Abstract
Amphibian metamorphosis is marked by dramatic, thyroid hormone (TH)-induced changes involving gene regulation by TH receptor (TR). It has been postulated that TR-mediated gene regulation involves chromatin remodeling. In the absence of ligand, TR can repress gene expression by recruiting a histone deacetylase complex, whereas liganded TR recruits a histone acetylase complex for gene activation. Earlier studies have led us to propose a dual function model for TR during development. In premetamorphic tadpoles, unliganded TR represses transcription involving histone deacetylation. During metamorphosis, endogenous TH allows TR to activate gene expression through histone acetylation. Here using chromatin immunoprecipitation assay, we directly demonstrate TR binding to TH response genes constitutively in vivo in premetamorphic tadpoles. We further show that TH treatment leads to histone deacetylase release from TH response gene promoters. Interestingly, in whole animals, changes in histone acetylation show little correlation with the expression of TH response genes. On the other hand, in the intestine and tail, where TH response genes are known to be up-regulated more dramatically by TH than in most other organs, we demonstrate that TH treatment induces gene activation and histone H4 acetylation. These data argue for a role of histone acetylation in transcriptional regulation by TRs during amphibian development in some tissues, whereas in others changes in histone acetylation levels may play no or only a minor role, supporting the existence of important alternative mechanisms in gene regulation by TR.
Amphibian metamorphosis is a postembryonic developmental switch directly initiated by thyroid hormone (TH; refs. 1, 2). TH and in particular the biologically more active form, 3,5,3′-triiodothyronine (T3), exerts its effects on target tissues via binding to TH receptors (TRs), which are transcription factors that belong to the nuclear receptor superfamily (3). TR modulates gene expression by binding to specific DNA sequences in target genes most often by forming a heterodimer with retinoid X receptors (RXRs or 9-cis-retinoic acid receptors). TRs have the capacity to both activate transcription in presence of ligand and repress transcription in its absence. Recent evidence supports the hypothesis that TRs switch, in a ligand-dependent manner, between the binding of a multicomponent corepressor complex and the binding of a coactivator complex (3). The observations that transcriptional activation may be associated with the recruitment of histone acetyl transferase and repression with the recruitment of histone deacetylase has led to a model in which chromatin remodeling targeted by TRs contributes in part to transcriptional control (4–7).
However, there is no evidence to date demonstrating whether such a mechanism of TR regulation of gene silencing and activation is involved in any physiological event. Amphibian metamorphosis provides an ideal model to address this aspect (2, 8). In Xenopus laevis, TRs are encoded by four genes (two TRα and two TRβ; ref. 9). TRβ genes are up-regulated at the transcriptional level during metamorphosis in response to endogenous TH synthesis (10–12). However, TRα genes are activated during late embryogenesis, well before the maturation of the thyroid gland and synthesis of endogenous TH (13). This expression pattern of TRα suggests a role for unliganded TR in gene silencing before TH-dependent gene activation during metamorphosis.
To investigate how TRs regulate gene expression during amphibian development, we examined in vivo TR binding and histone acetylation level on TH response gene promoters by using a chromatin immunoprecipitation assay with nuclei from whole embryos, tadpoles, or isolated tissues at various developmental stages. Our results indicate that TRs binds to TH response elements (TREs) in chromatin constitutively during development and that the modulation of histone acetylation is important for gene regulation by TRs.
Materials and Methods
Animals and Treatment.
Adults and stage 55 premetamorphic tadpoles of the South African clawed frog Xenopus laevis were obtained from Nasco (Fort Atkinson, WI). Embryos were prepared by in vitro fertilization as described (14). Approximately 100 embryos at stage 20 and 20 tadpoles at stage 47 were treated for 1 day with 100 nM T3 (Sigma), and/or 100 nM trichostatin A (TSA; Wako Biochemicals, Osaka), a specific histone deacetylase inhibitor (16). For analysis of gene regulation in the intestine, 12 stage 55 tadpoles were treated in 4 liters of dechlorinated tap water with 10 or 50 nM T3 and/or 100 nM TSA for 2 days without feeding. The animals were then killed by decapitation, after anesthesia, for intestine and tail isolation.
RNAs Extraction and PCR Analysis of Gene Expression.
RNAs were extracted from embryos, tadpoles, or isolated intestine or tail with RNAzol B (Tel-Test, Friendswood, TX) according to the manufacturer's instructions. RNAs were resuspended in diethyl pyrocarbonate-treated water and quantified by UV absorption. Analyzing the RNAs on an agarose gel with ethidium bromide staining further checked the RNAs quality and quantity. Reverse transcription reactions were performed by using 10 μg of total RNA in 20 μl as follows: RNAs and specific primers for the gene of interest and the internal control gene, the ribosomal protein gene rpl8 (2 μM each) were mixed in 10 μl, incubated at 65°C for 5 min, and allowed to cool down to room temperature. A mixture (10 μl) containing 5× first strand buffer (4 μl, GIBCO/BRL), DTT (2 μl, 0.1 M, GIBCO/BRL), dNTP mix (1 μl, 25 mM each, Pharmacia), RNAsin (0.1 μl, 10 units/μl, GIBCO/BRL), and reverse transcriptase SuperScript II (0.5 μl, 200 units/μl, GIBCO/BRL) was added to the annealed RNAs and primer solution before incubation at 42°C for 1 h. Two microliters of the resulting cDNA solution was used for PCR in 50 μl of reaction containing 10× Ex Taq buffer (5 μl, Takara Shuzo, Kyoto), dNTP mix (8 μl, 2.5 mM each, Takara Shuzo), four primers (reverse and forward primers for the gene of interest and rpl8, 2 μl of 2 μM solution for each, GIBCO/BRL), and Ex Taq polymerase (0.5 μl, 5 units/μl, Takara Shuzo). PCRs were done for 28 or 30 cycles each consisting of 94°C for 30 sec, 55°C for 30 sec, and 72°C for 30 sec. The primers used are, for the internal control rpl8 (17): forward 5′-AAAGAGAAACTGCTGGC-3′ and reverse 5′-GACGACCAGTACGACGA-3′; for TRα (9): forward 5′-ATGGCTTCCATGCCGGATGGG-3′ and reverse 5′-CTCTATCTTGTCCG-TGCAGAT-3′; for TRβ (9): forward 5′-ATAGTTAATGCGCCCGAGGGTGGA-3′ and reverse 5′-CTTTTCTATTCTCTCCACGCTAGC-3′; and for TH/bZip (18): forward 5′-TACTGGG-AAAAGAGGCGCAAGAAC-3′ and reverse 5′-CTTAAACCTCAGCTTAT-GTGGAAG-3′. PCR was also done on RNAs without reverse transcription as a control for genomic DNA contamination (data not shown). PCR products (10 μl) were electrophoresed on 2% agarose gels and stained with ethidium bromide.
Preparation of Nuclei and Chromatin Immunoprecipitation (ChIP).
Nuclei were isolated as described (19). The pellet was resuspended in 360 μl of nucleus isolation buffer (0.25 M sucrose/10 mM Tris⋅HCl, pH 7.5/3 mM CaCl2/1 mM PMSF/1 μg/ml aprotinin/1 μg/ml pepstatin). Proteins were crosslinked to DNA by adding formaldehyde (37%) directly to nuclear resuspension to a final concentration of 1% and incubated for 30 min at room temperature. The nuclei were pelleted and then resuspended in 200 μl of lysis buffer (1% SDS/50 mM Tris⋅HCl, pH 8.1/10 mM EDTA/1 mM PMSF/1 μg/ml aprotinin/1 μg/ml pepstatin) for 10 min on ice. The lysate was sonicated 15 times with 15-sec pulses by using a sonicator set to 70% of maximum power to reduce DNA length to between 200 and 1,000 bp. At this step, it is essential to keep the samples cold all of the time. Debris was removed by centrifugation for 10 min at 14,000 × g at 4°C. The DNA was quantified and adjusted to equal concentration for the resulting chromatin solution. ChIP assay was done using a kit from Upstate Biotechnology. Chromatin solution (1 ml) was used for each ChIP assay with 5 μl of anti-acetylated histone H4 antiserum (Upstate Biotechnology), 8 μl of anti-Xenopus TR antiserum (recognizing both TRα and TRβ), anti-Xenopus RXRα antiserum (which recognizes RXRα but not RXRγ and has not been tested on RXRβ) (20), or anti-Xenopus Rpd3 antiserum (21). Chromatin solution (500 μl) was used for the control of input DNA in the chromatin solution. After the ChIP protocol, the recovered DNA was resuspended in 20 μl of H2O for the ChIP samples and 40 μl for the input control. Semiquantitative PCR was performed in 50 μl with 5 μl of 10 times Ex Taq buffer (Takara Shuzo), 8 μl of dNTP mix (2.5 mM each, Takara Shuzo), 2 μl of each primer (2 μM each, GIBCO/BRL), 0.5 μl Ex Taq polymerase (5 units/μl, Takara Shuzo), 2 μl of the DNA sample, and 1 μl (1 μCi) of [α-32P]dCTP. The primers used for TRβ promoter: forward 5′-GTAAGCTGCCTGTGTCTATAC-3′ and reverse 5′-GACAGTCAGAGGAACTG-3′ (10); for TH/basic leucine zipper (bZIP) promoter: forward 5′-TCTCCCTGTTGTGTATAATGG-3′ and reverse 5′-CTCCCAACCCTACAGAGTTCA-3′ (22); for a segment of TRβ transcribed sequence: forward 5′-CAGAAACCTGAACCCACACAA-3′ and reverse 5′-CACTTTTCCACCCTCGGGCGCATT-3′ (located respectively in exons 3 and 4; ref. 23); and for the intestinal fatty acid binding protein (IFABP) promoter: forward 5′-ATAGCAGCAGGTGGTTGCG-3′ and reverse 5′-GGCCACAAGATCTACTCG-3′ (24). PCRs were done for 30–40 cycles as described above. PCR products were resolved on a 6% acrylamide-TBE gel (Novex) and visualized by autoradiography.
Results
Up-Regulation of TRα Genes at the End of Embryogenesis Correlates with TR/RXR Binding to TREs in Chromatin in Premetamorphic Tadpoles.
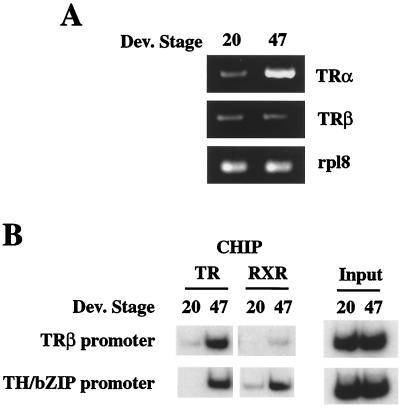
It has been proposed that Xenopus laevis embryos or tadpoles before feeding stage (stage 41) do not respond to exogenous TH because of the lack of sufficient TRs (25). In line with this hypothesis, Northern blot analysis of endogenous TRs failed to reveal TR mRNAs in tadpoles before stage 40 (13, 20). By using a more sensitive PCR assay, we compared the expression of TRα and TRβ genes in stage 20 embryos and stage 47 tadpoles. TRα gene expression was found to be low at stage 20 but high at stage 47 (Fig. 1A). On the other hand, little TRβ gene expression was detected at either stage 20 or 47 (Fig. 1A), in agreement with earlier reports.
Figure 1.
Up-regulation of TRα genes during late embryogenesis correlates with TR and RXR binding to TR target genes. (A) TRα mRNA expression increases between embryonic and tadpole stages. Total RNA was isolated from stage 20 embryos and stage 47 tadpoles and used for PCR analysis of TRα and TRβ mRNA levels. PCR products (10 μl) were electrophoresed on 2% agarose gels, and the gels were stained with ethidium bromide. The expression of ribosomal protein gene Rpl8 was used as an internal control. (B) TR and RXR binding to T3 response gene promoters increases between embryonic and tadpole stages. Chromatin from stage 20 embryo and stage 47 tadpole nuclei was immunoprecipitated with antibodies against TR or RXR and analyzed by PCR for the presence of the fragments containing the TREs of the two T3 response genes. Aliquots of the chromatin before immunoprecipitation were used directly for PCR as control (input). The figure represents one of three independent experiments with different batch of animals, all yielding same results.
To address directly whether this change in TR expression correlates with the binding of TR and RXR to chromatin, we analyzed TR and RXR binding to the promoters of two TH target genes by ChIP assay. Antibodies specific to TR (recognizing both TRα and TRβ) or RXR (recognizing RXRα but not RXRγ; it is worthwhile to point out that RXRα mRNA is expressed at much higher levels than RXRγ mRNA 20) were used to immunoprecipitate formaldehyde-crosslinked, sonicated chromatin fragments from nuclei isolated from embryos and tadpoles at different developmental stages. The TR or RXR-bound DNA fragments that were immunoprecipitated by the respective antibody were then analyzed by semiquantitative PCR by using primers flanking TH response elements in TH target genes.
For this purpose, we chose TRβA and TH/bZip genes, the only two Xenopus direct TH-response genes whose promoters have been characterized (10, 11, 22). The region selected for ChIP analysis contained the TRE (two direct repeats separated by 4 bp, 10, 11, 22) of the corresponding promoter but not any other nuclear receptor binding sites. As shown in Fig. 1B, ChIP analysis with antibodies to TR and RXR revealed little or no TR or RXR binding to the TRE fragment in either promoter in embryos at stage 20. The up-regulation of TRα in stage 47 tadpoles coincided with an increase in both TR and RXR binding to both promoters (Fig. 1B). As there is no detectable TH at this premetamorphic stage (15), these results indicate that unliganded receptor could bind to TREs in chromatin in vivo. We assume that this binding was most likely by TR/RXR heterodimers, as they are the preferred binding complexes for the TREs, although our assay could not distinguish the binding by TR/RXR heterodimers or that by TR/TR homodimers.
Differential Effects of T3 and TSA on the Regulation and Histone Acetylation Levels of TH Response Gene Promoters During Early Development.
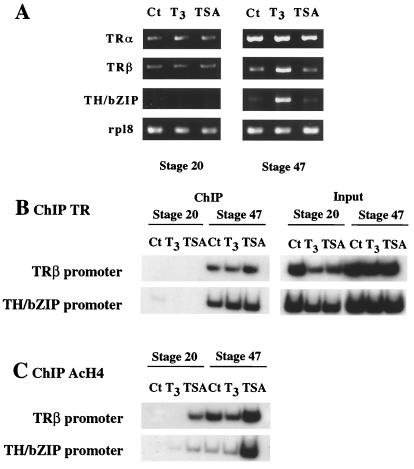
We next investigated whether the chromatin-bound TR/RXR regulate TH response genes in a T3- and histone acetylation-dependent manner. We treated embryos and tadpoles with T3 or histone deacetylase inhibitor TSA and then determined their effects on TRβ and TH/bZip gene regulation. At stage 20, neither T3 nor TSA was able to induce the expression of either T3 response gene (Fig. 2A). At stage 47, T3 treatment led to the activation of both TRβ and TH/bZip genes whereas TSA had no effect on either one (Fig. 2A). ChIP assay with TR antibody showed that the T3 or TSA treatment had no affect on TR binding to the TREs of TRβ and TH/bZip. Thus, TR is bound to the TREs constitutively in premetamorphic (stage 47) tadpoles but not in embryos at stage 20 (Fig. 2B), consistent with the very low level of TRs in embryos (Fig. 2A). ChIP assay with RXR antibody showed that like TR, RXR also was constitutively bound to TREs in stage 47 tadpoles (Fig. 1 and data not shown). Thus, TR/RXR binding to the TREs enables the TH response genes to respond to T3 treatment, whereas blocking histone deacetylase activity has little effect on the overall expression of the genes in the animals.
Figure 2.
Stage-dependent effects of T3 and TSA on transcription, DNA binding by TRs, and histone H4 acetylation at T3 response genes. (A) Premetamorphic tadpoles but not embryos are competent to respond to T3 treatment. Stage 20 embryos and stage 47 tadpoles were treated with 100 nM T3 or 100 nM TSA for 24 h. Total RNA was extracted from whole animals and used for PCR analysis of TRα, TRβ, and TH/bZIP expression. The expression of ribosomal protein gene Rpl8 was used as an internal control. Note that only TRβ and TH/bZIP genes are direct TH response genes, and they were induced only in tadpoles but not in embryos, which had little TR. (B) TR binding to TREs of T3 response genes was not affected by T3 or TSA treatment. Chromatin from animals treated as in A was immunoprecipitated with antibody against TR and analyzed by PCR for the presence of immunoprecipitated TRE-containing fragments. Aliquots of the chromatin before immunoprecipitation were used directly for PCR as control (input). (C) Histone H4 acetylation levels of the chromatin containing the TREs of the TH response genes are up-regulated between embryos and tadpoles by TSA but not T3 treatment. Chromatin isolated as above was immunoprecipitated with antibody against acetylated histone H4 and analyzed by PCR as above. As the same chromatin samples were used for TR and H4 ChIP assays, the input control was the same as shown in B. Note it is unclear why the intensity in the + T3 lane at stage 47 was slightly lower than the control, although the input intensity was also lower in the + T3 lane (see B). However, as we observed little correlation of histone acetylation with gene expression in whole animals, the result did not affect our conclusions. The figure represents one of two independent experiments with identical results.
To investigate whether T3 or TSA altered the status of histone acetylation on the chromatin of the promoter regions, we analyzed histone acetylation levels by ChIP assay. Antibodies specific to acetylated histone H4 were used to immunoprecipitate formaldehyde-crosslinked sonicated chromatin from nuclei isolated from stage 20 embryos and stage 47 tadpoles. The results showed that T3 alone had no effect on histone acetylation levels of either promoter in both the embryos and tadpoles (Fig. 2C). On the other hand, TSA caused remarkable increases in the acetylation levels on the promoters in both embryos and tadpoles (Fig. 2C). Interestingly, the acetylation levels at both TRβ and TH/bZip promoters were higher in premetamorphic tadpoles (stage 47), when TRα expression was high, than in embryos (stage 20), when TR expression was very weak (Fig. 2C). Thus, the binding by unliganded TR/RXR keeps the genes repressed despite the increase acetylation levels as the animal develops. On the other hand, increasing histone acetylation alone, by TSA treatment, is insufficient to alter the expression levels of TH-response genes in whole animals.
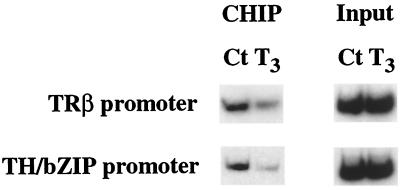
T3 Causes the Release of the Histone Deacetylase Rpd3 from TH-Response Genes.
Histone acetylation levels are determined by the action of histone acetylases and deacetylases. Unliganded, but not T3-bound, TR is known to interact with corepressor complexes containing histone deacetylases. Even though histone acetylation levels were found to be unchanged by T3 when analyzed in whole animals (Fig. 2C), it is possible that T3 treatment of premetamorphic tadpoles can influence the recruitment of histone acetylases/deacetylases to specific promoters. Thus, we next examined in tadpoles at stage 47 whether unliganded TR recruited histone deacetylases and whether T3 induced the release of histone deacetylases. ChIP assay with a polyclonal antibody to histone deacetylase Rpd3, the only characterized Xenopus histone deacetylase (21), revealed that Rpd3 was present, at least in some tissues, in the chromatin fragments containing the TREs of TRβ and TH/bZip genes (Fig. 3). More importantly, T3 treatment led to the release of Rpd3 from both TRE regions (Fig. 3). Thus, unliganded TR is capable of recruiting histone deacetylase in a T3-dependent manner in vivo, consistent with in vitro biochemical findings (26, 27).
Figure 3.
T3 treatment leads to the release of histone deacetylase Rpd3 from TH response gene promoters. Stage 47 tadpole nuclei were isolated after 100 nM T3 treatment for 24 h. Chromatin was immunoprecipitated with antibody against histone deacetylase Rpd3 and analyzed by PCR as in Fig. 2. The data represents one out of several independent experiments with identical result.
Constitutive Binding and Histone Deacetylase-Dependent Gene Repression by Unliganded TR in Premetamorphic Intestine or Tail.
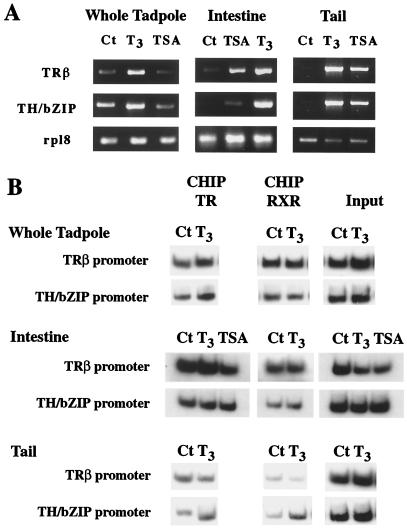
The lack of any detectable changes in histone acetylation levels analyzed on two T3-dependent promoters after T3 treatment in whole animals, does not rule out the possible presence of tissue specific changes. We chose the intestine and the tail to investigate this possibility. These two tissues are among the few well-characterized organs that undergo extensive remodeling and are known to have the most dramatic up-regulation of TH response genes during metamorphosis. Premetamorphic tadpole intestine consists predominantly of a single tissue, the larval epithelium, which undergoes apoptosis and is replaced by the adult epithelium (2, 28). The tail, on the other hand, completely disappears through an apoptotic pathway (2). Thus, these two organs offer relatively homogeneous tissues for study tissue specific changes in gene expression and chromatin remodeling.
First, we compared TRβ and TH/bZip gene expression in intestine, tail, and whole animal in premetamorphic tadpoles at stage 55 (Fig. 4A). Both genes were expressed very weakly in the intestine and the tail compare to whole tadpole. After T3 treatment for 2 days, TRβ and TH/bZip mRNA expression were up-regulated (Fig. 4A). Although TSA did not alter the expression of the genes in whole animals at stage 55 (Fig. 4), in the intestine and the tail, both genes were induced by TSA treatment (Fig. 4A). ChIP assay with TR and RXR antibodies showed that both receptors were bound to TRβ- and TH/bZip-promoter chromatin in premetamorphic intestine, tail, and whole animal, independent of T3 or TSA treatment (Fig. 4B and data not shown). Thus, the binding of unliganded TR to TREs leads to gene repression in a tissue specific manner, likely involving histone deacetylation.
Figure 4.
T3 and TSA induce transcription of T3 response genes without altering TR/RXR binding to TRE in premetamorphic tadpoles. (A) Differential effects of T3 and TSA on gene expression. Stage 55 tadpoles were treated for 2 days with T3 (10 nM) or TSA (100 nM). Total RNA was extracted from whole animals, intestine, or tail tissues and used for PCR analysis of TRβ and TH/bZIP expression. The expression of ribosomal protein gene Rpl8 was used as an internal control. Note that T3 treatment increased mRNA levels of T3 response genes in whole animals, intestine, and tail, whereas TSA treatment altered T3 response gene expression only in intestine and tail. (B) TR/RXR binds to TREs in chromatin constitutively. Chromatin isolated from whole tadpoles, the intestine, or the tail of the T3- or TSA-treated stage 55 tadpoles (A) was immunoprecipitated with antibodies against TR or RXR and analyzed by PCR as in Fig. 2. The figure represents one of two independent experiments with identical results.
Changes in Histone Acetylation Levels Correlate with Gene Regulation by TR During Intestine Metamorphosis.
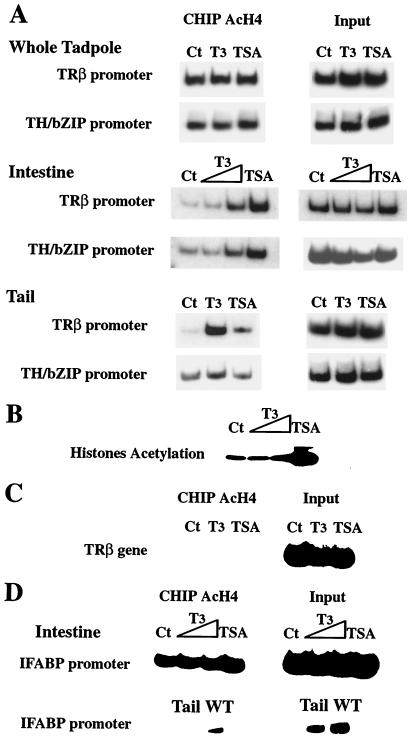
To further investigate the role of histone acetylation in regulation of the TH response genes, we examined whether T3 or TSA treatment altered the levels of histone H4 acetylation on TRβ- and TH/bZip-promoter chromatin. First, nuclei were isolated from intestine, tail, and whole tadpole at stage 55 after treatment with T3 or TSA and used for ChIP assay with an antibody specific to acetylated histone H4. As observed for stage 47, in whole tadpoles stage 55, the level of the acetylation levels on histone H4 in TRβ- and TH/bZip-promoter chromatin did not change (Fig. 5A). However, in the intestine and tail, T3 treatment led to an increase in the acetylation levels on histone H4 in chromatin of both promoters (Fig. 5A), as did TSA except for TH/bZIP in the tail (Fig. 5A). Second, by using an antibody against acetylated lysine, Western blotting of proteins isolated from nuclei of stage 55 tadpole intestine treated with the histone deacetylase inhibitor revealed that TSA induced hyperacetylation of histones (Fig. 5B). T3 treatment caused little change in overall core histone acetylation (Fig. 5B). This may not be surprising because T3 can both activate and repress, directly or indirectly, gene expression in various target tissues, and it would be expected to alter the histone acetylation levels of only its target genes but not globally.
Figure 5.
T3 treatment increases histone H4 acetylation specifically at the TRE regions of T3 response genes in premetamorphic tadpole intestine and tail. (A) Organ-specific changes in histone acetylation by T3 and TSA. Stage 55 tadpoles were treated for 2 days with T3 (10 nM) or TSA (100 nM). Nuclei extract from whole tadpole, intestine, or tail were used for ChIP assay by using an antibody against acetylated histone H4. Aliquots of the chromatin before immunoprecipitation were used directly in PCR as a DNA control (input). (B) TSA but not T3 increases overall histone acetylation level in the intestine. Nuclear proteins (20 μg) from intestine of the above animals were loaded on a Tris-glycine 18% acrylamide gel (Novex). Proteins were transferred to a poly(vinylidene difluoride) Immobilon-P membrane (Millipore), and rabbit anti-acetyl-lysine polyclonal antibody (Upstate Biotechnology) was used to analyze core histone acetylation states. (C) T3 and TSA treatment has no effects on histone H4 acetylation in the transcribed region of TRβ gene far from the promoter in the intestine. ChIP assay was performed as in A for an internal region of TRβ gene instead of the promoter region. (D) Histone H4 acetylation levels at the promoter of IFABP gene, which is not a directly T3 response gene, in the intestine, tail, and whole tadpole (WT). Note that histone acetylation of the promoter could be detected in the intestine or whole tadpoles but not in the tail, where the IFABP gene is not expressed. TSA but not T3 treatment increased slightly histone H4 acetylation level at the promoter of IFABP gene in the intestine. All experiments were done at least twice.
As a control, ChIP analysis of a transcribed region of TRβ gene between exons 3 and 4, which is >40 kb away from the TRβ promoter (9, 23), revealed that histone H4 acetylation level was very low and more importantly, not affected by either T3 or TSA treatment (Fig. 5C). Finally, as another control for specificity of the induced local histone hyperacetylation, we analyzed the acetylation level of IFABP promoter. The IFABP gene is expressed only in the intestinal epithelial cells (29). It is not a T3 direct response gene and its promoter lacks a TRE (24, 29). Consistently, we found that IFABP was not expressed in the tail but was expressed in the intestine (ref. 29 and data not shown). ChIP assay showed that the chromatin of its promoter contained acetylated histone H4 in the intestine but not in the tail (Fig. 5D). Furthermore, in the intestine, the level of histone H4 acetylation was not affected after a T3 treatment but slightly increased after TSA treatment (Fig. 5D). These results indicate that the observed change in histone acetylation levels is restricted to T3 target genes in specific tissues and is not a non-specific effect of the hormone treatment. Taken together, these data suggest that T3-induced gene expression requires histone hyperacetylation of chromatin at/near the TREs of target genes in vivo.
Discussion
In vitro biochemical and tissue culture transfection studies have shown that TR/RXR heterodimers can repress or activate gene transcription depending upon the absence or presence of TH. Our earlier work using the frog oocyte system indicates that such dual functions of the receptors persist even in the context of chromatin (12, 30–32, 42). Recent findings by us and others suggest that unliganded TR can recruit histone deacetylases, whereas T3-bound TR can recruit histone acetyltransferases (acetylases) to target genes (5, 7, 33, 42). This result has strengthened the connection between histone acetylation, chromatin remodeling, and hormone-induced gene regulation. However, there is a lack of in vivo evidence demonstrating the dual functions of TR/RXR and the physiological consequence of changes in histone acetylation as a mechanism in TR-mediated transcriptional regulation. Our studies here provide a critical link between in vitro studies and developmental/physiological roles of the receptors. Our major conclusions are (i) TR and RXR binds to TREs in chromatin constitutively during postembryonic development; (ii) in the absence of T3, histone deacetylase is present at TH response gene promoters, and (iii) changes in histone acetylation levels correlate with T3-dependent gene regulation in some but not all tissues.
Amphibian metamorphosis, a postembryonic process controlled by TH, is a unique model to study TR function in vivo during development. Before stage 35 (hatching stage) for Xenopus laevis, embryos are incapable of responding to exogenous T3 (34). The lack of competence to respond to T3 has been suggested to be attributable to the lack of adequate levels of TRs (25). TRα genes are activated only after tadpole hatching (stage 35) whereas TRβ genes are repressed until metamorphosis (13, 20). We have demonstrated here for the first time in vivo that there is little TR binding in a chromatin context to TREs of TH response genes in embryos. Moreover, the increase in TR binding to TREs as the embryos develop into tadpoles (e.g., from stage 20 to 47) associated with the up-regulation of TRα expression is directly correlated with the ability of TH response genes to be activated by T3 treatment in premetamorphic tadpoles. Our results further show that both TR and RXR are bound to the TREs in chromatin, although our assay does not allow us to distinguish between the binding of TR/TR homodimers and TR/RXR heterodimers to TREs. On the other hand, these results together with our earlier studies showing that over-expression of TRs and RXRs together but not individually in Xenopus embryos leads to specific regulation of TH response genes (14) provide a molecular basis, that is, the expression of TR and RXR genes, for tadpole competence (i.e., the ability to respond physiologically to T3).
TR/RXR appears to have dual functions, repressing TH response genes in premetamorphic tadpoles but activating them during metamorphosis. How TR represses genes in vivo during development is yet unclear. Here we have demonstrated that (i) T3 treatment lead to the release of histone deacetylase Rpd3 from the TRE regions of TH response genes in premetamorphic tadpoles, and (ii) treatment with the histone deacetylase inhibitor, TSA, leads to the activation of T3 response genes in vivo in the intestine and tail of premetamorphic tadpoles. These results suggest that during premetamorphic development, unliganded TRs are likely to repress transcription, in part by recruiting corepressor complexes containing histone deacetylases, although direct experimental proof remains be obtained.
The dynamics of histone acetylation provides an attractive mechanism for the reversible activation and repression of transcription (7, 35). Liganded TRs can recruit coactivator complex. Diverse coactivator proteins have been shown to have histone acetyltransferase activities (6, 36). Our in vivo data shows that T3 treatment leads to the release of histone deacetylase at T3 response gene promoters. We also show at least in the intestine and the tail, that T3 increases local histone H4 acetylation, which may be contributed by the release of deacetylase complexes and/or concurrent recruitment of acetylase complexes. These findings are consistent with the idea that histone acetylation is associated with transcriptionally competent chromatin and hypoacetylated histones with transcriptionally silent chromatin, and they support a role for alterations of histone acetylation levels in gene regulation by TRs.
It is worth pointing out that changes in histone acetylation are not necessarily always associated with alterations in gene expression. As we have observed here, T3 treatment led to the up-regulation of the TH response genes in whole animals or individual organs although no detectable changes in histone acetylation could be detected in whole animals. Likewise, the release of repression after TSA treatment was not observed in whole animals, even though it increased histone acetylation. One possible interpretation could be that in whole tadpoles even in presence of T3, the TH response genes may be active in certain tissues but repressed in others. This may lead to unaltered or minute changes in the overall levels of gene expression or histone acetylation after T3 or TSA treatment when analyzed on whole animals. Alternatively, but not mutually exclusively, histone acetylation may play only minor roles in gene repression in some organs/tissues or that multiple repression/activation pathways exists in many organs/tissues such that alterations in histone deacetylation levels alone is insufficient to change gene expression. For example, histone deacetylase-independent repression mechanisms have been suggested by the ability of some components of the TR-recruited corepressor complexes to interact with components of the basal machinery (37–39). Similarly, one TR-coactivators complex has been isolated and found to have no histone acetyltransferase activity (40). In addition, a growing number of non-histone proteins such as transcription factors or coactivators have been shown to be acetylated by histone acetyltransferases, leading to functional modifications (41). Thus, these observations together with our findings here also point to the existence of mechanisms other than those involving histone acetylation changes for transcriptional regulation by TR both in the presence and absence of TH.
In conclusion, our results show that in vivo during amphibian development, TRs are bound to TREs assembled into chromatin, whereas in the absence of hormone, they recruit histone deacetylase complexes to silence transcription in a tissue specific manner. Upon TH synthesis during metamorphosis, the receptors undergo conformational change that could lead to the release of deacetylase complexes and possible recruitment of acetyltransferase complexes, resulting in increased histone acetylation and gene activation. Although this model is likely an over-simplified one considering our current knowledge on the involvement of other cofactor complexes that do not alter histone acetylation levels and the ability of non-histone transcription factors and cofactor to be acetylated (40, 41), such a switch between transcriptional repression and activation involving chromatin remodeling by TRs provides one possible molecular mechanism for the control of the biphasic amphibian development.
Acknowledgments
We thank Drs. D. Guschin, P. Wade, and P. Jones for helpful discussions.
Abbreviations
- T3
3,5,3′-triiodothyronine
- TH
thyroid hormone
- TR
thyroid hormone receptor
- TRE
TH response element
- TSA
trichostatin A
- ChIP
chromatin immunoprecipitation
- RXR
retinoid X receptors
- IFABP
intestinal fatty acid binding protein
- bZIP
basic leucine zipper
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.260141297.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.260141297
References
- 1.Dodd M H I, Dodd J M. In: Physiology of the Amphibia. Lofts B, editor. New York: Academic; 1976. pp. 467–559. [Google Scholar]
- 2.Shi Y-B. Amphibian Metamorphosis: From Morphology to Molecular Biology. New York: Wiley; 1999. [Google Scholar]
- 3.Mangelsdorf D J, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans R M. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolffe A. Nature (London) 1997;387:16–17. doi: 10.1038/387016a0. [DOI] [PubMed] [Google Scholar]
- 5.Chen J D, Li H. Crit Rev Eukaryotic Gene Expression. 1998;8:169–190. doi: 10.1615/critreveukargeneexpr.v8.i2.40. [DOI] [PubMed] [Google Scholar]
- 6.McKenna N J, Lanz R B, O'Malley B W. Endocr Rev. 1999;20:321–344. doi: 10.1210/edrv.20.3.0366. [DOI] [PubMed] [Google Scholar]
- 7.Collingwood T N, Urnov F D, Wolffe A P. J Mol Endocrinol. 1999;23:255–275. doi: 10.1677/jme.0.0230255. [DOI] [PubMed] [Google Scholar]
- 8.Tata J R. BioEssays. 1993;15:239–248. doi: 10.1002/bies.950150404. [DOI] [PubMed] [Google Scholar]
- 9.Yaoita Y, Shi Y-B, Brown D D. Proc Natl Acad Sci USA. 1990;87:7090–7094. doi: 10.1073/pnas.87.18.7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ranjan M, Wong J, Shi Y-B. J Biol Chem. 1994;269:24699–24705. [PubMed] [Google Scholar]
- 11.Machuca I, Esslemont G, Fairclough L, Tata J R. Mol Endocrinol. 1995;9:96–107. doi: 10.1210/mend.9.1.7760854. [DOI] [PubMed] [Google Scholar]
- 12.Wong J, Shi Y-B, Wolffe A P. Genes Dev. 1995;9:2696–2711. doi: 10.1101/gad.9.21.2696. [DOI] [PubMed] [Google Scholar]
- 13.Yaoita Y, Brown D D. Genes Dev. 1990;4:1917–1924. doi: 10.1101/gad.4.11.1917. [DOI] [PubMed] [Google Scholar]
- 14.Puzianowska-Kuznicka M, Damjanovski S, Shi Y-B. Mol Cell Biol. 1997;17:4738–4749. doi: 10.1128/mcb.17.8.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leloup J, Buscaglia M. C R Acad Sci. 1977;284:2261–2263. [Google Scholar]
- 16.Yoshida M, Horinouchi S, Beppu T. BioEssays. 1995;17:423–430. doi: 10.1002/bies.950170510. [DOI] [PubMed] [Google Scholar]
- 17.Shi Y-B, Liang V C-T. Biochem Biophys Acta. 1994;1217:227–228. doi: 10.1016/0167-4781(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 18.Ishizuya-Oka A, Ueda S, Shi Y-B. Dev Genet (Amsterdam) 1997;20:329–337. doi: 10.1002/(SICI)1520-6408(1997)20:4<329::AID-DVG4>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 19.Almouzni G, Khochbin S, Dimitrov S, Wolffe A P. Dev Biol. 1994;165:654–669. doi: 10.1006/dbio.1994.1283. [DOI] [PubMed] [Google Scholar]
- 20.Wong J, Shi Y-B. J Biol Chem. 1995;270:18479–18483. doi: 10.1074/jbc.270.31.18479. [DOI] [PubMed] [Google Scholar]
- 21.Vermaak D, Wade P A, Jones P L, Shi Y-B, Wolffe A P. Mol Cell Biol. 1999;19:5847–5860. doi: 10.1128/mcb.19.9.5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Furlow J D, Brown D D. Mol Endocrinol. 1999;13:2076–2089. doi: 10.1210/mend.13.12.0383. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y-B, Yaoita Y, Brown D D. J Biol Chem. 1992;267:733–738. [PubMed] [Google Scholar]
- 24.Gao X, Sedgwick T, Shi Y-B, Evans T. Mol Cell Biol. 1998;18:2901–2911. doi: 10.1128/mcb.18.5.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y-B, Wong J, Puzianowska-Kuznicka M, Stolow M A. BioEssays. 1996;18:391–399. doi: 10.1002/bies.950180509. [DOI] [PubMed] [Google Scholar]
- 26.Heinzel T, Lavinsky R M, Mullen T M, Soderstrom M, Laherty C D, Torchia J, Yang W M, Brard G, Ngo S D, Davie J R, et al. Nature (London) 1997;387:43–48. doi: 10.1038/387043a0. [DOI] [PubMed] [Google Scholar]
- 27.Nagy L, Kao H Y, Chakravarti D, Lin R J, Hassig C A, Ayer D E, Schreiber S L, Evans R M. Cell. 1997;89:373–380. doi: 10.1016/s0092-8674(00)80218-4. [DOI] [PubMed] [Google Scholar]
- 28.Shi Y-B, Ishizuya-Oka A. Curr Top Dev Biol. 1996;32:205–235. doi: 10.1016/s0070-2153(08)60429-9. [DOI] [PubMed] [Google Scholar]
- 29.Shi Y-B, Hayes W P. Dev Biol. 1994;161:48–58. doi: 10.1006/dbio.1994.1006. [DOI] [PubMed] [Google Scholar]
- 30.Wong J, Shi Y-B, Wolffe A P. EMBO J. 1997;16:3158–3171. doi: 10.1093/emboj/16.11.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wong J, Li Q, Levi B-Z, Shi Y-B, Wolffe A P. EMBO J. 1997;16:7130–7145. doi: 10.1093/emboj/16.23.7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong J, Patterton D, Imhof A, Guschin D, Shi Y-B, Wolffe A P. EMBO J. 1998;17:520–534. doi: 10.1093/emboj/17.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu L, Glass C K, Rosenfeld M G. Curr Opin Genet Dev. 1999;9:140–147. doi: 10.1016/S0959-437X(99)80021-5. [DOI] [PubMed] [Google Scholar]
- 34.Tata J R. Dev Biol. 1968;18:415–440. doi: 10.1016/0012-1606(68)90050-x. [DOI] [PubMed] [Google Scholar]
- 35.Li Q, Sachs L, Shi Y-B, Wolffe A P. Trends Endocrinol Metab. 1999;10:157–164. doi: 10.1016/s1043-2760(98)00141-6. [DOI] [PubMed] [Google Scholar]
- 36.Torchia J, Glass C K, Rosenfeld M G. Curr Opin Cell Biol. 1998;10:373–383. doi: 10.1016/s0955-0674(98)80014-8. [DOI] [PubMed] [Google Scholar]
- 37.Baniahmad A, Ha I, Reinberg D, Tsai S Y, Tsai M J, O'Malley B W. Proc Natl Acad Sci USA. 1993;90:8832–8836. doi: 10.1073/pnas.90.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fondell J D, Roy A L, Roeder R G. Genes Dev. 1993;7:1400–1410. doi: 10.1101/gad.7.7b.1400. [DOI] [PubMed] [Google Scholar]
- 39.Wong C-W, Privalsky M L. Mol Cell Biol. 1998;18:5500–5510. doi: 10.1128/mcb.18.9.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rachez C, Freedman L P. Gene. 2000;246:9–21. doi: 10.1016/s0378-1119(00)00052-4. [DOI] [PubMed] [Google Scholar]
- 41.Cheung W L, Briggs S D, Allis D. Curr Opin Cell Biol. 2000;12:326–333. doi: 10.1016/s0955-0674(00)00096-x. [DOI] [PubMed] [Google Scholar]
- 42.Hu X, Lazar M A. Trends Endocrinol Metab. 2000;11:6–10. doi: 10.1016/s1043-2760(99)00215-5. [DOI] [PubMed] [Google Scholar]