Abstract
Pentavalent methylated arsenic (As) species such as monomethylarsonic acid [MMA(V)] and dimethylarsinic acid [DMA(V)] are used as herbicides or pesticides, and can also be synthesized by soil microorganisms or algae through As methylation. The mechanism of MMA(V) and DMA(V) uptake remains unknown. Recent studies have shown that arsenite is taken up by rice (Oryza sativa) roots through two silicon transporters, Lsi1 (the aquaporin NIP2;1) and Lsi2 (an efflux carrier). Here we investigated whether these two transporters also mediate the uptake of MMA(V) and DMA(V). MMA(V) was partly reduced to trivalent MMA(III) in rice roots, but only MMA(V) was translocated to shoots. DMA(V) was stable in plants. The rice lsi1 mutant lost about 80% and 50% of the uptake capacity for MMA(V) and DMA(V), respectively, compared with the wild-type rice, whereas Lsi2 mutation had little effect. The short-term uptake kinetics of MMA(V) can be described by a Michaelis-Menten plus linear model, with the wild type having 3.5-fold higher Vmax than the lsi1 mutant. The uptake kinetics of DMA(V) were linear with the slope being 2.8-fold higher in the wild type than the lsi1 mutant. Heterologous expression of Lsi1 in Xenopus laevis oocytes significantly increased the uptake of MMA(V) but not DMA(V), possibly because of a very limited uptake of the latter. Uptake of MMA(V) and DMA(V) by wild-type rice was increased as the pH of the medium decreased, consistent with an increasing proportion of the undissociated species. The results demonstrate that Lsi1 mediates the uptake of undissociated methylated As in rice roots.
Arsenic (As) contamination affects millions of people worldwide, particularly in South Asia where As-contaminated groundwater has been extracted for drinking (Chakraborti et al., 2002; Nordstrom, 2002). Recent studies have shown that foods, especially rice (Oryza sativa), are an important source of inorganic As to populations dependent on a rice diet (Kile et al., 2007; Ohno et al., 2007; Mondal and Polya, 2008). Paddy rice is more efficient than other cereal crops in accumulating As (Williams et al., 2007). This is because anaerobic conditions in submerged paddy soils lead to mobilization of arsenite [As(III); Takahashi et al., 2004; Xu et al., 2008], which is then taken up by rice roots mainly through the highly efficient transport pathway for silicon (Si; Ma et al., 2008). The relatively high accumulation of As in rice is of concern, as it may pose a significant health risk (Zhu et al., 2008; Meharg et al., 2009).
A number of As species may be present in soil depending on soil conditions and the history of As contamination. These include arsenate [As(V)], As(III), and methylated As species such as monomethylarsonic acid [MMA(V): CH3AsO(OH)2] and dimethylarsinic acid [DMA(V): (CH3)2AsO(OH)]. As(V) is the main species in aerobic soils, while As(III) dominates in anaerobic environments such as flooded paddy soils. Both MMA(V) and DMA(V) have been found in paddy soils (Takamatsu et al., 1982), which may have been derived from microbial and algal biomethylation and/or past uses of methylated As compounds. MMA(V), as sodium or calcium salt, and DMA(V), as sodium salt or free acid (also called cacodylic acid), are herbicides widely used for weed control on cotton (Gossypium hirsutum), orchards, and lawns, or as a defoliant of cotton (U.S. Environmental Protection Agency, 2006). Conversion of cotton fields for the production of paddy rice in the United States may be a reason for the high levels of methylated As reported for the U.S. rice (Meharg et al., 2009).
The mechanism of As(V) uptake by plants through the phosphate transport system has been well established (for review, see Zhao et al., 2009). In contrast, As(III) is taken up into the cells by aquaglyceroporins in Escherichia coli, yeast (Saccharomyces cerevisiae), and mammalian tissues (for review, see Bhattacharjee and Rosen, 2007). Recent studies have shown that several plant aquaporin channels belonging to the Nodulin 26-like Intrinsic Protein (NIP) subfamily are permeable to As(III) when expressed heterologously in yeast (Bienert et al., 2008; Isayenkov and Maathuis, 2008; Ma et al., 2008). The rice Si transporter Lsi1 (OsNIP2;1; Ma et al., 2006) is also permeable to As(III) when expressed in yeast or Xenopus laevis oocytes (Ma et al., 2008). Furthermore, the lsi1 rice mutant lost 57% of the influx capacity for As(III) compared to the wild type in short-term assays, suggesting that Lsi1 is an important entry route for As(III) (Ma et al., 2008). In rice roots, a second Si transporter, Lsi2, functions as an efflux carrier to transport Si efflux from the exodermis and endodermis cells toward the stele for xylem loading (Ma et al., 2007). This transporter also mediates As(III) efflux; two independent lsi2 mutants had 73% to 91% lower concentrations of As(III) in the xylem sap than their wild types (Ma et al., 2008). The shared uptake pathway between Si (silicic acid) and As(III) (arsenous acid) is consistent with their physiochemical properties; both are present predominantly as undissociated neutral molecules at the normal environmental and physiological pH range (pKa = 9.2, >99% undissociated at pH ≤ 7.0), and the two molecules have similar sizes.
The uptake mechanisms of methylated As species by plant roots are not known. Previous studies showed that both MMA(V) and DMA(V) can be taken up by roots and translocated to shoots in a number of plant species (Marin et al., 1992; Carbonell-Barrachina et al., 1998, 1999; Burló et al., 1999). Marin et al. (1992) found that uptake by rice followed the order of As(III) > MMA(V) > As(V) > DMA (V), although DMA(V) was more efficiently translocated from roots to shoots than other As species. Raab et al. (2007) reported large variations in the absorption and translocation efficiencies for As(V), MMA(V), and DMA(V) among the 46 plant species tested. On average, root absorption of As(V) was 2.5- and 5-times higher than MMA(V) and DMA(V), respectively. The translocation efficiency, defined as the shoot-to-root concentration ratio after 24-h exposure, was highest for DMA(V) (0.8), followed by MMA(V) (0.3) and As(V) (0.09). The concentration-dependent uptake kinetics of MMA(V) in rice roots could be described by the Michaelis-Menten equation, whereas the limited uptake of DMA(V) appeared to be linear in relation to the increasing concentration in the uptake medium (Abedin et al., 2002). Abbas and Meharg (2008) showed that DMA(V) uptake by maize (Zea mays) seedlings was enhanced by more than 10-fold by a pretreatment of phosphorus starvation; this compared with only 2-fold increase in As(V) uptake. They thought that DMA(V) might be taken up by the phosphate transporters, or that phosphorus starvation altered expression of a range of membrane transporters or even membrane permeability itself.
In addition to the root uptake of methylated As species, some plants appear to be able to biomethylate As, but the pathway and enzymology remains unclear (Wu et al., 2002; Zhao et al., 2009). In microbes, As methylation follows the Challenger pathway involving repeated steps of As reduction and oxidative methylation (Bentley and Chasteen, 2002). As(V) is first reduced to As(III), which is methylated by S-adenosylmethyltransferase using S-adenosyl-l-Met as the methyl donor. The product of this reaction is pentavalent MMA(V), which is reduced by a reductase to trivalent MMA(III) with thiols (e.g. glutathione). Methylation and reduction steps continue to produce di- and trimethyl As compounds. MMA(III) and DMA(III) are intermediates in the As methylation pathway, which is not very stable (Gong et al., 2001). In rice grain, DMA(V) is the main form of methylated As, and can account for up to 80% of the total As (Zavala et al., 2008; Meharg et al., 2009). In light of the significant presence of methylated As in rice, it is important to elucidate the transport and assimilation pathways of these As species in plants.
In this study, we present evidence that MMA(V) and DMA(V) are taken up by rice roots, at least partly, through the NIP aquaporin channel Lsi1, and that this process is strongly pH dependent. We also show that MMA(V) can be reduced to MMA(III) in planta.
RESULTS
As Speciation in Rice Plants Supplied with MMA(V) or DMA(V)
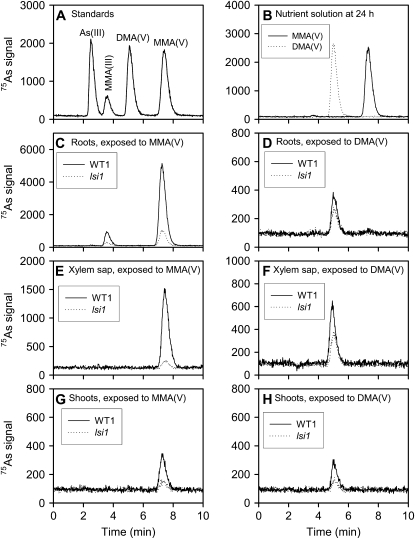
We first investigated if MMA(V) or DMA(V) supplied to plants undergoes chemical species transformation in the medium or inside plant tissues. Plants of the lsi1 mutant and its wild-type cultivar (WT1 = cv Oochikara) were exposed to 5 μm MMA(V) or DMA(V) in the nutrient solution for 24 h. As speciation in the nutrient solution, xylem sap, roots, and shoots was determined by HPLC-inductively coupled plasma (ICP)-mass spectrometry (MS; Fig. 1). Both MMA(V) and DMA(V) remained stable in the nutrient solution, with no transformation to other As species within 24 h (Fig. 1B).
Figure 1.
As speciation using HPLC-ICP-MS. Rice plants were exposed to 5 μm MMA(V) or DMA(V) for 24 h. A, Chromatogram of standards containing 25 ng As L−1 of As(III), MMA(V), and DMA(V) and 7.5 ng As L−1 MMA(III). B, Nutrient solutions after 24 h exposure. C and D, Root extracts of the wild type and lsi1 mutant exposed to MMA(V) (C) or DMA(V) (D). E and F, Xylem sap collected from the wild type and lsi1 mutant exposed to MMA(V) (E) or DMA(V) (F). G and H, Shoot extracts of the wild type and lsi1 mutant exposed to MMA(V) (G) or DMA(V) (H). 75As signal was normalized by the signal of the internal standard 72Ge.
In the roots of WT1 and lsi1 exposed to MMA(V), two As species, MMA(V) and MMA(III) [monomethylarsonous acid: CH3As(OH)2] were detected (Fig. 1C), indicating that MMA(V) was partly reduced to MMA(III) in rice roots. MMA(III) accounted for 13.6% ± 0.5% and 16.0% ± 0.4% of the extractable As in the roots of WT1 and lsi1, respectively, suggesting a similar extent of reduction of MMA(V) to MMA(III) in these two lines of rice. In contrast, only MMA(V) was detectable in the xylem sap and shoot extracts (Fig. 1, E and G).
In the root and shoot extracts and the xylem sap of both WT1 and lsi1 exposed to DMA(V), only DMA(V) was detectable, showing no transformation to other As species within 24 h (Fig. 1, D, F, and H).
It is clear from Figure 1 that the lsi1 mutant took up much less MMA(V) and DMA(V) than WT1; this was further investigated as described below.
Effects of Lsi1 and Lsi2 Mutation on the Uptake and Root-to-Shoot Translocation of MMA(V)
To investigate whether Lsi1 and Lsi2 are involved in the transport of MMA(V), we exposed the rice mutants lsi1 and lsi2, the double mutant lsi1lsi2, and their wild-type cultivars to 5 μm MMA(V) (pH 5.5) for 24 h. After preculture in the basal nutrient solution for 26 d, the root or shoot fresh weights were comparable between lsi1 and WT1, and between lsi2 and its wild type (WT2 = cv T-65).
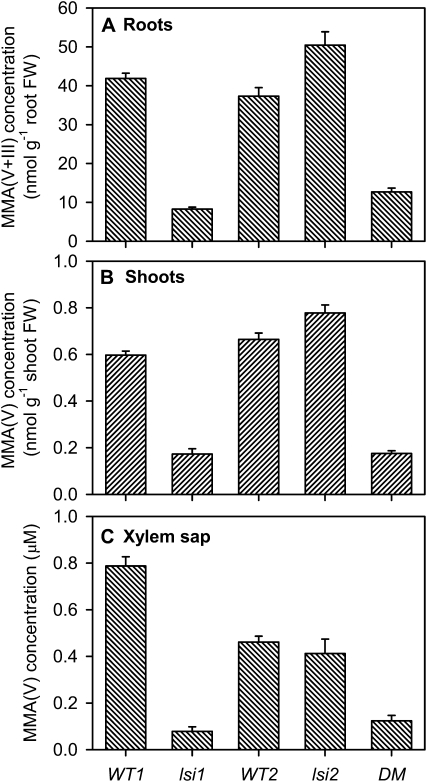
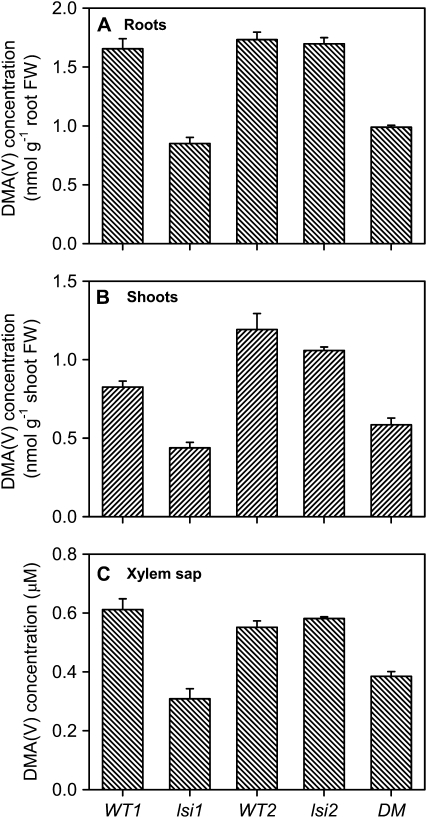
Compared with WT1, the lsi1 mutant had 80% lower concentration of MMA [the sum of MMA(V) and MMA(III)] in roots (P < 0.001 according to one-way ANOVA; Fig. 2). Similarly, the concentration of MMA(V) was 71% and 90% lower in shoots and xylem sap, respectively [P < 0.001; MMA(III) was not detected in these samples]. In contrast, the differences between WT2 and lsi2 were relatively small, with the latter having 35% higher concentration of MMA(V + III) in roots (P < 0.01) and 17% higher concentration of MMA(V) in shoots (P < 0.05) than the former (Fig. 2). The reasons for the slightly higher MMA accumulation in lsi2 are unclear. There was no significant difference between WT2 and the lsi2 mutant in the concentration of MMA(V) in the xylem sap. The concentrations of MMA(V + III) in roots, and MMA(V) in shoots and xylem sap of the double mutant lsi1lsi2 were similar to those of lsi1, and significantly (P < 0.001) lower than those of either WT1 or WT2.
Figure 2.
Concentrations of MMA(V + III) in roots (A), MMA(V) in shoots (B), and xylem sap (C) of the lsi1, lsi2, and lsi1si2 (double mutant [DM]) mutants and their wild-type rice. Plants were exposed to 5 μm MMA(V) for 24 h at pH 5.5. Error bars are +ses (n = 4). FW, Fresh weight.
Total As concentrations in roots and shoots were also determined. Their patterns (Supplemental Fig. S1) were essentially the same as those shown for the extractable MMA in Figure 2. The amount of MMA extracted by the phosphate buffer solution (PBS) accounted for on average 73% and 60% of the total As in roots and shoots, respectively.
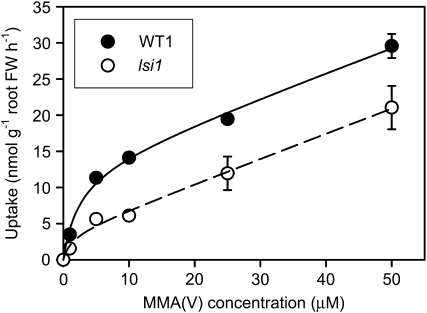
The data presented in Figure 2 indicate that the Lsi1 mutation resulted in a large loss in the ability of MMA(V) uptake, whereas the Lsi2 mutation did not. We further compared the short-term (30 min) uptake kinetics of MMA(V) by WT1 and lsi1 at a solution pH of 5.5. The uptake data (total As concentration in roots) can be described satisfactorily by a Michaelis-Menten model plus a linear equation (Fig. 3). The main difference between WT1 and lsi1 was found in Vmax, with the former being 3.5 times the latter (Table I). In contrast, there were no significant differences in Km or the linear slope.
Figure 3.
Kinetics of MMA(V) uptake into the roots of the wild-type rice and the lsi1 mutant. Plants were exposed to 0 to 50 μm MMA(V) for 30 min at pH 5.5. Lines are the fitted curves of the Michaelis-Menten plus linear model. Error bars are ±ses (n = 4). FW, Fresh weight.
Table I.
Kinetic parameters of MMA(V) and DMA(V) uptake by the lsi1 mutant and its wild-type rice
| As Species and Rice Line | Vmax | Km | Linear Slope | R2 |
|---|---|---|---|---|
| nmol g−1 root fresh weight h−1 | μm | |||
| MMA(V) uptake | ||||
| WT1 | 13.1 ± 1.9 | 2.4 ± 1.0 | 0.33 ± 0.04 | 0.99 |
| lsi1 | 3.7 ± 1.2 | 1.3 ± 1.5 | 0.35 ± 0.03 | 0.99 |
| DMA(V) uptake | ||||
| WT1 | 0.14 ± 0.006 | 0.99 | ||
| lsi1 | 0.052 ± 0.005 | 0.96 | ||
Because Lsi1 is a Si-transporting channel protein, we also investigated the effect of Si on MMA(V) uptake. The presence of 0.5 mm silicic acid in the nutrient solution did not significantly affect the short-term (30 min) uptake of MMA(V) (5 μm) by either WT1 or lsi1 (Supplemental Fig. S2A).
Effects of Lsi1 and Lsi2 Mutation on the Uptake and Root-to-Shoot Translocation of DMA(V)
After exposure to 5 μm DMA(V) (pH 5.5) for 24 h, the lsi1 mutant had 49%, 47%, and 50% lower concentrations of DMA(V) in the root and shoot extracts and xylem sap, respectively, than WT1 (P < 0.001; Fig. 4). In contrast, there was no significant difference between lsi2 and WT2 in either root, shoot, or xylem sap concentration. The lsi1lsi2 double mutant behaved similarly to the lsi1 single mutant, and had significantly (P < 0.001) lower concentrations of DMA(V) than either wild type. The patterns of total As concentrations in roots and shoots (Supplemental Fig. S3) resemble those of DMA(V) shown in Figure 4. The PBS extraction recovered 62% and 106% of the total As in roots and shoots, respectively.
Figure 4.
Concentrations of DMA(V) in roots (A), shoots (B), and xylem sap (C) of the lsi1, lsi2, and lsi1lsi2 (double mutant [DM]) mutants and their wild-type rice. Plants were exposed to 5 μm DMA(V) for 24 h at pH 5.5. Error bars are +ses (n = 4). FW, Fresh weight.
Comparing Figures 2 and 4, it is clear that rice roots took up much less (20- to 30-fold) DMA(V) than MMA(V). However, the concentrations of DMA(V) and MMA(V) in the xylem sap and in the shoots were comparable, even though their concentrations in the roots differed markedly.
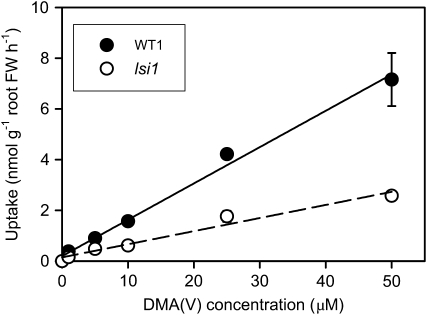
The kinetics of short-term DMA(V) uptake by both WT1 and lsi1 showed a linear pattern without saturation within the concentration range tested (1–50 μm; Fig. 5). The linear slope obtained for WT1 was 2.8 times that of lsi1 (Table I). Similar to MMA(V), the presence of silicic acid (0.5 mm) did not significantly inhibit DMA(V) uptake by either WT1 or lsi1 within the 30 min uptake period (Supplemental Fig. S2B).
Figure 5.
Kinetics of DMA(V) uptake into the roots of the wild-type rice and the lsi1 mutant. Plants were exposed to 0 to 50 μm DMA(V) for 30 min at pH 5.5. Lines are the fitted curves of a linear model. Error bars are ±ses (n = 4). FW, Fresh weight.
Uptake of MMA(V) and DMA(V) by Wild-Type Rice Was Sensitive to Solution pH
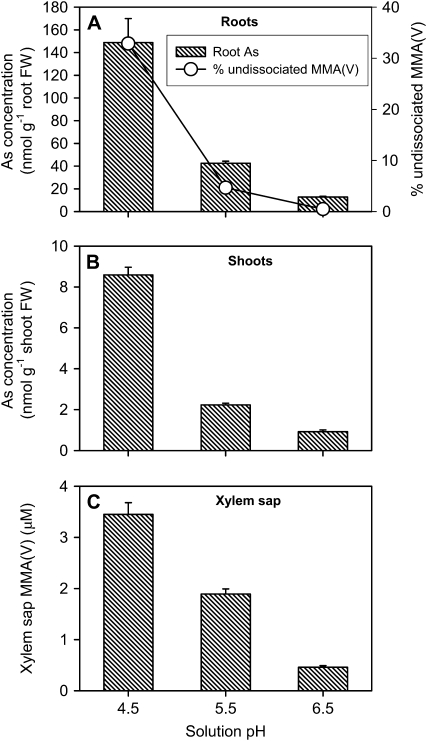
Aquaporins mediate fluxes of water and neutral molecules such as glycerol, urea, ammonia, silicic acid, and boric acid (Maurel et al., 2008). Compared with As(III) (pKa1 = 9.2), MMA(V) and DMA(V) have lower acid dissociation constants (pKa1 = 4.19 and 6.14, respectively; Zhang and Selim, 2008). Therefore, variation in the medium pH within the environmentally or physiologically relevant range may influence MMA(V) and DMA(V) uptake by affecting the extent of proton dissociation from the two As species. We investigated this hypothesis using WT1, which has a normal functional Lsi1. Plants were exposed to 5 μm MMA(V) or DMA(V) at three different pHs (4.5, 5.5, and 6.5) for 24 h. Solution pH was found to have a large effect on MMA(V) uptake (Fig. 6). Increasing pH from 4.5 to 5.5 and 6.5 decreased root As concentration by 71% and 91%, respectively (P < 0.001). Similarly, shoot As concentration was decreased by 74% and 89%, and xylem sap As by 45% and 87%, respectively, with increasing solution pH (P < 0.001). These patterns were broadly similar to the percentages of undissociated MMA(V) in the uptake solution, which decreased from 33% at pH 4.5 to 0.5% at pH 6.5 (Fig. 6A).
Figure 6.
Effect of medium pH on the proportion of undissociated MMA(V) in the medium and the uptake of MMA(V) by wild-type rice (WT1). Plants were exposed to 5 μm MMA(V) for 24 h at different pHs. Total As concentrations in roots (A) and shoots (B), and MMA(V) concentration in xylem sap (C). Error bars are +ses (n = 4). FW, Fresh weight.
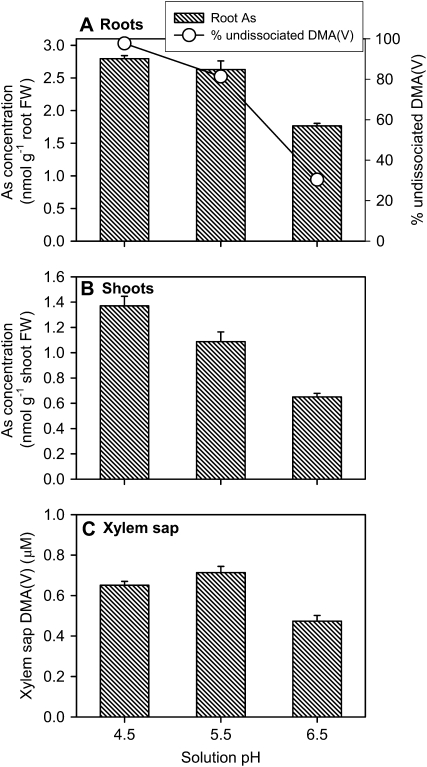
Solution pH had a smaller effect on DMA(V) uptake, which was most noticeable when pH was increased from pH 5.5 to 6.5 (34%–40% decrease in root, shoot, and xylem sap As concentrations, P < 0.001; Fig. 7). This pattern was generally consistent with the effect of pH on DMA(V) dissociation in the uptake solution (Fig. 7A). The undissociated DMA was the predominant species at pH 4.5 (98%) and 5.5 (81%), decreasing to 30% at pH 6.5.
Figure 7.
Effect of medium pH on the proportion of undissociated DMA(V) in the medium and the uptake of DMA(V) by wild-type rice (WT1). Plants were exposed to 5 μm DMA(V) for 24 h at different pHs. Total As concentrations in roots (A) and shoots (B), and DMA(V) concentration in xylem sap (C). Error bars are +ses (n = 4). FW, Fresh weight.
Assay of Transport Activity Using X. laevis Oocytes
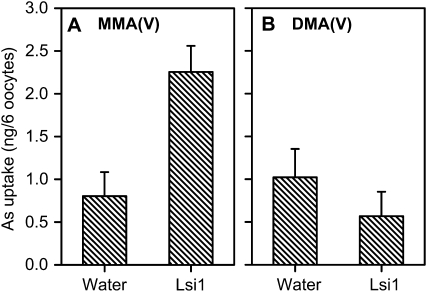
To obtain further evidence that Lsi1 can mediate influx of MMA(V) or DMA(V), we expressed the rice Lsi1 gene in X. laevis oocytes. When the pH of the medium was 4.5, MMA(V) uptake into the oocytes expressing Lsi1 was increased by 2.8-fold compared with the control (Fig. 8A). However, no enhancement in DMA(V) uptake was observed (Fig. 8B). At pH 7.8, which is the normal pH used for oocyte assays, no increase in either MMA(V) or DMA(V) uptake was observed from the expression of the Lsi1 gene (data not shown). This was not surprising because at this pH most of the MMA(V) (99.98%) and the DMA(V) (98%) were dissociated.
Figure 8.
Transport activity of the rice Lsi1 expressed in X. laevis oocytes. Oocytes were incubated with 100 μm MMA(V) (A) or DMA(V) (B) for 30 min. Injection of water serves as the control. Error bars are +ses (n = 3).
DISCUSSION
The results from this study demonstrate that the NIP aquaporin Lsi1 mediates the influx of MMA(V), and to a lesser extent, DMA(V), into rice roots. The lsi1 mutant, with a single mutation at the position of the 132nd amino acid (Ma et al., 2006), lost about 80% and 50% of the capacity to take up MMA(V) and DMA(V), respectively, in both 30-min and 1-d uptake experiments, compared with the wild-type rice (Figs. 2–5). When expressed heterologously in Xenopus oocytes, Lsi1 also showed transport activity for MMA(V) (Fig. 8). The lack of a detectable transport activity for DMA(V) in oocytes may be because its influx was much smaller than that of MMA(V), and thus difficult to detect within the relatively short period of transport assay. A significant effect of solution pH on the uptake of MMA(V) and DMA(V) by the roots of wild-type rice was observed, which is consistent with the pH effect on the dissociation of protons from both molecules. These data support the model of Lsi1-mediated uptake of protonated neutral molecules of MMA(V) and DMA(V). To our knowledge, our study is the first report that a plant aquaporin can transport pentavalent methylated As compounds. Previously, Liu et al. (2006) showed that the rat aquaglyceroporin AQP9 is able to facilitate the uptake of protonated MMA(III) into the yeast cells expressing the AQP9 gene, in addition to its permeability to As(III). They did not investigate whether AQP9 can transport MMA(V) or DMA(V). In our study, the possibility of MMA(V) being first reduced to MMA(III) prior to uptake via Lsi1 can be ruled out, as MMA(V) remained stable in the nutrient solution.
In rice roots, Lsi1 is highly expressed in the distal side of the plasma membranes in both exodermis and endodermis cells, where Casparian bands are formed (Ma et al., 2006). Its primary function is to facilitate the entry of silicic acid into root cells, Si being important for the resistance of rice against biotic and abiotic stresses (Ma and Yamaji, 2008). It appears that the pore structure of Lsi1 is also suitable for the passage of As(III) (Ma et al., 2008) as well as methylated As species. The molecular sizes of MMA(V) and DMA(V) are comparable to that of arsenous acid; all three As species are likely to be slightly smaller than that of silicic acid (Ma et al., 2008). However, methylation increases hydrophobicity. The more hydrophobic nature of DMA(V) may explain its much lower uptake than MMA(V) and As(III) (Figs. 2–5; also Abedin et al., 2002; Raab et al., 2007). A number of other NIP channels are permeable to As(III) (Bienert et al., 2008; Isayenkov and Maathuis, 2008; Ma et al., 2008; Kamiya et al., 2009). It would be interesting to examine if they are also permeable to methylated As. However, Lsi1 is much more highly expressed in rice roots than other NIP genes (Sakurai et al., 2005; Ma et al., 2008), and would be the main aquaporin involved in As transport in rice roots.
The lower pKa of MMA(V) and DMA(V) than As(III) means that the uptake of these methylated As will be much more sensitive to the environmental pH than As(III). The pH dependency observed in our study also explains the inconsistency in previous studies comparing uptake of methylated As versus inorganic As. For example, Marin et al. (1992) reported an efficient uptake of MMA(V) by rice, most likely because they used a low pH in the nutrient solution (pH 4.0), which favors the occurrence of protonated molecule, whereas others (Abedin et al., 2002; Raab et al., 2007) observed a low uptake efficiency of MMA(V) by rice using media with higher pHs. There remains an intriguing possibility that dissociated methylated As may be taken up by a different pathway, e.g. by phosphate transporters as speculated by Abbas and Meharg (2008). However, direct evidence for this speculation is still lacking.
If MMA(V) and DMA(V) uptake is mediated by Lsi1, why then did Si not inhibit their uptake by rice roots? One possible explanation is that aquaporins such as Lsi1 permit extremely fast flux of solutes (Maurel et al., 2008), and therefore competition between analogous substrates are less apparent. In yeast expressing plant NIP genes including Lsi1, As(III) uptake was not inhibited by Si (Bienert et al., 2008). Si also did not inhibit As(III) influx into Xenopus oocytes expressing Lsi1 (J.F. Ma and N. Mitani, unpublished data). The addition of Si did significantly decrease As(III) accumulation in rice shoots (Ma et al., 2008; Li et al., 2009), but this effect is attributed to competition for the efflux carrier Lsi2.
Interestingly, unlike Lsi1, Lsi2 does not appear to be involved in the transport of MMA(V) or DMA(V), since root uptake, xylem sap, and shoot concentrations of these methylated As did not decrease in the rice lsi2 mutant (Figs. 2 and 4). The Lsi2 protein, localized at the proximal side of both exodermis and endodermis cells of rice roots, mediates the efflux of silicic acid (Ma et al., 2007) and As(III) (Ma et al., 2008) from the exodermis and endodermis cells toward the stele for xylem loading, and plays a crucial role in the distribution of As(III) from roots to shoots. The lack of involvement of Lsi2 in the transport of methylated As may be because most of the MMA(V) and DMA(V) are dissociated at the cytoplasmic pH (approximately 7.5), and the negatively charged methylated As may be transported to the xylem via a different pathway. Alternatively, the Lsi2 efflux carrier protein may have a higher substrate specificity than the Lsi1 channel.
Our study provides in planta evidence that MMA(V) can be reduced to MMA(III) in rice roots (Fig. 1). This reduction is in accordance with the Challenger pathway of As methylation in microorganisms (Bentley and Chasteen, 2002). The enzyme responsible for MMA(V) reduction is unknown in plants. In human this reduction is catalyzed by the glutathione transferase (GST) omega with reduced glutathione as the electron donor (Aposhian et al., 2004). There are more than 50 GSTs in plants, though none of the omega class (Dixon et al., 2009). Presently it is not known if any plant GSTs can reduce MMA(V). MMA(III) has been shown to be much more toxic to human hepatocytes than MMA(V) and As(III) (Petrick et al., 2000). In plants MMA(III) can be complexed with thiol-rich phytochelatins (Raab et al., 2005), thus reducing its toxicity. Such complexation and possibly subsequent sequestration in vacuoles in the root may explain why MMA(III) was not detected in the xylem sap (Fig. 1). In contrast, MMA(V) and DMA(V) are not complexed by thiols and are readily loaded into xylem of rice. DMA(V), despite its very limited root uptake, is particularly mobile in the xylem translocation (Fig. 4). This finding is in agreement with previous studies (Marin et al., 1992; Raab et al., 2007).
MATERIALS AND METHODS
Plant Materials and Culture
Five lines of rice (Oryza sativa) were used, including the lsi1 mutant (Ma et al., 2002) and its wild-type cv Oochikara (WT1), the lsi2 mutant (Ma et al., 2007) and its wild-type cv T-65 (WT2), and the lsi1lsi2 double mutant. The double mutant was created by crossing lsi1 and lsi2 and isolated by genotyping the responsible genes using cleaved amplified polymorphic sequence markers. Seeds were surface sterilized with 0.5% NaOCl for 15 min, rinsed and soaked in deionized water overnight, and then placed on a nylon net floating on a 0.5 mm CaCl2 solution. After germination, seedlings were transferred to a 30-L container filled with the one-half-strength Kimura solution. The nutrient composition was as follows: 0.091 mm KNO3, 0.183 mm Ca(NO3)2, 0.274 mm MgSO4, 0.1 mm KH2PO4, 0.183 mm (NH4)2SO4, 0.5 μm MnCl2, 3 μm H3BO3, 0.1 μm (NH4)6Mo7O24, 0.4 μm ZnSO4, 0.2 μm CuSO4, 20 μm NaFe(III)-EDTA, and 2 mm MES (pH adjusted to 5.5 with NaOH). Nutrient solution was renewed every 3 d. The growth conditions were 16-h photoperiod with a light intensity of 350 μmol m−2 s−1, 25°C/20°C day/night temperatures, and 70% relative humidity.
Uptake of MMA(V) and DMA(V)
Seedlings (26 d old) of the five rice lines were transferred to 1-L pots containing 900 mL basal nutrient solution and 5 μm MMA(V) or DMA(V). Four plants were placed in each pot and each treatment was replicated in four pots. Solution pH was maintained at 5.5 with 2 mm MES buffer. Twenty-four hours later, stems were cut at about 1 cm above the roots with a sharp blade. The cut surfaces were rinsed with deionized water, blotted dry, and xylem exudates collected by pipette for 1 h after decapitation. Rice shoots were rinsed with deionized water, blotted dry, and frozen in liquid nitrogen. Rice roots were rinsed briefly in an ice-cold desorption solution containing 1 mm K2HPO4, 0.5 mm Ca(NO3)2, and 5 mm MES (pH 5.5), and immersed in 900 mL of the same solution for 10 min to remove apoplastic As. Root samples were blotted dry and frozen in liquid nitrogen. Shoots and roots were ground in a mortar and pestle with liquid nitrogen. Aliquots (0.2–0.5 g) of the ground materials were extracted with 20 mL PBS (2 mm NaH2PO4 and 0.2 mm Na2-EDTA, pH 6.0) for 1 h under sonication. The extract was filtered through four layers of muslin cloth, followed by filtration through a 0.45-micron filter before analysis of As speciation. Nutrient solutions were also sampled at 24 h for the analysis of As speciation. Both xylem sap and nutrient solutions were diluted with PBS solution, filtered through 0.45 micron, and stored on ice before analysis of As speciation.
Uptake Kinetics of MMA(V) and DMA(V)
The WT1 and lsi1 seedlings (18 d old) were used in this experiment. Three plants were transferred to a pot filled with 350 mL uptake solution containing 0.5 mm Ca(NO3)2, 5 mm MES (pH 5.5), and different concentrations (1–50 μm) of MMA(V) or DMA(V). Each treatment was replicated in four pots. The uptake solution was aerated continuously. After 30 min roots were rinsed and desorbed of the apoplastic As as described above, blotted dry, and weighed. Root samples were dried at 70°C for 48 h before analysis of total As. Shoots were not analyzed because a preliminary experiment showed negligible amounts of As in shoots after 30 min exposure to MMA(V) or DMA(V).
Effect of pH on the Uptake of MMA(V) and DMA(V)
WT1 was used in this experiment. Four 19-d-old seedlings were transferred to a 1-L pot filled with 900 mL basal nutrient solution and 5 μm MMA(V) or DMA(V). Solution pH was buffered at 4.5, 5.5, and 6.5 with 5 mm MES (pH adjusted with either NaOH or HCl). Each treatment was replicated in four pots. After 24 h, xylem sap and the root and shoot samples were collected as described in the first experiment. Solution pH was measured after 24 h uptake, which showed a small decrease (0.1–0.2 units) in all treatment solutions.
Transport Activity Assay Using Xenopus laevis Oocytes
The transport activities for MMA(V) and DMA(V) mediated by Lsi1 were assayed using Xenopus laevis oocytes. Oocytes were isolated from adult female X. laevis frogs, and placed in a modified Barth's saline (MBS) solution [88 mm NaCl, 1 mm KCl, 2.4 mm NaHCO3, 15 mm Tris-HCl pH 7.6, 0.3 mm Ca(NO3)2, 0.41 mm CaCl2, 0.82 mm MgSO4, 10 μg mL−1 sodium penicillin, 10 μg mL−1 streptomycin sulfate]. Oocytes were then treated with 0.1% collagenase type B (Roche Diagnostics) in a calcium-free MBS for 1.5 h to remove follicular cell layers, washed five times with MBS free of collagenase, and selected according to the size and development stage. Selected oocytes were incubated for 1 d in MBS at 18°C. The open reading frame of the Lsi1 gene was amplified and cloned as described before (Mitani et al., 2008) and the cRNA with cap analog was synthesized with mMASSAGGE mMACHINE High Yield Capped RNA transcription kit (Ambion) according to the manufacturer's instructions. Fifty nanoliters of cRNA (1 ng nL−1) were injected into the selected oocytes using a Nanoject II automatic injector (Drummond Scientific). As a negative control, 50 nL of RNase-free water were injected. After incubation in MBS at 18°C for 1 d, the oocytes were exposed to the MBS buffer at pH 4.5 containing 100 μm MMA(V) or DMA(V). Each treatment was replicated three times with each replicate containing six oocytes. After 30 min exposure, the oocytes were washed five times with MBS without As, homogenized with 0.5 mL of 0.1 n HNO3, and analyzed for As concentrations.
Analysis of As Concentration and Speciation
As speciation in nutrient solutions, xylem saps, and plant extracts was determined using HPLC-ICP-MS (Agilent LC1100 series and Agilent ICP-MS 7500ce, Agilent Technologies), as described previously (Xu et al., 2007) with modifications. As species [As(III), As(V), DMA(V), MMA(V), and MMA(III)] were separated by an anion-exchange column (Hamilton PRP X-100). DMA(V) was obtained from Aldrich and MMA(V) from Chem Service. MMA(III) was prepared by dissolving the solid oxide CH3AsO in deionized water. MMA(III) oxide was synthesized according to Cullen et al. (1989). Initially, As species were separated by a mobile phase containing 6.6 mm NH4H2PO4 and 6.6 mm NH4NO3 (pH 6.3), run isocratically at 0.6 mL min−1. This method allows a separation (in the order of retention time) of As(III), DMA(V), MMA(V), and As(V) within 10 min. However, DMA(V) and MMA(III) were not separated at the baseline. Because As(V) was not detected in any of the samples analyzed, we lowered the concentration of the eluant to 2 mm NH4H2PO4 and 2 mm NH4NO3 (pH 6.3, flow rate 0.8 mL min−1) to achieve a baseline separation of As(III), MMA(III), DMA(V), and MMA(V) (Fig. 1A). The outlet of the separation column was connected to a concentric nebulizer and a water-jacketed cyclonic spray chamber of the ICP-MS. An internal standard (germanium [Ge]) was mixed continuously with the post-column solution through a peristaltic pump. Signals at mass-to-charge ratio (m/z) 75 (As), 72 (Ge), and 35 (chlorine [Cl]) were collected with a dwell time of 500 ms for As and Ge and 200 ms for Cl. Possible polyatomic interference of 40Ar35Cl on m/z 75 was removed by the Agilent octopole reaction system operating in the helium gas mode. The As signal was normalized by the Ge signal to correct any signal drift during the analysis. Peaks were identified by comparisons with the retention times of standard compounds. As species in the samples were quantified by external calibration curves with peak areas. Analysis of As species was carried out immediately following sample collection or extraction. For each batch of samples, the analysis was completed within 12 h; no changes in As speciation were observed during this period of time.
Ground plant samples were digested in 5 mL high purity HNO3/HClO4 (87/13, v/v). Total As concentrations in the samples were determined by ICP-MS (Agilent 7500ce) operating in the helium gas mode to remove possible interference of ArCl on m/z 75. Certified reference materials (seaweed IAEA-140/TM and tomato leaves NIST1573a) and blanks were included for quality assurance. Repeated analysis of the two certified reference materials gave 43.6 ± 1.5 μg As g−1 for IAEA-140/TM (certified value 44.3 ± 2.1 μg As g−1) and 0.106 ± 0.0057 μg As g−1 for NIST1573a (certified value 0.112 ± 0.004 μg As g−1), respectively.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers AB222272 (Lsi1) and AB222273 (Lsi2).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Total As concentrations in roots and shoots of the lsi1, lsi2, and lsi1lsi2 mutants and their wild-type rice exposed to 5 μm MMA(V) for 24 h.
Supplemental Figure S2. Effect of Si (0.5 mm) on the concentration of As in the roots of the lsi1 and the wild-type rice exposed to 5 μm MMA(V) or DMA(V) for 30 min.
Supplemental Figure S3. Total As concentrations in roots and shoots of the lsi1, lsi2, and lsi1lsi2 mutants and their wild-type rice exposed to 5 μm DMA(V) for 24 h.
Supplementary Material
This work was supported by the Department for International Development and the Biotechnology and Biological Sciences Research Council (grant no. BB/F004087/1 to S.P.M. and F.-J.Z.), a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (grant no. 21248009 to J.F.M.), and a grant from the Ministry of Agriculture, Forestry and Fisheries of Japan (Genomics for Agricultural Innovation grant no. IPG–0006 to J.F.M.). Rothamsted research is an institute of the Biotechnology and Biological Sciences Research Council of the United Kingdom.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Fang-Jie Zhao (fangjie.zhao@bbsrc.ac.uk).
The online version of this article contains Web-only data.
References
- Abbas MHH, Meharg AA (2008) Arsenate, arsenite and dimethyl arsinic acid (DMA) uptake and tolerance in maize (Zea mays L.). Plant Soil 304 277–289 [Google Scholar]
- Abedin MJ, Feldmann J, Meharg AA (2002) Uptake kinetics of arsenic species in rice plants. Plant Physiol 128 1120–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aposhian HV, Zakharyan RA, Avram MD, Sampayo-Reyes A, Wollenberg ML (2004) A review of the enzymology of arsenic metabolism and a new potential role of hydrogen peroxide in the detoxication of the trivalent arsenic species. Toxicol Appl Pharmacol 198 327–335 [DOI] [PubMed] [Google Scholar]
- Bentley R, Chasteen TG (2002) Microbial methylation of metalloids: arsenic, antimony, and bismuth. Microbiol Mol Biol Rev 66 250–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee H, Rosen BP (2007) Arsenic metabolism in prokaryotic and eukaryotic microbes. In DH Nies, S Silver, eds, Molecular Microbiology of Heavy Metals, Vol Microbiol Monograph 6. Springer-Verlag, Berlin, pp 371–406
- Bienert GP, Thorsen M, Schüssler MD, Nilsson HR, Wagner A, Tamás MJ, Jahn TP (2008) A subgroup of plant aquaporins facilitate the bi-directional diffusion of As(OH)3 and Sb(OH)3 across membranes. BMC Biol 6 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burló F, Guijarro I, Carbonell-Barrachina AA, Valero D, Martinez-Sánchez F (1999) Arsenic species: effects on and accumulation by tomato plants. J Agric Food Chem 47 1247–1253 [DOI] [PubMed] [Google Scholar]
- Carbonell-Barrachina AA, Aarabi MA, DeLaune RD, Gambrell RP, Patrick WH (1998) The influence of arsenic chemical form and concentration on Spartina patens and Spartina alterniflora growth and tissue arsenic concentration. Plant Soil 198 33–43 [Google Scholar]
- Carbonell-Barrachina AA, Burlo F, Valero D, Lopez E, Martinez-Romero D, Martinez-Sanchez F (1999) Arsenic toxicity and accumulation in turnip as affected by arsenic chemical speciation. J Agric Food Chem 47 2288–2294 [DOI] [PubMed] [Google Scholar]
- Chakraborti D, Rahman MM, Paul K, Chowdhury UK, Sengupta MK, Lodh D, Chanda CR, Saha KC, Mukherjee SC (2002) Arsenic calamity in the Indian subcontinent—what lessons have been learned? Talanta 58 3–22 [DOI] [PubMed] [Google Scholar]
- Cullen WR, McBride BC, Manji H, Pickett AW, Reglinsky J (1989) The metabolism of methylarsine oxide and sulfide. Appl Organomet Chem 3 71–78 [Google Scholar]
- Dixon DP, Hawkins T, Hussey PJ, Edwards R (2009) Enzyme activities and subcellular localization of members of the Arabidopsis glutathione transferase superfamily. J Exp Bot 60 1207–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong ZL, Lu XF, Cullen WR, Le XC (2001) Unstable trivalent arsenic metabolites, monomethylarsonous acid and dimethylarsinous acid. J Anal At Spectrom 16 1409–1413 [Google Scholar]
- Isayenkov SV, Maathuis FJM (2008) The Arabidopsis thaliana aquaglyceroporin AtNIP7;1 is a pathway for arsenite uptake. FEBS Lett 582 1625–1628 [DOI] [PubMed] [Google Scholar]
- Kamiya T, Tanaka M, Mitani N, Ma JF, Maeshima M, Fujiwara T (2009) NIP1;1, an aquaporin homolog, determines the arsenite sensitivity of Arabidopsis thaliana. J Biol Chem 284 2114–2120 [DOI] [PubMed] [Google Scholar]
- Kile ML, Houseman EA, Breton CV, Smith T, Quamruzzaman O, Rahman M, Mahiuddin G, Christiani DC (2007) Dietary arsenic exposure in Bangladesh. Environ Health Perspect 115 889–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li RY, Stroud JL, Ma JF, McGrath SP, Zhao FJ (2009) Mitigation of arsenic accumulation in rice with water management and silicon fertilization. Environ Sci Technol 43 3778–3783 [DOI] [PubMed] [Google Scholar]
- Liu ZJ, Styblo M, Rosen BP (2006) Methylarsonous acid transport by aquaglyceroporins. Environ Health Perspect 114 527–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Ichii M, Wu GF (2002) A rice mutant defective in Si uptake. Plant Physiol 130 2111–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Tamai K, Yamaji N, Mitani N, Konishi S, Katsuhara M, Ishiguro M, Murata Y, Yano M (2006) A silicon transporter in rice. Nature 440 688–691 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N (2008) Functions and transport of silicon in plants. Cell Mol Life Sci 65 3049–3057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Tamai K, Konishi S, Fujiwara T, Katsuhara M, Yano M (2007) An efflux transporter of silicon in rice. Nature 448 209–212 [DOI] [PubMed] [Google Scholar]
- Ma JF, Yamaji N, Mitani N, Xu XY, Su YH, McGrath SP, Zhao FJ (2008) Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc Natl Acad Sci USA 105 9931–9935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin AR, Masscheleyn PH, Patrick WH (1992) The influence of chemical form and concentration of arsenic on rice growth and tissue arsenic concentration. Plant Soil 139 175–183 [Google Scholar]
- Maurel C, Verdoucq L, Luu DT, Santoni V (2008) Plant aquaporins: membrane channels with multiple integrated functions. Annu Rev Plant Biol 59 595–624 [DOI] [PubMed] [Google Scholar]
- Meharg AA, Williams PN, Adomako E, Lawgali YY, Deacon C, Villada A, Cambell RCJ, Sun G, Zhu YG, Feldmann J, et al (2009) Geographical variation in total and inorganic arsenic content of polished (white) rice. Environ Sci Technol 43 1612–1617 [DOI] [PubMed] [Google Scholar]
- Mitani N, Yamaji N, Ma JF (2008) Characterization of substrate specificity of a rice silicon transporter, Lsi1. Pflugers Arch 456 679–686 [DOI] [PubMed] [Google Scholar]
- Mondal D, Polya DA (2008) Rice is a major exposure route for arsenic in Chakdaha block, Nadia district, West Bengal, India: a probabilistic risk assessment. Appl Geochem 23 2987–2998 [Google Scholar]
- Nordstrom DK (2002) Public health—worldwide occurrences of arsenic in ground water. Science 296 2143–2145 [DOI] [PubMed] [Google Scholar]
- Ohno K, Yanase T, Matsuo Y, Kimura T, Rahman MH, Magara Y, Matsui Y (2007) Arsenic intake via water and food by a population living in an arsenic-affected area of Bangladesh. Sci Total Environ 381 68–76 [DOI] [PubMed] [Google Scholar]
- Petrick JS, Ayala-Fierro F, Cullen WR, Carter DE, Aposhian HV (2000) Monomethylarsonous acid (MMA(III)) is more toxic than arsenite in Chang human hepatocytes. Toxicol Appl Pharmacol 163 203–207 [DOI] [PubMed] [Google Scholar]
- Raab A, Schat H, Meharg AA, Feldmann J (2005) Uptake, translocation and transformation of arsenate and arsenite in sunflower (Helianthus annuus): formation of arsenic-phytochelatin complexes during exposure to high arsenic concentrations. New Phytol 168 551–558 [DOI] [PubMed] [Google Scholar]
- Raab A, Williams PN, Meharg A, Feldmann J (2007) Uptake and translocation of inorganic and methylated arsenic species by plants. Environ Chem 4 197–203 [Google Scholar]
- Sakurai J, Ishikawa F, Yamaguchi T, Uemura M, Maeshima M (2005) Identification of 33 rice aquaporin genes and analysis of their expression and function. Plant Cell Physiol 46 1568–1577 [DOI] [PubMed] [Google Scholar]
- Takahashi Y, Minamikawa R, Hattori KH, Kurishima K, Kihou N, Yuita K (2004) Arsenic behavior in paddy fields during the cycle of flooded and non-flooded periods. Environ Sci Technol 38 1038–1044 [DOI] [PubMed] [Google Scholar]
- Takamatsu T, Aoki H, Yoshida T (1982) Determination of arsenate, arsenite, monomethylarsonate, and dimethylarsinate in soil polluted with arsenic. Soil Sci 133 239–246 [Google Scholar]
- U.S. Environmental Protection Agency (2006) Revised reregistration eligibility decision for MSMA, DSMA, CAMA, and cacodylic acid. http://www.epa.gov/pesticides/reregistration/REDs/organic_arsenicals_red.pdf (June 26, 2009)
- Williams PN, Villada A, Deacon C, Raab A, Figuerola J, Green AJ, Feldmann J, Meharg AA (2007) Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ Sci Technol 41 6854–6859 [DOI] [PubMed] [Google Scholar]
- Wu JH, Zhang R, Lilley RM (2002) Methylation of arsenic in vitro by cell extracts from bentgrass (Agrostis tenuis): effect of acute exposure of plants to arsenate. Funct Plant Biol 29 73–80 [DOI] [PubMed] [Google Scholar]
- Xu XY, McGrath SP, Meharg A, Zhao FJ (2008) Growing rice aerobically markedly decreases arsenic accumulation. Environ Sci Technol 42 5574–5579 [DOI] [PubMed] [Google Scholar]
- Xu XY, McGrath SP, Zhao FJ (2007) Rapid reduction of arsenate in the medium mediated by plant roots. New Phytol 176 590–599 [DOI] [PubMed] [Google Scholar]
- Zavala YJ, Gerads R, Gürleyük H, Duxbury JM (2008) Arsenic in rice: II. Arsenic speciation in USA grain and implications for human health. Environ Sci Technol 42 3861–3866 [DOI] [PubMed] [Google Scholar]
- Zhang H, Selim HM (2008) Reaction and transport of arsenic in soils: equilibrium and kinetic modeling. Adv Agron 98 45–115 [Google Scholar]
- Zhao FJ, Ma JF, Meharg AA, McGrath SP (2009) Arsenic uptake and metabolism in plants. New Phytol 181 777–794 [DOI] [PubMed] [Google Scholar]
- Zhu YG, Williams PN, Meharg AA (2008) Exposure to inorganic arsenic from rice: a global health issue? Environ Pollut 154 169–171 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.