Abstract
Background
Lung injury promotes the expression of matrix metalloproteinase-7 (MMP7, matrilysin), which is required for neutrophil recruitment and re-epithelialization. MMP7 governs the lung inflammatory response through the shedding of syndecan-1. Because inflammation and repair are related events, we evaluated the role of syndecan-1 shedding in lung re-epithelialization.
Methodology/Principal Finding
Epithelial injury induced syndecan-1 shedding from wild-type epithelium but not from Mmp7−/− mice in vitro and in vivo. Moreover, cell migration and wound closure was enhanced by MMP7 shedding of syndecan-1. Additionally, we found that syndecan-1 augmented cell adhesion to collagen by controlling the affinity state of the α2β1 integrin.
Conclusion/Significance
MMP7 shedding of syndecan-1 facilitates wound closure by causing the α2β1 integrin to assume a less active conformation thereby removing restrictions to migration. MMP7 acts in the lungs to regulate inflammation and repair, and our data now show that both these functions are controlled through the shedding of syndecan-1.
Introduction
Being contiguous with the environment, mucosal surfaces are constantly exposed to toxic and pathogenic insults [1], [2]. As a first line of defense, mucosal epithelia have evolved to quickly respond to various forms of injury by coordinating the inflammatory response while repairing wounded tissue. MMP7 (matrilysin), a member of the matrix metalloproteinase (MMP) family, is expressed by all mucosal surfaces and is quickly upregulated in response to epithelial injury [3]–[11]. Accordingly, MMP7 functions to facilitate repair and regulate the acute inflammatory response [3]–[7].
The lungs are lined by a prototypical mucosal epithelium. Like other mucosal surfaces, injured lungs quickly initiate a pre-programmed response to recruit inflammatory cells and repair the damaged tissue. The roles of MMP7 in regulating repair and inflammation are particularly prominent in the lungs. In fact, the lung phenotypes are so pronounced that re-epithelialization and neutrophil recruitment into the alveolar space are almost completely abrogated in MMP7-deficient mice [3]–[6]. Our group previously reported that E-cadherin is shed in vivo from the injured lung epithelium by MMP7 [5]. Although we then proposed that E-cadherin shedding could promote repair by reorganizing cell-cell contacts, newer studies indicate that MMP7 shedding of E-cadherin functions in adaptive immune responses later in the response to injury (McGuire et al., unpublished observations). Early in the injury response, MMP7 promotes inflammation by shedding syndecan-1/KC (CXCL8) complexes that permit the transepithelial movement of neutrophils [4]. As shedding of syndecan-1 occurs coincident with the onset of re-epithelialization, we hypothesized that proteolysis of this membrane factor may also function in repair.
Syndecan-1 is one of four members of a family of transmembrane heparan sulfate proteoglycans with distinct expression patterns and functions [12]. Epithelial cells primarily express syndecan-1, and observations from various models indicate that it participates in wound healing [4], [13]–[17]. For example, suppression of syndecan-1 expression in epithelial cells induces a pro-migratory phenotype [18], [19], suggesting that intact syndecan-1 may moderate re-epithelialization. Consistent with this idea, syndecan-1 surface levels are decreased in injured cornea and skin during active repair [20], [21], and increased levels of syndecan-1 ectodomain are present in dermal wound fluid [22]–[24]. Syndecan-1 shedding from the cell surface is a MMP-dependent process in vitro and in vivo, and MMP shedding of syndecan-1 induces cell migration [25]. Moreover, MMP7 has been identified as the syndecan-1 sheddase in lung mucosa [4], [25]–[29].
Because it can modulate repair, we tested the idea that shedding of syndecan-1 from injured lung epithelium functions to promote re-epithelialization. Using in vitro and in vivo models, we found that syndecan-1 is shed from repairing epithelial cells after injury. Additionally, MMP7 shedding of syndecan-1 enhances cell migration and wound closure. Our data further demonstrates that syndecan-1 restrains cell migration by modifying the activation state of the α2β1 integrin. Our results establish that MMP7 shedding of syndecan-1 facilitates lung re-epithelialization and acts as a unified mechanism that regulates both acute inflammation and repair.
Results
MMP7 is required for cell migration
Air-liquid interface (ALI) cultures of airway epithelial cells differentiate into a complete mucociliary epithelium and act phenotypically similar to the in vivo epithelium thus providing a relevant organotypic culture system to study the airway mucosal epithelium [6]. We wounded wild-type (WT) and MMP7-null (Mmp7−/−) ALI cultures and observed the wound closure with time-lapse microscopy (Video S1). Whereas injured WT epithelium covered the wound in the field of view within 24 h, Mmp7−/− cells had a dramatic inability to close the wound. Wounded epithelium responds to injury by initially spreading over the wound followed by cell proliferation and migration over the damaged areas [30], [31]. Time-lapse microscopy revealed that the Mmp7−/− epithelium appeared to retain the ability to spread over the wound, as these cells formed extended lamellipodial fronts soon after wounding. However, only WT epithelium continued to migrate and complete the wound healing process. These data confirm that MMP7 is required for re-epithelialization.
MMP7 shedding of syndecan-1 in repair
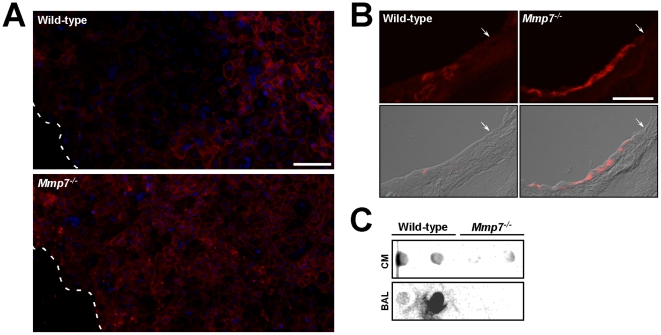
Because MMP7 sheds syndecan-1 from lung epithelium in response to injury [4], we evaluated if release of this proteoglycan functions in re-epithelialization. Immunofluorescence signal for syndecan-1 was decreased at the wound front of WT ALI cultures but remained in Mmp7−/− epithelium after injury (Figure 1A). Cells distal to the wound, representing uninjured epithelium, had equivalent syndecan-1 signal between WT and Mmp7−/− cultures. We also evaluated re-epithelialization in vivo using the naphthalene injury model. Naphthalene specifically kills Clara cells, which make up about 60% of airway epithelium in mice, while sparing other epithelial cell types and with minimal inflammation [6], [32]. In a well-described pattern of repair, the remaining epithelium becomes squamated and migrates to cover the denuded areas left by the sloughed Clara cells. By 14 days post-injury, the epithelium is repaired, and the Clara cell population is fully restored. Using this model, we observed syndecan-1 signal persisted in the Mmp7−/− epithelium after injury but was diminished in WT airway epithelium (Figure 1B). Vehicle-injected WT and Mmp7−/− mice had similar levels of syndecan-1 signal in an expected basolateral distribution (data not shown). Moreover, shed syndecan-1 was detected in the medium of injured WT cultures and in bronchoalveolar lavage fluid from naphthalene injured WT mice but not in Mmp7−/− samples (Figure 1C). These in vitro and in vivo findings confirm that MMP7 sheds syndecan-1 from injured lung epithelial cells.
Figure 1. Syndecan-1 shedding from injured lung epithelium.
(A) ALI cultures 24 h after wounding and (B) lungs two days after naphthalene injury were processed for syndecan-1 immunostaining (scale bar = 100 µm). ALI culture sections were counterstained with Dapi (blue). The white dashed line and the white arrows demarcate the wound front in ALI cultures and naphthalene-injured airway epithelium, respectively. Images are representative of consistent findings in several replicates (n≥3 ALI cultures or mice). (C) Syndecan-1 dot blot was performed using the 281-2 antibody (1∶1000) as previously described [4] on conditioned medium (CM) from injured ALI cultures and from bronchoalveolar lavage (BAL) fluid collected from lungs four days after naphthalene injury. Two independent samples were blotted from each genotype, but the leftmost WT BAL sample did not completely flow.
MMP7 releases syndecan-1 restrictions on wound closure
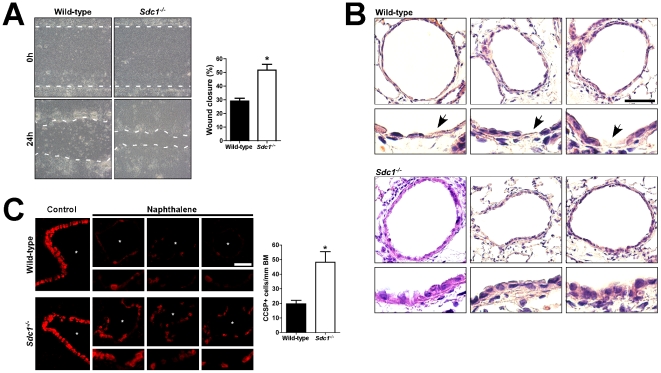
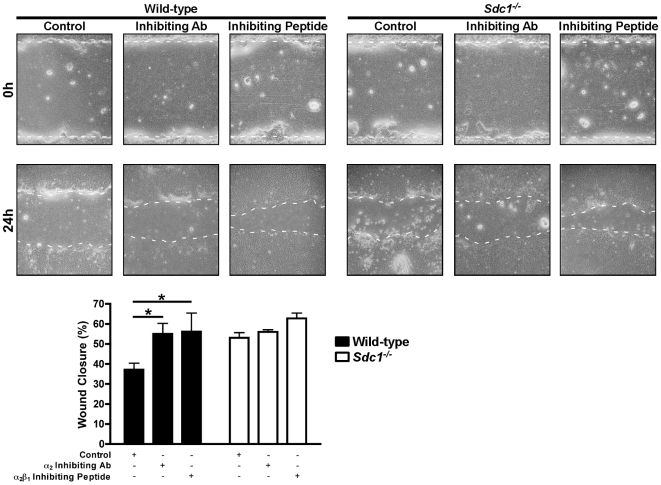
The observations that migration and syndecan-1 shedding were diminished in Mmp7−/− tissue and cells after injury suggested that release of syndecan-1 is needed to promote re-epithelialization. To study this idea, we injured syndecan-1 null (Sdc1−/−) ALI cultures, which grew and differentiated indistinguishable from WT cultures, and found wounds closed significantly faster than in WT cultures (Figure 2A). Additionally, following naphthalene injury, re-epithelialization in vivo was quantitatively faster in Sdc1−/− mice with cuboidal cells appearing sooner compared to WT airways, in which the lining remained patchy and squamated at this time (Figure 2B). To quantify repair in vivo, we immunostained for Clara-cell specific protein (CCSP) and found the number of CCSP-positive cells along the airways was 2.5 times greater in Sdc1−/− mice at 4 days post-naphthalene compared to WT mice (Figure 2C). The airway epithelium in WT and Sdc1−/− mice was equivalent in vehicle-injected controls and had similar degrees of injury after naphthalene injury (data not shown). The accelerated wound closure in Sdc1−/− cultures and airways indicate that MMP7 shedding of syndecan-1 releases restrictions to epithelial cell movement.
Figure 2. Syndecan-1 restrains lung re-epithelialization.
(A) Wild-type and Sdc1−/− ALI cultures were wounded, and the repair was quantified. Each experiment was performed in triplicate and repeated at least 6 times. Original magnification×100. *p<0.0005 by Student's T-Test. (B) Wild-type and Sdc1−/− mice 4 days after naphthalene injury were processed for (B) H&E staining and (C) CCSP immunostaining (scale bar = 100 µm). Each panel is from a different mouse and has an accompanying enlarged portion of the airway (i.e., airways from 3 different mice were shown). In H&E stained sections, Sdc1−/− airway epithelium was more cuboidal in appearance. In contrast, wild-type epithelium was predominantly squamous with persistently exposed substratum (arrows). Additionally, the number of CCSP+cells per linear length of basement membrane (BM) along the airways (asterisk) was determined to quantify the epithelial repair after naphthalene injury. n = 4 mice, *p<0.01 by Student's T-Test.
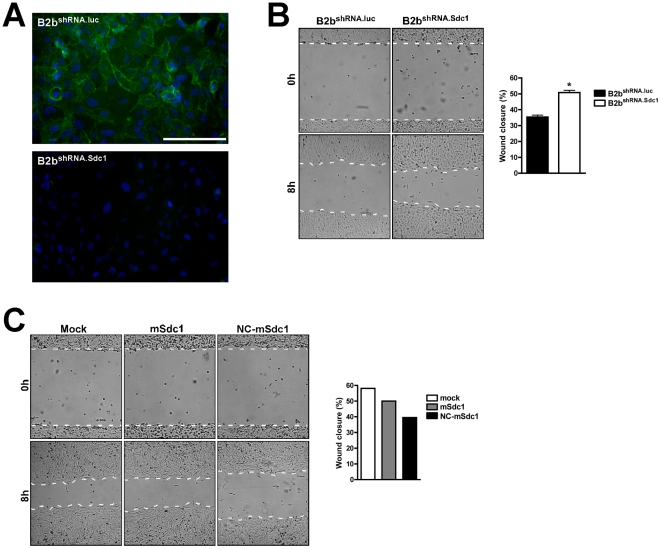
To understand better the mechanisms by which syndecan-1 restrains repair, we used a retroviral vector to create BEAS-2b cells (immortalized human bronchial airway epithelial cell line) that stably expressed shRNA that complements either human syndecan-1 mRNA (B2bshRNA.Sdc1) or a nonsense (luciferase) mRNA (B2bshRNA.luc). Syndecan-1 expression was markedly knocked down in B2bshRNA.Sdc1 cells but not altered in B2bshRNA.luc cells, which had the expected basolateral distribution of the proteoglycan (Figure 3A). Cell monolayers were injured and revealed significantly faster wound closure in B2bshRNA.Sdc1 compared to B2bshRNA.luc cells (Figure 3B). These findings recapitulated the more efficient re-epithelialization phenotype in Sdc1−/− ALI and naphthalene injury models and further support our conclusion that intact syndecan-1 functions to restrain migration.
Figure 3. MMP7 shedding of syndecan-1 releases restrictions to migration.
BEAS-2b cells were used to create stable knockdown cell lines using shRNA targeting either the luciferase gene as a control (B2bshRNA.luc) or syndecan-1 (B2bshRNA.Sdc1). Monolayers of B2bshRNA.luc and B2bshRNA.Sdc1 cells were (A) immunostained for syndecan-1 (green; scale bar = 100 µm), and (B) scratched and wound closure was quantified. n = 4, p<0.0001 by Student's T-Test. (C) Wound closure was determined for B2bshRNA.Sdc1 cells stably overexpressing MMP7 and transiently transfected with no plasmid (Mock), mouse syndecan-1 (mSdc1) or a MMP7-resistant syndecan-1 mutant (NC-Sdc1). The graph is a representative figure of reproducible results on Type I collagen.
Because MMP7 was undetectable by western blot in conditioned medium from BEAS-2b cells (data not shown), faster migration seen with targeted ablation of syndecan-1 suggest that release of this surface proteoglycan–and not other potential substrates–is the MMP7-mediated mechanism that promotes re-epithelialization. We stably overexpressed active MMP7 in B2bshRNA.Sdc1 cells and transiently transfected these cells with constructs that express either wild-type mouse syndecan-1 (mSdc1), which would not be affected by the shRNA targeting the human transcript, or a mutant mouse syndecan-1 resistant to MMP7 proteolysis (NC-mSdc1). Cells expressing NC-mSdc1 had reduced rates of wound closure compared to cells expressing cleavable WT syndecan-1 (Figure 3C). To assess the role of syndecan-1 in cell migration directly, we used a phagokinetic colloid gold migration assay [33] to measure the movement of individual cells over 24 h (Figure 4A). Consistent with rapid repair seen in Sdc1−/− ALI cultures and tissue, cells that lack syndecan-1 (B2bshRNA.Sdc1) migrated faster than control cells (B2bshRNA.luc) (Figure 4A). Together, these findings indicate that MMP7 cleavage of syndecan-1 releases restrictions to migration.
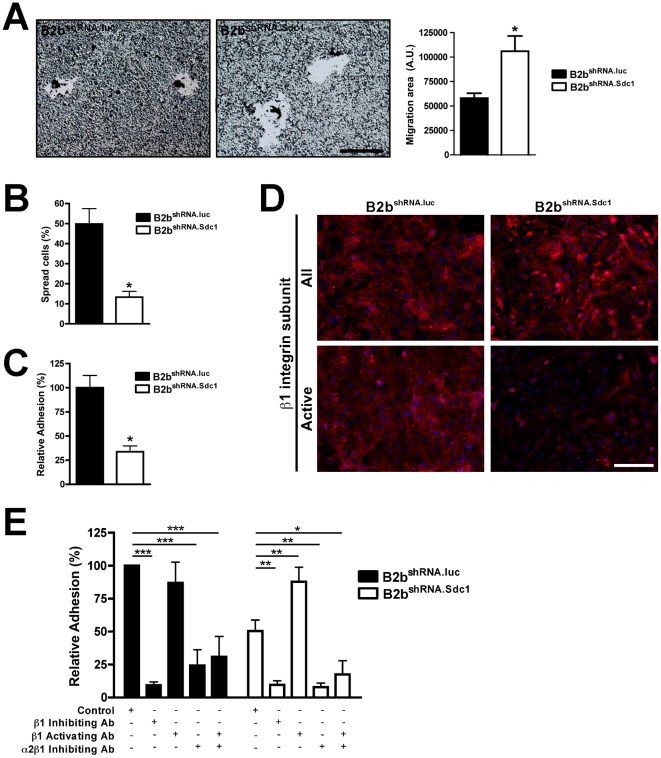
Figure 4. Syndecan-1 regulation of cell-matrix interactions.
(A) B2bshRNA.luc and B2bshRNA.Sdc1 cells were used in a gold colloid migration assay (scale bar = 100 µm). Total migration area was measured for cells plated on type I collagen. n = 4, *p<0.05 by Student's T-Test. (B) The percent of spread cells versus all cells was measured after plating on type I collagen. n = 5, *p<0.005 by Student's T-Test. (C) The relative adhesion percent for cells on type I collagen was determined. n = 6, *p<0.0005 by Student's T-Test. (D) Monolayers of B2bshRNA.luc and B2bshRNA.Sdc1 cells were immunostained for the β1 integrin subunit (red) using all (clone AIIB2) or active conformation-specific (clone 12G10) antibodies. Immunofluorescent images counterstained with Dapi (scale bar = 100 µm). (E) The relative adhesion percent for cells on type I collagen was measured in the presence of control, β1 subunit inhibiting antibody (clone AIIB2; 1 µg/ml), β1 subunit activating antibody (clone HUTS-21; 10 µg/ml) and/or α2β1 integrin inhibiting antibody (clone BHA2.1, 20 µg/ml). Isotype control antibodies were matched to specific antibody experiment. n≥3, *p<0.05, **p<0.01, ***p<0.001 by 2-way ANOVA and Bonferroni analysis.
Syndecan-1 affects cell adhesion and spreading
The ability of a cell to adhere and spread over a matrix substratum is essential for migration. Although they migrated faster, cell spreading was significantly blunted in B2bshRNA.Sdc1 cells compared to B2bshRNA.luc cells (13.3%±2.9 versus 49.8%±7.7, respectively; p<0.005; Figure 4B). Furthermore, B2bshRNA.Sdc1 cells were two-fold less adherent to type I collagen compared to B2bshRNA.luc cells after 30 min of contact (p<0.0001; Figure 4C). However, knock-down of syndecan-1 did not completely ablate the ability to ligate collagen and only changed the kinetics of adhesion as both B2bshRNA.Sdc1 and B2bshRNA.luc cell were attached to the substratum by 4 h (data not shown).
Syndecan-1 regulates α2β1 integrin affinity
Our findings consistently showed syndecan-1-dependent effects on collagen matrices indicating that this proteoglycan affects specific cell-matrix interactions to modulate its effect on cell migration. Syndecan-1 can associate with certain integrins [34], and we evaluated the β1 integrin subunit as it is common to all the fibrillar collagen binding integrins [35]. Deficiency of syndecan-1 did not affect the overall levels of β1 integrins (Figure 4D). However, the β1 integrin subunit can assume active and inactive conformations conferring dramatic differences in substrate affinity [36]. Using a conformation-specific antibody, we found active β1 present on the basolateral surface of B2bshRNA.luc cells but largely absent in B2bshRNA.Sdc1 cells lacking syndecan-1 (Figure 4D). Because α2β1 is the primary collagen binding integrin in most epithelia including the lungs [37], these data suggest that syndecan-1 governs the activation state of this receptor.
We tested the effects of syndecan-1 on the α2β1 integrin with cell adhesion assays in the presence of functional activating and inhibiting antibodies (Figure 4E). In the presence of isotype antibody, we again showed differential binding of B2bshRNA.luc and B2bshRNA.Sdc1 cells to collagen (100% vs 50.5±8.4%, respectively). Blocking antibodies against the β1 integrin subunit or specific to the α2β1 integrin abrogated binding of both B2bshRNA.luc and B2bshRNA.Sdc1 cell adhesion to collagen (β1: 9.3±2.5% vs. 9.7±3.0%, respectively; α2β1: 24.4±12.0% vs. 7.9±3.1%, respectively). In contrast, whereas addition of a β1 activating antibody did not significantly affect B2bshRNA.luc adhesion to collagen (87.0±15.7%), it did augment adhesion of syndecan-1-deficient B2bshRNA.Sdc1 cells compared to isotype control (87.9±11.0% vs. 50.5±8.4%, respectively). Forced β1 integrin subunit activation did not rescue cell adhesion in the presence of an α2β1 integrin inhibitor for either B2bshRNA.luc or B2bshRNA.Sdc1 cells (30.9±15.5% vs. 17.5±10.5%, respectively). These data indicate that syndecan-1 modulates cell adhesion to collagen via the α2β1 integrin and suggests that this regulation occurs by controlling the affinity state of the integrin.
Modulation of the α2β1 integrin during re-epithelialization
Because the presence of syndecan-1 appears to shift the α2β1 integrin to a higher affinity state, MMP7 shedding of syndecan-1 may release this control and deactivate the integrin to a lower affinity, thereby allowing more relaxed cell-matrix interactions that are permissive to cell migration. Indeed, whereas some degree of cell adhesion is needed to generate migratory traction, excessive adhesion impedes cell movement [38], [39]. In consideration of these concepts, our model would predict that in the presence of syndecan-1, the α2β1 integrin restrains cell migration during re-epithelialization. If true, forced activation of the β1 integrin subunit should slow re-epithelialization seen in Sdc1−/− ALI cultures. Moreover, inhibiting α2β1 integrin ligation should overcome the diminished migration seen in Mmp7−/− conditions where syndecan-1 persists at the cell surface.
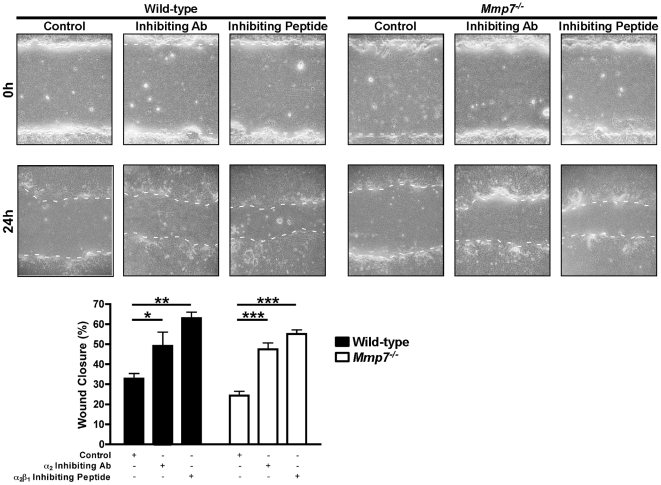
To test these ideas, we first established that blocking the α2β1 integrin enhances wound repair in ALI airway epithelial cultures. Wounded WT ALI cultures had enhanced wound closure in the presence of either an α2 subunit inhibiting antibody or an α2β1 inhibiting peptide (Figure 5 and 6). In contrast, Sdc1−/− ALI cultures had no additional augmentation of wound closure suggesting the α2β1 integrin contributed minimally to cell migration in the absence of syndecan-1 (Figure 5). However, and consistent with our hypothesis, the α2 subunit and α2β1 integrin inhibitors both increased the wound closure rate of injured Mmp7−/− ALI cultures (Figure 6). These data support our idea that in the presence of syndecan-1, the higher affinity state of the α2β1 integrin restrains migration of the repairing airway epithelium.
Figure 5. Wounded Sdc1−/− lung epithelium is unaffected byα2β1 integrin inhibition.
Wild-type and Sdc1−/− ALI cultures were injured in the presence of a control (hamster isotype IgG2; 10 µg/ml), α2 integrin subunit inhibiting antibody (clone Ha1/29; 10 µg/ml) or α2β1 integrin inhibiting peptide (5 mM). The percent wound closure was determined 24 h after injury. *p<0.05 by 2-way ANOVA and Bonferroni analysis. n = 4; Original magnification×100.
Figure 6. Wounded Mmp7−/− lung epithelium has augmented wound closure with α2β1 integrin inhibition.
Wild-type and Mmp7−/− ALI cultures were injured in the presence of a control (hamster isotype IgG2; 10 µg/ml), α2 integrin subunit inhibiting antibody (clone Ha1/29; 10 µg/ml) or α2β1 integrin inhibiting peptide (5 mM). The percent wound closure was determined 24 h after injury. *p<0.05, **p<0.01, ***p<0.001 by 2-way ANOVA and Bonferroni analysis. n = 4; Original magnification×100.
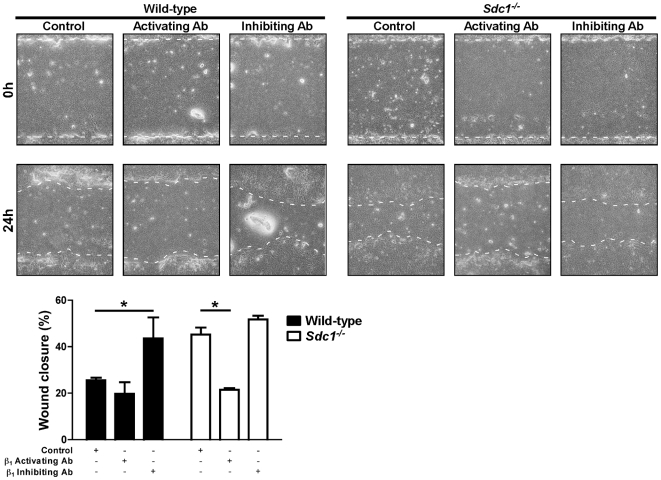
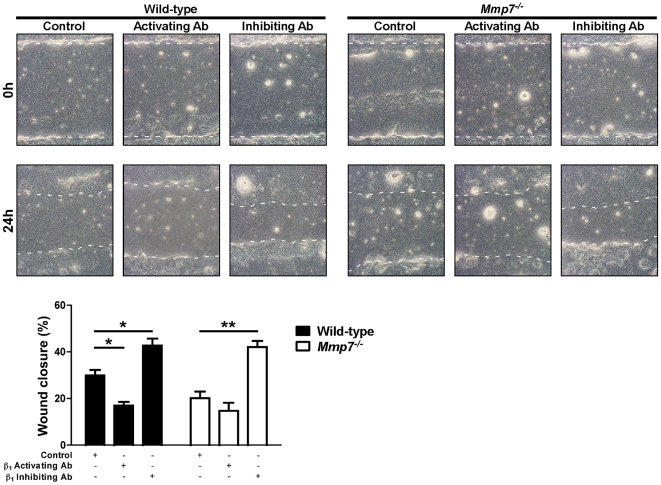
Next, we wounded WT and Sdc1−/− ALI cultures in the presence of control, β1-activating, or β1-inhibiting antibodies (Figure 7). Inhibition of β1 integrins augmented wound closure rates of injured WT ALI cultures to levels equivalent to Sdc1−/− cultures. Conversely, forced activation of the β1 integrin subunit slowed the wound closure rate of injured Sdc1−/− ALI cultures to that of WT conditions. Congruous with our hypothesis, inhibiting β1 integrin subunit restored wound closure rate of Mmp7−/− cultures to WT levels (Figure 8). In WT ALI cultures, the activating β1 integrin antibody significantly slowed wound closure relative to control conditions, but this effect was seen at the higher concentration used (compare Figures 7 and 8). In contrast, the higher concentration of β1 integrin activating antibody had no effect on Mmp7−/− wound closure rate suggesting that the β1 integrin subunit was already maximally activated.
Figure 7. Wounded Sdc1−/− lung epithelium has attenuated repair with forced β1 integrin subunit activation.
Wild-type and Sdc1−/− ALI cultures were injured in the presence of a control (rat isotype IgG, 1 µg/ml) or β1 integrin subunit activating (clone 9EG7, 1 µg/ml) or inhibiting (clone AIIB2, 1 µg/ml) antibodies. The percent wound closure was determined 24 h after injury. *p<0.05 by 2-way ANOVA and Bonferroni analysis. n = 4; Original magnification×100.
Figure 8. Wounded Mmp7−/− lung epithelium has enhanced repair with β1 integrin subunit inhibition.
Wild-type and Mmp7−/− ALI cultures were injured in the presence of a control (rat isotype IgG, 10 µg/ml) or β1 integrin subunit activating (clone 9EG7, 10 µg/ml) or inhibiting (clone AIIB2, 1 µg/ml) antibodies. The percent wound closure was determined 24 h after injury. *p<0.01, **p<0.001 by 2-way ANOVA and Bonferroni analysis. n = 4; Original magnification×100.
Discussion
Injury opens avenues for pathogenic entry across a breached barrier. Therefore, the body has evolved mechanisms to recruit inflammatory cells to combat potential pathogens while quickly repairing the damaged tissue. The lungs, in particular, have adapted its mucosal surface to utilize MMP7 in regulating both inflammation and repair. Various injuries stimulate a rapid and dramatic induction of MMP7 production by the wounded epithelium [3]–[11]. This expression is necessary for recruiting inflammatory cells while promoting re-epithelialization [3]–[6]. Our group previously reported MMP7 governs the inflammatory response through the shedding of syndecan-1 [4]. Here, we provide evidence that syndecan-1 shedding also serves to promote re-epithelialization.
Damaged epithelium start spreading within minutes after injury while the initiation of cell migration is delayed by several hours [30], [31]. Our data show that MMP7 regulates the migration component of the re-epithelialization process. Migration is a complex process that is affected by multiple interactions between the cell and its environment [40]. In particular, cell-matrix interaction must be tightly controlled to ensure affinity is adequate for traction but not so excessive as to prevent forward migration [38], [39]. Therefore, MMP7 seems to be required for proteolysis of a substrate that normally restrains cell migration. MMP7 is the primary sheddase of syndecan-1 in the lungs after injury [4]. Our findings demonstrate that MMP7 shedding of syndecan-1 also facilitates wound closure. Indeed, syndecan-1 restricts migration as demonstrated with the augmented wound closure in conditions lacking syndecan-1. Moreover, lung epithelial cells transfected with syndecan-1 resistant to MMP7 proteolysis attenuates cell migration compared to wild-type syndecan-1. The Sdc1−/− and Mmp7−/− conditions also have opposite repair phenotypes, and these effects are consistent with the idea that MMP7 shedding of syndecan-1 releases restraints to cell migration.
Our data show that syndecan-1 augments cell adhesion to collagen. This increase in cell-matrix binding, in turn, promotes cell spreading but represses cell migration. Numerous studies have documented a functional coupling of syndecan-1 with integrins [34]. Our results describe how syndecan-1 indirectly governs cell-matrix binding in the lungs by modulating α2β1 integrin affinity. Syndecan-1 shedding could promote cell migration by re-organizing cell interactions with the environment [25], [40]. Indeed, the loss of syndecan-1 has been shown to induce changes in the cellular machinery that promote migration [18], [19]. Integrin-mediated attachments can also promote cell spreading [41], but the cell may also require syndecan-1 as an additional signal to re-organize the actin cytoskeleton and facilitate cell spreading [42]–[49]. Certainly, changes in cell-matrix interaction can switch the functional outcome of the cell [50], [51]. Our findings indicate that syndecan-1 regulates the affinity state of the α2β1 integrin subunit, which in turn appears to coordinate cell attachments to the matrix that are required for spreading and migration. This activation event can occur quickly through a conformational change without any need for protein expression as the required components are already in place.
Syndecan-1 being functionally coupled to integrins has been demonstrated in other models [34]. For example, syndecan-1 regulates the activation state of αvβ3 integrin potentially as a way for breast carcinoma cells to acquire a more invasive phenotype [44], [45]. Additionally, syndecan-1 associates with and facilitates the activation of αvβ5 integrin in a fibroblast cell line [47]. Similarly, syndecan-1 was found to directly interact with and modulate the affinity state of αvβ3 and αvβ5 integrins in endothelial cells to facilitate angiogenesis in vivo [52]. Studies with keratinocytes show a migratory defect when deficient in syndecan-1, possibly through alterations in laminin 332 binding integrins [17], [53]–[55]. In contrast, Sdc1−/− dermal fibroblasts have increased migration, increased β1 and αv integrin subunit expression and augmentation of αv integrin subunit activity compared to WT conditions [56]. Recently, syndecan-1 was found to physically interact with the β1 integrin subunit [57] and augment cell adhesion to type I collagen by cooperating with α2β1 integrin [58]. Conversely, other studies specifically evaluating syndecan-1 effects on the β1 integrin subunit found no difference in expression and activation [19], [43], [46]. Syndecan-1 appears to have multiple possible interactions with various integrins, depending on the cell system that is used. This likely represents the fact that different lineages of cells express different repertoires of surface and intracellular proteins from which syndecan-1 can associate.
Syndecan-1 also appears to function in a tissue specific manner. Skin and corneal epithelium deficient in syndecan-1 have defective re-epithelialization in vivo apparently due to attenuated proliferative and migratory responses [16], [17], [59]. Interestingly, syndecan-1 overexpression also inhibits skin repair in vivo possibly through inhibitory actions from the syndecan-1 ectodomain [14]. One explanation for the different effects of syndecan-1 is that the skin and cornea have a stratified epithelium that is structurally and functionally distinct from the simple epithelium in the lungs.
A recent study using A549 cells, a carcinoma-derived alveolar type II cell line, reported that knockdown of syndecan-1 expression slowed cell migration [60]. The findings we present here directly contradict the results in the A549 cell line. A549 cell lines are cancerous in origin, and thus, changes associated with the transformed phenotype may have altered the functions of syndecan-1 and the pathways controlling cell movement, such as that seen with breast cancer cells [44], [45]. In contrast, we used a non-cancerous cell line (BEAS-2b) and organotypic cultures derived from primary epithelial cells, as well as in vivo models. Further investigation would be needed to fully understand the fundamental reasons for the different results found in these studies.
Together, our data support the conclusion that MMP7 facilitates re-epithelialization through the shedding of syndecan-1, which then changes the activation state of the α2β1 integrin to reduce cell affinity to collagen and remove restrictions to migration. MMP7 is induced in the lungs by injury and sheds syndecan-1 as a protective mechanism to recruit neutrophils while promoting re-epithelialization. Because inflammation and repair are two interrelated phenomena, MMP7 shedding of syndecan-1 could be the result of evolution co-opting two important biological processes into one unified mechanism.
Materials and Methods
Ethics Statement
All animal procedures were approved by the Institutional Animal Care and Use Committee at the University of Washington and the National Institute of Health Guide for the Care and Use of Laboratory animals.
Antibodies and Peptides
Clara cell specific protein (CCSP) was immunostained in mouse tissue with a rabbit polyclonal antibody (Upstate, Lake Placid, NY). Syndecan-1 antibodies were clone B-B4 for immunostaining on human samples (BD Biosciences, San Jose, CA) and clone 281.2 for mouse tissue (kindly provided by P.W. Park; Children's Hospital, Harvard Medical School, Boston, MA). Active conformation β1 integrin subunit was immunostained on human tissue with clone 12G10 (Millipore, Billerica, MA) as previously described [61]. All β1 integrin was immuostained with clone AIIB2 (Developmental Studies Hybridoma Bank, Iowa City, IA; Deposited by C. Damsky, UCSF). Appropriate Alexa Fluor labeled secondary antibodies were from Invitrogen (Carlsbad, CA).
Functional integrin activators and inhibitors were as follows. Clone BHA2.1 was used as a functional α2β1 integrin inhibitor in human cells [62], [63]. The α2β1 ligand peptide (Anaspec, Fremont, CA) inhibits integrin binding to collagen [64] and was used in mouse cells. Clone Ha1/29 (BD Biosciences) was used as a mouse α2 subunit inhibitor [65], [66]. Clones HUTS-21 [67] and 9EG7 [68], [69] were used respectively as human and mouse β1 subunit activating antibodies. Clone AIIB2 was used to inhibit β1 subunit function in both human and mouse systems [70]. Although the clone AIIB2 antibody was generated toward the human β1 subunit, cross-reactivity with several species have been established [71]–[73], and preliminary studies showed effect in blocking adhesion of murine cells (data not shown). All functional experiments were matched with isotype antibody controls from Santa Cruz Biotechnologies (general rat isotype), BD Biosciences (Armenian Hamster IgG2) and BioLegend (all mouse and rat monoclonal isotype controls). The one exception is when 9EG7 (rat IgG2a) and AIIB2 (rat IgG1) were both used (Figures 7 and 8), a general rat isotype IgG antibody (Santa Cruz Biotechnologies) was used as a common control. Preliminary experiments showed no difference between rat monoclonal IgG isotypes and the general rat IgG control (data not shown).
Lung injury models
Air-liquid interface (ALI) cultures were created from wild-type (WT), MMP7-null (Mmp7−/−) and syndecan-1-null (Sdc1−/−) mice all on a C57BL/6 background, and wound closure assays were performed as previously described [6]. Additionally, time-lapse microscopy of the repairing ALI culture obtained DIC images every 6 min over 24 h on a DeltaVision Olympus IX71 inverted microscope using a 20x/0.75 U plan Apo objective and a Photometric Coolsnap HQ camera (Applied Precision, Issaquah, WA).
The naphthalene injury model was used to study repair in vivo [6]. WT, Mmp7−/− and Sdc1−/− mice had intraperitoneal injections of 200 mg/kg sterile naphthalene dissolved in corn oil. All mice were injected between 8–10 am to minimize diurnal variations in naphthalene metabolism. Mice were sacrificed at the defined time point, bronchoalveolar lavage fluid was collected and the lung tissue was processed for histology.
Knockdown and Overexpression Cell Lines
The pSM2 vector containing shRNA specific for the human syndecan-1 gene or the luciferase gene was purchased from Open Biosystems (Huntsville, AL). Wild-type human MMP7 was mutated to contain a furin cleavage site at the junction of the pro- and catalytic domain (generously provided to us by D. Madtes; Fred Hutchinson Cancer Research Center, Seattle, WA). This construct was subcloned using BamHI and NotI restriction sites into the pBM-IRES-blastocidin retroviral expression plasmid (kindly provide by E. Raines; University of Washington, Seattle, WA). The full-length WT mouse syndecan-1 (mSdc1) was also PCR cloned from cDNA created from whole lung RNA, and the PCR product was inserted into the GFP Fusion TOPO TA expression kit (Invitrogen, Carlesbad, CA). A non-cleavable syndecan-1 (NC-mSdc1) was created by replacing the juxtamembrane MMP cleavage site with 15 amino acids from the human CD4 juxtamembrane sequence as previously described [25]–[27].
Stable knockdown cells were created using a retroviral transduction system. For both shRNA and overexpressing retroviral expression systems, retroviral particles were produced by transiently transfecting plasmid DNA into the PhiNx amphoteric packaging cell line (ATCC, Manassas, VA; Deposited by G. Nolan) [74]. Target cells were infected with retroviral conditioned medium containing 10 µg/ml DEAE-Dextran for 16 h at 33°C before being transferred to 37°C in fresh medium [75]. Two days after infection, medium containing the appropriate selection antibiotic was added and stably transduced cell lines were selected. Additionally, cells were transiently transfected to express mSdc1 or NC-mSdc1 with Lipofectamine 2000 (Invitrogen) following manufacturer's directions.
Histology and Immunostaining
All immunostaining of ALI cultures and of cell lines were performed on 100% methanol-fixed cultures. H&E staining and CCSP immunofluorescent staining was performed on 10% formalin-fixed, paraffin-embedded naphthalene injured tissue sections as previously described [6]. Epifluorescence images were captured using an Olympus BX-51 fluorescence/DIC microscope with U plan Apo 40x/0.85 and 20x/0.70 objectives and an Olympus DP25 5.5 megapixel digital camera. All immunofluorescent slides were processed with identical conditions. Images were captured with equal exposures and microscope settings. When necessary, minor linear changes to intensity were made equally with ImageJ software.
Migration, Spreading and Adhesion Assays
Assays to evaluate cell-matrix interactions effects on components of repair were employed. Cell spreading was evaluated as previously described [6]. Plated cells were allowed to spread for 60 min on various matrices, and the percent of cells spread compared to all plated cells was determined. We also evaluated cell migration using a modified colloid gold migration assay [33]. The protocol was modified so that experiments were performed on Permanox chamber slides (Nunc, Rochester, NY). In each experiment of the migration assay, at least 10 cells were randomly chosen, and the migration area was measured with ImageJ software. Scratch wound assays were also used as a method to measure migration and wound closure [5], [6].
Cell adhesion to a matrix was determined with a modified cell adhesion assay [76]. Cells were pre-labeled with 5 µM calcein AM (Alexis Biochemicals, San Diego, CA) in 10% FBS DMEM medium for 30 min. Cells were then washed and lifted from the culture plate by incubating in PBS-EDTA at 37°C for 10 min. All cells were collected and washed with PBS before plating onto matrix coated 96-well round-bottom plates in 1% FBS phenol-free DMEM (10,000 cells per well in 100 µl total volume). To synchronize the initial cell-matrix contact, plates were centrifuged at 30×g for 3 min. Total fluorescence (excitation 485, emission 530) was measured at 0 h as a baseline. After 30 min at 37°C, plates were washed with an electronic multichannel pipettor set at a consistent ejection force for all experiments to remove non-adherent cells. After three washes of PBS, 100 µl of 1% FBS phenol-free DMEM was replaced into each well and the overall fluorescence was measured. The percent adherence was determined by a ratio of the fluorescent signal at 30 min compared to baseline. When indicated, cell adhesion assays were performed in the presence of isotype and functional activating and inhibiting antibodies. To compare among different experiments, which may require different control antibodies, all data was normalized to the positive control condition and presented as a relative adhesion percent.
Supporting Information
Wound closure of wild-type and MMP7−/− ALI cultures. Wild-type and MMP7−/− ALI cultures were injured and wound closure was observed over 24 hours.
(21.22 MB MOV)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by the NIH grants (HL08396 to PC; HL077555 and HL029594 to WCP), the American Lung Association Biomedical Research Grant (PC) and the American Heart Association Beginning Grant-In-Aid (PC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med. 2008;359:2355–2365. doi: 10.1056/NEJMra0800353. [DOI] [PubMed] [Google Scholar]
- 2.Holgate ST. The Airway Epithelium is Central to the Pathogenesis of Asthma. Allergology International. 2008;57:1–10. doi: 10.2332/allergolint.R-07-154. [DOI] [PubMed] [Google Scholar]
- 3.Dunsmore SE, Saarialho-Kere UK, Roby JD, Wilson CL, Matrisian LM, et al. Matrilysin expression and function in airway epithelium. Journal of Clinical Investigation. 1998;102:1321–1331. doi: 10.1172/JCI1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Q, Park PW, Wilson CL, Parks WC. Matrilysin shedding of syndecan-1 regulates chemokine mobilization and transepithelial efflux of neutrophils in acute lung injury. Cell. 2002;111:635–646. doi: 10.1016/s0092-8674(02)01079-6. [DOI] [PubMed] [Google Scholar]
- 5.McGuire JK, Li Q, Parks WC. Matrilysin (Matrix Metalloproteinase-7) mediates E-cadherin ectodomain shedding in injured lung epithelium. American Journal of Pathology. 2003;162:1831–1843. doi: 10.1016/S0002-9440(10)64318-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen P, McGuire JK, Hackman RC, Kim K-H, Black RA, et al. Tissue Inhibitor of Metalloproteinase-1 Moderates Airway Re-Epithelialization by Regulating Matrilysin Activity. Am J Pathol. 2008;172:1256–1270. doi: 10.2353/ajpath.2008.070891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Swee M, Wilson C, Wang Y, McGuire JK, Parks W. Matrix metalloproteinase-7 (matrilysin) controls neutrophil egress by generating chemokine gradients. Journal of Leukocyte Biology. 2008;83:1404–1412. doi: 10.1189/jlb.0108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, et al. Regulation of intestinal à-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 9.López-Boado YS, Wilson CL, Hooper LV, Gordon JI, Hultgren SJ, et al. Bacterial exposure induces and activates matrilysin in mucosal epithelial cells. The Journal of Cell Biology. 2000;148:1305–1315. doi: 10.1083/jcb.148.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wilson CL, Heppner KJ, Rudolph LA, Matrisian LM. The metalloproteinase matrilysin is preferentially expressed by epithelial cells in a tissue-restricted pattern in the mouse. Molecular Biology of the Cell. 1995;86:113–117. doi: 10.1091/mbc.6.7.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saarialho-Kere UK, Vaalamo M, Puolakkainen P, Airola K, Parks WC, et al. Enhanced expression of matrilysin, collagenase, and stromelysin-1 in gastrointestinal ulcers. Am J Pathol. 1996;148:519–526. [PMC free article] [PubMed] [Google Scholar]
- 12.Couchman JR. Syndecans: Proteoglycan regulators of cell-surface microdomains? Nature Reviews Molecular Cell Biology. 2003;4:926–938. doi: 10.1038/nrm1257. [DOI] [PubMed] [Google Scholar]
- 13.Park PW, Pier GB, Hinkes MT, Bernfield M. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature. 2001;411:98–102. doi: 10.1038/35075100. [DOI] [PubMed] [Google Scholar]
- 14.Elenius V, Gotte M, Reizes O, Elenius K, Bernfield M. Inhibition by the Soluble Syndecan-1 Ectodomains Delays Wound Repair in Mice Overexpressing Syndecan-1. J Biol Chem. 2004;279:41928–41935. doi: 10.1074/jbc.M404506200. [DOI] [PubMed] [Google Scholar]
- 15.Xu J, Park PW, Kheradmand F, Corry DB. Endogenous Attenuation of Allergic Lung Inflammation by Syndecan-1. J Immunol. 2005;174:5758–5765. doi: 10.4049/jimmunol.174.9.5758. [DOI] [PubMed] [Google Scholar]
- 16.Stepp MA, Gibson HE, Gala PH, Iglesia DDS, Pajoohesh-Ganji A, et al. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J Cell Sci. 2002;115:4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- 17.Stepp MA, Liu Y, Pal-Ghosh S, Jurjus RA, Tadvalkar G, et al. Reduced migration, altered matrix and enhanced TGFbeta1 signaling are signatures of mouse keratinocytes lacking Sdc1. J Cell Sci. 2007;120:2851–2863. doi: 10.1242/jcs.03480. [DOI] [PubMed] [Google Scholar]
- 18.Leppä S, Vleminckx K, Van Roy F, Jalkanen M. Syndecan-1 expression in mammary epithelial tumor cells is E-cadherin-dependent. Journal of Cell Science. 1996;109 (Pt 6):1393–1403. doi: 10.1242/jcs.109.6.1393. [DOI] [PubMed] [Google Scholar]
- 19.Kato M, Saunders S, Nguyen H, Bernfield M. Loss of cell surface syndecan-1 causes epithelia to transform into anchorage-independent mesenchyme-like cells. Mol Biol Cell. 1995;6:559–576. doi: 10.1091/mbc.6.5.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grushkin-Lerner LS, Trinkaus-Randall V. Localization of integrin and syndecan in vivo in a corneal epithelial abrasion and keratectomy. Curr Eye Res. 1991;10:75–85. doi: 10.3109/02713689109007612. [DOI] [PubMed] [Google Scholar]
- 21.Oksala O, Salo T, Tammi R, Hakkinen L, Jalkanen M, et al. Expression of proteoglycans and hyaluronan during wound healing. J Histochem Cytochem. 1995;43:125–135. doi: 10.1177/43.2.7529785. [DOI] [PubMed] [Google Scholar]
- 22.Subramanian SV, Fitzgerald ML, Bernfield M. Regulated Shedding of Syndecan-1 and -4 Ectodomains by Thrombin and Growth Factor Receptor Activation. J Biol Chem. 1997;272:14713–14720. doi: 10.1074/jbc.272.23.14713. [DOI] [PubMed] [Google Scholar]
- 23.Kainulainen V, Wang H, Schick C, Bernfield M. Syndecans, Heparan Sulfate Proteoglycans, Maintain the Proteolytic Balance of Acute Wound Fluids. J Biol Chem. 1998;273:11563–11569. doi: 10.1074/jbc.273.19.11563. [DOI] [PubMed] [Google Scholar]
- 24.Penc SF, Pomahac B, Winkler T, Dorschner RA, Eriksson E, et al. Dermatan Sulfate Released after Injury Is a Potent Promoter of Fibroblast Growth Factor-2 Function. J Biol Chem. 1998;273:28116–28121. doi: 10.1074/jbc.273.43.28116. [DOI] [PubMed] [Google Scholar]
- 25.Endo K, Takino T, Miyamori H, Kinsen H, Yoshizaki T, et al. Cleavage of Syndecan-1 by Membrane Type Matrix Metalloproteinase-1 Stimulates Cell Migration. J Biol Chem. 2003;278:40764–40770. doi: 10.1074/jbc.M306736200. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Gotte M, Bernfield M, Reizes O. Constitutive and Accelerated Shedding of Murine Syndecan-1 Is Mediated by Cleavage of Its Core Protein at a Specific Juxtamembrane Site. Biochemistry. 2005;44:12355–12361. doi: 10.1021/bi050620i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of Syndecan-1 and -4 Ectodomains Is Regulated by Multiple Signaling Pathways and Mediated by a TIMP-3-Sensitive Metalloproteinase. J Cell Biol. 2000;148:811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brule S, Charnaux N, Sutton A, Ledoux D, Chaigneau T, et al. The shedding of syndecan-4 and syndecan-1 from HeLa cells and human primary macrophages is accelerated by SDF-1/CXCL12 and mediated by the matrix metalloproteinase-9. Glycobiology. 2006;16:488–501. doi: 10.1093/glycob/cwj098. [DOI] [PubMed] [Google Scholar]
- 29.Su G, Blaine S, Qiao D, Friedl A. Membrane Type 1 Matrix Metalloproteinase-Mediated Stromal Syndecan-1 Shedding Stimulates Breast Carcinoma Cell Proliferation. Cancer Research. 2008;68:9558–9565. doi: 10.1158/0008-5472.CAN-08-1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zahm JM, Chevillard M, Puchelle E. Wound repair of human surface respiratory epithelium. American Journal Of Respiratory Cell And Molecular Biology. 1991;5:242–248. doi: 10.1165/ajrcmb/5.3.242. [DOI] [PubMed] [Google Scholar]
- 31.Block ER, Matela AR, SundarRaj N, Iszkula ER, Klarlund JK. Wounding induces motility in sheets of corneal epithelial cells through loss of spatial constraints: role of heparin-binding epidermal growth factor-like growth factor signaling. J Biol Chem. 2004;279:24307–24312. doi: 10.1074/jbc.M401058200. [DOI] [PubMed] [Google Scholar]
- 32.Van Winkle LS, Buckpitt AR, Nishio SJ, Isaac JM, Plopper CG. Cellular response in naphthalene-induced Clara cell injury and bronchiolar epithelial repair in mice. Am J Physio. 1995;269:L800–L818. doi: 10.1152/ajplung.1995.269.6.L800. [DOI] [PubMed] [Google Scholar]
- 33.Pilcher BK, Dumin JA, Sudbeck BD, Krane SM, Welgus HG, et al. The activity of collagenase-1 is required for keratinocyte migration on a type I collagen matrix. The Journal of Cell Biology. 1997;137:1445–1457. doi: 10.1083/jcb.137.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan M, Humphries M, Bass M. Synergistic control of cell adhesion by integrins and syndecans. Nat Rev Mol Cell Biol. 2007;8:957–969. doi: 10.1038/nrm2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hynes RO. Integrins: Bidirectional, Allosteric Signaling Machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 36.Askari JA, Buckley PA, Mould AP, Humphries M. Linking integrin conformation to function. Journal of Cell Science. 2009;122 (Pt 2):165–170. doi: 10.1242/jcs.018556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheppard D. Functions of Pulmonary Epithelial Integrins: From Development to Disease. Physiological Reviews. 2003;83:673–686. doi: 10.1152/physrev.00033.2002. [DOI] [PubMed] [Google Scholar]
- 38.DiMilla PA, Barbee K, Lauffenberger DA. Mathematical model for the effects of adhesion and mechanics on cell migration speed. BiophysJ. 1991;60:15–37. doi: 10.1016/S0006-3495(91)82027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DiMilla PA, Stone JA, Quinn JA, Albelda SM, Lauffenberger DA. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. Journal of Cell Biology. 1993;122:729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, et al. Cell Migration: Integrating Signals from Front to Back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 41.Small JV, Rottner K, Kaverina I. Functional design in the actin cytoskeleton. Curr Opin Cell Biol. 1999;11:54–60. doi: 10.1016/s0955-0674(99)80007-6. [DOI] [PubMed] [Google Scholar]
- 42.Lebakken CS, Rapraeger AC. Syndecan-1 mediates cell spreading in transfected human lymphoblastoid (Raji) cells. The Journal of Cell Biology. 1996;132:1209–1221. doi: 10.1083/jcb.132.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lebakken CS, McQuade KJ, Rapraeger AC. Syndecan-1 Signals Independently of [beta]1 Integrins during Raji Cell Spreading. Experimental Cell Research. 2000;259:315–325. doi: 10.1006/excr.2000.4981. [DOI] [PubMed] [Google Scholar]
- 44.Beauvais DM, Rapraeger AC. Syndecan-1-mediated cell spreading requires signaling by [alpha]v[beta]3 integrins in human breast carcinoma cells. Experimental Cell Research. 2003;286:219–232. doi: 10.1016/s0014-4827(03)00126-5. [DOI] [PubMed] [Google Scholar]
- 45.Beauvais DM, Burbach BJ, Rapraeger AC. The syndecan-1 ectodomain regulates {alpha}v{beta}3 integrin activity in human mammary carcinoma cells. J Cell Biol. 2004;167:171–181. doi: 10.1083/jcb.200404171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burbach BJ, Ji Y, Rapraeger AC. Syndecan-1 ectodomain regulates matrix-dependent signaling in human breast carcinoma cells. Experimental Cell Research. 2004;300:234–247. doi: 10.1016/j.yexcr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 47.McQuade KJ, Beauvais DM, Burbach BJ, Rapraeger AC. Syndecan-1 regulates {alpha}v{beta}5 integrin activity in B82L fibroblasts. J Cell Sci. 2006;119:2445–2456. doi: 10.1242/jcs.02970. [DOI] [PubMed] [Google Scholar]
- 48.Carey DJ, Stahl RC, Cizmeci-Smith G, Asundi VK. Syndecan-1 expressed in Schwann cells causes morphological transformation and cytoskeletal reorganization and associates with actin during cell spreading. J Cell Biol. 1994;124:161–170. doi: 10.1083/jcb.124.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adams JC, Kureishy N, Taylor AL. A Role for Syndecan-1 in Coupling Fascin Spike Formation by Thrombospondin-1. J Cell Biol. 2001;152:1169–1182. doi: 10.1083/jcb.152.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flevaris P, Stojanovic A, Gong H, Chishti A, Welch E, et al. A molecular switch that controls cell spreading and retraction. The Journal of Cell Biology. 2007;179:553–565. doi: 10.1083/jcb.200703185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Margadant C, Raymond K, Kreft M, Sachs N, Janssen H, et al. Integrin a3b1 inhibits directional migration and wound re-epithelialization in the skin. Journal of Cell Science. 2009;122:278–288. doi: 10.1242/jcs.029108. [DOI] [PubMed] [Google Scholar]
- 52.Beauvais D, Ell B, Mcwhorter A, Rapraeger A. Syndecan-1 regulates v 3 and v 5 integrin activation during angiogenesis and is blocked by synstatin, a novel peptide inhibitor. Journal of Experimental Medicine. 2009:15. doi: 10.1084/jem.20081278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Okamoto O, Bachy S, Odenthal U, Bernaud J, Rigal D, et al. Normal human keratinocytes bind to the alpha3LG4/5 domain of unprocessed laminin-5 through the receptor syndecan-1. J Biol Chem. 2003;278:44168–44177. doi: 10.1074/jbc.M300726200. [DOI] [PubMed] [Google Scholar]
- 54.Bachy S, Letourneur F, Rousselle P. Syndecan-1 interaction with the LG4/5 domain in laminin-332 is essential for keratinocyte migration. J Cell Physiol. 2008;214:238–249. doi: 10.1002/jcp.21184. [DOI] [PubMed] [Google Scholar]
- 55.Ogawa T, Tsubota Y, Hashimoto J, Kariya Y, Miyazaki K. The Short Arm of Laminin {gamma}2 Chain of Laminin-5 (Laminin-332) Binds Syndecan-1 and Regulates Cellular Adhesion and Migration by Suppressing Phosphorylation of Integrin beta4 Chain. Mol Biol Cell. 2007;18:1621–1633. doi: 10.1091/mbc.E06-09-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jurjus R, Liu Y, Pal-Ghosh S, Tadvalkar G, Stepp M. Primary dermal fibroblasts derived from sdc-1 deficient mice migrate faster and have altered alphav integrin function. Wound repair and regeneration: official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16:649–660. doi: 10.1111/j.1524-475X.2008.00423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hayashida K, Stahl PD, Park PW. Syndecan-1 Ectodomain Shedding Is Regulated by the Small GTPase Rab5. Journal of Biological Chemistry. 2008;283:35435–35444. doi: 10.1074/jbc.M804172200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vuoriluoto K, Jokinen J, Kallio K, Salmivirta M, Heino J, et al. Syndecan-1 supports integrin alpha2beta1-mediated adhesion to collagen. Experimental Cell Research. 2008;314:3369–3381. doi: 10.1016/j.yexcr.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 59.Pal-Ghosh S, Tadvalkar G, Jurjus R, Zieske JD, Stepp M. BALB/c and C57BL6 mouse strains vary in their ability to heal corneal epithelial debridement wounds. Experimental Eye Research. 2008;87:478–486. doi: 10.1016/j.exer.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kliment CR, Englert JM, Gochuico BR, Yu G, Kaminski N, et al. Oxidative stress alters syndecan-1 distribution in lungs with pulmonary fibrosis. J Biol Chem. 2009;284:3537–3545. doi: 10.1074/jbc.M807001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ng T, Shima D, Squire A, Bastiaens PI, Gschmeissner S, et al. PKCalpha regulates beta1 integrin-dependent cell motility through association and control of integrin traffic. EMBO J. 1999;18:3909–3923. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hangan D, Uniyal S, Morris VL, MacDonald IC, von Ballestrem C, et al. Integrin VLA-2 (alpha2beta1) function in postextravasation movement of human rhabdomyosarcoma RD cells in the liver. Cancer Research. 1996;56:3142–3149. [PubMed] [Google Scholar]
- 63.Wang XQ, Frazier WA. The thrombospondin receptor CD47 (IAP) modulates and associates with alpha2 beta1 integrin in vascular smooth muscle cells. Molecular Biology of the Cell. 1998;9:865–874. doi: 10.1091/mbc.9.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Staatz WD, Fok KF, Zutter MM, Adams SP, Rodriguez BA, et al. Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. J Biol Chem. 1991;266:7363–7367. [PubMed] [Google Scholar]
- 65.Mendrick DL, Kelly DM. Temporal expression of VLA-2 and modulation of its ligand specificity by rat glomerular epithelial cells in vitro. Lab Invest. 1993;69:690–702. [PubMed] [Google Scholar]
- 66.Mendrick DL, Kelly DM, duMont SS, Sandstrom DJ. Glomerular epithelial and mesangial cells differentially modulate the binding specificities of VLA-1 and VLA-2. Lab Invest. 1995;72:367–375. [PubMed] [Google Scholar]
- 67.Luque A, Gómez M, Puzon W, Takada Y, Sánchez-Madrid F, et al. Activated conformations of very late activation integrins detected by a group of antibodies (HUTS) specific for a novel regulatory region (355-425) of the common beta 1 chain. J Biol Chem. 1996;271:11067–11075. doi: 10.1074/jbc.271.19.11067. [DOI] [PubMed] [Google Scholar]
- 68.Mould AP, Akiyama SK, Humphries MJ. The inhibitory anti-beta1 integrin monoclonal antibody 13 recognizes an epitope that is attenuated by ligand occupancy. Evidence for allosteric inhibition of integrin function. J Biol Chem. 1996;271:20365–20374. doi: 10.1074/jbc.271.34.20365. [DOI] [PubMed] [Google Scholar]
- 69.Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–25577. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- 70.Hall DE, Reichardt LF, Crowley E, Holley B, Moezzi H, et al. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. The Journal of Cell Biology. 1990;110:2175–2184. doi: 10.1083/jcb.110.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y, Chen BP, Lu M, Zhu Y, Stemerman MB, et al. Shear stress activation of SREBP1 in endothelial cells is mediated by integrins. Arteriosclerosis, Thrombosis, and Vascular Biology. 2002;22:76–81. doi: 10.1161/hq0102.101822. [DOI] [PubMed] [Google Scholar]
- 72.Yu W, Datta A, Leroy P, O'Brien LE, Mak G, et al. Beta1-integrin orients epithelial polarity via Rac1 and laminin. Molecular Biology of the Cell. 2005;16:433–445. doi: 10.1091/mbc.E04-05-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Masur SK, Idris A, Michelson K, Antohi S, Zhu LX, et al. Integrin-dependent tyrosine phosphorylation in corneal fibroblasts. Invest Ophthalmol Vis Sci. 1995;36:1837–1846. [PubMed] [Google Scholar]
- 74.Pear WS, Nolan GP, Scott ML, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kahn ML, Lee SW, Dichek DA. Optimization of retroviral vector-mediated gene transfer into endothelial cells in vitro. Circulation Research. 1992;71:1508–1517. doi: 10.1161/01.res.71.6.1508. [DOI] [PubMed] [Google Scholar]
- 76.Dumin JA, Dickeson SK, Stricker TP, Bhattacharyya-Pakrasi M, Roby JD, et al. Pro-collagenase-1 (Matrix Metalloproteinase-1) Binds the alpha 2beta 1 Integrin upon Release from Keratinocytes Migrating on Type I Collagen. J Biol Chem. 2001;276:29368–29374. doi: 10.1074/jbc.M104179200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wound closure of wild-type and MMP7−/− ALI cultures. Wild-type and MMP7−/− ALI cultures were injured and wound closure was observed over 24 hours.
(21.22 MB MOV)