Abstract
The Arabidopsis (Arabidopsis thaliana) gene BT2 encodes a 41-kD protein that possesses an amino-terminal BTB domain, a central TAZ domain, and a carboxyl-terminal calmodulin-binding domain. We previously demonstrated that BT2 could activate telomerase expression in mature Arabidopsis leaves. Here, we report its distinct role in mediating diverse hormone, stress, and metabolic responses. We serendipitously discovered that steady-state expression of BT2 mRNA was regulated diurnally and controlled by the circadian clock, with maximum expression in the dark. This pattern of expression suggested that BT2 mRNA could be linked to the availability of photosynthate in the plant. Exogenous sugars decreased BT2 expression, whereas exogenous nitrogen increased expression. bt2 loss-of-function mutants displayed a hypersensitive response to both sugar-mediated inhibition of germination and abscisic acid (ABA)-mediated inhibition of germination, thus supporting a role of ABA in sugar signaling in germination and development. Moreover, constitutive expression of BT2 imparted resistance to both sugars and ABA at germination, suggesting that BT2 suppresses sugar and ABA responses. In support of the previously described antagonistic relationship between ABA and auxin, we found that BT2 positively regulated certain auxin responses in plants, as revealed by knocking down BT2 expression in the high-auxin mutant yucca. Accumulation of BT2 mRNA was affected by a variety of hormones, nutrients, and stresses, and BT2 was required for responses to many of these same factors. Together, these results suggest that BT2 is a central component of an interconnected signaling network that detects and responds to multiple inputs.
Plants have evolved sophisticated mechanisms to perceive and transduce diverse environmental signals. Changes in light, the circadian clock, and nutrient status serve as major inputs to modulate the diurnal expression of networks of genes that regulate growth and development (Blasing et al., 2005; Gutierrez et al., 2008; Usadel et al., 2008). The circadian clock further serves as an input to regulate or “gate” the expression of multiple genes involved in metabolism, growth, and development, thereby rendering a physiological advantage for plant growth and survival (for review, see McClung, 2006). In addition to the intrinsic clock function, diurnal changes in nutrient status modulate the expression of several genes (Blasing et al., 2005). The availability of sugars activates “feast” genes involved in growth and biosynthesis, while low sugar concentrations activate “famine” genes that mobilize carbon from primary reserves or other cellular components (Koch, 1996; Yu, 1999). Similarly, changes in nitrogen status modulate the expression of numerous transcription factors, protein kinases/phosphatases, and enzymes involved in nitrate reduction and assimilation, amino acid biosynthesis, glycolysis, and iron and sulfate metabolism (Scheible et al., 2004; Wang et al., 2004).
Sugars and nitrates primarily affect plant growth by serving as building blocks for anabolic metabolism. They also function as signaling molecules that interact with light, hormones including abscisic acid (ABA) and ethylene, and stress signals to control vital processes of growth and development (Zhou et al., 1998; Stitt, 1999; Stitt and Krapp, 1999; León and Sheen, 2003; Wang et al., 2004). Sugars normally promote growth; however, high sugar concentrations suppress germination and postgermination development. Interestingly, these inhibitory effects are antagonized by nitrates, suggesting interplay between carbon and nitrogen status in the control of germination (Moore et al., 2003; Bi et al., 2005). Using screens for either resistance or sensitivity of germination to high sugar concentrations, sugar-insensitive or -hypersensitive mutants have been identified (Zhou et al., 1998; Laby et al., 2000; Pego et al., 2000; Rolland et al., 2002). Surprisingly, many sugar-insensitive mutants, such as sugar-insensitive4/glucose-insensitive1 (sis4/gin1) and sis5/gin6, are allelic to ABA synthesis (aba2) and ABA-insensitive (abi4) mutants, respectively (Arenas-Huertero et al., 2000; Cheng et al., 2002). Moreover, exogenous Glc specifically increases the expression of ABA synthesis genes and affects endogenous ABA concentrations, revealing an intimate connection between ABA and sugar signaling (Cheng et al., 2002; Price et al., 2003). ABA itself mediates seed dormancy, leaf senescence, stomatal closure, and several other plant stress responses (Fedoroff, 2002; Gubler et al., 2005). ABA signaling also has antagonistic interconnections with other hormones, including auxin and ethylene. The ABA-hypersensitive mutant hyponastic leaves1 (hyl1) is simultaneously resistant to auxin and cytokinin (Lu and Fedoroff, 2000). During lateral root initiation, auxin promotes initiation by down-regulating cell cycle inhibitors such as Kip-related proteins (Richard et al., 2001; Himanen et al., 2002). In contrast, ABA inhibits lateral root initiation by activating Kip-related proteins (Verkest et al., 2005). Also, several genes involved in promoting lateral root initiation, including AUXIN INDUCED IN ROOT CULTURES12 and INDOLE-3-ACETIC ACID19, are repressed by ABA (Hoth et al., 2002).
Although there has been significant progress in understanding how plants perceive light, nutrient, hormone, and stress signals, major questions persist regarding how plants simultaneously integrate and transduce these different signals. Global gene expression studies in Arabidopsis (Arabidopsis thaliana) have revealed that specific signals modulate extensive networks of genes. These networks typically include genes encoding putative transcription factors and protein kinases, along with genes involved in protein synthesis and ubiquitin-mediated protein degradation (Wang et al., 2004; Blasing et al., 2005; Gutierrez et al., 2008; Usadel et al., 2008). Members of the bZIP family of transcription factors characterized as G box (CACGTG)-binding factors (GBFs), such as bZIP2/GBF5 and bZIP11/ATB1, together with Snf1-related kinases (SnRK), KIN10/11, orchestrate synergistic transcriptional networks in response to sugar, energy deprivation, and diverse stresses (Baena-Gonzalez et al., 2007; Hanson et al., 2008). However, the molecular mechanisms of the components downstream of bZIP/SnRK that affect the adaptive responses remain elusive.
We previously identified BT2 (At3g48360) as an activator of telomerase in mature leaves of Arabidopsis (Ren et al., 2007). BT2 is an approximately 41-kD protein with an N-terminal BTB/POZ (for Broad-Complex, Tramtrack, and Bric-a-Brac/Poxvirus and Zinc finger) domain, a central TAZ (for Transcriptional Adaptor Zinc finger) domain, and a C-terminal calmodulin-binding domain. The Arabidopsis genome encodes four additional proteins with a similar domain structure (Du and Poovaiah, 2004). Recently, members of this BT family, including BT2, were demonstrated to play crucial roles in gametophyte development in Arabidopsis and were further shown to compensate for the loss of one another by reciprocal transcriptional regulation (Robert et al., 2009). Here, we report distinct and broader functions of BT2 in responding to changes in light signals, nutrient status, hormones, and certain stresses. During the diurnal cycle, BT2 expression peaked in the dark, and its expression was regulated by the circadian clock. Nutrient status also modulated BT2 expression: sugars repressed BT2 expression, while nitrates increased BT2 expression. Using bt2-null mutants and constitutively expressing BT2 lines, we demonstrated that BT2 modulated hormone responses. BT2 negatively regulates ABA- and sugar-mediated inhibition of germination. Loss of BT2 in the auxin-accumulating mutant yucca suppresses many of the phenotypes associated with high auxin concentrations. This result confirms our previous conclusion from BT2-overexpressing lines that BT2 potentiates auxin responses in postgermination and vegetative development (Ren et al., 2007). Furthermore, BT2 expression was modulated by multiple abiotic and biotic stresses, including ABA, cold, methyl jasmonate, and hydrogen peroxide (H2O2). Loss of BT2 function resulted in sensitivity to H2O2. Because BT2 expression is affected by multiple physiological and environmental conditions, and because it is also required for responses to many of these same conditions, the BT2 protein appears to be a key element in an interconnected network that detects and integrates responses to diverse signals.
RESULTS
Circadian and Light Regulation of BT2
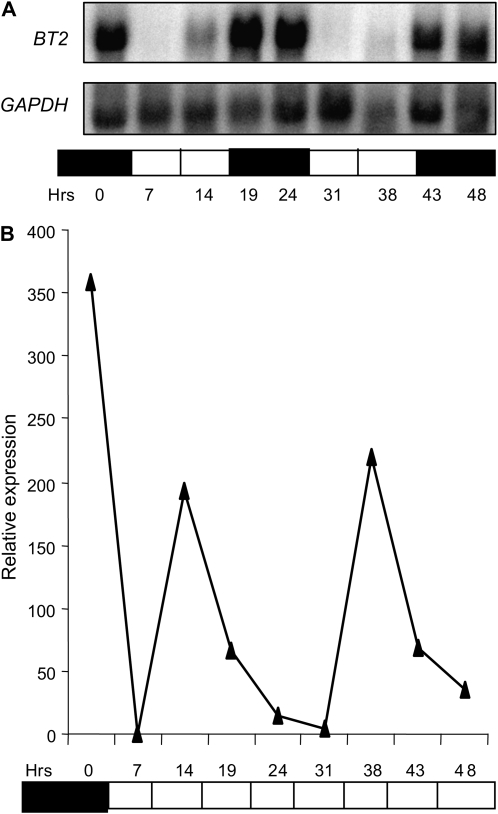
In our previous characterization of BT2's role in activating telomerase in mature leaves (Ren et al., 2007), we noticed that the level of BT2 message fluctuated among RNA samples harvested at different times. To uncover the cause of this fluctuation, we analyzed BT2 mRNA levels throughout the diurnal cycle. BT2 was highly expressed in the dark phase (19, 24, 43, and 48 h) and was almost undetectable in the light phase (7 and 31 h; Fig. 1A; Supplemental Fig. S1). However, its abundance increased slightly toward the end of the light phase (14 and 38 h), in apparent anticipation of the dark phase. Rhythmic expression of BT2 suggested that it may be under the control of a circadian clock. To test this hypothesis, 3-week-old light/dark-entrained plants were either transferred to continuous light or kept in a normal diurnal cycle, and RNA samples were subjected to quantitative real-time (qRT)-PCR analysis. The rhythmic pattern of BT2 expression seen in control plants under a normal diurnal cycle was maintained in plants transferred to continuous light (Fig. 1B).
Figure 1.
BT2 expression is diurnally regulated and controlled by a circadian clock. After entrainment of wild-type plants to 14-h/10-h light/dark cycles for 3 weeks, plants were either held in light/dark or transferred to continuous light. Total RNA was extracted from rosette leaves harvested at the indicated times and was subjected to RNA gel blot analysis (A) or qRT-PCR (B). mRNA of GAPDH was used as a loading control for the RNA gel blot. EIF-4A2 was used to normalize the qRT-PCR data. Expression values plotted for BT2 in B are averages of two biological replicates and are relative to the minimum value, which occurred at the 7-h time point. White and black bars at the bottom of each panel represent light and dark conditions, respectively.
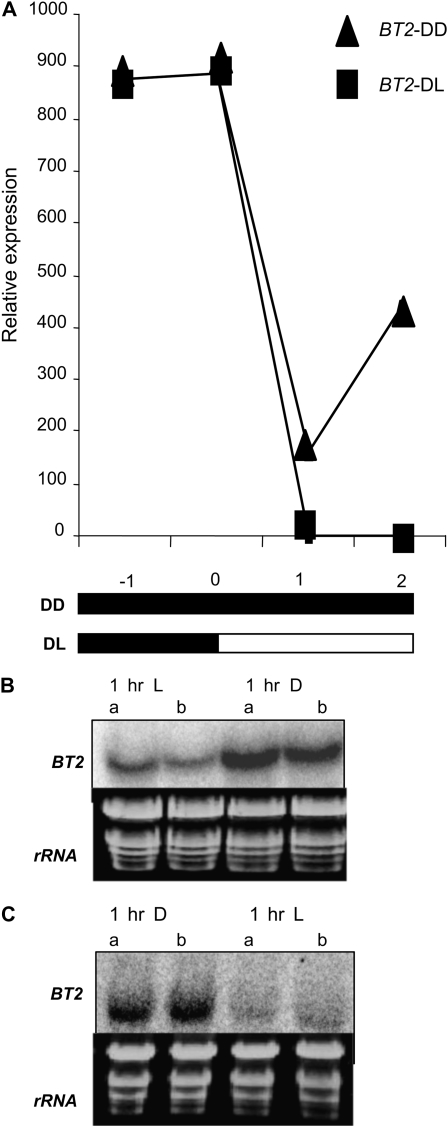
We then performed two experiments to determine whether light alone could modulate BT2 expression. First, we subjected 3-week-old light/dark-entrained plants to an extended dark treatment. RNA samples were harvested at −1, 0, +1, and +2 h into the extended dark phase, along with control samples that were harvested from plants in a normal light/dark cycle, and subjected to qRT-PCR analysis. BT2 expression was at its highest when the plants were in the dark at −1 and 0 h. As expected, BT2 expression was down-regulated at +1 and +2 h in control samples that were transferred to light. However, in plants that stayed in extended darkness, BT2 expression remained higher (Fig. 2A).
Figure 2.
Light modulates BT2 expression, independent of circadian regulation. A, After entrainment of wild-type plants to 14-h/10-h light/dark cycles for 3 weeks, plants were either held in extended darkness (DD) or transferred to light (DL). Total RNA was extracted from rosette leaves harvested at the indicated times and subjected to qRT-PCR analysis. 18S rRNA was used to normalize the qRT-PCR data, and the expression values plotted for BT2 are averages of two biological replicates. Values are relative to the minimum value, which occurred at the +1-h time point. White and black bars at the bottom represent light and dark conditions, respectively. B and C, Three-week-old wild-type plants were either exposed to 1 h of dark (D) during the middle of light phase (B) or 1 h of light (L) during the middle of dark phase (C). Rosette leaves were harvested and analyzed by RNA gel blots. The first two lanes in both blots represent controls that remained in their respective light and dark conditions. Replicate samples are indicated by a and b. Ethidium bromide-stained rRNA was used as a loading control.
In the second experiment, 3-week-old light/dark-entrained plants were exposed to either 1 h of dark during the light phase or 1 h of light during the dark phase and BT2 expression was analyzed by RNA gel blots. BT2 mRNA was increased by the brief exposure to dark during the light phase but decreased by the brief exposure to light during the dark phase (Fig. 2, B and C). Together, these results indicate that expression of BT2 is modulated by light and also is under the control of a circadian clock.
BT2 Responds to Changes in Nutrient Status of the Plant
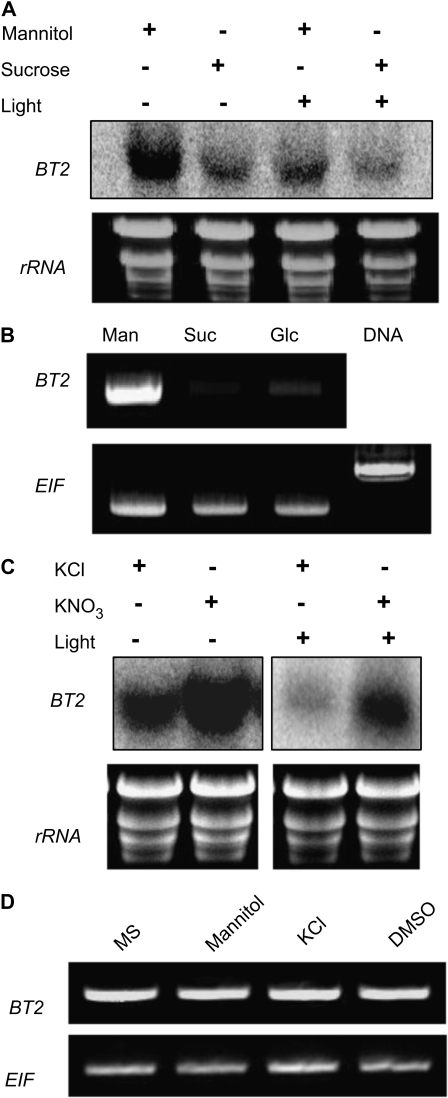
To understand the functional significance of the diurnal regulation of BT2, we investigated whether its expression was correlated with metabolic changes. One of the major metabolic changes associated with a diurnal cycle is the concentration of sugars, which peak during the light and diminish in the dark. To test whether BT2 expression responded to sugars, 3-week-old light/dark-entrained plants that were in the middle of a dark phase were treated with either Suc or mannitol (as an osmotic control) for 3 h in the dark. All samples were then subjected to RNA gel blot analysis. BT2 transcript was repressed by Suc even in the absence of the light (Fig. 3A), suggesting that the low-sugar status of plants is a strong signal for BT2 induction. Glc also repressed BT2 expression (Fig. 3B). Mannitol had no effect on BT2 mRNA concentrations (Fig. 3D).
Figure 3.
BT2 is repressed by sugars and induced by nitrates. A, Three-week-old wild-type plants were treated with mannitol or Suc in the dark or the light for 3 h. B, One-week-old seedlings, grown in continuous light, were treated with mannitol, Suc, or Glc for 3 h. Total RNA was isolated and subjected to RT-PCR analysis. EIF4-A2 expression was used as a loading control. C, Three-week-old wild-type plants were treated with KCl (control) or KNO3 in the dark (lanes 1 and 2) or the light (lanes 3 and 4) for 3 h. D, Conditions used as controls for this figure and for Figure 7A had no effect on BT2 mRNA concentrations. DMSO, Dimethyl sulfoxide.
Similar to sugars, changes in nitrogen status can affect resource allocation, growth, and development in plants. Nitrogen status also modulates the circadian clock by serving as an input (Scheible et al., 2004; Gutierrez et al., 2008). Moreover, the carbon-nitrogen ratio in plants is tightly regulated, with interconnected sensing and signaling mechanisms (Coruzzi and Zhou, 2001). For example, addition of nitrates reverses sugar-mediated repression of gene expression (Moore et al., 2003). Because BT2 expression was modulated by the circadian clock and responded to carbon signals, we wanted to determine whether nitrogen also modulated BT2 expression. Three-week-old light/dark-entrained plants were treated with either KNO3 or KCl for 3 h in the middle of a light phase. BT2 repression during the light phase was reversed by the addition of nitrates (Fig. 3C). Interestingly, nitrate induction of BT2 was also observed when plants were treated during their dark phase (Fig. 3C). KCl had no effect on BT2 mRNA concentrations (Fig. 3D).
BT2 Suppresses Sugar Signaling
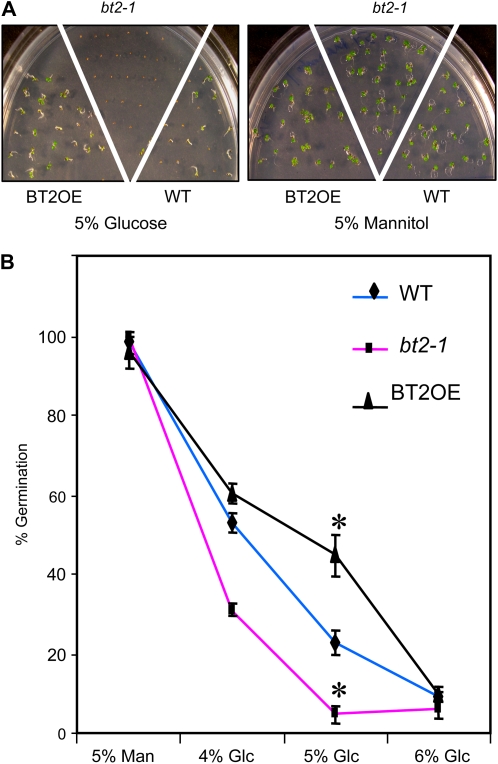
Sugars, in addition to their metabolic roles, act as signaling molecules and control key aspects of plant growth and development. High sugar levels early in plant development can inhibit germination and cotyledon emergence (Smeekens, 2000; Gazzarrini and McCourt, 2001; Moore et al., 2003). Because BT2 expression was modulated diurnally and by the sugar status of the plant, we predicted a role for BT2 in some aspects of sugar signaling. To test this hypothesis, seeds from bt2-null (bt2-1), constitutively expressing BT2 (BT2OE), and wild-type lines were germinated on various concentrations of Glc (4%, 5%, and 6%) or mannitol (5%) and the percentage of seedlings with normal cotyledon emergence was determined. All lines had nearly 100% germination on 5% mannitol; however, concentrations of 4% and 5% Glc were sufficient to inhibit wild-type germination. At similar concentrations, bt2-1 seeds were hypersensitive and BT2OE seeds were resistant to Glc inhibition of germination (Fig. 4). Higher concentrations of Glc (6%) inhibited germination of all lines equally. These results suggest that BT2 suppresses sugar signaling during germination and early vegetative development.
Figure 4.
BT2 suppresses sugar-mediated inhibition of germination. A, Visible phenotypes of 6-d-old wild-type (WT), bt2-1, and BT2OE lines grown on either 5% Glc or 5% mannitol. B, Quantification of germinated seedlings with normal cotyledons of wild-type, bt2-1, and BT2OE lines grown on various concentrations of Glc (4%, 5%, and 6%) or mannitol (5%). Approximately 30 seedlings per line per plate were assayed, and three plates per treatment were used. Error bars indicate sd. Asterisks indicate significant differences compared with the wild type (P < 0.05), as calculated by Student's t test.
BT2 Modulates Hormone Responses in Plants by Suppressing ABA Signaling while Enhancing Auxin Signaling
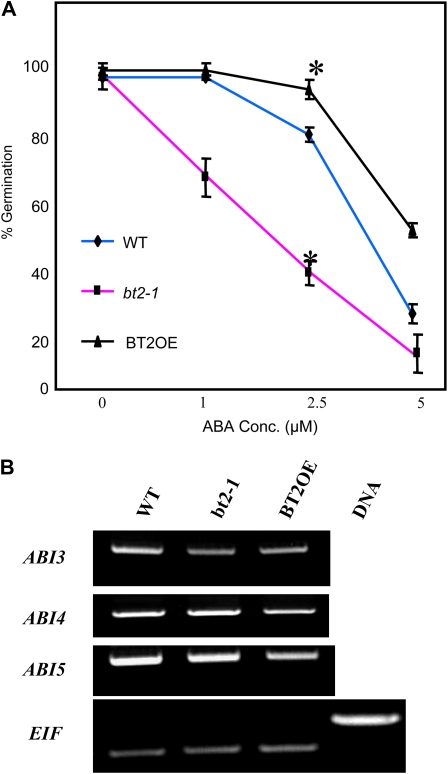
Because ABA inhibits germination in a manner similar to sugars (Arenas-Huertero et al., 2000; León and Sheen, 2003), we performed two experiments to determine whether BT2 also affected ABA signaling and responses at germination. First, we germinated seeds from bt2-1, BT2OE, and wild-type lines on various concentrations of ABA (0, 1, 2.5, and 5 μm) and determined the percentage of seedlings with normal cotyledon emergence. In parallel to the results obtained for sugars, ABA concentrations as low as 2.5 μm reduced germination of the wild type. However, at similar concentrations, the bt2-1 line was sensitive while the BT2OE line was resistant to ABA inhibition (Fig. 5A), leading us to conclude that BT2 suppresses certain ABA signals or responses at germination. Next, we asked whether selected ABA signaling genes were differentially expressed in bt2-1, BT2OE, and wild-type lines when grown in the presence of high sugars (5% Glc). The results from RT-PCR experiments performed on ABA-insensitive3 (ABI3), ABI4, and ABI5 indicate that the relative abundance of the respective transcripts in the different lines remained unaffected (Fig. 5B).
Figure 5.
BT2 suppresses ABA-mediated inhibition of germination. A, Quantification of the percentage of germinated seedlings with normal cotyledons of wild-type (WT), bt2-1, and BT2OE lines grown on various concentrations (Conc.) of ABA (0, 1, 2.5, and 5 μm). Approximately 30 seedlings per line per plate were assayed, and three plates per treatment were used. Error bars indicate sd. Asterisks indicate significant differences compared with the wild type (P < 0.05), as calculated by Student's t test. B, Ten-day-old wild-type, bt2-1, and BT2OE lines were grown on 5% Glc. Total RNA was isolated and subjected to RT-PCR analysis (25 cycles) to determine expression of ABI3, ABI4, and ABI5. EIF4-A2 expression was used as a loading control. [See online article for color version of this figure.]
We previously reported that BT2 potentiates some responses to auxin. bt2-1 seedlings are resistant to exogenous auxin, while constitutive expression of BT2 in the high-auxin mutant yucca exacerbates its phenotype (Ren et al., 2007). Here, we found that loss of BT2 in yucca specifically suppressed its characteristic high-auxin phenotype of epinastic cotyledons, epinastic leaves, shorter primary roots, excess root hair, and delayed development (Fig. 6). However, the elongated hypocotyl and petioles were less affected. Taken together, our findings here further strengthen the role of BT2 in enhancing certain auxin responses while suppressing ABA and sugar responses in plants.
Figure 6.
Loss of BT2 suppresses the high-auxin phenotypes in yucca. Suppression of the epinastic cotyledon and leaf morphology (A), shorter primary root (B), excess root hairs (C), and delayed flowering (D) of yucca in the double mutant, yucca × bt2-1. WT, Wild type.
BT2 Appears to Integrate Multiple Stress Signals
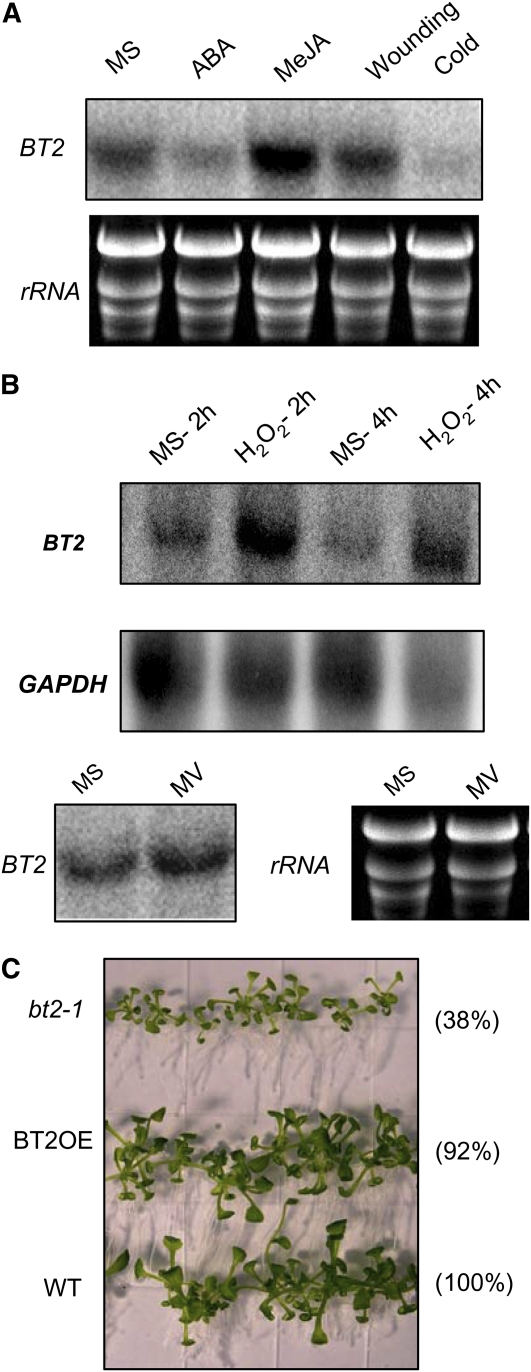
Because sugar and hormone signaling affect several responses to abiotic and biotic factors, and because BT2 has a role in both hormone and sugar signaling, we asked whether it also was required for stress responses. We started by analyzing changes in BT2 expression in response to different stress signals by treating 3-week-old plants with ABA, methyl jasmonate (Me-JA), cold, wounding, and H2O2. Treatment with ABA and cold lowered BT2 mRNA levels (Fig. 7A). Me-JA, which is antagonistic to ABA and mediates plant-pathogen defense signaling (Anderson et al., 2004), induced BT2 expression. Wounding did not affect the expression of BT2 (Fig. 7A). BT2 expression was induced by H2O2, a major reactive oxygen species (ROS) in plants, but not by treatment with methyl viologen, an electron transport inhibitor that also leads to oxidative stress (Fig. 7B). To ask whether loss of BT2 had any effect on plants challenged with free radical stress generated by H2O2, we grew bt2-1, BT2OE, and wild-type plants on media with and without 2 mm H2O2. Initially, H2O2 suppressed the growth of all lines equally (i.e. smaller leaves, shorter petioles, and an overall inhibition of vegetative growth). However, after a period of approximately 3 weeks in the presence of H2O2, bt2-1 plants were significantly smaller, whereas BT2OE plants were indistinguishable from wild-type plants (Fig. 7C).
Figure 7.
BT2 expression is modulated by multiple stress signals. A, Three-week-old wild-type plants were grown on MS or treated with either ABA (lane 2) or Me-JA (lane 3) for 3 h and subjected to either wounding stress (lane 4) or cold stress (lane 5) for 3 h. (B) The plants from A were then challenged with oxidative stress caused by either H2O2 or methyl viologen (MV) for the indicated times. Total RNA was extracted from rosette leaves and subjected to RNA-blot analysis. Ethidium bromide-stained rRNA and GAPDH were used as loading controls. C, Visible phenotypes of 3-week-old wild-type, bt2-1, and BT2OE lines grown on MS medium containing H2O2 (2 mm). Total fresh weight (mg) of 30 seedlings from two different plates and ratio of weights relative to the wild type (WT; in parentheses) are shown on the right.
DISCUSSION
We previously identified BT2 as an activator of telomerase activity in mature Arabidopsis leaves (Ren et al., 2007). Here, we report its roles in sugar signaling, its connections to hormone signaling, and its apparent function in integrating diverse biotic and abiotic stress signals. We showed that BT2 expression was diurnally regulated. However, this is not surprising, since 30% to 50% of Arabidopsis genes are diurnally regulated and the circadian clock and sugar status are the major inputs driving the diurnal regulation (Blasing et al., 2005). BT2 mRNA was more abundant (more than 100-fold) in the dark relative to the light (Fig. 1). BT2 expression was controlled by the circadian clock, because the diurnal anticipation of BT2 expression at 14 and 38 h (Fig. 1) in entrained plants persisted even in a continuous light cycle. In light/dark conditions, BT2 mRNA begins to accumulate at 14 and 38 h, in a possible anticipation of the dark phase (Fig. 1A). An initially puzzling feature of BT2 expression in continuous light was that, instead of peaking at the end of the dark period (24 and 48 h), the peaks of BT2 mRNA occurred at the end of the light period at 14 and 38 h (Fig. 1B). BT2 transcript failed to accumulate after the anticipatory period in continuous light, possibly because of the continuous presence of the abnormal light signal or another metabolic signal. We also found that BT2 was repressed and induced by brief exposure to light and dark alone, respectively (Fig. 2). These results suggest that light can also modulate BT2 expression independent of the circadian control. However, under prolonged exposure to an inappropriate signal, as in our continuous light experiment, the circadian regulation can override the abnormal signal, in an apparent attempt to restore an appropriate level of BT2 transcript.
Although the circadian clock drives the diurnal regulation of many genes, diurnal changes in sugar concentration also play a major role (Blasing et al., 2005) and BT2 expression was repressed by sugars (Fig. 3A). This result was also consistent with our finding that relative expression of BT2 was lowest during the light phase, possibly because of repression caused by higher levels of sugars produced by photosynthesis. In the dark, however, expression of BT2 was induced, possibly in response to sugar depletion. We also found that BT2 was induced by nitrates during both the light and dark phases (Fig. 3C). Addition of nitrates antagonizes sugar repression of gene expression (Moore et al., 2003). This antagonism could be due, in part, to competition for a limited amount of carbon in the cell that can be diverted either to produce organic acids and amino acids by nitrogen metabolism or to produce sugars and starches by carbon metabolism (Stitt and Krapp, 1999). Our results are consistent with gene expression databases, which indicate that BT2 is repressed by sugars and induced by nitrates (Scheible et al., 2004; Wang et al., 2004; Blasing et al., 2005; Usadel et al., 2008).
To determine whether BT2 was involved in responses to sugar signaling, we analyzed germination in the presence of inhibitory Glc concentrations and found that constitutive overexpression of BT2 imparted resistance to inhibition of germination and early vegetative development by Glc. In contrast, loss of BT2 resulted in significantly increased sensitivity to inhibitory Glc levels (Fig. 4).
High concentrations of sugars impart their inhibitory effect on germination by modulating ABA signaling (León and Sheen, 2003). Also, several mutations identified as Glc-insensitive or sugar-insensitive, such as gin1, gin5, sis4, sis7, and sis10, are allelic to ABA-insensitive/deficient mutations (Arenas-Huertero et al., 2000; Cheng et al., 2002). After identifying a role for BT2 in modulating sugar signaling/responses at germination, we wanted to determine whether ABA responses also were modulated by BT2. We found that, parallel to the sugar responses, bt2-1 was sensitive to ABA inhibition of germination, while BT2OE lines were resistant (Fig. 5A). Again, similar to sugars, ABA repressed the expression of BT2 (Fig. 7A). This pattern of reciprocal negative feedback (where BT2 suppresses ABA signaling and ABA suppresses BT2 expression) suggests that BT2 may normally function to prevent inappropriate signaling at low concentrations of ABA, but this function can be abrogated at higher concentrations of ABA.
BT2 does not appear to affect expression at the mRNA level for ABA signaling genes. This conclusion is supported by our previous microarray studies performed on the tac1-1d mutant line, which has increased BT2 expression (Ren et al., 2007). When compared with the wild type, tac1-1d lines did not display any significant changes in transcript levels for genes in ABA signaling pathways. This lack of influence on ABA signaling genes could be due to either insufficient expression of BT2 at the protein level in 35S∷BT2 and tac1-1d lines or the well-documented redundancy among BT family members (Robert et al., 2009) in the bt2-1 null mutant. Alternatively, BT2 itself may be a downstream target of the ABA signaling genes. Regardless of the mechanism, our results here strongly support a role for BT2 in modulating sugar and ABA responses at germination.
Cold signaling is intricately associated with ABA, and cold stress and treatment with ABA repressed BT2 expression (Fig. 7A). In fact, treatment with cold leads to an increase in the levels of ABA (Lee et al., 2001). Hence, it is possible that the cold repression of BT2 was an indirect effect of increased ABA levels and/or signaling. Jasmonate is antagonistic to ABA in modulating defense gene expression (Anderson et al., 2004) and salt stress-inducible gene expression in rice (Oryza sativa; Moons et al., 1997). In contrast to ABA and cold, Me-JA induced BT2 expression, thus suggesting a possible role of BT2 in jasmonate signaling/pathogen defense. However, further experiments are necessary to directly implicate BT2 in defense signaling.
ABA, often dubbed “the universal stress hormone,” is associated with the response to ROS and cross talks with multiple hormones and biotic and abiotic signals (Roitsch, 1999; Fedoroff, 2002; Couee et al., 2006). BT2 is induced by H2O2, a major ROS in plants, and loss of BT2 renders the plants sensitive to external H2O2 (Fig. 7). This sensitivity, however, was not observed when the bt2-1 lines were subjected to a different ROS stress, superoxide anion generated by methyl viologen (data not shown). Moreover, BT2 expression was not induced by ROS stress caused by methyl viologen (Fig. 7B), suggesting that the response of BT2 to H2O2 is specific and not due to general ROS-related stress.
We previously reported that BT2 enhances certain auxin responses (Ren et al., 2007). Here, we present additional evidence for its role in potentiating auxin responses in plants. Loss of BT2 in the high-auxin mutant yucca reversed several of its high-auxin phenotypes, including its characteristic epinastic cotyledons, epinastic leaves, shorter primary root, excess root hair, and delayed flowering (Fig. 6). Auxin is often antagonistic to ABA, perhaps because of its opposite effects on cytosolic pH and regulation of Ca2+ concentrations (Gehring et al., 1990). Mutations in genes such as HYPONASTIC LEAVES1 result in hypersensitivity to ABA while simultaneously rendering resistance to auxin (Lu and Fedoroff, 2000). From our results here and previous studies (Ren et al., 2007), BT2 seems to potentiate auxin responses and suppress ABA responses, consistent with antagonism between auxin and ABA.
Although BT2's initially described function was in regulating telomerase activity in mature leaves (Ren et al., 2007) and it was recently shown to function in gametophyte development along with other BT family genes (Robert et al., 2009), it now appears to play a much broader role. The gene itself responds to multiple biotic and abiotic signals, including light, circadian clock, phytohormones, and nutrients, and BT2 is required for the appropriate response to many of these same signals. We propose that BT2 occupies an integral position in a complex signaling network that perceives, integrates, and responds to multiple, and sometimes competing, signals. Preliminary results from our laboratory indicate that, similar to BT2 responses, expression of BT1 and BT5 is also responsive to sugars and nitrates (K.K. Mandadi, unpublished data), consistent with previous reports of functional redundancy in the BT gene family (Robert et al., 2009).
It is not yet clear how BT2 affects multiple signaling pathways. Earlier studies from other laboratories, using recombinant proteins, in vitro pull-down assays, or yeast two-hybrid screens, identified BT2, along with other BT family members, as interacting with either CULLIN3 (Figueroa et al., 2005) or with the BET9 and BET10 bromodomain proteins (Du and Poovaiah, 2004). Although BT2's in vivo partners are yet to be identified, we hypothesize that it assembles in multiprotein complexes. If the complex requires CULLIN3 or a similar protein, it may function as a ubiquitin ligase and target specific proteins for degradation. Alternatively, if the BT2 complex requires the BET9 or BET10 bromodomain proteins, the complex may work by recognizing the chromatin state of target gene promoters. Identification of proteins that interact with BT2 in vivo will be required to resolve the possible modes of action.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Wild-type Arabidopsis (Arabidopsis thaliana ecotype Columbia) or mutant plants were grown in soil in 14-h-light/10-h-dark cycles at 21°C and a light intensity of approximately 120 to 130 μmol m−2 s−1 with 70% relative humidity. For germination assays, seeds were surface sterilized with 50% (v/v) bleach and 0.1% (v/v) Triton X-100 for 7 min, cold treated at 4°C for 3 to 4 d, and then grown on Murashige and Skoog (MS) medium (Sigma) with 0.8% (w/v) phytagar under continuous low light (approximately 30 μmol m−2 s−1). All media contained 1% Suc, unless stated otherwise. BT2 overexpression lines and the bt2-1 null line were described previously (Ren et al., 2007). To examine the effect of loss of BT2 on the high-auxin phenotype of yucca (Zhao et al., 2001), we generated and examined the progeny of a bt2-1 × yucca cross.
Treatments and Expression Analysis
For circadian experiments and light/dark treatments, whole rosettes of 3-week-old wild-type plants (prior to flowering) were harvested at the indicated times of the diurnal cycle. Two biological replicates each containing two rosettes were harvested and subjected to RNA gel blot and qRT-PCR analysis. Total RNA was isolated using TRI reagent following the manufacturer's protocol (Ambion). For RNA gel blots, 20 μg of total RNA from each sample was separated on a 1.2% formaldehyde denaturing gel and transferred to a Hybond N+ membrane (Amersham). Blots were then probed with 32P-labeled PCR products obtained from amplification of BT2 cDNA using the primers listed (Supplemental Table S1). Subsequently, the blot was stripped and reprobed for GLYCERALDEHYDE PHOSPHATE DEHYDROGENASE (GAPDH) mRNA as a loading control. For qRT-PCR, 1 μg of RNA was used to make cDNA using the SuperScript first-strand cDNA synthesis kit (Invitrogen). Amplification by PCR was performed as described previously (Guo et al., 2008) using Power SYBR Green Master Mix (Applied Biosystems) and the ABI Prism 7500 sequence detection system (Applied Biosystems). The primers used for qRT-PCR are listed (Supplemental Table S1). EIF-4A2 (At1g54270) and 18S rRNA (At2g01010) were used to normalize the expression, and fold changes of BT2 expression were calculated following the ΔΔCT method (Livak and Schmittgen, 2001).
To determine the effects of sugars and nitrates, leaves from 3-week-old wild-type plants were excised at the indicated times. To maintain transpiration flow, petioles were immediately recut in liquid medium supplemented with 100 mm Suc or mannitol and 50 mm KNO3 or KCl (Chiou and Bush, 1998). Treatments were conducted for 3 h in the appropriate light conditions, and the samples were subsequently subjected to RNA gel-blot analysis as described above to detect BT2 expression. Ethidium bromide-stained rRNA was used as a loading control. For Glc treatments, seedlings were grown in MS liquid medium for 5 d in continuous light and later transferred into medium without any sugars for 2 d. After day 7, the medium was supplemented with 50 mm Glc, 50 mm Suc, or 50 mm mannitol and the seedlings were treated for 3 h (Scheible et al., 2004; Blasing et al., 2005). Subsequent analysis of BT2 expression was performed by RT-PCR using 5 μg of total RNA to prepare cDNA. To determine the expression of ABI3, ABI4, and ABI5, 10-d-old wild-type, bt2-1, and BT2OE lines were grown on 5% Glc and analyzed by RT-PCR (25 cycles). The primers used for RT-PCR are listed (Supplemental Table S1).
To determine the effects of various stresses on BT2 expression, 3-week-old wild-type plants were subjected to various stress stimuli during the light phase. Cold treatment was performed by floating leaves in MS liquid medium on ice for 3 h; for wounding, leaves were punctured with forceps at several places and transferred to MS liquid medium for 3 h; for stress hormones, leaves were treated for 3 h in MS liquid media consisting of ABA (100 μm, mixed isomers) and Me-JA (100 μm) or dimethyl sulfoxide (0.1%); for oxidative stress, leaves were treated for the indicated times in MS liquid media consisting of H2O2 (10 mm) and methyl viologen (100 μm). BT2 expression was analyzed by RNA gel-blot analysis using 20 μg of total RNA as described above. Ethidium bromide-stained rRNA was used as a loading control. All treatments were repeated at least twice at different periods, and the results described are representative of consistent data obtained in replicate experiments. Dimethyl sulfoxide did not affect BT2 expression.
Glc, ABA, and H2O2 Sensitivity Assays
For Glc inhibition assays, wild-type, bt2-1, and BT2OE lines were germinated on solid MS media with various concentrations of Glc (4%, 5%, and 6%, w/v) or mannitol (5%, w/v), as described previously (Bi et al., 2005). After 5 or 6 d, seedlings with normal cotyledons were counted. For ABA inhibition assay, wild-type, bt2-1, and BT2OE lines were germinated on MS solid media with various concentrations of ABA (0, 1, 2.5, and 5 μm), as described previously (Xiong et al., 2002). After 5 or 6 d, seedlings with normal cotyledons were counted. Three replicate plates for each treatment were used to calculate the percentage germination rates, and significant differences were determined by Student's t test. For the H2O2 sensitivity assay, wild-type, bt2-1, and BT2OE lines were germinated on MS solid medium with or without 2 mm H2O2 (Miao et al., 2006) and were kept vertically in continuous low light (approximately 30 μmol m−2 s−1) for 3 weeks. Mean fresh weight of the seedlings was determined from averages of two replicate plates.
Arabidopsis Genome Initiative locus numbers for the genes used in this article are as follows: BT1, At5g63160; BT2, At3g48360; BT3, At1g05690; BT4, At5g67480; BT5, At4g37610; ABI3, At3g24650; ABI4, At2g40220; ABI5, At2g36270; EIF-4A2, At1g54270; 18S rRNA, At2g01010; GAPDH, At3g04120.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Quantification of diurnal fluctuation of BT2 mRNA.
Supplemental Table S1. Primers used.
Supplementary Material
Acknowledgments
We thank Dr. Wayne Versaw for critical reading and comments on the manuscript and his laboratory members, especially Biwei Guo, for help with the circadian experiments and qRT-PCR analysis.
This work was supported by the National Science Foundation (grant no. MCB 0244159 to T.D.M.) and the Texas Water Resources Institute (grant no. 2008TX309B to K.K.M.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Thomas D. McKnight (mcknight@bio.tamu.edu).
Some figures in this article are displayed in color online but in black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Anderson JP, Badruzsaufari E, Schenk PM, Manners JM, Desmond OJ, Ehlert C, Maclean DJ, Ebert PR, Kazan K (2004) Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 16 3460–3479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arenas-Huertero F, Arroyo A, Zhou L, Sheen J, Leon P (2000) Analysis of Arabidopsis glucose insensitive mutants, gin5 and gin6, reveals a central role of the plant hormone ABA in the regulation of plant vegetative development by sugar. Genes Dev 14 2085–2096 [PMC free article] [PubMed] [Google Scholar]
- Baena-Gonzalez E, Rolland F, Thevelein JM, Sheen J (2007) A central integrator of transcription networks in plant stress and energy signaling. Nature 448 938–942 [DOI] [PubMed] [Google Scholar]
- Bi YM, Zhang Y, Signorelli T, Zhao R, Zhu T, Rothstein S (2005) Genetic analysis of Arabidopsis GATA transcription factor gene family reveals a nitrate-inducible member important for chlorophyll synthesis and glucose sensitivity. Plant J 44 680–692 [DOI] [PubMed] [Google Scholar]
- Blasing OE, Gibon Y, Gunther M, Hohne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible WR, Stitt M (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 17 3257–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A, Leon P, Nambara E, Asami T, Seo M, et al (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis abscisic acid biosynthesis and glucose signaling. Plant Cell 14 2723–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou TJ, Bush DR (1998) Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA 95 4784–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coruzzi GM, Zhou L (2001) Carbon and nitrogen sensing and signaling in plants: emerging ‘matrix effects.’ Curr Opin Plant Biol 4 247–253 [DOI] [PubMed] [Google Scholar]
- Couee I, Sulmon C, Gouesbet G, El Amrani A (2006) Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J Exp Bot 57 449–459 [DOI] [PubMed] [Google Scholar]
- Du L, Poovaiah BW (2004) A novel family of Ca2+/calmodulin-binding proteins involved in transcriptional regulation: interaction with fsh/Ring3 class transcription activators. Plant Mol Biol 54 549–569 [DOI] [PubMed] [Google Scholar]
- Fedoroff NV (2002) Cross-talk in abscisic acid signaling. Sci STKE 140 re10. [DOI] [PubMed] [Google Scholar]
- Figueroa P, Gusmaroli G, Serino G, Habashi J, Ma L, Shen Y, Feng S, Bostick M, Callis J, Hellmann H, et al (2005) Arabidopsis has two redundant Cullin3 proteins that are essential for embryo development and that interact with RBX1 and BTB proteins to form multisubunit E3 ubiquitin ligase complexes in vivo. Plant Cell 17 1180–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzarrini S, McCourt P (2001) Genetic interactions between ABA, ethylene and sugar signaling pathways. Curr Opin Plant Biol 4 387–391 [DOI] [PubMed] [Google Scholar]
- Gehring CA, Irving HR, Parish RW (1990) Effects of auxin and abscisic acid on cytosolic calcium and pH in plant cells. Proc Natl Acad Sci USA 84 9645–9649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler F, Millar AA, Jacobsen JV (2005) Dormancy release, ABA and pre-harvest sprouting. Curr Opin Plant Biol 8 183–187 [DOI] [PubMed] [Google Scholar]
- Guo B, Jin Y, Wussler C, Blancaflor EB, Motes CM, Versaw WK (2008) Functional analysis of the Arabidopsis PHT4 family of intracellular phosphate transporters. New Phytol 177 889–898 [DOI] [PubMed] [Google Scholar]
- Gutierrez RA, Stokes TL, Thum K, Xu X, Obertello M, Katari MS, Tanurdzic M, Dean A, Nero DC, McClung CR, et al (2008) Systems approach identifies an organic nitrogen-responsive gene network that is regulated by the master clock control gene CCA1. Proc Natl Acad Sci USA 105 4939–4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J, Hanssen M, Wiese A, Hendriks MMWB, Smeekens S (2008) The sucrose regulated transcription factor bZIP11 affects amino acid metabolism by regulating the expression of ASPARAGINE SYNTHETASE1 and PROLINE DEHYDROGENASE2. Plant J 53 935–949 [DOI] [PubMed] [Google Scholar]
- Himanen K, Boucheron E, Vanneste S, de Almeida Engler J, Inzé D, Beeckman T (2002) Auxin-mediated cell cycle activation during early lateral root initiation. Plant Cell 14 2339–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoth S, Morgante M, Sanchez JP, Hanafey MK, Tingey SV, Chua NH (2002) Genome-wide gene expression profiling in Arabidopsis thaliana reveals new targets of abscisic acid and largely impaired gene regulation in the abi1-1 mutant. J Cell Sci 115 4891–4900 [DOI] [PubMed] [Google Scholar]
- Koch KE (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47 509–540 [DOI] [PubMed] [Google Scholar]
- Laby RJ, Kincaid MS, Kim D, Gibson SI (2000) The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J 23 587–596 [DOI] [PubMed] [Google Scholar]
- Lee H, Xiong L, Gong Z, Ishitani M, Stevenson B, Zhu JK (2001) The Arabidopsis HOS1 gene negatively regulates cold signal transduction and encodes a RING finger protein that displays cold-regulated nucleocytoplasmic partitioning. Genes Dev 15 912–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León P, Sheen J (2003) Sugar and hormone connections. Trends Plant Sci 8 110–116 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- Lu C, Fedoroff N (2000) A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell 12 2351–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CR (2006) Plant circadian rhythms. Plant Cell 18 792–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Y, Lv D, Wang P, Wang XC, Chen J, Miao C, Song CP (2006) An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell 18 2749–2766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons A, Prinsen E, Bauw G, Van Montagu M (1997) Antagonistic effects of abscisic acid and jasmonates on salt stress-inducible transcripts in rice roots. Plant Cell 9 2243–2259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300 332–336 [DOI] [PubMed] [Google Scholar]
- Pego JV, Kortstee AJ, Huijser C, Smeekens S (2000) Photosynthesis, sugars and the regulation of gene expression. J Exp Bot 51 407–416 [DOI] [PubMed] [Google Scholar]
- Price J, Li TC, Kang SG, Na JK, Jang JC (2003) Mechanisms of glucose signaling during germination of Arabidopsis. Plant Physiol 132 1424–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren S, Mandadi KK, Boedeker AL, Rathore KS, McKnight TD (2007) Regulation of telomerase in Arabidopsis by BT2, an apparent target of TELOMERASE ACTIVATOR1. Plant Cell 19 23–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard C, Granier C, Inzé D, De Veylder L (2001) Analysis of cell division parameters and cell cycle gene expression during the cultivation of Arabidopsis thaliana cell suspensions. J Exp Bot 52 1625–1633 [PubMed] [Google Scholar]
- Robert HS, Quint A, Brand D, Vivian-Smith A, Offringa R (2009) BTB AND TAZ DOMAIN scaffold proteins perform a crucial function in Arabidopsis development. Plant J 58 109–121 [DOI] [PubMed] [Google Scholar]
- Roitsch T (1999) Source-sink regulation by sugar and stress. Curr Opin Plant Biol 2 198–206 [DOI] [PubMed] [Google Scholar]
- Rolland F, Moore B, Sheen J (2002) Sugar sensing and signaling in plants. Plant Cell (Suppl) 14 S185–S205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheible WR, Morcuende R, Czechowski T, Fritz C, Osuna D, Palacios-Rojas N, Schindelasch D, Thimm O, Udvardi MK, Stitt M (2004) Genome-wide reprogramming of primary and secondary metabolism, protein synthesis, cellular growth processes, and the regulatory infrastructure of Arabidopsis in response to nitrogen. Plant Physiol 136 2483–2499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51 49–81 [DOI] [PubMed] [Google Scholar]
- Stitt M (1999) Nitrate regulation of metabolism and growth. Curr Opin Plant Biol 2 178–186 [DOI] [PubMed] [Google Scholar]
- Stitt M, Krapp A (1999) The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ 22 583–621 [Google Scholar]
- Usadel B, Blasing OE, Gibon Y, Retzlaff K, Hohne M, Gunther M, Stitt M (2008) Global transcript levels respond to small changes of the carbon status during progressive exhaustion of carbohydrates in Arabidopsis rosettes. Plant Physiol 146 1834–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkest A, Weinl C, Inzé D, Veylder LD, Schnittger A (2005) Switching the cell cycle: Kip-related proteins in plant cell cycle control. Plant Physiol 139 1099–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Tischner R, Gutiérrez RA, Hoffman M, Xing X, Chen M, Coruzzi G, Crawford NM (2004) Genomic analysis of the nitrate response using a nitrate reductase-null mutant of Arabidopsis. Plant Physiol 136 2512–2522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong L, Lee H, Ishitani M, Tanaka Y, Stevenson B, Koiwa H, Bressan RA, Hasegawa PM, Zhu JK (2002) Repression of stress-responsive genes by FIERY2, a novel transcriptional regulator in Arabidopsis. Proc Natl Acad Sci USA 99 10899–10904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu SM (1999) Cellular and genetic responses of plants to sugar starvation. Plant Physiol 121 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Christensen SK, Fankhauser C, Cashman JR, Cohen JD, Weigel D, Chory J (2001) A role for flavin monooxygenase-like enzymes in auxin biosynthesis. Science 291 306–309 [DOI] [PubMed] [Google Scholar]
- Zhou L, Jang JC, Jones TL, Sheen J (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95 10294–10299 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.