further defined due to the repetitive nature of the DNA sequences within the centromeres. The remaining 28 (53.8%) breakpoints were positioned within euchromatic regions of chromosomes 9 and 20 and, with the exception of case 6789, had fixed cells available for further study. On 9p, FISH analysis indicated the involvement of PAX5 (4451, 5618 and 7550) and ZCCHC7 (1153, 2894, 7063 and 8901) in 3 and 4 cases, respectively. A further 2 cases showed breakpoints in the region 3’ of PAX5 (6897 and 10401). In their 7 dic(9;20) cases, Schoumans et al., reported that the 9p breakpoints mapped centromeric of PAX5, resulting in total deletion of the gene in all cases6 (Figure 1B). Furthermore, a recent study revealed intronic PAX5 breakpoints in 5/11 (45%) dic(9;20) cases.13
Figure 1.
In our study, breakpoints on 20q determined by FISH mapping, revealed three breakpoint clusters as detailed in Online Supplementary Table S4: Type I- 4 cases with breakpoints directly involving ASXL1/C20ORF112 (5618, 6897, 7550 and 10401), Type II- 12 cases with breakpoints proximal of ASXL1, resulting in deletion of this gene, and Type III- 10 cases with breakpoints distal to ASXL1, 5 of which were clustered between clones RP11-410N8 and WI2-250I3 (1553, 2894, 8901, 10061 and 10868) (Figure 1A). There are no known genes within this region. In total, ~61.5% (16/26) of the breakpoints on 20q resulted in disruption of the ASXL1 gene.
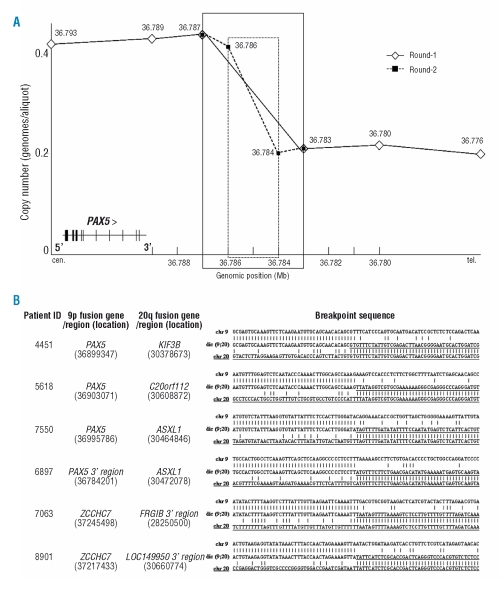
MCC was employed to refine, while LDI-PCR was used to amplify the translocation breakpoints for direct sequence analysis in 3 dic9;20 cases with material available (6897, 7063, 8901) (Figure 2). Each of the breakpoints identified by LDI-PCR was confirmed using standard PCR approaches. In case 6897, 1st and 2nd round MCC analyses mapped the breakpoint to a ~3.2kb (36.783–36.787Mb) and a ~1.4kb (36.784–36.786Mb) region 3’ of PAX5 (resulting in deletion of the entire PAX5 gene), respectively (Figure 2A). Sequence analysis showed that a region 3’ of PAX5 on 9p13.2 was juxtaposed with exons 1–4 of the ASXL1 gene on 20q11.21. The representative MCC data from case 6897 are illustrated in Figure 2A.
Figure 2.
MCC and sequence analyses of 6 ALL cases with dic(9;20) abnormality. (A) Representative MCC graph of case 6897. The genomic position of each MCC marker is shown above/beside the marker; the breakpoint region is indicated in boxes (solid line for round-1 and dotted line for round-2). MCC refines the position of the breakpoint in PAX5 3’ downstream region, from 3.2kb (round-1) to 1.4kb (round-2). (B) The dic(9;20) breakpoint sequence and the genomic location on 9p and 20q; alignment of the dic(9;20) breakpoint sequence (middle line) against the normal sequences of chromosome 9 (top line) and 20 (bottom line).
MCC markers (markers 39–44) were designed to cover a region of ~7kb (37.240–37.247Mb) within intron 2 of the ZCCHC7 gene. When applied to case 7063, they showed evidence of a copy number change between markers 43 and 44, indicating the location of the breakpoint to be within a ~1.2kb region (37.245–37.247Mb). As both cases displayed the same FISH pattern on 9p, the predicted breakpoint sequence of case 8901 was amplified directly by LDI-PCR, based on the MCC results of case 7063. LDI-PCR and sequencing analyses showed ZCCHC7 to be the partner of sequence 3’ of FRG1B (20q11.1) and LOC149950 (20q11.21) in patients 7063 and 8901, respectively.
In 3 previously published cases, 4451, 5618 and 7550, we showed that the PAX5 gene was juxtaposed to KIF3B (30.379Mb), C20ORF112 (30.609Mb) and ASXL1 (30.465Mb) sequences, respectively.7 Taken together with the dic(9;20) breakpoint sequences identified in this study, certain conclusions can be made: there is recurrent involvement, by a breakpoint within or a deletion of ZCCHC7 at 9p13.2 and ASXL1 at 20q11.21 in cases with dic(9;20). The cases with breakpoints within either ZCCHC7 or ASXL1 displayed common breakpoints within intron 2 (retained exons 1 and 2) and 4 (retained exons 1–4), respectively.
Due to lack of material, we were unable to perform further genomic or expression studies of these patients. The analysis of DNA sequence flanking the breakpoints provided no further insight into the mechanism by which these rearrangements occurred. ZCCHC7 has been previously shown to juxtapose MYC in B-cell lymphoma.14 ASXL1 (30.410–30.491Mb) encodes a protein product of 170 kDa, which is a mammalian homolog of Drosophila ASX (additional sex combs). It has been shown to act as a novel ligand-dependent coactivator of the retinoic acid (RA) receptor.15
The study of Schoumans et al. supports the involvement of ASXL1, as their cases show a similar pattern, as indicated in Figure 1B.6 Further studies should include investigation of the mutation and methylation status of ASXL1 and its expression in dic(9;20)-positive ALL patients.
Due to the breakpoint heterogeneity of 20q, and considering the location of the type III breakpoints clustering distal to ASXL1, the involvement of alternative genes telomeric to ASXL1 cannot be excluded. A recent array-based study identified 3 dic(9;20) cases with deletion of 9p and 20q. The molecular consequence of the 3 cases was a PAX5-C20ORF112 fusion, which specifically repressed the transcriptional activity of PAX5 in a dominant-negative fashion.13 Our current study identified a single case with the PAX5-C20ORF112 fusion (1/26, 3.8%), providing a frequency much lower than reported by Kawamata et al. of 27% (3/11).13 Although the involvement of ASXL1 is implicated in this study, the loss of other tumor suppressor genes from the region, such as DIDO1 (60.979–61.040Mb) and L3MBTL (41.576–41.604Mb), may represent important additional events.16,17
This study provides further evidence to support our hypothesis that specific gene loci can be the target of heterogeneous translocation breakpoints in human cancer, acting through a variety of mechanisms. The involvement of ASXL1 at 20q11.21 is highlighted both in our dataset and information published by others.6 Further evidence will likely emerge from the use of molecular analysis of larger patient cohorts. Future work should focus on expanding this type of approach to other chromosomal abnormalities, with the aims of identifying novel cancer-associated genes, improving the understanding of carcinogenesis and improving the management of cancer patients.
Supplementary Material
Acknowledgments
we thank the National Cancer Research Institute (NCRI) Childhood and Adult Leukaemia Working Parties and their members for having designed and coordinated the clinical trials through which these patients were identified and treated. We also wish to thank the UK Cancer Cytogenetics Group laboratories for providing cytogenetic and FISH data, and fixed cell suspensions.
Footnotes
Authorship and Disclosures
QA performed the experiments, analyzed the data and wrote the manuscript. SLW and HP performed the experiments and analyzed the data. AVM, MG, FMR, TD, CJH analyzed data and reviewed the manuscript. JCS supervised the research and wrote the manuscript.
The authors reported no potential conflicts of interest.
The online version of this article contains a supplementary appendix.
Funding: the work was supported by the Leukaemia Research, UK
References
- 1.Pan J, Xue Y, Wu Y, Wang Y, Shen J. Dicentric (7;9)(p11;p11) is a rare but recurrent abnormality in acute lymphoblastic leukemia: a study of 7 cases. Cancer Genet Cytogenet. 2006;169:159–63. doi: 10.1016/j.cancergencyto.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 2.Behrendt H, Charrin C, Gibbons B, Harrison CJ, Hawkins JM, Heerema NA, et al. Dicentric (9;12) in acute lymphocytic leukemia and other hematological malignancies: report from a dic(9;12) study group. Leukemia. 1995;9:102–6. [PubMed] [Google Scholar]
- 3.Rieder H, Schnittger S, Bodenstein H, Schwonzen M, Wormann B, Berkovic D, et al. dic(9;20): a new recurrent chromosome abnormality in adult acute lymphoblastic leukemia. Genes Chromosomes Cancer. 1995;13:54–61. doi: 10.1002/gcc.2870130109. [DOI] [PubMed] [Google Scholar]
- 4.Mitelman F, Mertens F, Johansson B. A breakpoint map of recurrent chromosomal rearrangements in human neoplasia. Nat Genet. 1997;15:417–74. doi: 10.1038/ng0497supp-417. [DOI] [PubMed] [Google Scholar]
- 5.Clark R, Byatt SA, Bennett CF, Brama M, Martineau M, Moorman AV, et al. Monosomy 20 as a pointer to dicentric (9;20) in acute lymphoblastic leukemia. Leukemia. 2000;14:241–6. doi: 10.1038/sj.leu.2401654. [DOI] [PubMed] [Google Scholar]
- 6.Schoumans J, Johansson B, Corcoran M, Kuchinskaya E, Golovleva I, Grander D, et al. Characterisation of dic(9;20)(p11–13;q11) in childhood B-cell precursor acute lymphoblastic leukaemia by tiling resolution array-based comparative genomic hybridisation reveals clustered breakpoints at 9p13.2 and 20q11.2. Br J Haematol. 2006;135:492–9. doi: 10.1111/j.1365-2141.2006.06328.x. [DOI] [PubMed] [Google Scholar]
- 7.An Q, Wright SL, Konn ZJ, Matheson E, Minto L, Moorman AV, et al. Variable breakpoints target PAX5 in patients with dicentric chromosomes: A model for the basis of unbalanced translocations in cancer. Proc Natl Acad Sci USA. 2008;105:17050–4. doi: 10.1073/pnas.0803494105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strefford JC, Worley H, Barber K, Wright S, Stewart ARM, Robinson HR, et al. Genome complexity in acute lymphoblastic leukaemia is revealed by array-based comparative genomic hybridization. Oncogene. 2007;26:4306–18. doi: 10.1038/sj.onc.1210190. [DOI] [PubMed] [Google Scholar]
- 9.Daser A, Thangavelu M, Pannell R, Forster A, Sparrow L, Chung G, et al. Interrogation of genomes by molecular copy-number counting (MCC) Nat Methods. 2006;3:447–53. doi: 10.1038/nmeth880. [DOI] [PubMed] [Google Scholar]
- 10.Jalali GR, An Q, Konn ZJ, Worley H, Wright SL, Harrison CJ, et al. Disruption of ETV6 in intron 2 results in upregulatory and insertional events in childhood acute lymphoblastic leukaemia. Leukemia. 2008;22:114–23. doi: 10.1038/sj.leu.2404994. [DOI] [PubMed] [Google Scholar]
- 11.Slater R, Smit E, Kroes W, Bellomo MJ, Muhlematter D, Harbott J, et al. A non-random chromosome abnormality found in precursor-B lineage acute lymphoblastic leukaemia: dic(9;20)(p1?3;q11) Leukemia. 1995;9:1613–9. [PubMed] [Google Scholar]
- 12.Forestier E, Gauffin F, Andersen MK, Autio K, Borgström G, Golovleva I, et al. Clinical and cytogenetic features of pediatric dic(9;20) (p13.2;q11.2)-positive B-cell precursor acute lymphoblastic leukemias: a Nordic series of 24 cases and review of the literature. Genes Chromosomes Cancer. 2008;47:149–58. doi: 10.1002/gcc.20517. [DOI] [PubMed] [Google Scholar]
- 13.Heerema NA, Maben KD, Bernstein J, Breitfeld PP, Neiman RS, Vance GH. Dicentric (9;20)(p11;q11) identified by fluorescence in situ hybridization in four pediatric acute lymphoblastic leukemia patients. Cancer Genet Cytogenet. 1996;92:111–5. doi: 10.1016/s0165-4608(96)00172-0. [DOI] [PubMed] [Google Scholar]
- 14.Kawamata N, Ogawa S, Zimmermann M, Niebuhr B, Stocking C, Sanada M, et al. Cloning of genes involved in chromosomal translocations by high-resolution single nucleotide polymorphism genomic microarray. Proc Natl Acad Sci USA. 2008;105:11921–6. doi: 10.1073/pnas.0711039105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bertrand P, Bastard C, Maingonnat C, Jardin F, Maisonneuve C, Courel MN, et al. Mapping of MYC breakpoints in 8q24 rearrangements involving non-immunoglobulin partners in B-cell lymphomas. Leukemia. 2007;21:515–23. doi: 10.1038/sj.leu.2404529. [DOI] [PubMed] [Google Scholar]
- 16.Cho YS, Kin EJ, Park UH, Sin HS, Um SJ. Additional sex comb-like 1 (ASXL1), in cooperation with SRC-1, acts as a ligand-dependent coactivator for retinoic acid receptor. J Biol Chem. 2006;281:17588–98. doi: 10.1074/jbc.M512616200. [DOI] [PubMed] [Google Scholar]
- 17.Fütterer A, Campanero MR, Leonardo E, Criado LM, Flores JM, Hernández JM, et al. Dido gene expression alterations are implicated in the induction of hematological myeloid neoplasms. J Clin Invest. 2005;115:2351–62. doi: 10.1172/JCI24177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bench AJ, Li J, Huntly BJ, Delabesse E, Fourouclas N, Hunt AR, et al. Characterization of the imprinted polycomb gene L3MBTL, a candidate 20q tumour suppressor gene, in patients with myeloid malignancies. Br J Haematol. 2004;127:509–18. doi: 10.1111/j.1365-2141.2004.05278.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.