Abstract
Diversity is one major factor driving plant productivity in temperate grasslands. Although decomposers like earthworms are known to affect plant productivity, interacting effects of plant diversity and earthworms on plant productivity have been neglected in field studies. We investigated in the field the effects of earthworms on plant productivity, their interaction with plant species and functional group richness, and their effects on belowground plant competition. In the framework of the Jena Experiment we determined plant community productivity (in 2004 and 2007) and performance of two phytometer plant species [Centaurea jacea (herb) and Lolium perenne (grass); in 2007 and 2008] in a plant species (from one to 16) and functional group richness gradient (from one to four). We sampled earthworm subplots and subplots with decreased earthworm density and reduced aboveground competition of phytometer plants by removing the shoot biomass of the resident plant community. Earthworms increased total plant community productivity (+11%), legume shoot biomass (+35%) and shoot biomass of the phytometer C. jacea (+21%). Further, phytometer performance decreased, i.e. belowground competition increased, with increasing plant species and functional group richness. Although single plant functional groups benefited from higher earthworm numbers, the effects did not vary with plant species and functional group richness. The present study indicates that earthworms indeed affect the productivity of semi-natural grasslands irrespective of the diversity of the plant community. Belowground competition increased with increasing plant species diversity. However, belowground competition was modified by earthworms as reflected by increased productivity of the phytometer C. jacea. Moreover, particularly legumes benefited from earthworm presence. Considering also previous studies, we suggest that earthworms and legumes form a loose mutualistic relationship affecting essential ecosystem functions in temperate grasslands, in particular decomposition and plant productivity. Further, earthworms likely alter competitive interactions among plants and the structure of plant communities by beneficially affecting certain plant functional groups.
Keywords: Aboveground–belowground interactions, Diversity–productivity relationship, Grassland, Positive interactions
Introduction
The relationship between plant diversity and productivity as an essential ecosystem function has been a focus of contemporary ecological studies (reviewed in Loreau et al.2002; Hooper et al. 2005; Balvanera et al. 2006; Cardinale et al. 2006). Two mechanisms responsible for positive diversity–productivity relationships are distinguished. Sampling or selection effects are due to the increased probability of presence of highly productive species at high diversity levels (Huston 1997; Wardle 1999), whereas complementarity effects are due to tradeoffs in species’ efficiency in using different resources, in colonization and competitive abilities, or in their fitness under different environmental conditions (Loreau 2000; Loreau and Hector 2001). However, following the resource-based competition theory (Tilman et al. 1997) and the niche dimension theory (Harpole and Tilman 2007), the positive diversity–productivity relationship has mostly been attributed to complementary resource use, particularly in long-term experiments (Cardinale et al. 2007).
Although decomposers are known to profoundly impact nutrient cycling and, thereby, resource availability for plants (Scheu 2003; Brown et al. 2004; Wardle et al. 2004), the role of decomposers for the diversity–productivity relationship has been largely neglected in previous biodiversity experiments. Earthworms are a major component of the decomposer fauna in many terrestrial ecosystems (Lee 1985; Edwards and Bohlen 1996). Through burrowing, casting and mixing of litter and soil (bioturbation) they influence physical and chemical characteristics of the topsoil (Edwards and Bohlen 1996; Tiunov and Scheu 1999; Eisenhauer et al. 2007) with important consequences for plant growth and competitive interactions among plant species and plant functional groups (Wurst et al. 2005; Partsch et al. 2006; Eisenhauer and Scheu 2008a). Since belowground plant competition is an essential factor structuring plant communities (Cahill 1999; Weigelt et al. 2007), it is suggested that earthworms drive plant competition and plant community assembly (Kreuzer et al. 2004; Wurst et al. 2005; Eisenhauer and Scheu 2008a).
In order to study the impacts of earthworms on plant productivity and belowground competition in a plant diversity gradient, we measured aboveground biomass production (in 2004 and 2007) and the performance of two phytometer species [Centaurea jacea (herb) and Lolium perenne (grass); in 2007 and 2008] in earthworm subplots and in subplots with reduced earthworm density in the framework of the Jena Experiment. We hypothesized that: (1) earthworms enhance plant productivity, (2) belowground plant competition increases with plant diversity, and (3) earthworms alter belowground plant competition.
Materials and methods
Experimental setup
The study was conducted in the framework of the Jena Experiment, a large field experiment investigating the role of biodiversity (plant species and functional group richness) for element cycling and trophic interactions in grassland plant communities (Fig. 1a; Roscher et al. 2004). The study site is located on the floodplain of the Saale river at the northern edge of Jena (Thuringia, Germany). Mean annual air temperature 3 km south of the field site is 9.3°C and annual precipitation is 587 mm (Kluge and Müller-Westermeier 2000). The site had been used as an arable field for the last 40 years and the soil is an Eutric Fluvisol (FAO-Unesco 1997). The experiment was established in May 2002 and the studied system represents Central European mesophilic grassland traditionally used as hay meadow (Arrhenatherion community). A pool of 60 native plant species was used to establish a gradient of plant species (1, 2, 4, 8, 16, and 60) and plant functional group richness (one, two, three, and four) in a total of 82 plots of 20 × 20 m (Table 1; Roscher et al. 2004). Plant species were aggregated into four plant functional groups: grasses (16 species), small herbs (12 species), tall herbs (20 species), and legumes (12 species; Roscher et al. 2004). Experimental plots were mown twice a year (in June and September), as is typical for hay meadows, and weeded twice a year (in April and July) to maintain the target species composition. Plots were assembled into four blocks following a gradient in soil characteristics, each block containing an equal number of plots of all plant species and plant functional group richness levels. Further information on the design and setup of the Jena Experiment is given in Roscher et al. (2004).
Fig. 1.
a Photograph of the field site of the Jena Experiment taken in 2007 showing the main experimental plots (20 × 20 m) varying in plant species richness (1, 2, 4, 8, 16, and 60) and plant functional group richness (one, two, three, and four). The field site is located on the floodplain of the Saale river at the northern edge of Jena (Thuringia, Germany; background). Photograph by A. Weigelt. b Photograph of one exemplary earthworm plot (2 × 4 m) showing the subplots for earthworm density manipulations (1 × 1 m; earthworm and earthworm reduction), and the four octet devices used for the earthworm extraction by electro-shocking. Photograph by N. Eisenhauer. c Photograph of one earthworm subplot showing the planting pattern for phytometer plants (Lolium perenne and Centaurea jacea; five individuals per species and subplot), the removed shoot material of the resident plant community within a radius of 10 cm around the phytometer individuals to reduce aboveground plant competition. Black arrows indicate a distance of 10 cm. Photograph by N. Eisenhauer. d Photograph of the phytometer plants (L. perenne, 1–5; C. jacea, α-ε) of one exemplary earthworm subplot in late April 2008. Photograph by N. Eisenhauer
Table 1.
The design of the Jena Experiment
| Plant species richness | Plots | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 8 | 16 | 60 | |||
| Plant functional group richness | 1 | 16 | 8 | 4 | 4 | 2 | – | 30(22) |
| 2 | – | 8 | 4 | 4 | 4 | – | 20(8) | |
| 3 | – | – | 4 | 4 | 4 | – | 12(8) | |
| 4 | – | – | 4 | 4 | 4 | 4 | 16(8) | |
| Plots | 16 | 16 | 16 | 16 | 14 | 4 | 82 (46) | |
Combinations of plant species richness and plant functional group richness levels and the number of plots (given in italics) per diversity level. Earthworm subplots were established on plots with 1, 4 and 16 plant species (n = 46, given in bold). For more details on the experimental design see Roscher et al. (2004). Both phytometer species (five individuals each) were planted into all 92 subplots of the 46 plots of the earthworm experiment after establishment of the plant mixtures
Earthworm densities were manipulated on the one (16 replicates), four (16 replicates) and 16 plant species richness plots (14 replicates) starting in September 2003 (Table 1). On each plot, two randomly selected subplots of 1 × 1 m were used to establish the following treatments: earthworm and earthworm reduction (Fig. 1b). Subplots were enclosed with PVC shields aboveground (20 cm) and belowground (15 cm) to reduce the escape or colonization of earthworms. Earthworm subplots (+ew) received 25 adult individuals of L. terrestris (average fresh weight with gut content 4.10 ± 0.61 g) per year (15 individuals in spring and ten in autumn). The earthworm addition treatment was established since the earthworm density was low after establishment of the Jena Experiment which involved repeated disk cultivation to reduce weed density, a practice which is known to detrimentally affect earthworms (Edwards and Bohlen 1996). Further, two earthworm extraction campaigns were performed per year (spring and autumn) on the adjacent earthworm reduction subplots (−ew) by electro-shocking (Fig. 1b). A combination of four octet devices (DEKA 4000; Deka Gerätebau, Marsberg, Germany) was used (Thielemann 1986). In each subplot, earthworm extraction was performed for 35 min, by increasing the voltage from 250 V (10 min) to 300 V (5 min), 400 V (5 min), 500 V (5 min), and 600 V (10 min). Extracted earthworms were identified, counted and weighed in the laboratory (data not shown).
The success of earthworm density manipulations was measured via the soil surface activity of L. terrestris which is known to bury plant seeds irrespective of seed size and shape (Milcu et al. 2006; Eisenhauer and Scheu 2008b). In May 2006, we performed a seed dummy experiment to determine earthworm soil surface activity (Eisenhauer et al. 2008). While earthworm soil surface activity in earthworm subplots did not differ from that in control subplots (not considered in the present study), it was decreased considerably in earthworm reduction subplots (−38%; Eisenhauer et al. 2008).
Plant community biomass
Plant community biomass was harvested from earthworm subplots using two metal frames of 20 × 50 cm (∑ 0.2 m²) cutting shoots 3 cm above the soil surface in May 2004 and 2007, i.e. 1 and 3 years after the establishment of the treatments. In 2007, plant shoot biomass was separated for the plant functional groups grasses, herbs, and legumes, dried (5 days, 80°C) and weighed. Plant community biomass was calculated as g dry weight/m².
Phytometers
We used L. perenne (grass) and C. jacea (herb) as phytometer (model) species in order to investigate earthworm and belowground competition effects on plant performance in a standardized way. These two plant species were chosen since they were well studied in previous greenhouse experiments (Eisenhauer and Scheu 2008a, b; Sabais A, unpublished data). Plant seeds were obtained from Rieger-Hofmann (Blaufelden-Raboldshausen, Germany). In late February 2007, seeds of both phytometer species were germinated in soil from the field site of the Jena Experiment in a temperature-controlled greenhouse with day/night cycles of 16/8 h (20/18°C). After 3 weeks, individual seedlings (height 3–5 cm) were transplanted into separate plant pots (diameter 5 cm, height 5 cm) and hardened for another 4 weeks in the botanical garden in Darmstadt (Hesse, Germany). In late April 2007, five phytometer plants (height 5–10 cm, n = 460) per species were transplanted with the soil of the plant pots into the earthworm subplots 10 cm apart from each other (Fig. 1c). Further, plant shoot biomass of the resident plant community was cut 3 cm above soil surface level within a radius of 10 cm to reduce the competition for light (aboveground competition) and improve phytometer establishment. Removal of the shoot biomass of the resident plant community was repeated every 2 months.
In late August 2007 (after 4 months) and in late April 2008 (after 12 months), the phytometers (Fig. 1d) were harvested by cutting shoot material 3 cm above the soil surface, dried (3 days, 80°C) and weighed. The number of surviving phytometer plants was determined (% of initially transplanted) and the individual phytometer biomass per plant species was calculated (g dry weight/individual). Further, the height [L. perenne and C. jacea, (cm)] and number of flower heads (C. jacea) of phytometer plants were determined in 2007. The height of the phytometers was determined by measuring the perpendicular distance from the soil at the base to the highest point reached with all parts in the natural position by the highest plant individual using a ruler (cm; Heady 1957).
Statistical analyses
Normal distribution and homogeneity of variance were improved by log-transformation. Split plot ANOVA (GLM, type I sum of squares) was used to analyse the effects of block (BL), plant species richness (SR), plant functional group richness (FR), and presence of grasses (GR), small herbs (SH), tall herbs (TH), legumes (LE), and earthworms (EW; earthworm and earthworm reduction), EW × SR, EW × FR, time (TI; community biomass, 2004 and 2007; phytometers, 2007 and 2008), TI × SR, TI × FR, TI × EW, TI × Plot, TI × EW × SR, and TI × EW × SR on total shoot biomass of the plant community, survival of phytometer plants (L. perenne and C. jacea), and on the shoot biomass per phytometer individual (L. perenne and C. jacea) in a hierarchical order. Earthworm treatments were represented by subplots and sampling times by sub-subplots in the split plot ANOVA (Scheiner and Gurevitch 2001). Since treatment effects did not change with time, interactions with time are not shown. Further, split plot ANOVA was used to analyse the effects of BL, SR, FR, GR, SH, TH, LE, and EW on the shoot biomass of grasses (2007), herbs (2007), and legumes (2007), on the height of the phytometer plants (2007; L. perenne and C. jacea), and on the number of flower heads per C. jacea individual (2007).
F-values given in the text refer to those where the respective factor (and interaction) was fitted first (Schmid et al. 2002). BL was always fitted first, followed by SR and FR. Then, the effects of presence of certain plant functional groups were calculated followed by Plot, EW, EW × SR, EW × FR, Subplot, and TI with the respective interactions. Treatments analysed at the plot scale (BL, SR, FR, GR, SH, TH, and LE) were tested against the variance between plots to avoid pseudoreplication, whereas treatments analysed on the subplot scale (EW, EW × SR, and EW × FR) were tested against the variance between subplots (Scheiner and Gurevitch 2001). While TI, TI × SR, and TI × FR were tested against the variance of TI × Plot, TI × EW × SR and TI × EW × SR were tested against the total variance (TI × Subplot).
Moreover, split plot analysis of covariance (ANCOVA) was used to analyse the effects BL, SR, FR, GR, SH, TH, LE, EW, and the covariate total shoot biomass of the plant community in 2007 (cBM) on the survival and shoot biomass of the phytometer L. perenne and on the shoot biomass and number of flower heads of the phytometer C. jacea (all 2007). This was done to eliminate the variance of aboveground competition from the analysis since removal of the resident plant community (to reduce aboveground plant competition) may not have been completely successful.
ANOVA and comparisons of means (Tukey’s HSD test, α < 0.05) were performed using SAS 9.1 (SAS Institute, Cary, N.C.). To identify associations between total shoot biomass of the plant community and the survival and shoot biomass of the phytometer L. perenne and the shoot biomass and number of flower heads of the phytometer C. jacea, respectively, correlations were carried out using STATISTICA 7.1 (StatSoft, Tulsa, Okla.). Means presented in text and figures represent back-transformed means of the log-transformed data.
Results
Plant community
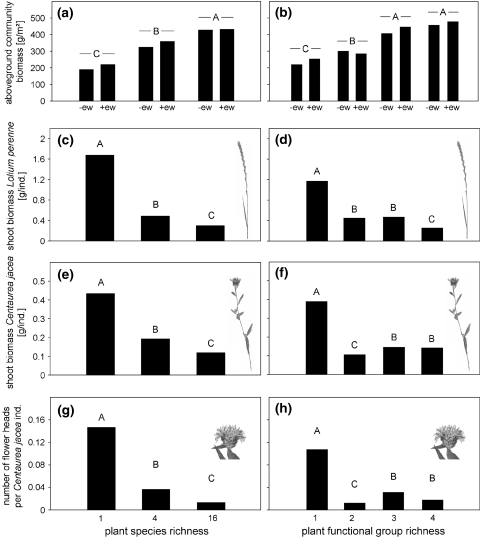
On average, 284.7 g/m² total shoot biomass was harvested in May 2004 and 331.1 g/m² in May 2007. Neither shoot biomass differed between 2004 and 2007 nor were there significant interactions between sampling time and any of the treatments (not shown). Total shoot biomass increased significantly with increasing plant species and functional group richness (Table 2; Fig. 2a, b). Further, total shoot biomass increased significantly in the presence of small herbs (+11%) and legumes (+77%). Moreover, total shoot biomass was increased in earthworm subplots compared to subplots with reduced earthworm density (+11%). The earthworm effect did not depend on plant species and functional group richness, and therefore these interactions were excluded from the statistical model (Table 2; Fig. 2a, b).
Table 2.
ANOVA table of F- and P-values on the effects of block (BL), plant species richness (SR), plant functional group richness (FR), presence/absence of grasses (GR), small herbs (SH), tall herbs (TH) and legumes (LE), and earthworms (EW) on the shoot biomass of grasses, herbs, legumes, and total community biomass
| Plant community biomass | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grasses (g/m²) | Herbs (g/m²) | Legumes (g/m²) | Total biomass (g/m²) | |||||||||
| df | F | P | df | F | P | df | F | P | df | F | P | |
| BL | 3 | 2.82 | 0.0804 | 3 | 0.64 | 0.5947 | 3 | 1.14 | 0.3741 | 3 | 0.30 | 0.8233 |
| SR | 2 | 2.89 | 0.0915 | 2 | 2.48 | 0.1062 | 2 | 2.17 | 0.1606 | 2 | 16.19 | <0.0001a |
| FR | 3 | 4.15 | 0.0288b | 3 | 1.14 | 0.3549 | 3 | 0.90 | 0.4716 | 3 | 10.17 | <0.0001a |
| GR | 1 | nc | nc | 1 | 1.68 | 0.2083 | 1 | 0.27 | 0.6151 | 1 | 0.33 | 0.5701 |
| SH | 1 | 0.00 | 0.9620 | 1 | nc | nc | 1 | 0.59 | 0.4583 | 1 | 10.47 | 0.0029a |
| TH | 1 | 0.78 | 0.3917 | 1 | nc | nc | 1 | 0.11 | 0.7434 | 1 | 0.12 | 0.7302 |
| LE | 1 | 0.83 | 0.3799 | 1 | 0.49 | 0.4910 | 1 | nc | nc | 1 | 7.69 | 0.0093a |
| EW | 1 | 0.95 | 0.3426 | 1 | 0.07 | 0.7947 | 1 | 4.85 | 0.0426a | 1 | 3.78 | 0.0586a |
| EW × SR | 2 | 0.13 | 0.8797 | 2 | 1.26 | 0.2981 | 2 | 1.09 | 0.3584 | 2 | excl | excl |
| EW × FR | 3 | 0.40 | 0.7537 | 3 | 1.50 | 0.2372 | 3 | 0.30 | 0.8272 | 3 | excl | excl |
| Plot | 13 | 2.99 | 0.0165 | 23 | 5.10 | <0.0001 | 11 | 13.48 | <0.0001 | 31 | 4.40 | <0.0001 |
| Subplot | – | nc | nc | – | nc | nc | – | nc | nc | 42 | 0.91 | 0.6224 |
| Error | 18 | 28 | 16 | 42 | ||||||||
nc Not calculated, excl excluded from analysis, df degrees of freedom
Significant effects (P ≤ 0.05) and distinct tendencies (P ≤ 0.06) are given in bold
aIncrease (with increasing diversity level or in the presence of the respective factor)
bDecrease (with increasing diversity level or in the presence of the respective factor)
Fig. 2.
Variations in aboveground community biomass as affected by a plant species richness (1, 4, 16) and earthworms [earthworm reduction subplots (−ew) and earthworm subplots (+ew)] and b plant functional group richness (one, two, three, four) and earthworms. Variations in c and d shoot biomass of the phytometer plant species Lolium perenne [g/individual. (ind.)], e and f shoot biomass of the phytometer plant species Centaurea jacea (g/ind.), g and h number of flower heads per Centaurea jacea individual as affected by plant species richness (c, e, g) and plant functional group richness (d, f, h). Bars with different letters vary significantly (Tukey’s HSD test, α < 0.05)
The shoot biomass of grasses (overall mean 183.2 g/m²) tended to decrease with increasing plant species richness and decreased significantly with increasing plant functional group richness (Table 2). Contrarily, the shoot biomass of herbs (overall mean 162.2 g/m²) was not affected by our treatments. The shoot biomass of legumes (overall mean 191.8 g/m²) was increased considerably in earthworm subplots compared to subplots with reduced earthworm density (+35%; Table 2).
Phytometers
Generally, 91% of L. perenne and 87% of C.jacea phytometers survived the first 4 months and 76 and 71% of the phytometer plants after 12 months of field exposure, respectively (time effect; F1,38 (Lolium) = 5.27, P = 0.027 and F1,38 (Centaurea) = 13.07, P = 0.0009). While survival of L. perenne phytometers decreased with increasing plant functional group richness, the survival of C. jacea phytometers was not affected by the treatments (Table 3).
Table 3.
ANOVA table of F- and P-values on the effects of BL, SR, FR, GR, SH, TH and LE, and EW on the survival, shoot biomass, and shoot height of the phytometer Lolium perenne, and on the survival, shoot biomass, shoot height, and number of flower heads of the phytometer Centaurea jacea
| Phytometer plants | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. perenne | C. jacea | ||||||||||||||||||||
| Survival (%) | Shoot biomass (g/ind.) | Shoot height (cm) | Survival (%) | Shoot biomass (g/ind.) | Shoot height (cm) | Flower heads (no./ind.) | |||||||||||||||
| df | F | P | df | F | P | df | F | P | df | F | P | df | F | P | df | F | P | df | F | P | |
| BL | 3 | 0.77 | 0.5203 | 3 | 0.48 | 0.6984 | 3 | 0.60 | 0.6196 | 3 | 1.45 | 0.2468 | 3 | 3.25 | 0.0348 | 3 | 1.04 | 0.3881 | 3 | 1.74 | 0.1773 |
| SR | 2 | 1.83 | 0.1762 | 2 | 19.58 | <0.0001b | 2 | 2.37 | 0.1086 | 2 | 0.89 | 0.4189 | 2 | 4.99 | 0.0132 b | 2 | 0.07 | 0.9314 | 2 | 7.44 | 0.0021b |
| FR | 3 | 5.06 | 0.0056 b | 3 | 9.40 | 0.0001b | 3 | 1.18 | 0.3301 | 3 | 0.30 | 0.8259 | 3 | 3.03 | 0.0439b | 3 | 0.13 | 0.9426 | 3 | 3.97 | 0.0158b |
| GR | 1 | 0.82 | 0.3718 | 1 | 1.77 | 0.1923 | 1 | 2.25 | 0.1426 | 1 | 0.15 | 0.7055 | 1 | 0.02 | 0.9025 | 1 | 0.41 | 0.5271 | 1 | 2.48 | 0.1244 |
| SH | 1 | 0.00 | 0.9845 | 1 | 3.02 | 0.0920 | 1 | 8.31 | 0.0068b | 1 | 0.02 | 0.8759 | 1 | 0.38 | 0.5404 | 1 | 0.21 | 0.6516 | 1 | 0.62 | 0.4379 |
| TH | 1 | 0.04 | 0.8358 | 1 | 0.15 | 0.7005 | 1 | 0.65 | 0.4252 | 1 | 1.18 | 0.1885 | 1 | 0.00 | 0.9888 | 1 | 0.16 | 0.6891 | 1 | 3.21 | 0.0819 |
| LE | 1 | 0.53 | 0.4714 | 1 | 10.66 | 0.0026a | 1 | 25.46 | <0.0001a | 1 | 3.14 | 0.0857 | 1 | 0.51 | 0.4794 | 1 | 0.34 | 0.5652 | 1 | 0.68 | 0.4168 |
| EW | 1 | 0.34 | 0.5637 | 1 | 0.06 | 0.8007 | 1 | 0.15 | 0.7025 | 1 | 0.16 | 0.6917 | 1 | 4.78 | 0.0352a | 1 | 0.49 | 0.4901 | 1 | 0.06 | 0.8132 |
| EW × SR | 2 | 0.55 | 0.5840 | 2 | 0.10 | 0.9078 | 2 | 0.83 | 0.4428 | 2 | 1.80 | 0.1793 | 2 | 0.35 | 0.7047 | 2 | 0.42 | 0.6631 | 2 | 0.23 | 0.7992 |
| EW × FR | 3 | 0.11 | 0.9544 | 3 | 1.37 | 0.2680 | 3 | 0.35 | 0.7921 | 3 | 1.75 | 0.1729 | 3 | 2.11 | 0.1155 | 3 | 0.14 | 0.9323 | 3 | 0.08 | 0.9211 |
| Plot | 32 | 1.15 | 0.3370 | 32 | 5.12 | <0.0001 | 34 | 1.66 | 0.0628 | 32 | 14.92 | <0.0001 | 31 | 27.01 | <0.0001 | 34 | 38.61 | <0.0001 | 34 | 3.18 | 0.0003 |
| Subplot | 38 | 1.10 | 0.3902 | 38 | 2.10 | 0.0124 | – | nc | nc | 38 | 1.16 | 0.3207 | 37 | 2.21 | 0.0102 | – | nc | nc | – | nc | nc |
| Error | 38 | 38 | 40 | 38 | 35 | 40 | 40 | ||||||||||||||
Individual; for other abbreviations, see Table 2
Significant effects (P ≤ 0.05) are given in bold
aIncrease (with increasing diversity level or in the presence of the respective factor)
bDecrease (with increasing diversity level or in the presence of the respective factor)
Shoot biomass of L. perenne was significantly higher in 2008 (0.89 g/ind.) than in 2007 (0.47 g/ind.; F1,38 = 19.90, P < 0.0001) but, on the contrary, shoot biomass of C. jacea was significantly higher in 2007 (0.48 g/ind.) than in 2008 (0.10 g/ind.; F1,38 = 122.37, P < 0.0001). Shoot biomass of both phytometer plants decreased significantly with increasing plant species (Fig. 2c, e) and functional group richness (Table 3; Fig. 2d, f). Moreover, shoot biomass of L. perenne was increased significantly in the presence of legumes (+35%). Further, shoot biomass of C. jacea was increased significantly in earthworm subplots compared to subplots with reduced earthworm density (+21%; Table 3).
Shoot height of the phytometer L. perenne (overall mean 16.6 cm) was decreased in the presence of small herbs (−20%) but increased in the presence of legumes (+25%; Table 3). Conversely, shoot height of the phytometer C. jacea was not affected by our treatments (Table 3).
The number of flower heads per C. jacea individual (overall mean 0.043) decreased significantly with increasing plant species (Fig. 2g) and functional group richness (Table 3; Fig. 2h).
Plant shoot biomass as covariate
The survival of L. perenne phytometers after 4 months was negatively correlated with total shoot community biomass (r = −0.25); nevertheless, fitting this variable (ANCOVA) did not eliminate the significant effect of plant functional group richness (Table 4). Similarly, the shoot biomass of L. perenne phytometers after 4 months was negatively correlated with total shoot community biomass (r = −0.26); nevertheless, fitting this variable did not eliminate the effects of plant species and functional group richness. Further, shoot biomass of C. jacea phytometers after 4 months was negatively correlated with total shoot community biomass (r = −0.14), but fitting this variable did not eliminate the distinct trend of decreasing C. jacea shoot biomass with increasing plant species richness. Moreover, the number of C. jacea flower heads in 2007 was correlated negatively with total shoot community biomass (r = −0.33). While fitting this variable did not eliminate the significant effect of plant species richness, the effect of plant functional group richness disappeared.
Table 4.
ANOVA and analysis of covariance (ANCOVA) table of F- and P-values on the effects of SR, FR, and the covariate total shoot community biomass (cBM) on the survival and shoot biomass of the phytometer L. perenne, and shoot biomass and number of flower heads of the phytometer C. jacea
| Phytometer plants | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L. perenne | C. jacea | |||||||||||||||||||||||
| Survival 2007 (%) | Shoot biomass 2007 (g/ind.) | Shoot biomass 2007 (%) | Flower heads 2007 (no./ind.) | |||||||||||||||||||||
| ANOVA | ANCOVA | ANOVA | ANCOVA | ANOVA | ANCOVA | ANOVA | ANCOVA | |||||||||||||||||
| df | F | P | df | F | P | df | F | P | df | F | P | df | F | P | df | F | P | df | F | P | df | F | P | |
| cBM | – | nc | nc | 1 | 6.68 | 0.0140a | – | nc | nc | 1 | 18.91 | 0.0001a | – | nc | nc | 1 | 18.96 | 0.0001a | – | nc | nc | 1 | 23.51 | <0.0001a |
| SR | 2 | 2.26 | 0.1202 | 2 | 0.08 | 0.921 | 2 | 14.03 | <0.0001a | 2 | 11.49 | 0.0002a | 2 | 2.67 | 0.0843a | 2 | 2.62 | 0.0880a | 2 | 7.44 | 0.0021a | 2 | 3.71 | 0.0355a |
| FR | 3 | 7.46 | 0.0006a | 3 | 5.48 | 0.004a | 3 | 5.48 | 0.0036a | 3 | 3.42 | 0.0283a | 3 | 2.17 | 0.1099 | 3 | 1.95 | 0.14 | 3 | 3.97 | 0.0158a | 3 | 1.95 | 0.141 |
| Error | 33 | 32 | 33 | 32 | 33 | 32 | 33 | 32 | ||||||||||||||||
For other abbreviations, see Table 2
Significant effects (P ≤ 0.05) are given in bold
aDecrease with increasing diversity level
Discussion
Hypothesis 1: earthworms enhance plant productivity
The results of the present study underline the positive diversity–productivity relationship (e.g. Tilman et al. 1997; Hector et al. 1999; Hooper et al. 2005) by showing increasing shoot biomass of the plant community with increasing plant species and functional group richness. A previous study using all experimental plots of the Jena Experiment reported transgressive overyielding due to both complementarity and selection effects (Roscher et al. 2005). However, total shoot biomass of the plant community was increased in subplots with higher earthworm density (+11%) which was primarily due to an increased shoot biomass of legumes (+35%). Remarkably, this did not depend on the diversity of the plant community. Moreover, shoot biomass of the phytometer C. jacea was increased significantly on subplots with higher earthworm density (+21%). Generally, earthworms are assumed to be beneficial soil animals promoting plant growth (Scheu 2003; Brown et al. 2004). They were shown to fragment litter, mix litter and soil, stimulate microbial activity, and thereby drive decomposition and soil nutrient availability (Lee 1985; Edwards and Bohlen 1996; Eisenhauer and Scheu 2008a). However, the majority of studies performed in this context concentrated on the performance of single arable plant species, i.e. on the yield of crop plants (Scheu 2003). The impact of earthworms on plant performance in natural habitats and on more complex plant communities was widely neglected. Recent greenhouse experiments suggested strong effects of earthworms on the productivity of more complex plant communities (Partsch et al. 2006; Eisenhauer and Scheu 2008a). However, field studies in calcareous grassland reported inconsistent results by showing that although numerous, particularly graminoid plant species were associated with earthworm casts (Zaller and Arnone 1999a), earthworm activity had no effect on the shoot biomass production of the plant community (Zaller and Arnone 1999b). Earthworm manipulation in the present study only reduced earthworm density (rather than eliminating earthworms; Eisenhauer et al. 2008), the observed effects therefore are minimum effects. Thus, although plant diversity effects on plant productivity were more pronounced than earthworm effects, hypothesis 1 is confirmed. Earthworm effects were likely due to enhanced nutrient availability in earthworm subplots since earthworms were shown to drive litter decomposition, particularly that of legumes, on the field site of the Jena Experiment (Milcu et al. 2008) and since N availability drives the competition between grassland plant species there (Temperton et al. 2007).
Hypothesis 2: belowground competition increases with increasing plant diversity
Performance of both phytometer plants (shoot biomass, number of C. jacea flower heads) decreased considerably with increasing plant species and functional group richness. This was likely due to increasing belowground competition, i.e. competition for soil nutrients and water, since we reduced aboveground competition, i.e. competition for light, by removing the shoot biomass of the resident plant community within a radius of 10 cm around each phytometer individual. Moreover, we excluded the variance explained by aboveground competition by fitting total shoot biomass of the plant community as covariate. Thereby, the significance of plant diversity effects on phytometer performance was reduced but not eliminated. Similar results (increased mortality, decreased number and size of inflorescences) were reported for the phytometer plant Rumex acetosa investigated in the Jena Experiment (Scherber et al. 2006); however, aboveground competition was not eliminated in this study. The authors concluded that decreased plant productivity was not due to increased invasion resistance of more diverse plant communities, but caused by reduced resource allocation to reproductive tissues when growing in mixtures. Furthermore, Lambers et al. (2004) reported overall negative effects of plant species richness on individual plant species’ performance in the Cedar Creek biodiversity experiment. Von Felten and Schmid (2008) found that belowground competition was more important than aboveground plant competition in a pot diversity experiment, and Cahill (1999), by experimentally differentiating above- and belowground competition, concluded that belowground competition plays an essential role for plant community assembly. Root biomass and thereby the uptake capacity for soil nutrients was shown to increase with plant diversity (Spehn et al. 2005; Fargione and Tilman 2005), presumably resulting in increased belowground plant competition and decreased availability of open niches (Fargione et al. 2003). This was supported by results of Temperton et al. (2007) showing that phytometer plants suffered from the presence of plant species in the resident plant community belonging to the same plant functional group (legumes). Further, Fargione and Tilman (2005) argued that root biomass and soil nitrate concentrations are measures of the efficiency of exploitative competition for N, suggesting that a community’s ability to efficiently consume soil nutrients leads to invasion resistance. Indeed, our results show for the first time that belowground competition in a plant diversity gradient strongly impacted phytometer performance and, therefore, likely shapes plant community assembly under field conditions. The fact that phytometer performance can primarily be attributed to belowground competition was supported by the result that shoot height of phytometers, which is often increased when competing for light, was not affected by plant species and functional group richness. However, removing the aboveground biomass in the vicinity of phytometers might also have influenced the competition between the resident plant community and the phytometers. Belowground competition might have been reduced as a consequence of our treatment by removing photosynthetic tissue and thereby the amount of C compounds produced. On the other hand, our treatment might have increased belowground competition due to enhanced allocation of resources belowground. Nevertheless, since all subplots were treated uniformly the results of the present study most likely show the general fact of enhanced belowground competition with increasing plant diversity and, therefore, hypothesis 2 is confirmed.
Hypothesis 3: earthworms alter belowground plant competition
Earthworms affected single plant functional groups by increasing shoot biomass of legumes and of the phytometer C. jacea. Surprisingly, earthworms had no effect on grass biomass and on the performance of the phytometer L. perenne. Previous greenhouse experiments reported pronounced earthworm effects particularly on grasses (Kreuzer et al. 2004; Wurst et al. 2005; Eisenhauer and Scheu 2008a). The authors concluded that earthworms drive the competition between grasses and legumes by increasing soil N availability and, thereby, enhancing the competitive strength of grasses. Further, Zaller and Arnone (1999a) reported that particularly graminoid species were associated with earthworm casts in calcareous grassland. Moreover, earthworms were shown to increase the invasibility of plant communities for grass weeds (Eisenhauer et al. 2008). Therefore, missing earthworm impacts on grasses in the present study might have been due to low earthworm numbers in plots with grasses in general (Milcu et al. 2008; Eisenhauer N, unpublished data).
In contrast to L. perenne, the shoot biomass of C. jacea was increased significantly on subplots with higher earthworm density. This is in line with other studies focussing on the effects of earthworms on herb species (Wurst et al. 2005, 2006; Partsch et al. 2006) showing that earthworms not only alter herb performance but also secondary metabolism (Wurst et al. 2006). The authors of the above-mentioned studies concluded that beside the increased soil nutrient availability and plant performance this likely affects defence mechanisms against herbivores.
Remarkably, earthworms increased particularly shoot biomass of legumes in the present study. This is in contrast to previous greenhouse experiments indicating that legumes suffer from plant competition in the presence of earthworms (Wurst et al. 2005; Eisenhauer and Scheu 2008a). Unlike previous greenhouse experiments where earthworm densities generally were kept constant in all plant species mixtures, contrasting effects in the field might have been due to feedback mechanisms of the presence of grasses and legumes in the plant community. Field studies conducted in the framework of the Jena Experiment showed that earthworm performance was increased considerably in the presence of legumes (Milcu et al. 2008; Eisenhauer N, unpublished data). Further, legume litter decomposition was increased in earthworm subplots compared to subplots where the earthworm density was reduced (Milcu et al. 2008). Therefore, results of the present study and the study of Milcu et al. (2008) indicate that both legumes and earthworms benefit from each other’s presence. While N-rich legume litter and root exudates beneficially affect earthworm performance (Milcu et al. 2006, 2008), legumes likely benefit from earthworm presence through an increased decomposition of plant residues and the associated increase in soil nutrient availability (Eisenhauer and Scheu 2008a). Although legumes are able to satisfy their N supply through N2 fixation of associated root-nodule bacteria, they were shown to also depend on mineralized N in soil (Eisenhauer and Scheu 2008a). We therefore suggest that earthworms and legumes which function as keystone organisms in temperate grasslands (Milcu et al. 2008) have a loose mutualistic relationship. In contrast to legumes, grasses were shown to have detrimental feedback effects on earthworm performance by changing the habitat structure thereby hampering earthworms, i.e. impeding exploitation of patchily distributed resources in soil by earthworms, and by providing plant residues of poor quality, i.e. low N availability (Eisenhauer N, unpublished data). Consequently, the above-mentioned greenhouse studies only reported short-term effects of earthworms on plant competition but did not account for long-term feedback effects of plant community characteristics on earthworm performance and, in turn, their consequences for plant performance.
Indeed, earthworms likely altered belowground competition by only affecting the productivity of single plant functional groups, i.e. legumes and the phytometer C. jacea (herb) confirming hypothesis 3; however, impacts were rather weak and did not differ with varying plant diversity. Thus, earthworms did not modify the increased belowground competition at higher plant diversity.
Conclusion
The present study highlights that earthworms indeed affect the productivity of semi-natural grassland plant communities. Belowground competition increased with increasing plant diversity; however, it was modified by earthworms by beneficially affecting certain plant species and plant functional groups. Particularly legumes benefited from earthworm presence. Considering also previous studies, we suggest that earthworms and legumes form a loose mutualistic relationship affecting essential ecosystem functions in temperate grasslands. Since both legumes and earthworms increase decomposition (Milcu et al. 2008) and total plant community productivity (Roscher et al. 2005), the present study accentuates the positive interaction and importance of these key functional groups for belowground/aboveground interrelationships.
Acknowledgments
This research was supported by the German Science Foundation and was conducted in the framework of the Jena Experiment. We thank C. M. Pusch, T. Keil, V. Hörsch, M. Klier, S. Gass and the gardener team (Darmstadt University of Technology) for support during the experiment. Comments of two anonymous referees helped to improve the manuscript. The present experiment complies with current German laws.
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Balvanera P, Pfisterer AB, Buchmann N, He JS, Nakashizuka T, Raffaelli D, Schmid B (2006) Quantifying the evidence for biodiversity effects on ecosystem functioning and services. Ecol Lett 9:1146–1156 [DOI] [PubMed]
- Brown GG, Edwards CA, Brussaard L (2004) How earthworms affect plant growth: burrowing into the mechanisms. In: Edwards CA (ed) Earthworm ecology, 2nd edn. CRC, Boca Raton, pp 13–49
- Cahill JF (1999) Fertilization effects on interactions between above- and belowground competition in an old field. Ecology 80:466–480
- Cardinale BJ, Srivastava DS, Duffy JE, Wright JP, Downing AL, Sankaran M, Jouseau C (2006) Effects of biodiversity on the functioning of trophic groups and ecosystems: a meta-analysis. Nature 443:989–992 [DOI] [PubMed]
- Cardinale BJ, Wright JP, Cadotte MW, Carroll IT, Hector A, Srivastava DS, Loreau M, Weiss JJ (2007) Impacts of plant diversity on biomass production increase through time because of species complementarity. Proc Natl Acad Sci USA 104:18123–18128 [DOI] [PMC free article] [PubMed]
- Edwards CA, Bohlen PJ (1996) Biology and ecology of earthworms, 3rd edn. Chapman and Hall, London
- Eisenhauer N, Scheu S (2008a) Earthworms as the drivers of the competition between grasses and legumes. Soil Biol Biochem 40:2650–2659 [DOI]
- Eisenhauer N, Scheu S (2008b) Invasibility of experimental grassland communities: the role of earthworms, plant functional group identity and seed size. Oikos 117:1026–1036 [DOI]
- Eisenhauer N, Partsch S, Parkinson D, Scheu S (2007) Invasion of a deciduous forest by earthworms: changes in soil chemistry, microflora, microarthropods and vegetation. Soil Biol Biochem 39:1099–1110 [DOI]
- Eisenhauer N, Milcu A, Sabais ACW, Scheu S (2008) Animal ecosystem engineers modulate the diversity–invasibility relationship. PLoS ONE 3:e3489 [DOI] [PMC free article] [PubMed]
- FAO-Unesco (1997) Soil map of the world. Revised legend with corrections and update, ISRIC, Wageningen
- Fargione JE, Tilman D (2005) Diversity decreases invasion via both sampling and complementarity effects. Ecol Lett 8:604–611 [DOI]
- Fargione JE, Brown C, Tilman D (2003) Community assembly and invasions: an experimental test of neutral versus niche processes. Proc Natl Acad Sci USA 100:8916–8920 [DOI] [PMC free article] [PubMed]
- Harpole WS, Tilman D (2007) Grassland species loss resulting from reduced niche dimension. Nature 446:791–793 [DOI] [PubMed]
- Heady HF (1957) The measurement and value of plant height in the study of herbaceous vegetation. Ecology 38:313–320 [DOI]
- Hector A, Schmid B, Beierkuhnlein C, Caldeira MC, Diemer M, Dimitrakopoulos PG, Finn J, Freitas H, Giller PS, Good J, Harris R, Högberg P, Huss-Danell K, Joshi J, Jumpponen A, Körner C, Leadley PW, Loreau M, Minns A, Mulder CPH, O’Donovan G, Otway SJ, Pereira JS, Prinz A, Read DJ, Scherer-Lorenzen M, Schulze E-D, Siamantziouras ASD, Spehn EM, Terry AC, Troumbis AY, Woodward FI, Yachi S, Lawton JH (1999) Plant diversity and productivity experiments in European grasslands. Science 286:1123–1127 [DOI] [PubMed]
- Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, Schmid B, Setälä H, Symstadt AJ, Vandermeer J, Wardle DA (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35 [DOI]
- Huston MA (1997) Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia 108:449–460 [DOI] [PubMed]
- Kluge G, Müller-Westermeier G (2000) Das Klima ausgewählter Orte der Bundesrepublik Deutschland: Jena. Berichte des Deutschen Wetterdienstes 213. Offenbach/Main, Germany
- Kreuzer K, Bonkowski M, Langel R, Scheu S (2004) Decomposer animals (Lumbricidae, Collembola) and organic matter distribution affect the performance of Lolium perenne (Poaceae) and Trifolium repens (Fabaceae). Soil Biol Biochem 36:2005–2011 [DOI]
- Lambers JHR, Harpole WS, Tilman D, Knops J, Reich PB (2004) Mechanisms responsible for the positive diversity–productivity relationship in Minnesota grasslands. Ecol Lett 7:661–668 [DOI]
- Lee KE (1985) Earthworms: their ecology and relationships with soils and land use. Academic Press, Sydney
- Loreau M (2000) Biodiversity and ecosystem functioning: recent theoretical advances. Oikos 91:3–17 [DOI]
- Loreau M, Hector A (2001) Partioning selection and complementarity in biodiversity experiments. Science 412:72–76 [DOI] [PubMed]
- Loreau M, Naeem S, Inchausti P (2002) Biodiversity and ecosystem functioning: synthesis and perspectives. Oxford University Press, New York
- Milcu A, Partsch S, Langel R, Scheu S (2006) The response of decomposers (earthworms, springtails and microorganisms) to variations in species and functional group diversity of plants. Oikos 112:513–524 [DOI]
- Milcu A, Partsch S, Scherber C, Weisser WW, Scheu S (2008) Earthworms and legumes control litter decomposition on a plant diversity gradient. Ecology 89:1872–1882 [DOI] [PubMed]
- Partsch S, Milcu A, Scheu S (2006) Decomposers (Lumbricidae, Collembola) affect plant performance in model grasslands of different diversity. Ecology 87:2548–2558 [DOI] [PubMed]
- Roscher C, Schumacher J, Baade J, Wilcke W, Gleixner G, Weisser WW, Schmid B, Schulze E-D (2004) The role of biodiversity for element cycling and trophic interactions: an experimental approach in a grassland community. Basic Appl Ecol 5:107–121 [DOI]
- Roscher C, Temperton VM, Scherer-Lorenzen M, Schmitz M, Schumacher J, Schmid B, Buchmann N, Weisser WW, Schulze E-D (2005) Overyielding in experimental grassland communities–irrespective of species pool or spatial scale. Ecol Lett 8:419–429 [DOI]
- Scheiner SM, Gurevitch J (2001) Design and analysis of ecological experiments, 2nd edn. Oxford University Press, New York
- Scherber C, Milcu A, Partsch S, Scheu S, Weisser WW (2006) The effects of plant diversity and insect herbivory on performance of individual plant species in experimental grassland. J Ecol 94:922–931 [DOI]
- Scheu S (2003) Effects of earthworms on plant growth: patterns and perspectives. Pedobiologia 47:1–11 [DOI]
- Schmid B, Hector A, Huston MA, Inchausti P, Nijs I, Leadley PW, Tilman D (2002) The design and analysis of biodiversity experiments. In: Loreau M, Naeem S, Inchausti P (eds) Biodiversity and ecosystem functioning. Oxford University Press, Oxford, pp 61–75
- Spehn EM, Hector A, Joshi J, Scherer-Lorenzen M, Schmid B, Bazeley-White E, Beierkuhnlein C, Caldeira MC, Diemer M, Dimitrakopoulos PG, Finn JA, Freitas H, Giller GS, Good J, Harris R, Högberg P, Huss-Danell K, Jumpponen A, Koricheva J, Leadley PW, Loreau M, Minns A, Mulder CPH, O’Donovan G, Otway SJ, Palmborg C, Pereira JS, Pfisterer AB, Prinz A, Read DJ, Schulze E-D, Siamantziouras ASD, Terry AC, Troumbis AY, Woodward FI, Yachi S, Lawton JH (2005) Ecosystem effects of biodiversity manipulations in European grasslands. Ecol Monogr 75:37–63 [DOI]
- Temperton VM, Mwangi PN, Scherer-Lorenzen M, Schmid B, Buchmann N (2007) Positive interactions between nitrogen-fixing legumes and four different neighbouring species in a biodiversity experiment. Oecologia 151:190–205 [DOI] [PubMed]
- Thielemann U (1986) The octet-method for sampling earthworm populations. Pedobiologia 29:296–302
- Tilman D, Lehman CL, Thomson KT (1997) Plant diversity and ecosystem productivity: theoretical considerations. Proc Natl Acad Sci USA 94:1857–1861 [DOI] [PMC free article] [PubMed]
- Tiunov AV, Scheu S (1999) Microbial respiration, biomass, biovolume and nutrient status in burrow walls of Lumbricus terrestris L. (Lumbricidae). Soil Biol Biochem 31:2039–2048 [DOI]
- Von Felten S, Schmid B (2008) Complementarity among species in horizontal versus vertical rooting space. J Plant Ecol 1:33–41 [DOI]
- Wardle DA (1999) Is “sampling effect” a problem for experiments investigating biodiversity-ecosystem function relationships? Oikos 87:403–407 [DOI]
- Wardle DA, Bardgett RD, Klironomos JN, Setälä H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633 [DOI] [PubMed]
- Weigelt A, Schumacher J, Walther T, Bartelheimer M, Steinlein T, Beyschlag W (2007) Identifying mechanisms of competition in multi-species communities. J Ecol 95:53–64 [DOI]
- Wurst S, Langel R, Scheu S (2005) Do endogeic earthworms change plant competition? A microcosm study. Plant Soil 271:123–130 [DOI]
- Wurst S, Langel R, Rodger S, Scheu S (2006) Effects of belowground biota on primary and secondary metabolites in Brassica oleracea. Chemoecology 16:69–73 [DOI]
- Zaller JG, Arnone JA III (1999a) Interactions between plant species and earthworm casts in a calcareous grassland under elevated CO2. Ecology 80:837–881
- Zaller JG, Arnone JA III (1999b) Earthworm and soil moisture effects on the productivity and structure of grassland communities. Soil Biol Biochem 31:517–523 [DOI]