Abstract
Purpose
To evaluate the impact of subtle progesterone (P4) rise on the day of HCG on pregnancy outcome in ICSI patients stimulated with long agonist protocol.
Methods
One hundred forty-nine consecutive controlled ovarian hyperstimulation cycles for ICSI using long luteal agonist protocol.
Results
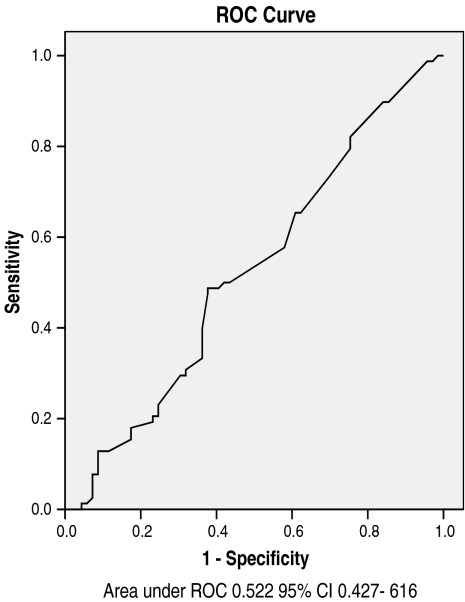
Mean serum progesterone on day of hCG was 0.88±0.51 ng/mL values ≥1 ng/mL were found in 34.2% of cycles. Serum E2 on day of hCG and number of oocytes retrieved were significantly higher in the group with P4 ≥ 1 ng/mL. The area under ROC for serum progesterone in prediction of pregnancy was 0.52, indicating that within the values studied, serum progesterone on day of hCG is not predictive of pregnancy outcome.
Conclusion
P4 values ≥1 ng/mL on day of hCG are common in long agonist ICSI cycles particularly with high response. Within the P4 values encountered in this study, implantation and pregnancy rates are not adversely affected.
Keywords: Pre-HCG, Progesterone, Long-agonist, ICSI, Pregnancy
Introduction
Prior to the introduction of gonadotropin releasing hormone agonists (GnRHa), premature luteinization [1] was common, affecting up to 20% of controlled ovarian hyperstimulation cycles [2]. In non-agonist protocols, premature luteinization is common in cases with lower ovarian reserve [3, 4] where LH rise occurs despite lower response to stimulation and lower estradiol values. A deficiency of the so called gonadotropin surge attenuating factor (Gn-SAF) has been incriminated [5].
Nowadays when agonist and antagonist protocols are in routine use, genuine premature luteinization is rarely encountered. The term, however, despite being a misnomer, is still used to indicate the appreciable rise in serum progesterone which sometimes occurs before hCG administration [6–8]. Premature progesterone rise (PPR), a more precise term, is defined as serum progesterone ≥1.0 ng/mL (about 3.2 nmol/L) or P4/E2 ratio ≥ 1 on the day of hCG administration [9, 10]. Such premature rise in serum progesterone was found to correlate with relatively high ovarian response to stimulation as indicated by large number of oocytes and high estradiol levels [11]. The reported incidence of PPR varies between 5–30% of long agonist cycles [12, 13]. A pituitary escape from the suppressive effect of GnRHa is unlikely because LH concentrations are invariably low. Exposure to large amounts of exogenous gonadotropins [14], increased LH sensitivity of granulosa cells, and pooled contribution of numerous mature follicles were suggested as causes for this PPR [15, 16].
The debate whether this subtle PPR negatively affects ICSI cycle outcome is still ongoing.
Subjects and methods
One hundred and forty nine consecutive cycles of controlled ovarian hyperstimulation (COH) for ICSI because of male factor infertility were included. Criteria for inclusion were female age <37 years, regularly menstruating, and basal FSH <10 mIU/mL. Long luteal agonist protocol was used using Buserline acetate. Recombinant FSH injections in a fixed dose of 225 IU/day (Gonal-F 75 IU; Serono, Italy) were started once down regulation is confirmed (serum E2 <50 pg/mL). Ovarian response was monitored with vaginal ultrasonography as well as serum estradiol. Criteria for hCG were at least three follicles ≥18 mm in size with a serum E2 level of >150 pg/mL per follicle. Serum estradiol (E2) and progesterone (P) levels were assessed on the day of hCG administration. Oocyte retrieval (OR) was carried out 34–36 h after hCG using single lumen needle and automatic aspiration system. Cumulus removal was carried out after 2 h of pre-incubation by repeated pipetting in hyaluronidase (20 IU/mL), then corona was removed by repeated pipetting into 130 μ strippers. Semen was prepared by density gradient centrifugation and regular ICSI was carried out on metaphase II oocytes thereafter. Fertilization was assessed 16–18 h after ICSI and was confirmed by the presence of two pronuclei. Ultrasound-guided embryo transfer (ET) was done 52 h after OR. Class A embryos were those with equal blastomeres and less than 10% fragmentation. Vaginal micronized progesterone 800 mg/day was used for luteal support. Statistical analysis was done using SPSS software, version 14.0.
Results
Among the 149 included cycles, serum progesterone (P4) on day of hCG ranged between 0.08 and 2.9 (0.88 ± 0.51). P4 level was <1 ng/mL in 98 cycles (65.8%) while values ≥1 ng/mL were found in 51 cycles (34.2%) among them values ≥2 ng/mL were found only in five cases (3%) within which one pregnancy occurred. Cycles were classified using P4 values ≥1 as cut-off into two groups. The group with P4 ≥ 1was considered to have progesterone rise. Age, stimulation days, number of ampoules, number of embryos transferred, implantation rates and pregnancy rates were not different between the two groups. Serum E2 on day of hCG, and number of oocytes retrieved, however, were significantly higher in the group with P4 ≥ 1 ng/mL. (P values 0.019 and 0.042 respectively) (Table 1). The area under ROC for serum progesterone in prediction of pregnancy was 0.52, 95% confidence interval 0.427 and 0.616 indicating that within the values studied, serum progesterone on day of hCG is not predictive of pregnancy outcome.(Fig. 1)
Table 1.
Clinical and laboratory data classified by serum progesterone on day of HCG
| Clinical data | P4 < 1 | P4 ≥ 1 | P value |
|---|---|---|---|
| Age | 31.24 ± 4.365 | 30.02 ± 4.19 | 0.180 |
| Stimulation days | 11.9±1.7 | 11.7±1.1 | 0.193 |
| No. of ampoules | 47.6±12.6 | 44.5±11.8 | 0.073 |
| E2 on day of hCG pg/mL | 2,240±1,062 | 2,777±1,366 | 0.019* |
| No. of follicles | 12.3±4.3 | 13.4±4.6 | 0.077 |
| No. of oocytes | 12.1±4.15 | 13.63±4.7 | 0.042* |
| % of mature oocytes | 89.6±15.6 | 92.1±8.8 | 0.09 |
| Fertilization rate | 77.9±16.2 | 76.6±19.7 | 0.34 |
| Cleavage rate | 80.1±23.5 | 79.1±22.4 | 0.31 |
| Embryos transferred | 2.0±0.7 | 1.96±0.8 | 0.71 |
| Implantation rate | 25.2±31.1 | 24.6±32.9 | 0.41 |
| Clinical pregnancy rate | 44% | 47% | 0.63 |
*Significant p < 0.05
Fig. 1.
Discussion
The subject is not new but the issue is still unresolved. Schoolcraft et al [8] started the debate in 1991 when they reported that pregnancy rate is adversely affected by this progesterone rise. Shortly after this report Silverberg and colleagues [17], and Mio et al [18] reported similar results. Contemporary to these early studies, several other reports fueled the debate by refuting the finding and denying any effect of PPR on cycle outcome [19–21].
Because embryo quality was found to be improved rather than impaired in these cases, as tested in donor oocytes [22], and frozen-thawed ET cycles [23], impaired endometrial receptivity was suspected as a primary cause of decreased pregnancy rates in these cycles.
In this study, serum levels of progesterone on day of hCG were ≥1 ng/mL in 51 cycles (34.2%), while values ≥2 ng/mL were found only in five cases (3%), among which one pregnancy occurred. According to most of the literature, serum progesterone ≥1.0 ng/mL (about 3.2 nmol/L) or P4/E2 ratio ≥ 1 on the day of hCG is defined as premature progesterone rise [9, 10]. Therefore, cycles in this study were classified using P4 values ≥1 ng/mL as cut-off into two groups, one with PPR defined as values ≥1 ng/mL, and one with values <1 ng/mL. Age, stimulation days, number of ampoules, number of embryos transferred, implantation rates and pregnancy rates, were not different between the two groups. Serum E2 on day of hCG, and number of oocytes retrieved however, were significantly higher in the group with P4 ≥ 1. The area under the receiver operating characteristic (ROC) curve was calculated to assess the predictive value of the progesterone for the probability of pregnancy. The area under the ROC curve was 0.522 [95% confidence interval (CI) 0.427–0.616] which favors the null hypothesis meaning that progesterone values are not predictive of pregnancy. Indeed, data are still conflicting regarding the issue; while Li et al [24] in 2008 reported a negative association between serum progesterone levels ≥3.97 nmol/L and pregnancy rate, a recent meta-analysis in 2007 [25] considered that the best available evidence does not support any association between progesterone elevation on the day of hCG administration and the probability of clinical pregnancy in women undergoing ovarian stimulation with GnRH analogues and gonadotrophins for IVF. An even more confusing report was that of Papanikolaou et al [26] in which progesterone rise on the day of hCG was found to impair pregnancy outcome in day 3 single embryo transfer, while having no effect on day 5 single blastocyst transfer. In a recent trial to explain and evaluate PPR done by Nikolettos et al [27], patients with serum progesterone above 0.9 ng/mL on day of hCG had elevated serum concentrations of IL-6, VEGF, and bFGF, as well as elevated intrafollicular concentrations of IL-6. They suggested that these cytokines might influence certain enzymes in steroidogenic pathway. In their report, the outcome of ICSI cycles was not associated with premature elevation of progesterone when the cut-off value is set at 0.9 ng/mL.
We believe that using different methodologies for hormone measurement, different progesterone cut-off levels in different patient populations with different stimulation protocols and different responses. The issue would remain unresolved. We also agree with de-Ziegler in his letter [28] that until proven otherwise, the clinical consequence of pre-hCG progesterone elevation should be analyzed within the context of the ovarian response to COH in which it is encountered. Only when pre-hCG progesterone elevation is observed in case of low response to COH, poor prognosis might be predicted.
Acknowledgement
We acknowledge the participation of all members of Miami IVF center in Alexandria in monitoring and follow up of subjects of the study. We also extend our gratitude to our patients who agreed to participate in the study and signed the required consent.
Contribution to authorship Design of the study, acquisition of data, analysis of data, reviewing and finalizing the manuscript: Hisham Ali Saleh. Participation in acquisition and analysis of data, drafting and editing the manuscript: Mervat sheikh Elarab. Contribution to collection and analysis of data Mohamed Draz. All authors have approved the final version of manuscript.
Details of ethics approval The trial was performed in accordance with the declaration of Helsinki, and subsequent revisions, and approved by the ethical institutional review board. Written informed consent was obtained from all included cases before entering in study.
Funding and disclosure of interest The study did not receive any funding from any organization or pharmaceutical company, and no author of this study has conflict of interest.
Footnotes
Capsule
Pre-hCG, subtle progesterone rise is common in high response long agonist ICSI cycles, and is not associated with lower pregnancy rate.
References
- 1.Wildt L, Diedrich K, van der Ven H, et al. Ovarian hyperstimulation for I n-vitro fertilization controlled by GnRH agonist administered in combination with human menopausal gonadotropins. Hum Reprod. 1986;1:15–9. [DOI] [PubMed]
- 2.de Ziegler D, Cedars MI, Randle D, et al. Suppression of the ovary using gonadotropin releasing hormone agonist prior to stimulation for oocyte retrieval. Fertil Steril. 1987;48:807–10. [DOI] [PubMed]
- 3.Hoff JD, Quingley ME, Yen SSC. Hormonal dynamics at midcycle: a re-evaluation. J Clin Endocrinol Metab. 1983;57:792–6. [DOI] [PubMed]
- 4.Younis JS, Matilsky M, Radin O, Ben-Ami M. Increased progesterone estradiol ratio in late follicular phase could be related to low ovarian reserve in in-vitro fertilization/embryo transfer cycles with along gonadotropin releasing hormone agonist. Fertil Steril. 2001;76(2):294–9. doi:10.1016/S0015-0282(01)01918-5. [DOI] [PubMed]
- 5.Younis JS, Haddad S, Matilsky M, Ben-Ami M. Premature luteinization: could it be an early manifestation of low ovarian reserve. Fertil Steril. 1998;69(3):461–5. doi:10.1016/S0015-0282(97)00561-X. [DOI] [PubMed]
- 6.Martinez F, Barri PN, Coroleu B, Tur R, Sorsa-Leslie T, Harris WJ, et al. Women with poor response to IVF have lowered circulating gonadotropin surge attenuating factor (Gn-SAF) bioactivity during spontaneous and stimulated cycles. Hum Reprod. 2002;17(3):634–40. doi:10.1093/humrep/17.3.634. [DOI] [PubMed]
- 7.Sharma V, Ryder T, Whitehead M, et al. Influence of superovulation on endometrial and embryonic development. Fertil Steril. 1990;53:822–8. [DOI] [PubMed]
- 8.Schoolcraft W, Huynh D, Sinton E, Hamilton F, Schlenker T, Meldrum DR. Lower pregnancy rate with premature luteinization during pituitary suppression with leuprolide acetate. Fertil Steril. 1991;55:563–6. [PubMed]
- 9.Eldstein MC, Seltman HJ, Cox BJ, et al. Progesterone level on the day of human chorionic gonadotropin administration in cycles with gonadotropin releasing hormone agonist suppression are not predictive of pregnancy outcome. Fertil Steril. 1990;54:853–7. [DOI] [PubMed]
- 10.Kably Ambe A, Medez Epinosa G, Barron Vallejo J. A new parameter for the evaluation of premature luteinization: the pre-ovulatory molecular relation between serum concentrations of progesterone and estradiol. Ginecol Obstet Mex. 1998;66:208–13. [PubMed]
- 11.Simon C, Cano F, Valbuena D, Remohi J, Pellicer A. Clinical evidence for a detrimental effect on uterine receptivity of high serum estradiol concentrations in high and normal responders. Hum Reprod. 1995;10:2432–7. [DOI] [PubMed]
- 12.Hugues JN, Durnerin IC. Revisiting gonadotropin releasing hormone agonist protocols and management of poor ovarian responses to gonadotropins. Hum Reprod Update. 1998;4(1):83–101. doi:10.1093/humupd/4.1.83. [DOI] [PubMed]
- 13.Hazout A, deZiegler D, Cornel C. Comparison of short 7 day and prolonged treatment with gonadotropin releasing hormone agonist desensitization for controlled ovarian hyperstimulation. Fertil Steril. 1993;59:596–600. [DOI] [PubMed]
- 14.Fanchin R, de Ziegler D, Castracane VD, et al. Pathophysiology of premature progesterone elevation. Fertil Steril. 1995;64:796–801. [DOI] [PubMed]
- 15.Ubaldi F, Bennink HC, van Steeirteghem A, Smitz J, Devroey P. Premature luteinization in in-vitro fertilization cycles using gonadotropin releasing hormone agonist (GnRH-a) and recombinant follicle stimulating hormone (FSH), and GnRH-a and urinary FSH. Fertil Steril. 1996;66:275–80. [DOI] [PubMed]
- 16.Ubaldi F, Albano C, Peukert M, et al. Subtle progesterone rise after administration of the gonadotropin releasing hormone antagonist Cetrorelix in intra-cytoplasmic sperm injection cycles. Hum Reprod. 1996;11:1405–7. [DOI] [PubMed]
- 17.Silverberg KM, Burns WN, Olive DL, Riehl RM, Shenken RS. Serum progesterone levels predict success of the IVF/ET in patients stimulated with leuprolide acetate and human menopausal gonadotropins. J Clin Endocrinol Metab. 1991;73:797–803. [DOI] [PubMed]
- 18.Mio Y, Onahara Y, Sekijima A, Harada T, Iwabe T, Terakawa N. Subtle rise in serum progesterone during the follicular phase as a predictor of the outcome of in-vitro fertilization. Fertil Steril. 1992;58:159–65. [DOI] [PubMed]
- 19.Abuzeid MI, Sasy MA. Elevated progesterone levels in the late follicular phase do not predict success of in-vitro fertilization embryo transfer. Fertil Steril. 1996;65:981–5. [DOI] [PubMed]
- 20.Givens CR, Shirock ED, Dendekar PV, Martin MC. Elevated serum progesterone levels on the day of human chorionic gonadotropin administration do not predict outcome in assisted reproduction cycles. Fertil Steril. 1994;62:1011–7. [DOI] [PubMed]
- 21.Bustillo M, Stern JJ, Coulam CB. Serum progesterone at the time of human chorionic gonadotropin administration does not predict pregnancy in in-vitro fertilization and embryo transfer. Hum Reprod. 1995;10:2862–7. [DOI] [PubMed]
- 22.Legro RS, Ary BA, Paulson RJ, Stanczyk FZ, Sauer MV. Premature luteinization as detected by serum progesterone is associated with higher pregnancy rate in donor oocyte in vitro fertilization. Hum Reprod. 1993;8:1506–11. [DOI] [PubMed]
- 23.Silverberg KM, Burns WN, Martin M, Shenken RS, Olive DL. Elevated serum progesterone level on the day of hCG administration in in-vitro fertilization cycles do not adversely affect embryo quality. Fertil Steril. 1994;61:508–13. RB. [DOI] [PubMed]
- 24.Li R, Qiao J, Wang L, Zhen X, Lu Y. Serum progesterone concentration on day of HCG administration and IVF outcome. Reprod Biomed Online. 2008;16:5627–31. [DOI] [PubMed]
- 25.Venetis CA, Kolibianakis EM, Papanikolaou E, Bontis J, Devroey P, Tarlatzis BC. Is progesterone elevation on the day of human chorionic gonadotrophin administration associated with the probability of pregnancy in in vitro fertilization? A systematic review and meta-analysis. Hum Reprod Update. 2007;13(4):343–55. doi:10.1093/humupd/dmm007. [DOI] [PubMed]
- 26.Papanikolaou EG, Kolibianakis EM, Pozzobon C, Tank P, Tournaye H, Bourgain C, Van Steirteghem A, Devroey P. Progesterone rise on the day of human chorionic gonadotropin administration impairs pregnancy outcome in day 3 single-embryo transfer, while has no effect on day 5 single blastocyst transfer. Fertil Steril. 2007 Jun 5. [DOI] [PubMed]
- 27.Nikolettos N, Asimakopoulos B, Köster F, Schöpper B, Schulz C, Caglar GS, et al. Cytokine profile in cases with premature elevation of progesterone serum concentrations during ovarian stimulation. Physiol Res. 2008;57(2):215–24. [DOI] [PubMed]
- 28.de Ziegler D, Bijaoui G, Chapron C. Pre-hCG elevation of plasma progesterone: good, bad or otherwise. Hum Reprod Update. 2008;14(4):393. doi:10.1093/humupd/dmn020. [DOI] [PubMed]