Abstract
Loosening of cell walls is an important developmental process in key stages of the plant life cycle, including seed germination, elongation growth, and fruit ripening. Here, we report direct in vivo evidence for hydroxyl radical (·OH)-mediated cell wall loosening during plant seed germination and seedling growth. We used electron paramagnetic resonance spectroscopy to show that ·OH is generated in the cell wall during radicle elongation and weakening of the endosperm of cress (Lepidium sativum; Brassicaceae) seeds. Endosperm weakening precedes radicle emergence, as demonstrated by direct biomechanical measurements. By 3H fingerprinting, we showed that wall polysaccharides are oxidized in vivo by the developmentally regulated action of apoplastic ·OH in radicles and endosperm caps: the production and action of ·OH increased during endosperm weakening and radicle elongation and were inhibited by the germination-inhibiting hormone abscisic acid. Both effects were reversed by gibberellin. Distinct and tissue-specific target sites of ·OH attack on polysaccharides were evident. In vivo ·OH attack on cell wall polysaccharides were evident not only in germinating seeds but also in elongating maize (Zea mays; Poaceae) seedling coleoptiles. We conclude that plant cell wall loosening by ·OH is a controlled action of this type of reactive oxygen species.
The plant cell protoplast is surrounded by the cell wall, a highly complex composite permeated by water and composed mainly of cellulose microfibrils embedded in a matrix of hemicellulosic and pectic polysaccharides, also containing proteins and phenolic compounds (Fry, 2000; Cosgrove, 2005; Knox, 2008). Inorganic ions and enzymes secreted into the plant cell walls, collectively called the apoplast, can be bound to specific wall components and contribute to the dynamic nature of this compartment. Plant cell growth is driven by water uptake and restricted by the cell wall: the structural properties and mechanical strength of the plant cell wall determine the shape and the rate and direction of growth of individual cells as well as the mechanical resistance of whole tissues (Cosgrove, 2005; Schopfer, 2006). Cell wall loosening, therefore, is an important process in all stages of plant development requiring elongation growth or tissue weakening. These include pollen tube elongation (Eckardt, 2005), root hair development (Foreman et al., 2003; Monshausen et al., 2007), fruit ripening (Brummell and Harpster, 2001; Fry et al., 2001), seedling elongation, and seed germination (Finch-Savage and Leubner-Metzger, 2006; Müller et al., 2006), which is the focus of this study.
In the mature seeds of most angiosperms, the embryo is covered by two envelopes: the living endosperm and the dead testa. In order for seeds to complete germination successfully (germination being defined as the events between seed imbibition and radicle emergence), cell wall loosening is required for radicle elongation growth driven by water uptake and for weakening of the covering envelopes (Bewley, 1997b; Finch-Savage and Leubner-Metzger, 2006; Nonogaki, 2006). A developmental switch from seed germination to seedling growth takes place after radicle emergence (Lopez-Molina et al., 2001). As these two stages of plant growth are based on different developmental programs, it is not known whether initial radicle elongation within the seed is driven by the same mechanisms as seedling elongation growth after radicle emergence.
Cell wall loosening requires structural changes in the wall, as load-bearing bonds must be broken. Known wall-modifying mechanisms in plants include enzymatic hydrolysis, transglycosylation, and expansin action (Cosgrove, 2005). In seeds in particular, enzymatic hydrolysis of endosperm cell walls by endoglycanases such as β-1,3-glucanase (Leubner-Metzger, 2002) and β-1,4-mannanase (Nonogaki et al., 2000; Toorop et al., 2000; da Silva et al., 2004) has been shown to play a role during seed germination (for a detailed discussion and references, see Bewley, 1997a; Finch-Savage and Leubner-Metzger, 2006). Expansins and xyloglucan endotransglucosylase/hydrolases are expressed in the endosperm cap of tomato (Solanum lycopersicum) seeds during germination (Chen et al., 2002), where they can contribute to endosperm weakening.
Hydroxyl radicals (·OH) have been proposed as an additional plant cell wall-loosening agent (Schopfer, 2001). These extremely reactive molecules can, if produced directly in the apoplast, attack cell wall polysaccharides and lead to breakage of load-bearing structures. While this process has been hypothesized to play a role in a variety of contexts, such as seed germination (Bailly, 2004) and seedling growth (Schopfer, 2001), it has so far only been shown directly in ripening pear (Pyrus communis) fruits (Fry et al., 2001). Schopfer (2001) showed that extension can be induced in dead coleoptiles by exposing them to ·OH and that exposure to ·OH accelerates the growth of living seedlings. However, cell wall oxidation was not investigated in seedlings.
We investigated in vivo ·OH production and oxidation of cell wall polysaccharides in defined tissues of germinating cress (Lepidium sativum) seeds and maize (Zea mays) seedlings. Germination and seedling elongation represent distinct key developmental processes that require wall loosening for elongation growth or tissue weakening. Production of reactive oxygen species (ROS), including ·OH and superoxide (O2·−), has been reported in seeds and seedlings of various plant species during development (Bailly, 2004; Oracz et al., 2009) and the alleviation of dormancy (Oracz et al., 2007), but their role is not yet understood. Their known mode of action could be either indirect (cellular signaling; Oracz et al., 2009) or direct (e.g. scission of polymers), but the latter was often regarded as a “negative role,” causing toxicity and deterioration (Bailly, 2004; Winterbourn, 2008). Here, we report direct in vivo evidence for a “positive” developmental role and a novel direct action of apoplastic ROS during seed germination and seedling growth. Our approach is, to our knowledge, the first to combine direct biochemical and biophysical detection of ROS with an investigation of their in vivo action on the cell wall and alterations to biomechanical tissue properties.
RESULTS AND DISCUSSION
Tissue Weakening during Seed Germination: Hydrogen Peroxide Inhibits and ·OH Generation Promotes Weakening of the Endosperm Envelope
Seed germination of garden cress comprises two sequential steps, testa and endosperm rupture, as does germination of the model plant Arabidopsis (Arabidopsis thaliana; Müller et al., 2006). Arabidopsis is a close relative of cress, and both species share a highly similar seed anatomy and germination physiology. As cress seeds are much larger than the tiny seeds of Arabidopsis, they are better suited to biochemical and biomechanical approaches at the tissue or organ level.
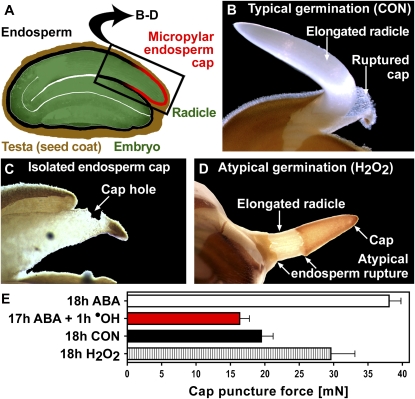
Typically, cress embryos emerge from their covering layers by the elongating radicle penetrating the weakened endosperm cap, which covers the radicle after the testa has ruptured (Fig. 1, A and B). Weakening of the cap, which consists of one to two cell layers, is required for typical germination and is inhibited by the germination-inhibiting hormone abscisic acid (ABA; Müller et al., 2006). Indirect evidence supports the view that cap weakening also occurs in Arabidopsis seeds and is regulated by hormones in the same manner (Finch-Savage and Leubner-Metzger, 2006; Bethke et al., 2007).
Figure 1.
Developmental factors and ROS affect the weakening of the cress endosperm cap. A, A mature cress seed consists of an embryo surrounded by two covering tissues: the testa and the endosperm. The cap covers the radicle tip. B, Typical germination. Cress seeds germinate in two steps. After approximately 8 h, the brown testa ruptures, revealing the radicle still covered by the cap, which weakens until at approximately 18 h it is penetrated by the elongating radicle. CON, Control (medium without additions). C, In caps incubated after dissection at 18-h CON (“isolated caps”), tissue weakening led to the formation of a hole at the place where the radicle would have emerged after 1 to 2 d. D, Atypical germination after addition of H2O2 to the medium. E, Effects of H2O2, ABA, and ABA followed by ·OH (Fenton reaction initiated by 100 μm H2O2 and ascorbic acid in caps loaded with Fe2+) on cap weakening quantified by puncture force measurements. In a control treatment with Fe2+ and ascorbic acid but without H2O2, the puncture force did not decline significantly (34.9 ± 1.5 mN). Mean values ± se of at least three replicates of 25 caps are presented. A and B are modified from Müller et al. (2006).
Hydrogen peroxide (H2O2) treatment is known to stimulate germination of dormant seeds by releasing dormancy and by degradation of endogenous inhibitors such as ABA (Bailly, 2004). Our cress seed batch exhibits only a very shallow dormancy when fresh and none in the after-ripened state, which the seeds used in this study were in. For these nondormant cress seeds, the addition of 10 mm H2O2 to the medium did not change the germination kinetics but led to atypical germination in approximately 10% of the seeds (Fig. 1D): the endosperm cap was torn off at its base instead of being penetrated by the radicle. Measurements of the tissue resistance of caps exposed to 10 mm H2O2 showed that cap weakening is inhibited (Fig. 1E). This might be caused by cell wall-tightening reactions that H2O2 is known to cause by cross-linking extraprotoplasmatic polymers (Brisson et al., 1994; Schopfer, 1996; Encina and Fry, 2005). We cannot rule out cytotoxic effects of a 10 mm H2O2 treatment, although we observed that the seeds developed into normal-looking and healthy seedlings. It seems likely that the radicle, whose elongation was not influenced by 10 mm H2O2 (data not shown), elongates as usual while cap weakening fails to keep up, causing the atypical germination described above. This effect shows that the cap can act as a restraint to radicle elongation despite its thinness. These conclusions are in agreement with work on the thin lettuce (Lactuca sativa) endosperm, for which chemical inhibition of weakening increases the percentage of seeds that exhibit either embryo expansion without protrusion (embryo buckling within the endosperm envelope) or atypical endosperm rupture (Pavlista and Haber, 1970).
While the addition of H2O2 alone to the medium thus inhibited cap weakening, the generation of ·OH in the cap cell walls via a Fenton reaction (Fe2+ + H2O2 → Fe3+ + OH− + ·OH) strongly accelerated it (Fig. 1E). We quantified this effect directly by puncture force measurements in which the tissue resistance of cress endosperm caps preincubated in ABA and then exposed to apoplastic ·OH was determined. During typical germination, the force it took to rupture the endosperm tissue declined prior to endosperm rupture and radicle emergence from approximately 38 mN to approximately 20 mN (18h CON). This cap weakening was inhibited by ABA (18h ABA) and by H2O2 (18h H2O2). Incubation in ABA for 17 h followed by only 1 h of exposure to apoplastic ·OH led to a decline in tissue resistance: the puncture force was approximately 17 mN (Fig. 1E).
In caps that were incubated separately from radicles after dissection of the seeds, this decline was followed by local tissue dissolution and the formation of a hole at the tip of the cap where radicle emergence would usually occur (Fig. 1C). This developmentally regulated hole formation was inhibited by H2O2 as well as by ABA, in agreement with these substances' influence on tissue resistance: after 1 d, four out of five caps incubated without H2O2 and ABA had a hole, while none of the caps incubated in the presence of 10 mm H2O2 or 10 μm ABA did. Taken together, these results suggest a positive role for ·OH in cell wall loosening during cress seed germination (i.e. in the developmentally and hormonally controlled processes of hole formation and cap weakening required for seed germination).
·OH and O2·− Are Produced in Vivo in the Apoplast during Cress Seed Germination
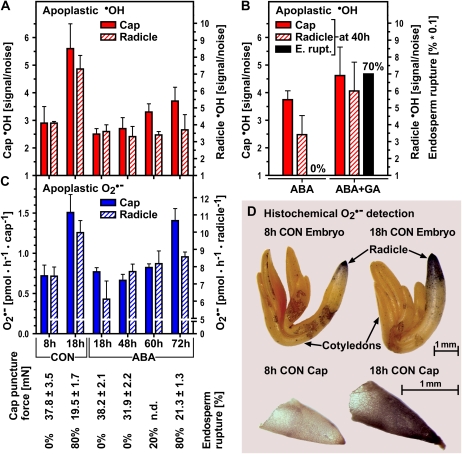
In order to have a cell wall-loosening effect in vivo, ·OH must be produced in the direct vicinity of wall polysaccharides: the radicals' mobility range is extremely limited owing to their high reactivity and short life span (Fry et al., 2001; Schopfer, 2001). We used a spin trap that reacts with ·OH, forming a stable adduct to detect in vivo ·OH production by electron paramagnetic resonance (EPR) spectroscopy. This method is specific for apoplastic ·OH, as Heyno et al. (2008) showed when they used the technique to detect the inhibitory influence of cadmium on ·OH produced apoplastically at the plasma membrane independently of its stimulatory effect on intracellular ·OH produced in mitochondria. It has been successfully used to detect ·OH in Arabidopsis and cucumber (Cucumis sativus) seedling roots: in cucumber seedlings, but not in the small Arabidopsis seedlings, even localization to the growing zone was possible (Renew et al., 2005). We investigated in vivo apoplastic ·OH production in cress seeds (Fig. 2), whose size made it possible to work with separate seed parts (Müller et al., 2006).
Figure 2.
In vivo detection of apoplastic ·OH and O2·− production in cress caps and radicles during seed germination. A, Quantification of EPR signal sizes indicative of in vivo-generated apoplastic ·OH in caps and radicles dissected at the times indicated. Seeds were incubated in medium without (control [CON]) or with 10 μm ABA (ABA) added. Note the different scales of the y axes for cap and radicle. For comparison with the germination response, the puncture force and endosperm rupture values are given below graph C. B, ABA-GA antagonism. In vivo-generated apoplastic ·OH (EPR as in A) in caps and radicles treated with ABA or ABA + GA (5 μm ABA + 10 μm GA4+7). Means of radicle samples (ABA versus ABA + GA) differ significantly (P < 0.05) as calculated by one-way ANOVA followed by Tukey's multiple comparison test (GraphPad Prism software). E. rupt., Endosperm rupture. C, Quantification of apoplastic O2·− in intact caps and radicles by photometric determination of the reduction of XTT. D, Histochemical staining of O2·− production with nitroblue tetrazolium chloride in embryos (10 min of staining) and endosperm caps (15 min of staining). In A to C, mean values ± se of at least four replicates of 100 radicles and caps are shown.
In vivo apoplastic ·OH production in cress endosperm caps and radicles increased strongly between 8 and 18 h (Fig. 2A). The 8-h time point is characterized by a still unweakened endosperm and nonelongating radicle (8h CON), while at 18 h, cap weakening has progressed, tissue resistance is halved, and radicle elongation starts (18h CON). ABA inhibited these physiological processes between 8 and 18 h and inhibited ·OH production in both tissues as well.
A tissue-specific ABA effect could be observed as the seeds progressed to germination in the presence of ABA: caps showed an increase of ·OH production toward 72 h, while radicles showed an ·OH production equal to that at 18 h of ABA (Fig. 2A). Possible interpretations of this phenomenon are (1) that cell wall loosening and thereby radicle elongation mechanisms differ in the presence and absence of ABA, and (2) that the increase in ·OH production takes place during a very narrow time window, as it leads to immediate cell wall loosening and radicle growth driven by water uptake (Müller et al., 2006).
The reversion of the inhibitory ABA effect on germination and endosperm weakening by its antagonist gibberellin (GA; Müller et al., 2006) could also be observed at the level of in vivo apoplastic ·OH production in radicles and endosperm caps (Fig. 2B). While we observed an increase in variance between the samples, which is possibly due to the fact that hormone interactions tend to vary strongly within a population, the overall effect was an obvious increase of ·OH production on reversion of the ABA inhibition of germination with GA. These observations support our hypothesis that hormone-sensitive ·OH-mediated effects in the cell wall contribute to endosperm weakening and radicle elongation.
We conclude that the increase in apoplastic ·OH production might be a mechanism for endosperm cap weakening and radicle elongation during germination and that the ABA-mediated inhibition of these processes might at least in part be caused by the ABA inhibition of the apoplastic ·OH production, which can be reversed by GA. ABA and GA are known for their antagonistic effects on the expression of cell wall hydrolases in the endosperm cap during weakening just prior to endosperm rupture (Finch-Savage and Leubner-Metzger, 2006). Examples include β-1,3-glucanase in tobacco (Nicotiana tabacum; Leubner-Metzger, 2002) and β-1,4-mannanase in tomato (Nonogaki et al., 2000; Toorop et al., 2000; da Silva et al., 2004) and coffee (Coffea arabica), where in addition an inhibitory effect of ABA on embryo growth potential has been demonstrated (da Silva et al., 2004).
Two hypotheses, which are not mutually exclusive, have been put forward to explain the source of ·OH production in the cell wall: natural Fenton reactions dependent on a reductant (e.g. ascorbate), transition metal ions (e.g. copper), and a source of H2O2 (e.g. O2 or O2·−) in the cell wall (Fry, 1998); and peroxidase-mediated Haber-Weiss reactions (H2O2 + O2·− → ·OH + OH− + O2; Schopfer, 2001). We found that O2·−, a precursor of ·OH according to either of the hypotheses and a product of the reaction of ·OH with polysaccharides (Deeble et al., 1990), was produced in the apoplast of radicles and endosperm caps (Fig. 2C). In both seed parts, its production increased from 8 to 18 h (CON) and was inhibited by ABA. With ABA, we observed a delayed increase of O2·− production in the endosperm cap, whose temporal pattern was highly similar to the ABA regulation of ·OH production. In the radicle, only a minor increase was observed, which is in accordance with the ·OH production pattern.
Figure 2D shows histochemical O2·− detection, in which we observed that O2·− production in embryos localized most strongly to the radicle, the part of the embryo that elongates first and strongest and that comes into contact with the cap. While in 8-h radicles the staining was exclusively localized to the very tip, by 18 h it had spread to additional adjacent parts of the elongation zone. The intensity of the staining, but not the spread of the localization, was inhibited by ABA (data not shown). O2·− production by the 18-h endosperm cap occurred over its entire surface, while 8-h caps stained very weakly (Fig. 2D). The ROS production in the apoplast by any of the proposed mechanisms, therefore, appears to be spatially as well as temporally regulated.
In our system, different inhibitors of O2·− production had tissue-specific effects (Table I). O2·− production, which is required for the peroxidase-mediated mechanism of ·OH production (Schopfer, 2001) and potentially contributes to the Fenton-mediated mechanism (since O2·− is rapidly dismutated to H2O2 and O2), was more sensitive to inhibition by cyanide (KCN) in the endosperm cap than in the radicle, while diphenyleneiodonium chloride (DPI) led to inhibition in both seed parts. KCN is known to inhibit peroxidases, which can produce O2·− (Minibayeva et al., 2000), as well as ascorbate oxidase, which is hypothesized to play a role in ROS generation (Green and Fry, 2005) and other heme-containing enzymes, while DPI is an inhibitor of membrane-located NADPH oxidases and other flavoenzymes (Doussiere and Vignais, 1992). Our inhibitor results are in agreement with the hypothesis that NADPH oxidases, as well as apoplastic peroxidases or ascorbate oxidases, play a role in O2·− production in germinating cress seeds. Highly specific inhibitors of these different enzymes are not known, but the observed differences in the inhibition sensitivities between radicle and endosperm caps imply that the mechanisms differ qualitatively between the two seed tissues. It should be noted that differences in the permeability of the tissues might account for part of the difference between inhibitor effects.
Table I.
Inhibitors of O2·− production have tissue-specific effects on germinating cress seeds
O2·− production was measured in radicles and endosperm caps of cress seeds incubated for 18 h. Two photometric assays were used: oxidation of epinephrine and reduction of XTT. The addition of CuZn-superoxide dismutase (SOD; from bovine erythrocytes) led to an inhibition of around two-thirds of the total signal, indicating that this fraction is specifically caused by O2·−-mediated reactions. As SOD can only reach the surface of the tissues, it is possible that the remaining fraction is (at least in part) also caused by O2·−, with the reaction taking place in regions inaccessible to SOD but accessible to XTT and epinephrine. Mean values ± se at 18 h of at least three replicates (n = 120) are presented in comparison with the untreated control. n.d., Not determined.
| Treatment | Radicle
|
Endosperm Cap
|
||
|---|---|---|---|---|
| Inhibition | se | Inhibition | se | |
| % | % | |||
| Epinephrine method | ||||
| Control | 0.0 | 0.0 | ||
| KCN (0.1 mm) | 20.4 | 12.6 | 60.5 | 0.9 |
| KCN (1 mm) | 45.2 | 6.3 | 57.2 | 2.2 |
| DPI (15 μm) | 5.2 | 3.0 | 0.0 | 4.2 |
| DPI (50 μm) | 38.9 | 16.0 | 34.8 | 9.1 |
| CuZn-SOD (150 units mL−1) | 60.1 | 2.9 | 68.5 | 2.2 |
| XTT method | ||||
| Control | 0.0 | 0.0 | ||
| KCN (0.1 mm) | 25.0 | 7.5 | n.d. | |
| KCN (1 mm) | 33.1 | 6.1 | n.d. | |
| DPI (50 μm) | 26.4 | 7.3 | n.d. | |
| CuZn-SOD (150 units mL−1) | 60.3 | 1.2 | 73.4 | 1.9 |
The production of O2·− by NADPH oxidases has been linked to growth processes in various stages of plant, animal, and fungal development: tobacco pollen tube elongation (Potocký et al., 2007), root hair tip growth in Arabidopsis (Foreman et al., 2003; Monshausen et al., 2007), ear development of mouse embryos (Kiss et al., 2006), fungal spore germination and appressorium formation in the rice (Oryza sativa) pathogen Magnaporthe grisea (Egan et al., 2007), and vegetative growth and ascospore germination of the fungus Podospora anserina (Malagnac et al., 2004). Liszkay et al. (2004) found that O2·− and ·OH production are associated with maize root elongation and proposed that ·OH causes cell wall loosening, but a direct in vivo action of these ROS on cell walls was not investigated. It has only recently begun to emerge that ROS play an important role in cell signaling throughout the kingdoms (Bailly, 2004; Laloi et al., 2004; D'Autréaux and Toledano, 2007). ROS signaling has recently also been investigated in the context of seed germination (Oracz et al., 2009). For the various modes of ROS action, therefore, it is important to carefully distinguish between signaling and direct mechanisms. We demonstrate in the next section that developmentally targeted in vivo ·OH production in seeds and seedlings causes tissue-specific scission of cell wall polysaccharides in vivo.
·OH Loosens Cell Walls in Vivo and Has Tissue-Specific and Hormonally Controlled Target Polysaccharides
Having established that apoplastic ·OH is produced in vivo following a developmental pattern, we applied 3H fingerprinting (Fry et al., 2001) to cell walls from the most informative sample comparisons (Figs. 3–5). This technique is the only accepted method that can demonstrate direct in vivo ·OH action on cell wall polysaccharides. Such action can, depending on which atoms of the polysaccharide the ·OH targets, (1) cause immediate chain scission, (2) convert glycosidic bonds to unstable ester bonds, and (3) introduce relatively stable oxo groups, thus forming glycosulose residues (Miller and Fry, 2001). The fingerprinting method is based on 3H labeling of the oxo groups, whose presence in polysaccharides (other than at the reducing terminus) is diagnostic of recent ·OH attack (Fry et al., 2001; Miller and Fry, 2001). It has been shown that these 3H fingerprints differ characteristically between unripe and ripe fruits and that in vivo ·OH attack increases during fruit ripening and may be an important mechanism of fruit cell wall loosening (Fry et al., 2001). ·OH attack on cell wall polymers potentially leads to the breakage of load-bearing polysaccharides and could thereby cause cell wall loosening, but direct evidence that ·OH attack on cell wall polysaccharides occurs in vivo and increases during seed germination and seedling growth was lacking.
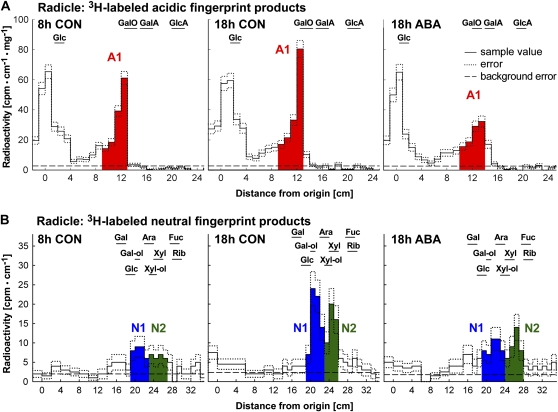
Figure 3.
Detection of in vivo ·OH attack on cress seed radicle cell walls by 3H fingerprinting (Fry et al., 2001). A, Representative 3H fingerprints of acidic products from radicle samples. Signal intensity in the scintillation count is plotted against distance from the origin after high-voltage paper electrophoresis (PE) at pH 3.5. Monosaccharide markers were run with the samples. B, Representative 3H fingerprints of neutral products from radicle samples. Neutral material (which comigrated with Glc during paper electrophoresis) was eluted and rerun by PC in an acidic solvent. Peaks N1 and N2 remain unidentified: they did not comigrate exactly with any monosaccharide tested, and when eluted and rerun in a basic solvent, they migrated within the disaccharide zone (more slowly than the slowest monosaccharide; data not shown). Therefore, they may have been disaccharides containing unusual sugar residues not susceptible to Driselase digestion. Markers are as follows: GalA, galacturonic acid; GalO, galactonic acid; Gal-ol, galactitol; GlcA, glucuronic acid; Xyl-ol, xylitol. CON, Control.
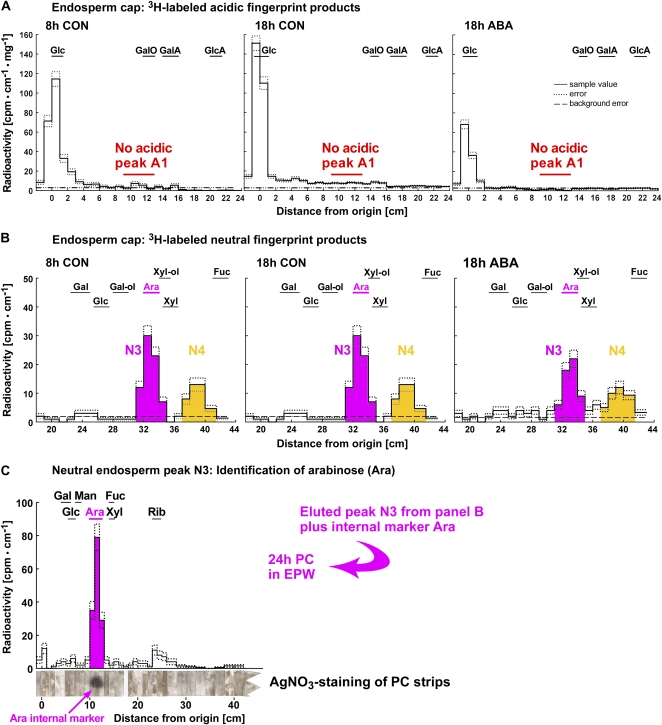
Figure 4.
Detection of in vivo ·OH attack on cress seed endosperm cap cell walls by 3H fingerprinting and identification of the neutral compound observed as peak N3 as Ara. 3H fingerprints of cap samples (A and B) differ quantitatively and qualitatively from those of radicle samples (see Fig. 3). A, Representative electrophoretic 3H fingerprints of 3H-labeled products from cap samples. Signal intensity in the scintillation count is plotted against distance from the origin after high-voltage paper electrophoresis at pH 3.5. No acidic peak was detected. B, Representative chromatographic 3H fingerprints of 3H-labeled products from cap samples. The samples were eluted from the fraction that comigrated with Glc during paper electrophoresis at pH 3.5 and rerun by PC. B and C, The neutral compound observed as peak N3 was identified as Ara by PC in different solvents with reference to internal and external markers (Fry, 2000). Peak N4 remains unidentified. Peak N3 comigrates with an external Ara standard in butanol:acetic acid:water (12:3:5 [v/v]). C, Peak N3 was eluted and rerun by PC in ethyl acetate:pyridine:water (EPW; 8:2:1 [v/v]). Again, the peak comigrates with the external marker Ara. The internal marker Ara (arrow) comigrated with the EPW peak, as shown by AgNO3 staining of the strips of chromatography paper after recovery from the scintillation fluid. For abbreviations of acidic and neutral markers, see Figure 3 legend. CON, Control.
Figure 5.
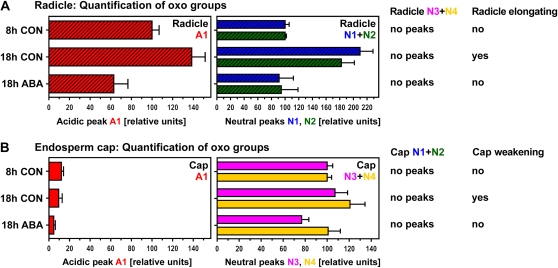
Quantification of peak areas indicative of ·OH attack on polysaccharides in the radicle (A) and the endosperm cap (B). While a distinct acidic peak (A1) was only present in the radicle, neutral peaks were detected in all samples but differed qualitatively between radicles (N1 and N2; see Fig. 3) and caps (N3 and N4; see Fig. 4). N3 was identified as [3H]Ara (see Fig. 4, B and C). Areas under peaks were normalized by setting the value at 8 h to 100. The physiological state of the seeds at the time of dissection is indicated. Mean values ± se of at least four replicates (200 radicles and 1,000 caps used for extraction) are presented. CON, Control.
In germinating cress seeds, the onset of radicle elongation was associated with increased ·OH attack on cell wall polysaccharides in the radicle that upon enzymic digestion yielded acidic as well as neutral 3H-labeled products (Figs. 3 and 5): We found an increase in 3H labeling of the acidic product A1 and the two neutral products N1 and N2. This increase between 8-h CON and 18-h CON was approximately 1.5-fold for A1 and approximately 2-fold for N1 and N2. ABA completely inhibited this increase in in vivo ·OH attack (Fig. 5A).
Our 3H fingerprinting results for the corresponding endosperm caps (Figs. 4 and 5) differed qualitatively and quantitatively from those of the radicle: the most striking difference was the lack of a clearly defined 3H-labeled acidic peak in the endosperm cap samples (Fig. 4A). The component that yields acidic product A1, therefore, is either absent from cap cell wall polymers or not attacked by ·OH in the cap. In 3H-labeled cap samples, the neutral peaks N1 and N2 were not detected, but two other neutral peaks (N3 and N4; Figs. 4B and 5B) with different migration patterns were evident. We identified the radioactive peak N3 in the endosperm cap samples as [3H]Ara by its exact comigration with an Ara internal marker during paper chromatography (PC) in several different solvents (Fig. 4C). That the product is [3H]Ara rather than [3H]arabinitol indicates that we were detecting oxidized midchain or nonreducing terminal sugar residues, not reducing terminal Ara moieties. A sugar residue that upon ·OH attack forms an oxo derivative that is reducible by NaB3H4 to a [3H]Ara residue could originally have been either an Ara residue or one of its epimers (e.g. a nonreducing terminal xylopyranose residue; Miller and Fry, 2001). The unidentified neutral products present in our 3H fingerprints (N1, N2, and N4) may include rare epimeric monosaccharides (Miller and Fry, 2001) such as lyxose or disaccharides resistant to enzymic digestion.
There was no increase in the in vivo ·OH attack leading to [3H]Ara between 8-h CON and 18-h CON, but ABA decreased the in vivo ·OH attack that leads to [3H]Ara (Fig. 5B). A small increase between 8-h CON and 18-h CON was evident for the in vivo ·OH attack leading to N4. This increase was inhibited by ABA. Thus, seed germination is associated with developmentally regulated, qualitatively and quantitatively distinct patterns of in vivo ·OH attack on cell wall polymers in radicle and cap tissues.
Our findings are in accordance with current knowledge summarized by Knox (2008) that plant cell wall polymers are extensively regulated developmentally and differ in structure and function between organs, tissues, and taxa; for example, endosperm cell walls are known to contain more hemicellulose than somatic cell walls (Bewley, 1997a). Cress radicles and caps would be expected to have distinct cell wall composition, potentially yielding different “fingerprints” after ·OH attack. In contrast to cell wall hydrolases, which tend to have high substrate specificity, ·OH radicals can attack any polysaccharide (Fry, 1998), although not necessarily uniformly; for example, the fingerprint obtained from ·OH-attacked xyloglucan contained 25 times more [3H]Xyl than [3H]Glc (Miller and Fry, 2001). Bethke et al. (2007) observed in seeds of Arabidopsis that endosperm cell walls become thinner during germination. The thinning is most obvious in the cap. Based on their physiological/microscopical experiments, the authors suggest that ROS is an attractive mechanism of cell wall loosening during germination. This hypothesis is in agreement with our direct biochemical evidence for a developmental role of in vivo ·OH attack in cell wall loosening during germination.
In addition to seeds, we investigated in vivo ·OH attack on cell walls in maize seedling coleoptiles, a classical and well-characterized system for cell elongation. In vivo ·OH production has been shown in this system (Schopfer, 2001), but a role for in vivo ·OH attack of cell wall polysaccharides during elongation growth has never been demonstrated in this model system or in any other seedlings during elongation growth. We found a strong increase in in vivo ·OH cell wall attack between slowly and rapidly elongating coleoptiles (Fig. 6). This trend is similar to the one we observed for nonelongating and elongating radicles.
Figure 6.
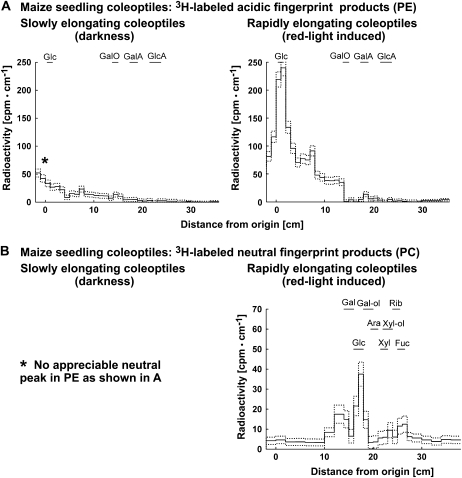
Detection of in vivo ·OH attack on maize seedling coleoptile cell walls by 3H fingerprinting during elongation growth. A, Representative 3H fingerprint of labeled products from segments of slowly and rapidly elongating coleoptiles. Signal intensity in the scintillation count (i.e. 3H labeling of former oxo groups) is plotted against distance from the origin after high-voltage paper electrophoresis (PE) at pH 3.5. Only the rapidly elongating coleoptiles showed an appreciable neutral peak (comigrating with Glc), which was eluted and rerun by PC. B, Representative 3H fingerprint of labeled neutral products from segments of rapidly elongating coleoptiles. The sample was eluted from the fraction that comigrated with Glc during paper electrophoresis and rerun on PC. For abbreviations of acidic and neutral markers, see Figure 3 legend.
Maize coleoptiles that had been induced to elongate by a red light pulse showed an over 5-fold increase in labeling of neutral compounds but no acidic peak. The associated four neutral peaks differed qualitatively from the seed-derived peaks N1 to N3 as judged by their RF values, while N4 could be present. Previous 3H fingerprinting work on maize coleoptile cell walls produced [3H]Gal (Fry, 1998) but no differences in 3H fingerprints between auxin (20 μm indole acetic acid)-treated and control coleoptiles.
Endosperm cap weakening (this work) and fruit softening (Fry et al., 2001) are developmental processes that involve in vivo ·OH cell wall attack and cell separation but not cell elongation (Bewley, 1997b). Radicle elongation during seed germination is a growth process involving cell elongation that likely includes an increase in in vivo ·OH cell wall attack as well (Fig. 3). In addition, our results support a role of ·OH in elongation growth of maize coleoptiles and complement the work by Schopfer (2001) with direct evidence for a corresponding mechanism. Taken together, our results suggest that in vivo ·OH production during tissue weakening and elongation growth leads to ·OH cell wall attack of tissue- and/or species-specific polysaccharide target sites.
CONCLUSION
We provide direct evidence that in vivo ·OH production in the apoplast causes in vivo scission of specific cell wall polysaccharides in elongating maize coleoptiles as well as the radicles and endosperm caps of germinating cress seeds. This constitutes a novel mechanism for cell wall loosening during seed germination. The direct action of ·OH on cell wall polysaccharides has tissue-specific target sites, is temporally, hormonally (GA-ABA antagonism), and developmentally regulated, and appears to be a mechanism of general importance, as it is evident in diverse developmental processes during the plant life cycle. Our findings shed new light on the role of ROS in plants and provide a novel interpretation frame for ROS production during seed germination.
In vivo ·OH attack of cell wall polysaccharides appears to be a mechanism by which ROS mediate diverse developmental processes of plants. An intriguing issue of this mechanism is that its specificity is determined by the dynamic structural organization of the apoplast. Direct ·OH attack on extraprotoplasmatic polymers would require a tight control of the amount and site of ROS production, the mechanism of which is still unclear at this point. However, the fact that we could detect only a small number of distinct peaks in the 3H fingerprinting (Figs. 3–5) strongly suggests that ·OH attack of cell wall polysaccharides, and therefore also its generation, is not randomly distributed over all cell wall polysaccharides. This suggests that ·OH is produced at specific sites, such as peroxidases, that can be preferentially associated with particular polysaccharides (Carpin et al., 2001), or at transition metal ions that are known to be complexed by specific cell wall polymers (Fry et al., 2002). The positive effects of a tightly controlled production of ·OH may also play a role in other living systems, where developmental processes require the loosening of extracellular matrices. As ROS have been detected in the context of growth or weakening in organisms from bacteria and fungi to plants and mammalian embryos (Gapper and Dolan, 2006), it seems likely that this mechanism can be found throughout the kingdoms.
MATERIALS AND METHODS
Plant Material, Germination, and Puncture Force Measurements
For germination, cress seeds (Lepidium sativum ‘Gartenkresse einfache’; Juliwa) were imbibed in petri dishes on two layers of filter paper with 6 mL of one-tenth-strength Murashige and Skoog salts in continuous white light (101.2 μmol m−2 s−1) at 18°C as described (Müller et al., 2006). Where indicated, cis-S(+)-ABA, GA4/7, or H2O2 was added to the medium in the concentrations indicated. Tissue resistance was determined with the puncture force method as described (Müller et al., 2006). For ·OH treatment, isolated intact caps were incubated in 1 mm FeSO4 in 10 mm phosphate buffer, pH 6.0, for 30 min and washed for 10 min in the buffer. Subsequently, to initiate the Fenton reaction, a freshly prepared mixture of H2O2 and ascorbic acid was added to give a final concentration of 100 μm each. Maize (Zea mays ‘Perceval’; Asgrow) seedlings were grown in plastic boxes on vermiculite and deionized water at 25°C in darkness. Fast coleoptile growth was induced after 4 d by a 10-min red light pulse (2.6 μmol m−2 s−1), after which the seedlings were transferred back to darkness. Coleoptile segments for 3H labeling were harvested 5 d after imbibition. These segments were taken 5 mm below the coleoptile tip and were 5 mm long.
·OH Detection by EPR Spectroscopy
Isolated radicles or endosperm caps (100) were incubated for 3 h in spin-trapping solution [50 mm α-(4-pyridyl-1-oxide)-N-tert-butylnitrone containing 4% (v/v) ethanol] on a rotary shaker. EPR spectra were recorded for the incubation solution at room temperature in a flat cell with an ESR-300 X-band spectrometer from Bruker at 9.7-GHz microwave frequency, 100-kHz modulation frequency, modulation amplitude of 1 G, and 63-mW microwave power as described (Renew et al., 2005). Representative spectra are shown in Supplemental Fig. S1. Signal size was calculated as signal-to-noise ratio.
O2·− Detection
O2·− production was measured by photometric determination of the reduction of XTT (for 3′-[1-phenylamino-carbonyl]-3,4-tetrazolium]-bis[4-methoxy-6-nitro] benzenesulfonic acid hydrate; Polysciences). Radicles or caps (100) were collected in 10 mm phosphate buffer, pH 6.5, on ice. Tissues were left for 20 min in order for wounding effects to subside (Roach et al., 2008). The reaction was started by adding 0.5 mm XTT followed by incubation on a rotary shaker at 300 rpm for 3 h. Absorption spectra of the incubation medium were measured. A470 – A650 (reference wavelength) was used to calculate XTT reduction. Copper/zinc (CuZn)-superoxide dismutase (from bovine erythrocytes) was purchased from Sigma-Aldrich.
In addition, O2·− production was measured by photometric determination of the oxidation of epinephrine to adrenochrome. Radicles or caps (120) were collected in 10 mm phosphate buffer, pH 7.0, on ice. After the last dissection, tissues were left for 20 min in order for wounding effects to subside (Roach et al., 2008). The reaction was started by adding 1 mm epinephrine followed by incubation on a rotary shaker at 300 rpm for 3 h. A480 was then measured in the incubation medium. Tissues that had been incubated for 18 h were used for inhibitor studies. Inhibitors were added at the indicated concentrations before the colorimetric reaction was started.
For the histochemical detection of O2·−, cress seeds were dissected and the embryos and endosperm caps equilibrated for 10 min in 50 mm phosphate buffer, pH 6.0. Nitroblue tetrazolium chloride (10 mm) was then added. When staining was visible, seed parts were removed from the staining solution, washed for 1 min in phosphate buffer, and photographed.
3H Fingerprinting
Fingerprinting of ·OH-attacked polysaccharides was modified from Fry et al. (2001). Radicles or endosperm caps or maize coleoptile tissue (100 mg fresh weight) were ground on ice in 1.5 mL of buffered ethanol (ethanol:pyridine:acetic acid:water, 75:2:2:19 [v/v]) containing 10 mm sodium thiosulfate. Ethanol is an excellent scavenger of ·OH, preventing any postmortem action of ·OH on polysaccharides; thiosulfate blocks the Fenton reaction, preventing further ·OH production (Fry, 1998). After washing in 75% (v/v) ethanol, part of the suspension was used for dry weight determination and an equal part for 3H labeling. For the latter portion, the suspension was centrifuged at 2,300g for 10 min and the pellet was washed twice with 10 mL of 75% (v/v) ethanol. For saponification of pectin methyl esters, the pellet was suspended in 200 μL of 0.2 m NaOH. After 5 min, 0.5 mL of labeling solution (1 m NH3 containing either 1 mm NaB3H4 at 1.95 MBq μmol−1 [for radicles and coleoptiles] or 5 mm NaB3H4 at 0.39 MBq μmol−1 [for endosperm caps]) was added to each sample. Samples were left on a rotary shaker for 2 d. Excess NaB3H4 was scavenged with 10 mg of Xyl at 20°C overnight, after which NH3 was evaporated in a draft of air. The solution was then acidified with 100 μL of acetic acid; polysaccharides were precipitated with 3.5 mL of ethanol and washed three times with 75% (v/v) ethanol. The ethanolic solution contained [3H]xylitol, indicating that a suitable excess of NaB3H4 had been used (PC; data not shown). The dried pellets were digested in 200 μL of 1% (w/v) partially purified Driselase (Fry, 2000) in a volatile buffer (pyridine:acetic acid:water, 1:1:98 [v/v], pH 4.7) containing a volatile antimicrobial agent (0.5% [w/v] chlorobutanol) for 5 d. Digestion was stopped with 35 μL of 90% (v/v) formic acid. Samples were briefly centrifuged, and 40 μL of supernatant was run by high-voltage electrophoresis at pH 3.5 on Whatman 3MM paper (2.5 kV, 1 h; Fry, 2000). Strips of the electrophoretogram were assayed for 3H by scintillation counting. Material that comigrated with marker Glc (i.e. the neutral fraction) was eluted from the paper with water (after removal of scintillation fluid by washing in toluene and drying) and rerun by PC on Whatman No. 1 in butanol:acetic acid:water (12:3:5 [v/v]). Markers were stained with AgNO3 after removal of any scintillation fluid from the paper by washing in toluene.
Photographic Documentation
All photographs were taken with a Leica DCF480 digital camera attached to a stereomicroscope (Leica Mz 12,5).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Representative EPR spectra for radicle samples.
Supplementary Material
Acknowledgments
We thank Anita Rott (University Freiburg) and Janice Miller (University of Edinburgh) for expert technical help. We also thank Ilse Kranner and Thomas Roach (Millennium Seed Bank) and Eiri Heyno and Christiane Groß (Commissariat à l'Energie Atomique) for expert advice in the O2·− and EPR techniques, respectively. We are grateful to Peter Schopfer (University of Freiburg) for critical comments and suggestions.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. DFG LE720/6) and the Deutscher Akademischer Austauschdienst (grant nos. D/0628197 and D/07/09926) to G.L.-M. and by the Biotechnology and Biological Sciences Research Council to S.C.F.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Gerhard Leubner-Metzger (gerhard.leubner@biologie.uni-freiburg.de).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Bailly C (2004) Active oxygen species and antioxidants in seed biology. Seed Sci Res 14 93–107 [Google Scholar]
- Bethke PC, Libourel IGL, Aoyama N, Chung YY, Still DW, Jones RL (2007) The Arabidopsis aleurone layer responds to nitric oxide, gibberellin and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol 143 1173–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD (1997. a) Breaking down the walls: a role for endo-β-mannanase in release from seed dormancy? Trends Plant Sci 2 464–469 [Google Scholar]
- Bewley JD (1997. b) Seed germination and dormancy. Plant Cell 9 1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson LF, Tenhaken R, Lamb C (1994) Function of oxidative cross-linking of cell wall structural proteins in plant disease resistance. Plant Cell 6 1703–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummell DA, Harpster MH (2001) Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol Biol 47 311–339 [PubMed] [Google Scholar]
- Carpin S, Crevecoeur M, de Meyer M, Simon P, Greppin H, Penel C (2001) Identification of a Ca2+-pectate binding site on an apoplastic peroxidase. Plant Cell 13 511–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Nonogaki H, Bradford KJ (2002) A gibberellin-regulated xyloglucan endotransglycosylase gene is expressed in the endosperm cap during tomato seed germination. J Exp Bot 53 215–223 [DOI] [PubMed] [Google Scholar]
- Cosgrove DJ (2005) Growth of the plant cell wall. Nat Rev Mol Cell Biol 6 850–861 [DOI] [PubMed] [Google Scholar]
- da Silva EAA, Toorop PE, van Aelst AC, Hilhorst HW (2004) Abscisic acid controls embryo growth potential and endosperm cap weakening during coffee (Coffea arabica) seed germination. Planta 220 251–261 [DOI] [PubMed] [Google Scholar]
- D'Autréaux B, Toledano MB (2007) ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 8 813–824 [DOI] [PubMed] [Google Scholar]
- Deeble DJ, Bothe E, Schuchmann HP, Parsons BJ, Phillips GO, von Sonntag C (1990) The kinetics of hydroxyl-radical induced strand breakage of hyaluronic acid: a pulse radiolysis study using conductiometry and laser-light scattering. Z Naturforsch [C] 45 1031–1043 [DOI] [PubMed] [Google Scholar]
- Doussiere J, Vignais P (1992) Diphenylene iodonium as an inhibitor of the NADPH oxidase complex of bovine neutrophils: factors controlling the inhibitory potency of diphenylene iodonium in a cell-free system of oxidase activation. Eur J Biochem 208 61–71 [DOI] [PubMed] [Google Scholar]
- Eckardt NA (2005) VANGUARD1: at the forefront of pollen tube growth. Plant Cell 17 327–329 [Google Scholar]
- Egan MJ, Wang ZY, Jones MA, Smirnoff N, Talbot NJ (2007) Generation of reactive oxygen species by fungal NADPH oxidases is required for rice blast disease. Proc Natl Acad Sci USA 104 11772–11777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encina A, Fry S (2005) Oxidative coupling of a feruloyl-arabinoxylan trisaccharide (FAXX) in the walls of living maize cells requires endogenous hydrogen peroxide and is controlled by a low-Mr apoplastic inhibitor. Planta 223 77–89 [DOI] [PubMed] [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G (2006) Seed dormancy and the control of germination. New Phytol 171 501–523 [DOI] [PubMed] [Google Scholar]
- Foreman J, Demidchick V, Bothwell JHF, Mylona P, Miedema H, Torres MA, Linstead P, Costa S, Brownlee C, Jones JDG, et al (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422 442–446 [DOI] [PubMed] [Google Scholar]
- Fry SC (1998) Oxidative scission of plant cell wall polysaccharides by ascorbate-induced hydroxyl radicals. Biochem J 332 507–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC (2000) The Growing Plant Cell Wall: Chemical and Metabolic Analysis. Blackburn Press, Caldwell, NJ
- Fry SC, Dumville JC, Miller JG (2001) Fingerprinting of polysaccharides attacked by hydroxyl radicals in vitro and in the cell walls of ripening pear fruit. Biochem J 357 729–737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry SC, Miller JG, Dumville JC (2002) A proposed role for copper ions in cell wall loosening. Plant Soil 247 57–67 [Google Scholar]
- Gapper C, Dolan L (2006) Control of plant development by reactive oxygen species. Plant Physiol 141 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MA, Fry SC (2005) Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-l-threonate. Nature 433 83–87 [DOI] [PubMed] [Google Scholar]
- Heyno E, Klose C, Krieger-Liszkay A (2008) Origin of cadmium-induced reactive oxygen species production: mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol 179 687–699 [DOI] [PubMed] [Google Scholar]
- Kiss PJ, Knisz J, Zhang Y, Baltrusaitis J, Sigmund CD, Thalmann R, Smith RJ, Verpy E, Bánfi B (2006) Inactivation of NADPH oxidase organizer 1 results in severe imbalance. Curr Biol 16 206–213 [DOI] [PubMed] [Google Scholar]
- Knox JP (2008) Revealing the structural and functional diversity of plant cell walls. Curr Opin Plant Biol 11 308–313 [DOI] [PubMed] [Google Scholar]
- Laloi C, Apel K, Danon A (2004) Reactive oxygen signalling: the latest news. Curr Opin Plant Biol 7 323–328 [DOI] [PubMed] [Google Scholar]
- Leubner-Metzger G (2002) Seed after-ripening and over-expression of class I β-1,3-glucanase confer maternal effects on tobacco testa rupture and dormancy release. Planta 215 659–698 [DOI] [PubMed] [Google Scholar]
- Liszkay A, van der Zalm E, Schopfer P (2004) Production of reactive oxygen intermediates by maize roots and their role in wall loosening and elongation growth. Plant Physiol 136 3114–3123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Molina L, Mongrand SB, Chua NH (2001) A postgermination developmental arrest checkpoint is mediated by abscisic acid and requires the ABI5 transcription factor in Arabidopsis. Proc Natl Acad Sci USA 98 4782–4787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malagnac F, Lalucque H, Lepere G, Silar P (2004) Two NADPH oxidase isoforms are required for sexual reproduction and ascospore germination in the filamentous fungus Podospora anserina. Fungal Genet Biol 41 982–997 [DOI] [PubMed] [Google Scholar]
- Miller JG, Fry SC (2001) Characteristics of xyloglucan after attack by hydroxyl radicals. Carbohydr Res 332 389–403 [DOI] [PubMed] [Google Scholar]
- Minibayeva FV, Gordon IK, Kolesnikov OP, Chasov AV (2000) Role of extracellular peroxidases in the superoxide production by wheat roots. Protoplasma 217 125–128 [DOI] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S (2007) Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA 104 20996–21001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K, Tintelnot S, Leubner-Metzger G (2006) Endosperm-limited Brassicaceae seed germination: abscisic acid inhibits embryo-induced endosperm weakening of Lepidium sativum (cress) and endosperm rupture of cress and Arabidopsis thaliana. Plant Cell Physiol 47 864–877 [DOI] [PubMed] [Google Scholar]
- Nonogaki H (2006) Seed germination: the biochemical and molecular mechanisms. Breed Sci 56 93–105 [Google Scholar]
- Nonogaki H, Gee OH, Bradford KJ (2000) A germination-specific endo-β-mannanase gene is expressed in the micropylar endosperm cap of tomato seeds. Plant Physiol 123 1235–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oracz K, Bouteau HE, Farrant JM, Cooper K, Belghazi M, Job C, Job D, Corbineau F, Bailly C (2007) ROS production and protein oxidation as novel mechanisms for seed dormancy alleviation. Plant J 50 452–465 [DOI] [PubMed] [Google Scholar]
- Oracz K, El-Maarouf-Bouteau H, Kranner I, Bogatek R, Corbineau F, Bailly C (2009) The mechanisms involved in seed dormancy alleviation by hydrogen cyanide unravel the role of reactive oxygen species as key factors of cellular signaling during germination. Plant Physiol 150 494–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlista A, Haber AH (1970) Embryo expansion without protrusion in lettuce seeds. Plant Physiol 45 636–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocký M, Jones AM, Bezvoda R, Smirnoff N, Zárský V (2007) Reactive oxygen species produced by NADPH oxidase are involved in pollen tube growth. New Phytol 174 742–751 [DOI] [PubMed] [Google Scholar]
- Renew S, Heyno E, Schopfer P, Liszkay A (2005) Sensitive detection and localization of hydroxyl radical production in cucumber roots and Arabidopsis seedlings by spin trapping electron paramagnetic resonance spectroscopy. Plant J 44 342–347 [DOI] [PubMed] [Google Scholar]
- Roach T, Ivanova M, Beckett RP, Minibayeva FV, Green I, Pritchard HW, Kranner I (2008) An oxidative burst in embryonic axes of recalcitrant sweet chestnut seeds as induced by excision and desiccation. Physiol Plant 133 131–139 [DOI] [PubMed] [Google Scholar]
- Schopfer P (1996) Hydrogen peroxide-mediated cell-wall stiffening in vitro in maize coleoptiles. Planta 199 43–49 [Google Scholar]
- Schopfer P (2001) Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J 28 679–688 [DOI] [PubMed] [Google Scholar]
- Schopfer P (2006) Biomechanics in plant growth. Am J Bot 93 1415–1425 [DOI] [PubMed] [Google Scholar]
- Toorop PE, van Aelst AC, Hilhorst HWM (2000) The second step of the biphasic endosperm cap weakening that mediates tomato (Lycopersicon esculentum) seed germination is under control of ABA. J Exp Bot 51 1371–1379 [PubMed] [Google Scholar]
- Winterbourn CC (2008) Reconciling the chemistry and biology of reactive oxygen species. Nat Chem Biol 4 278–286 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.