Abstract
Truffles are symbiotic fungi that form ectomycorrhizas with plant roots. Here we present evidence that at an early stage of the interaction, i.e. prior to physical contact, mycelia of the white truffle Tuber borchii and the black truffle Tuber melanopsorum induce alterations in root morphology of the host Cistus incanus and the nonhost Arabidopsis (Arabidopsis thaliana; i.e. primary root shortening, lateral root formation, root hair stimulation). This was most likely due to the production of indole-3-acetic acid (IAA) and ethylene by the mycelium. Application of a mixture of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid and IAA fully mimicked the root morphology induced by the mycelium for both host and nonhost plants. Application of the single hormones only partially mimicked it. Furthermore, primary root growth was not inhibited in the Arabidopsis auxin transport mutant aux1-7 by truffle metabolites while root branching was less effected in the ethylene-insensitive mutant ein2-LH. The double mutant aux1-7;ein2-LH displayed reduced sensitivity to fungus-induced primary root shortening and branching. In agreement with the signaling nature of truffle metabolites, increased expression of the auxin response reporter DR5∷GFP in Arabidopsis root meristems subjected to the mycelium could be observed, confirming that truffles modify the endogenous hormonal balance of plants. Last, we demonstrate that truffles synthesize ethylene from l-methionine probably through the α-keto-γ-(methylthio)butyric acid pathway. Taken together, these results establish the central role of IAA and ethylene as signal molecules in truffle/plant interactions.
Ectomycorrhizal symbioses are mutualistic interactions between filamentous fungi and plant roots. Truffles, which are ascomycete fungi renown for their aromatic fruiting bodies, form ectomycorrhizas (ECM) in temperate climates predominantly with trees (i.e. hazel [Corylus avellana], oaks [Quercus spp.]).
In soil, microorganisms communicate with plants by exchanging chemical signals throughout the rhizosphere. Depending on the nature of the interaction, these molecules can be either volatiles or solutes (dissolved solids). For example, rhizobacteria induce growth promotion in Arabidopsis (Arabidopsis thaliana) through the action of the volatile compound 2,3-butanediol (Ryu et al., 2003). The sesquiterpene volatile (E)-β-caryophyllene is produced by maize (Zea mays) roots fed upon by arthropods, and serves as attractant to natural enemies of the insects (Rasmann et al., 2005). Nonvolatile signal molecules can also dissolve in water and diffuse in the soil. Indeed the nitrogen-fixing bacteria Rhizobium secrete a nodulation factor that induces changes in root morphology of legumes (Dénarié and Cullimore, 1993; Heidstra and Bisseling, 1996). Mycelium branching is induced in the mycorrhizal fungus Gigaspora margarita by 5-deoxy-strigol, a strigolactone exuded from the roots of the legume host Lotus japonicus (Akiyama et al., 2005).
Also ectomycorrhizal fungi engage in a molecular dialogue with plants and produce chemical signals that modulate plant root/ECM morphogenesis. The indole alkaloid hypaphorine produced by ectomycorrhizal fungus Pisolithus tinctorius inhibits root hair elongation in the host Eucalyptus globules and the nonhost Arabidopsis (Béguiristain et al.,1995; Reboutier et al., 2002). Hormones, mainly indole-3-acetic acid (IAA), have also been often implicated in symbiotic interactions (Barker and Tagu, 2000; Martin et al., 2001; Sirrenberg et al., 2007; Contreras-Cornejo et al., 2009). Most studies involving hormones have focused at a late stage of interaction, when ECMs were developing or already formed (for review, see Barker and Tagu, 2000). Indeed IAA-overproducing mutants of the ectomycorrhizal fungus Hebeloma cylindrosporum form significantly more mycorrhizas with the host Pinus pinaster than the wild type (Gay et al., 1994). Similarly applying exogenous IAA to the ectomycorrhizal system Piloderma croceum/Quercus robur also resulted in a more intense ECM colonization of the host compared to controls with no additional IAA (Herrmann et al., 2004).
Truffles form ECM with a variety of hosts such as oaks, hazels, but also some shrubs (i.e. Cistus). Under laboratory conditions, the establishment of the symbiotic phase takes 2 to 3 months (Sisti et al., 1998; Miozzi et al., 2005). Strains of the same truffle species vary in their capacity to colonize a single host (Giomaro et al., 2000). Volatile organic compounds have been implicated in the signaling between truffle and plants. Splivallo et al. (2007) discussed the phytotoxic activity of fruiting body volatiles and Menotta et al. (2004) highlighted their potential role as mycorrhization signals. Fruiting bodies volatiles shortened primary roots of plants (Splivallo et al., 2007) while the effect of mycelial metabolites has not been addressed.
Our aim here was to investigate how truffle mycelia modify plant root architecture. We focus our effort on an early stage of interaction (10 d) to explain changes in root morphology prior to ECM formation and highlight the action of diffusible signals on the host plant Cistus incanus and the nonhost Arabidopsis. Using Arabidopsis mutants, we did not aim to investigate the mechanism behind the IAA/ethylene cross talk but rather to show that the fungal metabolites are perceived in planta through both auxin and ethylene signaling pathways. We illustrate how truffle metabolites modify the auxin response of the root meristem using the auxin reporter line DR5∷GFP. We further demonstrate that both hormones are produced by truffles at concentrations that fully explain the root phenotypic responses of the host and nonhost plants. Last, we elucidate ethylene biosynthesis in truffles.
RESULTS
Truffles Modify Root Morphology and Architecture of Both Host and Nonhost Plants
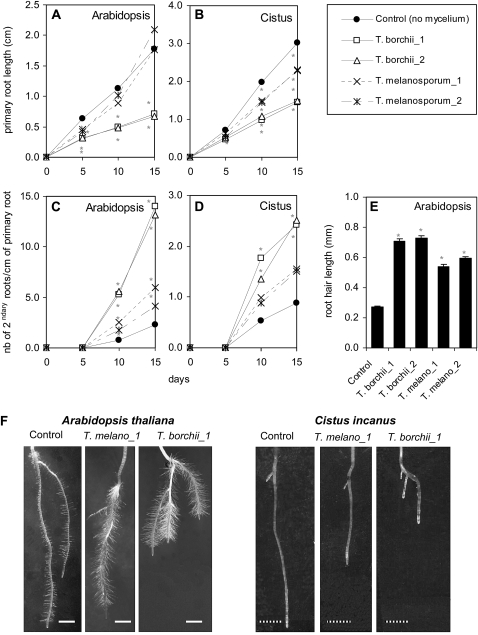
The effect of signals released by truffle mycelia on root architecture of the host C. incanus or the nonhost Arabidopsis was investigated. Petri dishes used to cocultivate plants and fungi (Fig. 1) were inoculated with either mycelium of two strains of Tuber melanosporum, two strains of Tuber borchii, or mock inoculated with an agar plug for the control. Root morphology (root length, branching, and root hair length) was recorded every 5 d up to 15 d (Fig. 2). During the period of cocultivation plant roots and mycelium did not enter in direct contact (Supplemental Fig. S1). Changes in root morphology can consequently be attributed to diffusible fungal signals.
Figure 1.
Uncompartmented bioassay. Dual bioassay used to test the effect of truffle mycelial exudates/volatiles on plants. The lower half of the petri dish is filled with malt extract and inoculated with either mycelium or an agar plug (mock control) and kept in the dark at 23°C ± 2°C. After 10 d (to allow biomass buildup) seeds of the test plants are added to the upper part of the petri dish (on Murashige and Skoog agar). Note that the mycelium and plant roots are not in direct contact (in this picture, C. incanus seedlings are 10-d-old). Scale: Petri dish is 10 × 10 cm2. [See online article for color version of this figure.]
Figure 2.
Changes in root morphology of Arabidopsis and C. incanus induced by truffle mycelia metabolites. Using the bioassay setup of Figure 1, two truffle species (T. melanosporum_1 = strain 1, T. melanosporum_2 = strain 2; and T. borchii_1 = strain 1, T_borchii_2 = strain 2) reduced primary root length of Arabidopsis (A and F) and C. incanus (B and F), and increased root branching (C, D, and F) and increased root hair length in Arabidopsis (E and F). F, Root morphology of 10-d-old seedlings (scale Arabidopsis 1.0 mm, scale Cistus 2.5 mm). Statistics: Asterisk (*) indicates statistically different results from control (P < 0.05, ANOVA on ranks with Dunn's posthoc test; for root length and branching n > 30 seedlings/treatment; for root hair length n = 300). Bars in E represent ses.
The presence of the two strains of T. borchii inhibited primary root growth of Arabidopsis while T. melanosporum inhibited primary root growth only transiently at 5 d postinoculation and resumed normal growth afterward. For the host plant C. incanus all strains of T. borchii and T. melanosporum significantly inhibited primary root growth (Fig. 2, A, B, and F). Root branching was stimulated with all truffle strains for Arabidopsis, but only with the two strains of T. borchii for C. incanus (Fig. 2, C, D, and F). Root hair length of Arabidopsis was also increased by all the truffle strains/species tested here (Fig. 2, E and F)—C. incanus did not develop any root hair (Fig. 2F). In summary, we observed that the presence of truffle mycelia induces profound changes in root morphology and architecture. These changes are partly dependent on the truffle species involved but can occur in a host as well as in a nonhost plant.
Truffles Release IAA in the Culture Medium
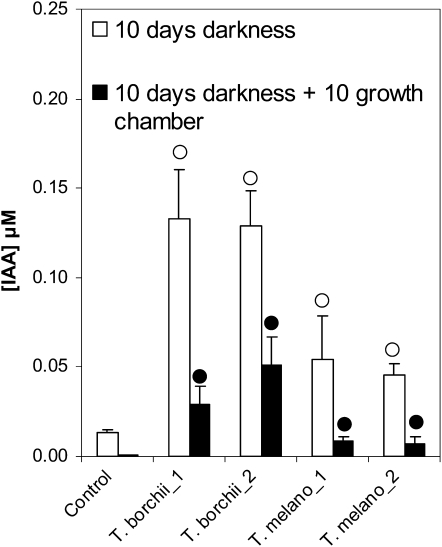
The morphological changes observed upon coculturing Arabidopsis seedlings with fungal mycelia (Fig. 2) are reminiscent of plants treated with the phytohormones auxin (IAA) or ethylene. Since both IAA and ethylene can be produced by ectomycorrhizal fungi (Graham and Linderman, 1980; Strzelczyk et al., 1994; Barker and Tagu, 2000), we investigated whether truffles produced IAA and ethylene under the conditions of the bioassay described in Figure 1. IAA exuded by truffles was quantified in petri dishes without plants to assert that the IAA was indeed of fungal origin (Supplemental Fig. S2). Quantification was done by HPLC-electrospray ionization (ESI)-tandem mass spectrometry (MS/MS) from the Murashige and Skoog agar portion of the petri dishes (Supplemental Fig. S2) after 10 d of mycelial growth (which corresponds to the time when the seeds of the test plants were added to the petri dishes in Fig. 1) and after 10 additional days in the growth chamber (corresponding to 10-d-old seedlings in Fig. 1). After 10 d in the dark, IAA was present in the Murashige and Skoog portion of the control petri dishes (probably due to diffusion of the IAA contained in malt extract; see Dix and Van Staden, 1982) but its concentration was significantly higher in the petri dishes that contained mycelia of T. borchii or T. melanosporum (Fig. 3). After 10 d in the growth chamber (day/night cycles), IAA concentrations had dropped compared to the earlier time point, nevertheless remaining significantly higher than in the control for the strains of T. borchii (Fig. 3). We conclude that T. borchii and T. melanosporum release IAA into the culture medium. IAA accumulates in the medium in the dark, and is partially degraded by light (Dunlap and Robacker, 1988) under the growth chamber conditions (16-h photoperiods).
Figure 3.
IAA concentrations in agar resulting from the exudation of truffle mycelia. After 10 d of growth in the dark, strains of T. borchii and T. melanosporum increased the IAA concentration from 3 to 10 times compared to the control petri dish containing no mycelium. IAA concentration after 10 more days in the growth chamber had dropped in all samples due to degradation by light (n = 3 or 4 petri dish/treatment, bars indicate sd; circles [○ for day 10 and • for day 10 + 10] indicate statistical differences compared to the respective controls, P < 0.05, Mann-Whitney).
Truffles Produce Ethylene from l-Met Probably via the α-Keto-γ-(Methylthio)Butyric Acid Pathway
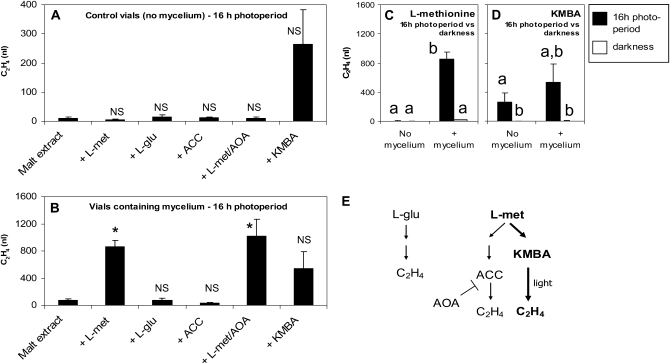
Ethylene production by truffle mycelium was measured by gas chromatography (GC)-flame ionization detector. T. borchii (strain 1) was grown on pure malt extract or malt extract containing various ethylene precursors/inhibitor for 10 d, either in growth chamber conditions with 16-h photoperiods (as in the bioassays depicted in Fig. 1) or in darkness. No plants were present in the experimental setup to make sure that the ethylene detected was indeed produced by the mycelium (Supplemental Fig. S2). Under the bioassay conditions (16-h photoperiods), the malt extract itself released small amounts of ethylene, but significantly more ethylene was produced by the mycelium (P < 0.01, Mann-Whitney test).
Three ethylene biosynthesis pathways have been described in microorganisms (Chagué et al., 2002). We investigated which one might be functional in truffles. α-Keto-γ-(methylthio)butyric acid (KMBA) is known to be degraded to ethylene by light (Yang, 1967; Billington et al., 1979). Not knowing to which extent this photodegradation occurred under our bioassay conditions, we first tested KMBA's and the other ethylene precursors/inhibitor's stability in malt extract agar. After 10 d under a 16-h photoperiod, malt extract itself released tiny amounts of ethylene (Fig. 4A). Under the same photoregime, supplementing malt extract with KMBA did not significantly increase the ethylene level (Fig. 4A). However, significantly more ethylene was released from KMBA-supplemented malt extract kept in 16-h photoperiod compared to KMBA-supplemented malt extract kept in the dark (Fig. 4D). This demonstrates that KMBA was photodegraded to ethylene under our experimental conditions.
Figure 4.
Ethylene biosynthesis in truffles. The occurrence of three ethylene biosynthesis pathways described in microorganisms was investigated in T. borchii using various ethylene precursors/inhibitor. Under 16-h photoperiods and without mycelium, addition of the ethylene precursor/inhibitors to malt extract did not significantly increase ethylene concentration (A). Under 16-h photoperiods, significantly higher ethylene levels were detected from mycelium grown on l-Met compared to the mycelium grown on unsupplemented medium (B). The other ethylene precursors (l-Gln, ACC, KMBA) did not increase the ethylene concentration compared to the mycelium grown on unsupplemented medium (B). Addition of AOA, an inhibitor of the ACC pathway, did not reduce ethylene concentration, confirming that l-Met was not transformed through the ACC pathway (B). The mycelium could only transform l-Met to ethylene under 16-h photoperiods, but not in the dark (C). In the contrary, KMBA was photodegraded to ethylene regardless of the presence/absence of the mycelium, while the molecule was not degraded in the dark (D). Taken together, these results demonstrate that ethylene is synthesized from l-Met probably through the KMBA pathway (marked in bold in E). Statistics: P < 0.05, Mann-Whitney. For A and B, asterisk (*) indicates difference from the unsupplemented malt extract; NS = nonsignificant. For C and D, different letters indicate statistically different results. For each treatment, n ≥ 3. Bars = ses (A–D).
A second set of experiments was performed to investigate which ethylene precursor can be used by the mycelium to produced ethylene. l-Met and l-Gln are two known ethylene precursors in microorganisms (Chagué et al., 2002). l-Met transformation to ethylene can proceed via two different intermediates, namely 1-aminocyclopropane-1-carboxylic acid (ACC) and KMBA (Chagué et al., 2002).
Under 16-h photoperiods, significantly higher ethylene levels were detected from mycelium grown on l-Met compared to the mycelium grown on unsupplemented medium (Fig. 4B). The other ethylene precursors (l-Glu, ACC, KMBA) did not significantly increase ethylene concentration as compared to the mycelium grown on unsupplemented medium (Fig. 4B). Furthermore, addition of (aminooxy)acetic acid hemihydrochloride (AOA; an inhibitor of the ACC pathway) to malt extract supplemented with l-Met did not reduce ethylene concentration, confirming that l-Met was not transformed through the ACC pathway (Fig. 4B).
Taken together, these results suggest that ethylene synthesis proceeds either through KMBA or via an undiscovered pathway. We further tested the KMBA pathway hypothesis by incubating mycelium in the dark with l-Met. No ethylene production was observed (Fig. 4C), suggesting that l-Met was actually transformed to an intermediate that required photodegradation to release ethylene. Since KMBA can be photodegraded to ethylene (Fig. 4D), it might actually be the intermediate produced by the mycelium, yet the presence of another pathway or of a different intermediate cannot be excluded.
Ethylene Released by Truffles Increases Root Hair Length
To confirm that ethylene produced by truffles was responsible for the alteration of root morphology in Arabidopsis seedlings, we modified the bioassay setup depicted in Figure 1 as follows (compartmented bioassy): Fungal mycelium was grown inside a small petri dish, itself contained in a larger one with plants (Supplemental Fig. S3). In this manner, nonvolatile metabolites produced by mycelium such as IAA could not reach the seedlings. Since both IAA and ethylene are able to increase root hair length (Pitts et al., 1998) in a concentration-dependent manner, the compartmented bioassay permitted us to check whether ethylene was produced at a high enough concentration to induce root hair elongation. In the compartmented bioassay, mycelium grown on pure malt extract stimulated root hair growth (Supplemental Fig. S4). Root hair growth was further stimulated by supplying mycelium with the ethylene precursor l-Met (Supplemental Fig. S4, C and E). When an ethylene trap was included in the bioassay, root hair elongation was reduced (Supplemental Fig. S5D).
In the compartmented bioassay, primary root length was only inhibited when ethylene production was induced by supplying the mycelium with 10 mm l-Met (Supplemental Fig. S4, A and D), and the inhibition was reduced in the presence of an ethylene trap (Supplemental Fig. S5B). Root branching was also slightly increased (approximately 1.6 times) by volatiles released by the mycelium grown on malt extract (not supplemented with l-Met; Supplemental Fig. S4B).
In summary, in the compartmented bioassay root hair length was affected by mycelial volatiles and the extent of the effect could be modulated with an ethylene precursor and a trap for volatiles. This corroborates the hypothesis that Arabidopsis seedlings are sensing ethylene released by the mycelium.
Response of the Host and Nonhost to IAA and ACC
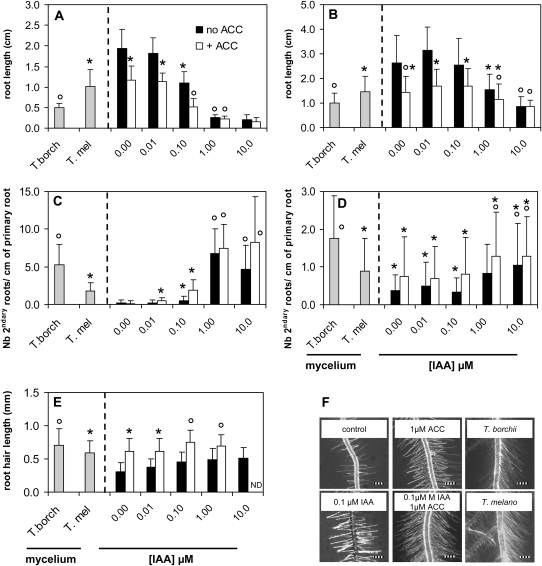
To investigate whether IAA and ethylene could account for the morphological changes observed with host and nonhost plants, dose-response curves for both hormones were established. Ethylene is difficult to handle in the bioassays because of its volatility, and consequently ACC, its direct nonvolatile precursor in plants was used. ACC was chosen rather than l-Met because it is converted to ethylene in a single enzymatic step compared to three steps for l-Met. The bioassay setup shown in Figure 1 (without mycelium) was supplied with varying concentrations of IAA (from 10 to 0.01 μm) alone, or including the ethylene precursor ACC (1 μm). The results are displayed in Figure 5. The extent of root growth inhibition, lateral root density, and root hair elongation caused by T. borchii (strain 1) and T. melanosporum (strain 1) were mimicked by IAA concentrations in the medium ranging between 1 and 0.1 μm and 0.1 and 0.01 μm, respectively (Fig. 5, A–D), well in agreement with the IAA amounts exuded by those two species into the medium (0.13 ± 0.03 μm for T. borchii strain 1 and 0.05 ± 0.02 μm for T. melanosporum strain 1; Fig. 3). While IAA treatment alone often mimicked the primary root shortening and branching response observed with both truffle species, the additive action of IAA and ACC was needed to fully restore Arabidopsis's root hair elongation induced by the mycelium (Fig. 5, E and F). In summary, a combined exogenous application of IAA and ethylene induced root responses equivalent to the presence of truffle mycelium in both host and nonhost plant.
Figure 5.
Comparison of root morphology induced by truffles or IAA ± ACC. Root length (A and B), branching (C and D), and root hair length (E) of Arabidopsis (A, C, and E) or Cistus (B and D) subjected to mycelial metabolites of T. borchii strain 1 or T. melanosporum strain 1 (gray bars) or synthetic IAA alone (black bars), or IAA and ACC (1 μm; white bars). Error bars represent ses; n > 15 seedlings per treatment. The same symbols (*, ○) represent values that are statistically the same (P < 0.05, ANOVA on ranks with posthoc Dunn's test). Note that the root hair phenotype induced by the mycelium in Arabidopsis was only fully restored by supplying IAA and ACC together (E and F: root hair of 15-d-old seedlings, scale bars in F = 0.25 mm; ND = not determined).
Truffle Metabolites Are Perceived by Arabidopsis through IAA and Ethylene Signaling Pathways
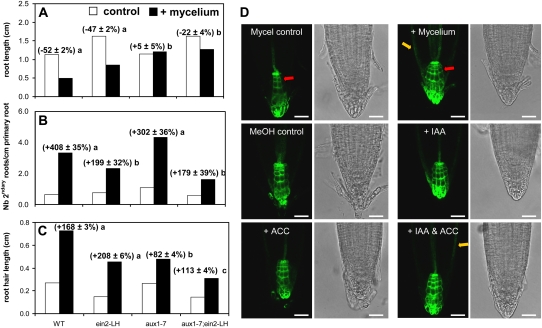
To address more specifically whether Arabidopsis reacts on mycelial auxin or ethylene we made use of several Arabidopsis mutants with reduced sensitivity toward these two phytohoromones. Seedlings of Arabidopsis, including the auxin influx mutant aux1-7 that has reduced auxin and ethylene sensitivity (Pickett et al., 1990), the ethylene-insensitive mutant ein2-LH, and the double mutant aux1-7;ein2-LH were grown for 10 d either in the presence of T. borchii (strain 1) or mock inoculated with an agar plug (controls) as described in Figure 1. Compared to their respective controls, the mycelium inhibited primary root development to similar levels in the wild-type and ein2-LH mutant, while no or significantly smaller inhibition was observed for aux1-7 or the double mutant (Fig. 6A). Lateral root density was equally strongly induced by T. borchii mycelium in aux1-7 as in wild type. However, the ein2-LH and the double mutant aux1-7;ein2-LH were significantly less sensitive (both to similar levels) to lateral root induction than the wild type (Fig. 6B). The shortened root hair phenotype of aux1-7 and aux1-7;ein2-LH double mutants was partially rescued by the presence of T. borhii mycelium (Fig. 6C). Additional auxin and ethylene mutants, including the null mutants aux1-T and ein2-T (Fischer et al., 2006) and the auxin signaling mutants axr1-3 and axr4-1, were also tested and gave comparable results (Supplemental Fig. S6). In summary the changes in root morphology induced by truffle metabolites in Arabidopsis can be attributed partly to auxin transport and signaling (inhibition of primary root growth and induction of root hair growth) and to ethylene signaling (induction of lateral root formation).
Figure 6.
Screening Arabidopsis mutants for resistance to truffle metabolites. Using the bioassay setup of Figure 1, the auxin aux1-7, ethylene ein2-LH, and double mutant aux1-7;ein2-LH were tested for their sensitivity to truffle (T. borchii strain 1) metabolites and compared to the wild type. A to C, Primary root length (A), root branching (B), and root hair length (C). Bars represent average values for 10-d-old seedlings, n > 20 seedlings/treatment; for root hair length n = 300, 15-d-old seedlings. Percentages represent changes versus respective controls (±ses). Different letters indicates statistically different values (P < 0.05, ANOVA on ranks and posthoc Dunn's test). D, Root tips of the Arabidopsis auxin reporter line DR5∷GFP. The mycelium increased the GFP signal intensity at the level of the first tier columnella cells (red arrows) and at the level of the epidermis (yellow arrows) compared to the mycelium control in DR5∷GFP roots. Hormones including IAA (0.1 μm) and ACC (1.0 μm) mimicked this increase in signal intensity fully when applied together (+IAA and ACC; D). For quantification of the GFP signal, refer to Supplemental Figure S7. MeOH control = control for the IAA, ACC, and IAA and ACC treatments. Scale bars in D = 25 μm.
To assess whether truffle metabolites modified auxin response levels in Arabidopsis roots, a reporter line transformed with the auxin-responsive DR5 promoter driving GFP (DR5∷GFP) was treated as in the bioassay of Figure 1, either coinoculated with T. borchii (strain 1) or mock inoculated with an agar plug (control). Five-day-old seedlings subjected to the mycelium showed a significant signal increase in the first tier columnella cells and epidermis compared to the untreated seedlings (Fig. 6D; Supplemental Fig. S7). Furthermore the signal increase in the first tier columnella cells was mimicked by ACC (1 μm) applied alone or in combination with IAA (0.1 μm; Supplemental Fig. S7). On the contrary only the additive effect of both hormones triggered the GFP signal observed with the mycelium in the epidermis (Supplemental Fig. S7).
DISCUSSION
Previous work established the role of fungal IAA in ECM interactions, while the role of ethylene has been suggested but not proven (Barker and Tagu, 2000). We report that IAA and ethylene produced by truffles act additively on plant roots to induce changes such as root shortening, increased branching, and root hair elongation.
ECM colonize plants by producing a fungal mantle enveloping short secondary roots and a Hartig net consisting of intercellular hyphae that develop between root cells (Smith and Read, 1997). ECM formation is known to induce changes in root morphology, including formation of lateral roots and in some plants root dichotomous branching in meristems (Peterson and Bonfante, 1994).
Our data highlights that lateral root formation can already be stimulated before contact between an ECM fungus and plant roots, demonstrating that the increase in branching is not the consequence of the mantel and Hartig net formation but rather the response of the root to diffusible fungal metabolites.
In our bioassays, IAA and ethylene were the major signals controlling root development before contact with the mycelium. Other signals might however be involved either at this early interaction stage or later on. This is highlighted by the fact that the two strains of T. borchii used in this study produce ECM with different efficiency and anatomical traits (Giomaro et al., 2000; Sisti et al., 2003), however in our bioassays they affected root morphology to a comparable extent (Fig. 2). Root morphology was also affected by volatiles released from the mycelium (Supplemental Fig. S4). As ethylene generally decreases root branching (Negi et al., 2008), the increased branching observed in our compartmented system (Supplemental Fig. S4B; 0 mm l-Met) suggests the presence of a volatile signal different from ethylene. Carbon dioxide is known to induce such root branching in Arabidopsis while stimulating primary root elongation (Crookshanks et al., 1998). Primary root elongation was, however, not observed in our setup (Supplemental Fig. S4, A and B), suggesting that either the CO2 concentration was relatively low or that the unidentified volatile was different from CO2.
Ethylene had been implicated in ECM establishment mainly on a speculative basis (Barker and Tagu, 2000). Its production by ectomycorrhizal fungi has been documented for long (Graham and Linderman, 1980; Strzelczyk et al., 1994), but its effect on root morphology has only been investigated in pine (Pinus spp.; Scagel and Linderman, 1998). The latter authors highlighted that in vitro ethylene production by various ectomycorrhizal species positively correlated to root IAA content but poorly correlated with plant morphological responses.
Because the role of ethylene as compared to IAA was neglected, we addressed ethylene biosynthesis by the fungus. Fungi can synthesize ethylene through three routes. The ACC pathway, most common in plants, has been described in Penicillium citricum (Jia et al., 1999). The 2-oxogluatarate pathway is known to operate in different Penicillium species (Fukuda et al., 1986; Pazout and Pazoutova, 1989) and in Fusarium oxysporum (Hottiger and Boller, 1991). The last pathway, in which l-Met is deaminated to produce KMBA, has been reported for Saccharomyces cerevisiae and Penicillium digitatum (Billington et al., 1979). Strzelczyk et al. (1994) proved that l-Met could be successfully transformed to ethylene by six mycorrhizal fungi of pine, suggesting that its biosynthesis precedes either through the KMBA or ACC pathways that both share l-Met as a common precursor (Fig. 4C). Our results indicate that ethylene is synthesized in truffles from l-Met, probably through the KMBA pathway (Fig. 4, A–C). Interestingly, the ascomycete Botrytis cinerea is able to exudate KMBA in the culture medium where it is photooxidized by light, and without the action of any enzyme to form ethylene (Chagué et al., 2002). Similarly the mycelium of T. borchii did not produce any ethylene in the dark (Fig. 4, C and D), suggesting that photooxidation by light might also be necessary and corroborating the KMBA hypothesis.
Considering the underground habitat of truffles and the instability of KMBA to photooxidation, the latter molecule could act as a signal in the first centimeters of soil where light can still penetrate, however whether that light intensity would suffice to degrade KMBA to ethylene is unknown. Another hypothesis is that the ethylene intermediate produced by truffle could actually be degraded enzymatically, either after being taken up inside roots or by other organisms in the rhizosphere. Supporting this possibility, peroxidases can indeed degrade KMBA into ethylene (Chagué et al., 2002), and certainly they abound in the rhizosphere.
IAA production has been reported for numerous but not all ectomycorrhizal fungi. Based on the occurrence of IAA intermediates described in plants, three different pathways that share Trp as a common precursor have been reported in fungi (Reineke et al., 2008). In truffles the IAA synthesis has not yet been investigated. In our study, IAA was produced by all truffle species and strains, and accumulated in the medium in the dark. Since Slankis's (1973) auxin theory stating that auxin is responsible of ECM morphogenesis, many studies have demonstrated that even if partly correct, the theory was an oversimplification (Smith and Read, 1997). One advantage of this investigation compared to earlier ones (Scagel and Linderman, 1998; Sirrenberg et al., 2007) was to quantify IAA directly in the bioassays. By doing so and establishing a dose response of the test plants to synthetic IAA, we could demonstrate that IAA alone could explain many of the changes observed in root morphology, but not all (i.e. root hair; Fig. 5E). Ethylene (or ACC) was necessary to fully express the morphological changes induced on plants by truffle mycelia. Scagel and Linderman (1998) had examined the effect of both hormones on conifers. They highlighted that in vitro fungal IAA and ethylene production positively correlated with root IAA content, suggesting that both hormones could act on the root endogenous IAA level. Two years later Barker and Tagu (2000) hypothesized that a local increase in IAA concentration due to the inhibition of IAA transport by ethylene could trigger ECM-like root morphogenesis. In this study we focused on an early interaction stage, when both partners are not yet in physical contact. We demonstrated that IAA and ethylene indeed act together on plants to induce changes in root morphology. As a matter of fact the action of both hormones is sometimes difficult to distinguish as both IAA and ethylene are, for instance, known to increase root hair length (Pitts et al., 1998). A well-documented cross talk between both hormones further increases the level of complexity (Swarup et al., 2002). For example at the molecular level, CTR1, a negative regulator of the ethylene signaling pathway acts upstream of two antranilate synthase genes (ASA1, ASB1) themselves involved in the IAA-precursor Trp biosynthesis (Stepanova, et al., 2005; Ikeda et al., 2009). These two antranilate synthases are strongly induced by exogenous ACC application, leading to an increased auxin response in the root tip (Stepanova et al., 2005) similar to the one observed in our bioassays (Fig. 6D). At the physiological and morphological level ethylene regulates auxin transport and lateral root formation in Arabidopsis (Ivanchenko et al., 2008; Negi et al., 2008). The latter finding points in the same direction as our data, indicating that the highest resistance to truffle metabolites in terms of root branching was obtained with the ethylene-insensitive ein2-LH and the double mutant aux1-7;ein2-LH (Fig. 6B). In opposition to root branching, primary root inhibition was essentially due to IAA (Fig. 5, A and B), the finding also supported by the aux1-7 and aux1-7;ein2-LH mutants (Fig. 6A), hence indicating that both hormones shape different aspects of root morphology during the interaction with truffles.
Both hormones identified in this study, besides being plant hormones, are produced by a large variety of organisms (Cristescu et al., 2002; Reineke et al., 2008) that makes one wonder about their specificity as mycorrhizal signals. Additionally both host and nonhost plants responded to truffle metabolites in similar ways in terms of increased root branching and reduced primary root. Two interpretations are possibly in line with those results: (1) either the metabolites serve as early mycorrhization signals and are released constitutively, (2) or these signals are not specific and are produced only under given conditions such as our laboratory bioassays.
Regarding the first point, a similar response to the mycorrhization metabolite hypaphorine was obtained in the nonhost Arabidopsis and the host E. globules (Reboutier et al., 2002), suggesting that a similar response between host and nonhost does not contradict signal specificity. However in our bioassays both test plants responded in different ways at the level of root hairs (Fig. 2, E and F). Unlike in ericoid mycorrhizas, root hairs are not involved in ECM colonization (Peterson and Massicotte, 2004), and are in the contrary repressed by some ECM fungi (Jambois et al., 2005). Interestingly, Arabidopsis developed longer root hairs in the presence of truffles. Longer root hairs might mechanically hinder root colonization by ECM fungi, but whether this is specific of a nonhost is unclear considering that the host plant Cistus did not develop any root hair. In conclusion, truffles might use hormonal signals that are recognized by most plants. Such a strategy could be more cost effective for the fungus than producing more complex and specific signals, especially if the fate of ECM formation is controlled by the plant.
Regarding the second point, one can conceive that truffles produce IAA and ethylene in the field at a stage of their life cycle different from the symbiotic one. For instance the burnt, a zone around the host plant devoid of vegetal cover observed with some truffle species (Pacioni, 1991; Splivallo, 2007) could be explained by IAA and ethylene. Because at high concentrations these molecules act as potent herbicides (Hansen and Grossmann, 2000; Grossmann, 2003), their production by truffle mycelium could explain the scarcity of the herbaceous cover observed inside the burnt. In this case both hormones might not have a symbiotic signaling role but could rather reflect a change in the metabolism of the fungus. This is further supported by the observation that the burnt generally appears 1 to 2 years before the formation of fruiting bodies (Splivallo, 2007).
CONCLUSION
Ethylene and IAA produced by ECM fungi have long been suspected to act together to induce ECM formation. We have demonstrated that in laboratory bioassays and at an early stage of interaction (without direct contact), truffles release both hormones at levels that indeed explain all morphological changes induced in the roots of the host Cistus and the nonhost Arabidopsis. Is KMBA (or another ethylene precursor) secreted in the soil before being photooxidized or is it degraded enzymatically/chemically to ethylene inside the mycelium? In this study, metabolites potentially involved in truffle-plant signaling were identified by coculturing both organisms on agarized sterile media. In future work we intend to test the occurrence of these metabolites in truffle fields.
MATERIALS AND METHODS
Biological Material
Cistus incanus was chosen as a model of host plant because of its capacity to form ECM with truffles (Comandini et al., 2006). Arabidopsis (Arabidopsis thaliana) was selected as a nonhost model. Arabidopsis was chosen as a model of nonhost plant because of the mutant and reporter lines available (accession nos. are given for the Nottingham Arabidopsis Stock Centre [http://Arabidopsis.info/]). Arabidopsis ecotype Columbia-0 was tested as wild type (N1092), ethylene and auxin mutants ein2-T (N586500) and aux1-T (N520355; Fischer et al., 2006), ein2-LH (N9719; Hobbie et al., 2000), aux1-7 (N9583; Pickett et al., 1990), axr4-1 (N8018; Hobbie and Estelle, 1995), and axr1-3 (N3075; Lincoln et al., 1990), double mutant aux1-7;ein2-LH (N8843; Hobbie et al., 2000), and the auxin reporter line carrying the DR5rev∷GFP construct (Friml et al., 2003).
Truffle mycelia of Tuber borchii (strains ATCC 96540 = strain 1, and 43BO = strain 2) were donated by Prof. Bonfante (University of Turin, Italy), while Dr. Chevalier (Institut National de la Recherche Agronomique, Clermont, France) provided Tuber melanosporum strains Bal1 (strain 1) and Rey_t (strain 2). Mycelial strains were grown in the dark at 23°C ± 2°C and regularly subcultured on malt extract agar (10 g L−1 malt extract broth, 15 g L−1 agar-agar, pH 7.0).
Bioassays
Dual Culture Mycelium/Plants, Uncompartmented
The experimental setup is exemplified in Figure 1. Square petri dishes (10 × 10 cm2) were filled with 40 mL malt extract (10 g L−1 malt extract broth, Difco Laboratories; 15 g L−1 agar-agar, Carl Roth Gmbh; pH adjusted to 7.0 with KOH). After solidification half the malt extract agar was removed with a spatula and replaced by Murashige and Skoog agar (2.2 g L−1 Murashige and Skoog medium including vitamins, Duchefa Biochemie B.V.; 15 g L−1 Suc, Sigma Aldrich Biochemie GmbH; 15 g L−1 agar-agar; pH adjusted to 5.8–6.0). The malt extract side of the petri dishes was inoculated with a 1.0-cm mycelial plug of the different strains of T. melanosporum and T. borchii (taken from the colony margin of a approximately 1-month-old colony grown on malt extract) or with a malt extract agar plug (mock control). Inoculated plates (including the control plates without fungus) were kept horizontally in darkness for 10 d (23°C ± 2°C) to allow mycelial biomass buildup and exudation in the medium. Ten sterilized seeds of Arabidopsis (wild type or mutants) or of Cistus incanus were placed on the Murashige and Skoog agar part of the petri dish, which was then positioned vertically in a growth chamber kept at a constant 20°C ± 1°C with a 16-h photoperiod and a light intensity of 95 μmol m−2 s−1.
Dual Culture Mycelium/Plants, Compartmented without Volatile Trap
The experimental setup is shown in Supplemental Figure S1. For the media composition, refer to the uncompartmented bioassay. Ten seeds of Arabidopsis were placed on Murashige and Skoog agar in the upper part of a 10 × 10 cm2 petri dish. A round open petri dish filled with malt extract agar (supplemented with 0 mm, 1 mm, or 10 mm l-Met) and inoculated either with truffle mycelium (pregrown for 10 d on malt extract agar on a cellophane membrane) or with an agar plug (mock control) was placed in the bottom part of the square petri dish. In this setup the mycelial exudates do not diffuse in the Murashige and Skoog agar and only the mycelial volatiles can reach the seedlings. Seeds of Arabidopsis (wild type) were placed on the Murashige and Skoog agar part of the petri dish, which was then positioned vertically in a growth chamber kept at a constant 20°C ± 1°C with a 16-h photoperiod and a light intensity of 95 μmol m−2 s−1.
Dual Culture Mycelium/Plants, Compartmented with Volatile Trap
A volatile trap was included in some of the compartmented bioassays as exemplified in Supplemental Figure S5A. A single trap was made of 62.5 ± 0.2 mg activated charcoal (60-100 mesh [powder, Sigma-Aldrich] wrapped in filter paper). The traps were activated by autoclaving followed by 3 h drying at 150°C, and positioned in the petri dishes (no contact with the agar) by hanging them on an iron pin at the beginning of the bioassay (Supplemental Fig. S5A). The growth conditions for the mycelium and the plants were exactly the same as for the other compartmented/uncompartmented bioassays.
Bioassays with Synthetic Hormones (ACC, IAA)
IAA (98%, Carl Roth Gmbh) and the ethylene precursor ACC (98%, Sigma Aldrich Biochemie GmbH) were used to establish a dose response of Arabidopsis and C. incanus to IAA and ethylene. The metabolites were added separately or together to the agar of the uncompartemnetd bioassay, reaching final concentrations of 10−8 to 10−5 m (IAA) and 1 μm (ACC) in the agar.
Determination of Root Length, Branching, and Root Hair Length
Primary root length and root branching (secondary roots) were monitored every 5 d up to 15 d. Primary root length was determined with the image analysis software ImageJ (http://rsb.info.nih.gov/nih-image) after scanning the petri dishes at a resolution of 400 dpi. Root branching was determined for each seedling by counting the number (abbreviated nb in the figures) of secondary roots visible under a stereo microscope at a magnification of 30 times (Leica Wild M10). Root hair length was quantified with ImageJ from digital images obtained from the primary root section located 1.0 cm above the root tip under the stereo microscope at a magnification of 40 times (a Leica DFC300FX digital camera was mounted on the stereo microscope; image acquisition with Leica IM, version 4 software).
Confocal Laser-Scanning Microscopy on DR5∷GFP Root Tips
Confocal laser-scanning microscopy was performed using a Leica TCS SP5 scanning system mounted on a Leica DM5000CS inverted microscope with a water-corrected 63× objective. The GFP was excited with an argon laser (488 nm) and fluorescence was detected between 496 and 551 nm. Seedlings were individually mounted in water and immediately subjected to analysis. The GFP signal was quantified by (1) counting the number of first tier columnella cells where the GFP was expressed and (2) calculating how frequently a signal was visible in the epidermis (data pooled from three petri dishes, n ≥ 12 seedlings).
IAA Quantification by HPLC-ESI-MS/MS
IAA Extraction from Agar
IAA was quantified in the upper Murashige and Skoog agar part of the dual culture mycelium/plant (uncompartmented) bioassays at two time points: 10 d after inoculation (equivalent to the beginning of the bioassay with plants) and after 10 additional days in the growth chamber. A quantity of 10 to 15 g Murashige and Skoog agar was removed from each plate with a scalpel, placed in a 50 mL falcon tube, and shortly homogenized in a mixer with 7.0 mL water:MeOH (1:1, v/v) containing 37.5 ng mL−1 of deuteriated IAA ([2,4,5,6,7-2H5]IAA, Eurisotop) as an internal standard. The sample was acidified with 50 μL fuming acetic acid, mixed with 7.0 mL ethyl acetate, vortexed for 30 s, and centrifuged for at 4,500 rcf for 10 min to force phase separation. The upper phase was concentrated to dryness at 40°C under vacuum, redissolved in 1.0 mL of water:MeOH containing 7.0 mm acetic acid, and filtered on a 0.2 μm polytetrafluoroethylene filter for HPLC-MS analysis.
Quantification of IAA by HPLC-ESI-MS/MS
The protocol was modified from Sirrenberg et al. (2007). In short, analyses were performed on a Varian system equipped with an autosampler (ProStar 430), a binary pump system (ProStar 210), a column oven, and a reversed-phase column (Polaris C18-A, 100 × 2mm; particle size 5 μm, mounted with a ChromSep guard column, both from Varian). Samples (10 μL injection volume) were eluted at 40°C with a flow rate of 0.2 mL/min as follows. Hold for 1 min, 90% solvent A (95:5 water:ACN) and 10% solvent B (MeOH). Ramp to 98% solvent B in 7 min and hold for 7 min. The detector was a 1200LC triple quadrupole mass spectrometer (Varian) equipped with an electrospray interface. Drying gas (nitrogen) pressure was 18 psi. Nebulizing gas (air) was set to 50 psi, and the pressure of collision gas (argon) was 1.4 mTorr. Voltage was set to −4,400 V (needle), −600 V (shield), and 40 V (capillary). Multiple reaction monitoring modus was used to operate the mass spectrometer. Mass transitions were: for IAA 173.9/130.0 (collision energy 9.0 eV), and for D5-IAA 178.8/134.0 (collision energy 11.5 eV). A calibration curve of the ratio of peak areas of unlabeled standards to peak area of deuterium-labeled standard was used for the quantification of IAA.
Ethylene Quantification by GC
Truffle mycelium (T. borchii strain 1) was cultured in a 20 mL solid-phase microextraction vial containing 10 mL malt extract agar and sealed air tight with a silicon/polytetrafluoroethylene septum. Control vials were inoculated with an agar plug. The samples were kept either under the same conditions in which the bioassays were performed (growth chamber conditions) or in complete darkness.
Various ethylene precursors/inhibitors were added to the medium to investigate the ethylene biosynthetic pathway in truffles. l-Met and l-Gln were tested as potential ethylene precursors. ACC (98%, Sigma Aldrich Biochemie GmbH) and KMBA (≥97%, Sigma-Aldrich GmbH, Germany) were used as possible intermediates and AOA (98%, Sigma-Aldrich GmbH) as an inhibitor of the ACC pathway (Cristescu et al., 2002). All chemicals, except the heat unstable KMBA, were mixed in 0.5 mL malt extract agar at 60°C to reach a final concentration of 10 mm and then added as a 2.0 mm layer on the top of 9.5 mL malt extract agar in the solid-phase microextraction vial. KMBA was added (50 μL of a 10 mg mL−1 solution in water) to vials containing 10 mL malt extract agar once the agar had solidified and cooled down to room temperature. Vials containing the chemicals but mock inoculated with an agar plug were used as controls.
Ethylene measurements were performed on a GC equipped with a flame ionization detector (Agilent Technologies GC 6980 N) and a GC-GASPRO J&W column (30 m × 320 μm). Helium was used as a carrier gas at 2.0 mL min−1. A volume of 100 μL taken from the headspace of 10-d-old samples was injected in the inlet heated to 150°C (split 0.2:1). Temperature program was 60°C for 2 min, ramp at 20°C min−1 to 150°C, and hold 1 min.
For quantification a calibration curve was established with pure ethylene (≥99.95%, Sigma-Aldrich GmbH). Average ethylene production was calculated for each treatment using four replicates.
Data and Statistical Analysis
Each bioassay was repeated at least three times independently. All data were first checked for normality (Kolmogorov-Smirnov test) and depending on the result analyzed with a parametric or nonparametric test. All morphological data (primary root length, branching, root hair length) were analyzed with a nonparametric ANOVA on ranks followed by Dunn's test that allows multiple comparisons of group of unequal sizes. The remaining data (IAA, ethylene, and GFP) were analyzed by a t test in case of normal distribution and otherwise with the nonparametric Mann-Whitney test (SigmaPlot 11.0, Systat Software Inc.).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Uncompartmented bioassay setup: no contact between the plant roots and mycelium.
Supplemental Figure S2. Experimental setup for IAA and ethylene determination.
Supplemental Figure S3. Compartmented bioassay.
Supplemental Figure S4. Effect of mycelial volatiles on Arabidopsis.
Supplemental Figure S5. Effect of activated charcoal in the compartmented bioassay.
Supplemental Figure S6. Screening Arabidopsis mutants for resistance to truffle metabolites.
Supplemental Figure S7. GFP quantification in root tips of DR5∷GFP Arabidopsis.
Supplementary Material
Acknowledgments
We wish to acknowledge Caroline Gutjahr for editing and commenting on the manuscript. We would like to thank the following people for providing biological material: Prof. Paola Bonfante (T. borchii mycelia); Dr. Gérard Chevalier (T. melanosporum mycelia); Dr. Raffaella Balestrini (C. incanus); Prof. Christiane Gatz, Hella Tappe, and Christoph Weiste (axr mutants of Arabidopsis); and Dr. Thomas Teichmann for the auxin reporter line carrying the DR5∷GFP construct. We are also thankful to Prof. Peter Schu for providing access to the confocal microscope and to Michael Reusche for introducing us to the machine.
This work was supported by the Swiss National Fund (grant no. PBSKA–118998/1) and the Deutsche Forschungsgemeinschaft (grant no. SP 1191/1–1).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Richard Splivallo (ricsi17@hotmail.com).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open access articles can be viewed online without a subscription.
References
- Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435 824–827 [DOI] [PubMed] [Google Scholar]
- Barker SJ, Tagu D (2000) The roles of auxins and cytokinins in mycorrhizal symbioses. J Plant Growth Regul 19 144–154 [DOI] [PubMed] [Google Scholar]
- Béguiristain T, Côté R, Rubini P, Jay-Allemand C, Lapeyrie F (1995) Hypaphorine accumulation in hyphae of the ectomycorrhizal fungus Pisolithus tinctorius. Phytochemistry 40 1089–1091 [Google Scholar]
- Billington D, Golding B, Primrose S (1979) Biosynthesis of ethylene from methionine. Biochem J 182 827–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagué V, Elad Y, Barakat R, Tudzynski P, Sharon A (2002) Ethylene biosynthesis in Botrytis cinerea. FEMS Microbiol Ecol 40 143–149 [DOI] [PubMed] [Google Scholar]
- Comandini O, Contu M, Rinaldi AC (2006) An overview of Cistus ectomycorrhizal fungi. Mycorrhiza 16 381–395 [DOI] [PubMed] [Google Scholar]
- Contreras-Cornejo HA, Macias-Rodriguez L, Cortes-Penago C, López-Bucio J (2009) Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol 149 1579–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristescu SM, De Martinis D, te Lintel Hekkert S, Parker DH, Harren FJM (2002) Ethylene production by Botrytis cinerea in vitro and in tomatoes. Appl Environ Microbiol 68 5342–5350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crookshanks M, Taylor G, Dolan L (1998) A model system to study the effects of elevated CO2 on the developmental physiology of roots: the use of Arabidopsis thaliana. J Exp Bot 49 593–597 [Google Scholar]
- Dénarié J, Cullimore J (1993) Lipo-oligosaccharide nodulation factors: a new class of signalling molecules mediating recognition and morphogenesis. Cell 74 951–954 [DOI] [PubMed] [Google Scholar]
- Dix L, Van Staden J (1982) Auxin and gibberellin-like substances in coconut milk and malt extract. Plant Cell Tissue Organ Cult 1 239–245 [Google Scholar]
- Dunlap JR, Robacker KM (1988) Nutrient salts promote light-induced degradation of indole-3-acetic acid in tissue culture media. Plant Physiol 88 379–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Ikeda Y, Ljung K, Serralbo O, Singh M, Heidstra R, Palme K, Scheres B, Grebe M (2006) Vectorial information for Arabidopsis planar polarity is mediated by combined AUX1, EIN2, and GNOM activity. Curr Biol 16 2143–2149 [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jurgens G (2003) Efflux dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426 147–153 [DOI] [PubMed] [Google Scholar]
- Fukuda H, Fujii T, Ogawa T (1986) Preparation of a cell-free ethylene forming system from Penicilium digitatum. Agric Biol Chem 50 977–981 [Google Scholar]
- Gay G, Normand L, Marmeisse R, Sotta B, Debaud JC (1994) Auxin overproducer mutants of Hebeloma cylindrosporum Romagnési have increased mycorrhizal activity. New Phytol 128 645–657 [Google Scholar]
- Giomaro G, Zambonelli A, Sisti D, Cecchini M, Evangelista V, Stocchi V (2000) Anatomical and morphological characterization of mycorrhizas of five strains of Tuber borchii Vittad. Mycorrhiza 10 107–114 [Google Scholar]
- Graham JH, Linderman RG (1980) Ethylene production by ectomycorrhizal fungi, Fusarium oxysporum f.sp. pini, and by asceptically synthesized ectomycorrhizae and Fusarium-infected Douglas-fir roots. Can J Microbiol 26 1340–1347 [DOI] [PubMed] [Google Scholar]
- Grossmann K (2003) Mediation of herbicide effects by hormone interactions. J Plant Growth Regul 22 109–122 [Google Scholar]
- Hansen H, Grossmann K (2000) Auxin-induced ethylene triggers abscisic acid biosynthesis and growth inhibition. Plant Physiol 124 1437–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidstra R, Bisseling T (1996) Nod factor induced hosts responses and mechanisms of Nod factor perception. New Phytol 133 25–43 [Google Scholar]
- Herrmann S, Oelmuller R, Buscot F (2004) Manipulation of the onset of ectomycorrhiza formation by indole-3-acetic acid, activated charcoal or relative humidity in the association between oak microcuttings and Piloderma croceum: influence on plant development and photosynthesis. J Plant Physiol 161 509–517 [DOI] [PubMed] [Google Scholar]
- Hobbie L, Estelle M (1995) The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J 7 211–220 [DOI] [PubMed] [Google Scholar]
- Hobbie L, McGovern M, Hurwitz LR, Pierro A, Liu NY, Bandyopadhyay A, Estelle M (2000) The axr6 mutants of Arabidopsis thaliana define a gene involved in auxin response and early development. Development 127 23–32 [DOI] [PubMed] [Google Scholar]
- Hottiger T, Boller T (1991) Ethylene biosynthesis in Fusarium oxysporum sp tulipae proceeds from glutamate/2-oxoglutarate and requires oxygen and ferrous ions in vivo. Arch Microbiol 157 18–22 [Google Scholar]
- Ikeda Y, Men S, Fischer U, Stepanova AN, Alonso JM, Ljung K, Grebe M (2009) Local auxin biosynthesis modulates gradient-directed planar polarity in Arabidopsis. Nat Cell Biol 11 731–738 [DOI] [PubMed] [Google Scholar]
- Ivanchenko MG, Muday GK, Dubrovsky JG (2008) Ethylene-auxin interactions regulate lateral root initiation and emergence in Arabidopsis thaliana. Plant J 55 335–347 [DOI] [PubMed] [Google Scholar]
- Jambois A, Dauphin A, Kawano T, Ditengou FA, Bouteau F, Legué V, Lapeyrie F (2005) Competitive antagonism between IAA and indole alkaloid hypaphorine must contribute to regulate ontogenesis. Physiol Plant 123 120–129 [Google Scholar]
- Jia YJ, Kakuta Y, Sugawara M, Igarashi T, Oki N, Kisaki M, Shoji T, Kanetuna Y, Horita T, Matsui H, et al (1999) Synthesis and degradation of 1-aminocyclopropane-1-carboxylic acid by Penicilium citricum. Biosci Biotechnol Biochem 63 542–549 [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M (1990) Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F, Dupleissis S, Ditengou F, Lagrange H, Voiblet C, Lapeyrie F (2001) Developmental cross talking in the ectomycorrhizal symbiosis: signals and communication genes. New Phytol 151 145–154 [DOI] [PubMed] [Google Scholar]
- Menotta M, Gioacchini AM, Amicucci A, Buffalini M, Sisti D, Stocchi V (2004) Headspace solid-phase microextraction with gas chromatography and mass spectrometry in the investigation of volatile organic compounds in an ectomycorrhizae synthesis system. Rapid Commun Mass Spectrom 18 206–210 [DOI] [PubMed] [Google Scholar]
- Miozzi L, Balestrini R, Bolchi A, Novero M, Ottonello S, Bonfante P (2005) Phospholipase A(2) up-regulation during mycorrhiza formation in Tuber borchii. New Phytol 167 229–238 [DOI] [PubMed] [Google Scholar]
- Negi S, Ivanchenko MG, Muday GK (2008) Ethylene regulates lateral root formation and auxin transport in Arabidopsis thaliana. Plant J 55 175–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacioni G (1991) Effects of Tuber metabolites on the rhizospheric environment. Mycol Res 95 1355–1358 [Google Scholar]
- Pazout J, Pazoutova S (1989) Ethylene is synthesised by vegetative mycelium in surface cultures of Penicillium-cyclopium westling. Can J Microbiol 35 384–387 [Google Scholar]
- Peterson RL, Bonfante P (1994) Comparative structure of vesicular arbuscular mycorrhizas and ectomycorrhizas. Plant Soil 159 79–88 [Google Scholar]
- Peterson RL, Massicotte HB (2004) Exploring structural definitions of mycorrhizas with enphasis on nutrient-exchange interfaces. Can J Bot 82 1074–1088 [Google Scholar]
- Pickett FB, Wilson AK, Estelle M (1990) The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol 94 1462–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RJ, Cernac A, Estelle M (1998) Auxin and ethylene promote root hair elongation in Arabidopsis. Plant J 16 553–560 [DOI] [PubMed] [Google Scholar]
- Rasmann S, Kollner TG, Degenhardt J, Hiltpold I, Toepfer S, Kuhlmann U, Gershenzon J, Turlings TCJ (2005) Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 434 732–737 [DOI] [PubMed] [Google Scholar]
- Reboutier D, Bianchi M, Brault M, Roux C, Dauphin A, Rona JP, Legué V, Lapeyrie F, Bouteau F (2002) The indolic compound hypaphorine produced by ectomycorrhizal fungus interferes with auxin action and evokes early responses in nonhost Arabidopsis thaliana. Mol Plant Microbe Interact 15 932–938 [DOI] [PubMed] [Google Scholar]
- Reineke G, Heinze B, Schirawski J, Buettner H, Kahmann R, Basse CW (2008) Indole-3-acetic acid (IAA) biosynthesis in the smut fungus Ustilago maydis and its relevance for increased IAA levels in infected tissue and host tumour formation. Mol Plant Pathol 9 339–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu CM, Farag MA, Hu CH, Reddy MS, Wei HX, Pare PW, Kloepper JW (2003) Bacterial volatiles promote growth in Arabidopsis. Proc Natl Acad Sci USA 100 4927–4932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scagel CF, Linderman RG (1998) Relationships between in vitro indole acetic acid or ethylene production capacity by ectomycorrhizal fungi and conifer seedling responses in symbiosis. Symbiosis 24 13–34 [Google Scholar]
- Sirrenberg A, Göbel C, Grond S, Czempinski N, Ratzinger A, Karlovsky P, Santos P, Feussner I, Pawlowski K (2007) Piriformospora indica affects plant growth by auxin production. Physiol Plant 131 581–589 [DOI] [PubMed] [Google Scholar]
- Sisti D, Giomaro G, Cecchini M, Faccio A, Novero M, Bonfante P (2003) Two genetically related strains of Tuber borchii produce Tilia mycorrhizas with different morphological traits. Mycorrhiza 13 107–115 [DOI] [PubMed] [Google Scholar]
- Sisti D, Zambonelli A, Giomaro G, Rossi I, Ceccaroli P, Citterio B, Benedetti PA, Stocchi V (1998) In vitro mycorrhizal synthesis of micropropagated Tilia platyphyllos Scop. plantlets with Tuber borchii Vittad. mycelium in pure culture. Acta Hortic 457 379–387 [Google Scholar]
- Slankis V (1973) Hormonal relationships in mycorrhizal development. In GC Marks, TT Kozlowski, eds, Ectomycorrhizae: Their Ecology and Physiology. Academic Press, New York, pp 231–298
- Smith SA, Read D (1997) Mycorrhizal Symbiosis, Ed 2. Academic Press, London
- Splivallo R (2007) Biological significance of truffle secondary metabolites. In P Karlovsky, ed, Secondary Metabolites in Soil Ecology. Soil Biology 14. Springer-Verlag, Berlin, pp 141–166
- Splivallo R, Novero M, Bertea CM, Bossi S, Bonfante P (2007) Truffle volatiles inhibit growth and induce an oxidative burst in Arabidopsis thaliana. New Phytol 175 417–424 [DOI] [PubMed] [Google Scholar]
- Stepanova AN, Hoyt JM, Hamilton AA, Alonso JM (2005) A link between ethylene and auxin uncovered by the characterization of two root-specific ethylene-insensitive mutants in Arabidopsis. Plant Cell 17 2230–2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strzelczyk E, Kampert M, Pachlewski R (1994) The influence of pH and temperature on ethylene production by mycorrhizal fungi of pine. Mycorrhiza 4 193–196 [Google Scholar]
- Swarup R, Parry G, Graham N, Allen T, Bennett M (2002) Auxin cross-talk: integration of signaling pathways to control plant development. Plant Mol Biol 49 411–426 [DOI] [PubMed] [Google Scholar]
- Yang S (1967) Biosynthesis of ethylene: ethylene formation from methional by horse radish peroxidase. Arch Biochem Biophys 122 481–487 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.