Flowers are the reproductive structure of angiosperms. They are composed of four distinct types of organs: sepals, petals, stamens, and carpels, which typically develop on four concentric rings, or whorls (Fig. 1A). In Arabidopsis (Arabidopsis thaliana), floral organ identity relies on the combinatorial action of four classes of flower-specific transcription factors, expressed in partially overlapping domains, and inferred to form four distinct protein complexes (for review, see Ferrario et al., 2004; Krizek and Fletcher, 2005). Sepals are specified by complexes composed of class A (APETALA1 [AP1]) and E (SEPALLATA [SEP]) proteins, petals by a combination of class A, B (AP3 and PISTILLATA [PI]), and E proteins, stamens by a combination of class B, C (AGAMOUS [AG]), and E proteins, and carpels are specified by class C and E proteins. This model can be extrapolated to other taxa, except for the A function whose generalization remains ambiguous (for review, see Ferrario et al., 2004).
Figure 1.
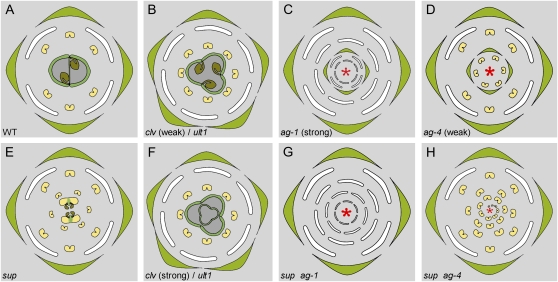
Diagrams of wild-type and mutant Arabidopsis flowers displaying supernumerary organs. A, Wild-type flowers consist of four whorls bearing four sepals, four petals, six stamens, and two fused carpels, respectively. B, Weak clv and ult1 mutant flowers exhibit supernumerary organs contained within the four primary whorls. C, Strong ag-1 mutant flowers are indeterminate, with numerous supernumerary whorls of organs and homeotic transformations of reproductive organs into perianth ones. D, Weak ag-4 mutant flowers are also indeterminate, with numerous supernumerary whorls of stamens and sepals. E, sup mutant flowers display a limited number of supernumerary whorls of stamens and sometimes staminoid carpels in the center. F, Strong clv and some ult1 mutant flowers display supernumerary organs in each primary whorl but also exhibit a weak loss of FM termination, with a limited number of supernumerary whorls within the gynoecium. G, sup ag-1 double mutant flowers display an indefinite number of petals inside the first whorl of sepals. H, sup ag-4 double mutants have fully indeterminate male flowers, with the two inner whorls replaced by an indeterminate number of stamens borne on a spiral. Red asterisks indicate totally indeterminate FMs.
Floral organs are generated by a flower meristem (FM), a pool of pluripotent, dividing cells, which is itself produced by, or derives from the transformation of another meristem: the shoot apical meristem (SAM). SAM and FM share many regulators, which appear to be widely conserved among angiosperms, or at least among eudicots (for review, see Nardmann and Werr, 2007). KNOX I homeodomain transcription factors (SHOOT MERISTEMLESS [STM] and KNAT1, 2, and 6 in Arabidopsis) keep meristematic cells in an undifferentiated state, while the WUSCHEL/CLAVATA (WUS/CLV) negative feedback loop maintains a constant population of stem cells in the meristem center (for review, see Williams and Fletcher, 2005; Sablowski, 2007a). These genes are expressed in a similar way in the FM compared to the SAM, and their mutations cause similar defects in both SAM and FM: stm and wus meristems terminate prematurely, whereas clv meristems are enlarged and produce more organs than those of wild-type plants. Thus, during its first developmental stages, FM homeostasis seems to be achieved by roughly the same molecular mechanisms as it is in the SAM.
However, the FM differs from the SAM in several ways: it produces floral organs instead of leaves, and neither generates axillary meristems nor elongating internodes. Also, FM growth pattern is determinate. Unlike the SAM, which maintains stem cells in its center and keeps producing new organs throughout the life of the plant, stem cells are only transiently maintained within the FM. In Arabidopsis, stem cell maintenance is disrupted at stage 6 of flower development (stages as described in Smyth et al., 1990), when carpel primordia are initiated, making the flower determinate, with a fixed number of whorls and organs per whorl. No more floral organs are produced after the carpels, which are congenitally fused, forming the placenta at their junction, where ovules develop (Figs. 1A and 2A). A similar disruption of stem cell maintenance is seen in most angiosperms, although some minor timing differences do exist between species with different placentation types (discussed by Colombo et al., 2008).
Figure 2.
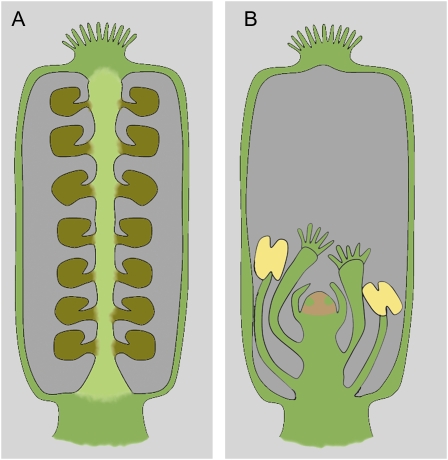
Siliques of wild-type and indeterminate flowers. A, Silique of a wild-type, determinate flower: Placenta develops between the fused carpels and bears ovules, and no floral organs are found within the gynoecium. B, Silique of an indeterminate flower, exhibiting supernumerary floral organs within the gynoecium, due to maintenance of the FM after stage 6; placenta usually develops (not shown), but at a much lesser extent than in wild type, and the seed set is greatly reduced due to the development of these supernumerary organs.
In several species, mutants with flowers displaying more organs than the wild type have been described. However, production of these extra organs may result from distinct processes generating different phenotypes. Mutants such as clv and ultrapetala1 (ult1) in Arabidopsis have enlarged meristems, including FMs, which consequently produce more floral organs in each whorl (Fig. 1B; Clark et al., 1993, 1995; Kayes and Clark, 1998; Fletcher, 2001). This phenomenon is associated with an increase of the stem cell population (Schoof et al., 2000; Carles et al., 2005), and therefore results from spatial alterations of the FM. Conversely, mutants such as ag in Arabidopsis (Figs. 1, C and D, and 2B), plena (ple) in Antirrhinum (Antirrhinum majus), or drooping leaves in rice (Oryza sativa) exhibit a prolonged maintenance of stem cells within the FM, resulting in the production of extra organs borne on supernumerary whorls (Bowman et al., 1989, 1991; Yanofsky et al., 1990; Bradley et al., 1993; Sieburth et al., 1995; Nagasawa et al., 2003). This phenotype, referred to as flower indeterminacy or loss of FM termination, is thus due to a temporal, rather than spatial, alteration of flower development. Interestingly, supernumerary whorls are also seen in clv and sometimes ult1 mutant flowers, though to a much lesser extent than in ag and ple flowers (Fig. 1F; Clark et al., 1993, 1995; Kayes and Clark, 1998; Fletcher, 2001; Carles et al., 2005), showing that spatial and temporal alterations of the stem cell population are not exclusive.
This review deals with the molecular mechanisms that control FM termination, which are closely linked to that of floral organ identity. Most of the discussion will be focused on Arabidopsis, but there will be reference to work with other plant species when available evidence exists that either extends or challenges models established in Arabidopsis.
AG IS THE MAIN DEVELOPMENTAL SWITCH TOWARD FM TERMINATION
AG Triggers FM Termination by Turning WUS Off at Stage 6
AG does not only control floral organs identity. Strong ag alleles (ag-1 to -3) cause homeotic transformations of stamens into petals, but also a total loss of FM termination (Fig. 1C; Bowman et al., 1989, 1991; Yanofsky et al., 1990). Carpels are not simply transformed into sepals: instead the whole fourth whorl is replaced by a new flower bud that in turn develops into a new abnormal flower. Thus new flowers are indefinitely produced within the initial flower as stem cells are maintained in the center of the FM. Weaker ag alleles (ag-4 [Fig. 1D] and AG-Met-205) have few or no defects in floral organ identity, but still exhibit a total loss of FM termination (Sieburth et al., 1995).
AG's role in flower determinacy appears widely conserved among angiosperms (for review, see Ferrario et al., 2004). However, two AG orthologs are found in several species, and they sometimes underwent subfunctionalization. For instance, PLE alone appears necessary for sexual organ identity and FM termination in Antirrhinum: ple mutants display an ag-like phenotype (except that nested flowers arise inside the fourth whorl instead of the third in strong ag mutants), while farinelli (far) mutants are unaffected (Bradley et al., 1993; Davies et al., 1999).
AG disrupts stem cell maintenance within the FM by turning WUS expression off at stage 6 of flower development (Fig. 3): WUS mRNA becomes undetectable at this stage in wild-type flowers (Mayer et al., 1998), but remains continuously expressed in the everlasting FM of ag mutant flowers, a phenomenon that is both necessary and sufficient to oppose FM termination (Lenhard et al., 2001; Lohmann et al., 2001). It is worth noting that this effect is specific to WUS, as prolonged expression of another promeristematic gene, STM, contrary to that of WUS, does not affect FM termination (Lenhard et al., 2001). WUS repression by AG occurs at least partly non-cell autonomously and is therefore probably indirect (Sieburth et al., 1998).
Figure 3.
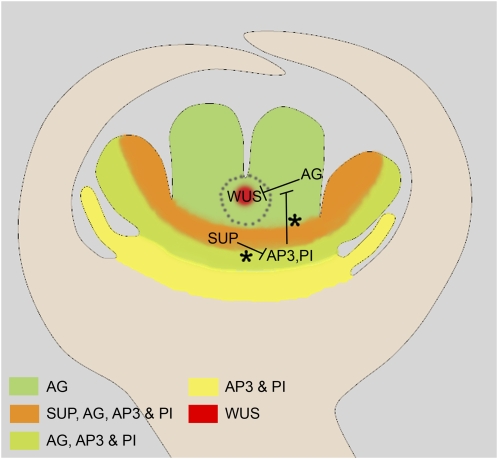
Main genetic pathways promoting FM termination. FM termination is due to the extinction of WUS expression at stage 6 of flower development, due to its repression by the MADS-box transcription factor AG. To perform this function, AG is required in a subdomain of whorl 4, marked off by gray dots. FM termination also requires the previous exclusion of AP3 and PI, which appear to oppose WUS repression by AG, from whorl 4. This exclusion is mediated by SUP. This may explain why AG, while expressed from stage 3 onwards, fails to shut WUS expression down before stage 6, when carpel primordia emerge. However, this delay was also proposed to be due to a progressive increase in AG expression level during flower development, a high dose of AG being specifically required for FM termination. This sketch represents a stage 6 flower bud, but it is worth noting that SUP's functions occur during earlier developmental stages, but condition the subsequent disruption of stem cell maintenance by AG. Asterisks indicate interactions that are still debated.
Interestingly, AG remains so far the only single gene strictly required for FM termination in Arabidopsis, making it the main switch toward stem cell arrest within the FM. However, a total loss of FM termination was more recently observed in several double mutants (Alvarez and Smyth, 1999; Prunet et al., 2008) and transgenic plants (Chen, 2004; Zhao et al., 2007), while some mutants, including clv, ult1, perianthia (pan), and superman (sup) only display a delay of FM termination, and produce a limited number of supernumerary whorls before stem cell maintenance eventually stops (Fig. 1, E and F; Schultz et al., 1991; Bowman et al., 1992; Clark et al., 1993, 1995; Kayes and Clark, 1998; Fletcher, 2001; Carles et al., 2004; Das et al., 2009; Maier et al., 2009). However, like in ag mutants, these phenotypes are always associated with a transient or persistent maintenance of WUS expression beyond stage 6, depending on the strength of the loss of FM termination (Schoof et al., 2000; Carles et al., 2004; Zhao et al., 2007; Prunet et al., 2008; Das et al., 2009; Maier et al., 2009).
FM Termination Requires AG Expression at High Levels and in the Very Center of the FM
A progressive decrease in AG levels triggers a wide range of phenotypes, from nearly normal flowers without any homeotic conversion but with delayed FM termination—their gynoecium enclosing a secondary flower—to perfect phenocopies of strong ag mutant flowers (Mizukami and Ma, 1995; Chuang and Meyerowitz, 2000). Thus AG's three functions (promoting stamen and carpel identity and FM termination) are separable depending on the dose, and FM termination requires the highest amount of protein.
AG's functions can also be separated on a spatial basis. pAP3::AG ag-3 transgenic plants, which lack functional AG in the whole fourth whorl, but still express it in the third, exhibit flowers with stamens but no carpel, and utterly lacking FM termination (Jack et al., 1997), showing the importance of AG expression in the fourth whorl to switch WUS expression. Moreover, the delay or loss of FM termination without alteration of floral organ identity observed in clv, ult1, and pan single mutants as well as in crabs claw squint (crc sqn) and crc ult1 double mutants is associated with a transient or persistent defect in AG expression in an inner subdomain of whorl 4 (Clark et al., 1993; Fletcher, 2001; Prunet et al., 2008; Das et al., 2009; Maier et al., 2009). A similar, persistent defect of AG expression in the very center of the fourth whorl is also seen in the totally indeterminate flowers of transgenic plants constitutively expressing a miR172-resistant version of AG's repressor AP2 (35S::AP2m3; Zhao et al., 2007). AG therefore appears to be required specifically in the very center of the FM (Fig. 3), where WUS is expressed, to switch it off and arrest stem cell maintenance.
FM Termination Relies on a Complex, Multilevel Activation of AG
Other mutations causing defects in FM termination were described, most of which affect genes encoding direct or indirect posttranscriptional activators of AG, thus unraveling the complex, multilevel regulation of this gene.
A modifier screen for enhancers of the weak ag-4 allele led to the identification of HUA1 and 2 (Chen and Meyerowitz, 1999; Li et al., 2001). Both proteins, together with HUA ENHANCER (HEN) 2 and 4 (that were themselves identified in a screen for enhancers of hua1 hua2) are involved in the control of AG pre-mRNA correct splicing (Cheng et al., 2003).
Mutation of HEN1, which encodes a protein involved in microRNAs biosynthesis, causes pleiotropic phenotypes and sometimes triggers a strong loss of FM termination in a hua1 hua2 background, while disruption of DICER LIKE1 (DCL1, also named CARPEL FACTORY), which also acts in the miRNAs processing pathway, has an even stronger effect on FM termination (Jacobsen et al., 1999; Chen et al., 2002; Park et al., 2002). Both proteins are necessary for the accumulation of miR172, which in turn enables AG activation by down-regulating AP2 (Chen et al., 2002; Aukerman and Sakai, 2003; Chen, 2004). Interestingly, the increase in AP2 protein level in hen1 and dcl1 plants is similar to that in miR172-resistent AP2 overexpressing lines, but their flowers exhibit a much weaker loss of FM termination (Jacobsen et al., 1999; Chen et al., 2002; Chen, 2004; Zhao et al., 2007). This could be due to a defect in the accumulation of other miRNAs in hen1 and dcl1 plants, some of them having an antagonistic role to that of miR172 (Chen, 2004). Indeed, another family of miRNA, miR169, was shown to dampen the expression of AG orthologs in whorls 3 and 4 of Antirrhinum and petunia (Petunia hybrida) by repressing AG activators (Cartolano et al., 2007). Thus the weak loss of FM termination of hen1 and dcl1 flowers may be due to the lack of miR169 that would counterbalance that of miR172.
Finally, to carry on its functions, AG also needs to interact with SEP proteins. Indeed, flowers from plants mutant simultaneously for three SEP genes still display a normal AG expression pattern, but entirely lack FM termination (Pelaz et al., 2000; Ditta et al., 2004). A similar commitment of class E proteins in FM termination was described in several other species, including petunia, gerbera (Gerbera hybrida), and tomato (Solanum lycopersicum; Angenent et al., 1994; Pnueli et al., 1994; Vandenbussche et al., 2003; Uimari et al., 2004). It is worth noting that in both petunia and rice, ovule identity (also called class D) genes also appear to promote FM termination (Ferrario et al., 2006; Dreni et al., 2007). This raises the possibility of a similar but yet uncharacterized role of ovule identity genes in Arabidopsis, which could partly account for the delay between the onset of AG expression and WUS repression (Ferrario et al., 2006). Conversely, the requirement of class D proteins in FM termination could be specific to species with central placentation: In petunia and rice, the placenta and ovule, respectively, directly arise from the FM, which therefore terminates slightly later than in Arabidopsis flowers, at a stage corresponding to placenta/ovule rather than carpel initiation (discussed by Colombo et al., 2008).
FM TERMINATION IS CLOSELY LINKED TO THE FEMALE DEVELOPMENTAL PROGRAM
Control of FM Termination by SUP Is Independent of AG Transcription, But May Be Mediated by Class B Gene Repression
SUP, a C2H2 zinc-finger transcription repressor (Sakai et al., 1995; Hiratsu et al., 2002), also regulates floral organ number. Its mutation triggers the development of extra whorls of stamens inside the third whorl, usually at the expense of the gynoecium, which is often missing or replaced by staminoid carpels (Fig. 1E; Schultz et al., 1991; Bowman et al., 1992), although some alleles of sup also exhibit a mild increase in carpel number (Jacobsen and Meyerowitz, 1997; Rohde et al., 1999). Mutation of the ortholog of SUP in petunia triggers a similar phenotype, but also affects other aspects of flower development (Nakagawa et al., 2004), suggesting that SUP function in flower development is conserved to some extent. The octandra (oct) mutation (whose mapping has been elusive so far) also exhibits a sup-like phenotype in Antirrhinum, but interestingly, the far ple double mutant exhibits a phenotype similar to that of ag sup double mutant in Arabidopsis, showing that AG orthologs share OCT's role in this process (Davies et al., 1999).
Yet the origin of the sup phenotype remains a debated matter. It was first proposed that the sup phenotype is heterochronic, the switch from male program to female program being delayed. Thus, the FM seems transiently stuck in developmental time and keeps producing stamens instead of generating carpels and disrupting stem cell maintenance (compare Fig. 4, C and D; Schultz et al., 1991; Bowman et al., 1992). This first model suggests that SUP indirectly promotes FM termination. Interestingly, however, AG expression pattern remains unaltered in sup mutant flowers (Bowman et al., 1992), and no alteration of AG function has been shown so far. Indeed, the sup phenotype may be mediated by class B genes. The production of extra whorls of stamens in sup mutant flowers is associated with an expansion of AP3 and PI expression toward the center of the FM (compare Fig. 4, A and B; Bowman et al., 1992; Goto and Meyerowitz, 1994). Additionally, the class B genes seem to be necessary for the establishment of the sup phenotype: AP3 and PI were first described as epistatic to SUP, as ap3 sup and pi sup double mutant flowers are very similar to single ap3 and pi flowers (Schultz et al., 1991; Bowman et al., 1992). Corroborating these data, flowers of plants overexpressing AP3 alone (p35S::AP3) or together with PI (p35S::AP3/PI), as well as plants overexpressing AP3's activator UNUSUAL FLORAL ORGAN (p35S::UFO) phenocopy the sup phenotype (Jack et al., 1994; Krizek and Meyerowitz, 1996; Lee et al., 1997). On the other hand, ap3 or pi mutant flowers are often overdeterminate: Third whorl stamens are converted to carpels, but the overall number of organs in the two inner whorls is fewer than in wild-type flowers (2.7 carpels per flower on average in pi-1, instead of eight stamens and carpels; Bowman et al., 1989, 1991). Overdeterminacy is even more obvious in flowers of Antirrhinum mutant for either DEFICIENS or GLOBOSA, the orthologs of AP3 and PI, which entirely lack a fourth whorl (Sommer et al., 1990; Trobner et al., 1992). Thus, the class B genes AP3 and PI appear to oppose FM termination by AG (Fig. 3), and their exclusion from the fourth whorl by SUP appears necessary for floral determinacy.
Figure 4.
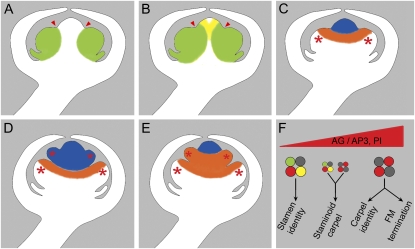
Control of flower determinacy by SUP and class B proteins. A and B, Effects of the sup-1 mutation on class B genes expression pattern. Green staining marks simultaneous expression of AP3 and PI; yellow staining marks that of PI alone. Red arrowheads mark the boundary between whorls 3 and 4. A, mRNA distribution pattern of class B genes AP3 and PI in a wild-type flower at stage 5. At that stage, AP3 and PI are coexpressed in the third whorl, but are excluded from the fourth whorl. B, mRNA distribution pattern of class B genes AP3 and PI in a sup-1 mutant flower at stage 5. Compared to wild type, expression of AP3 and PI is expanded in the fourth whorl, toward the FM center. PI mRNA expansion appears wider than that of AP3. C to E, Two models of production of supernumerary stamens in sup-1 mutant flowers. The sketches represent sup-1 mutant flowers at stage 5 (C) and 7 (D and E). Different colors are used to follow the evolution of two cell populations, which correspond to two distinct domains of the flower at stage 5, during flower development. Red asterisks mark stamen primordia. C, Blue staining marks the center of the flower, which at this stage corresponds to the FM. Orange staining marks the inner part of whorl 3, which corresponds to SUP expression domain. D, First model: supernumerary stamens are produced from the FM whose termination is delayed. The identity of these supernumerary organs results from the ectopic expression of class B genes in the fourth whorl. E, Second model: supernumerary stamens are produced from the inner part of whorl 3 that overproliferates. Conversely, proliferation within the FM is strongly reduced. The broadening of AP3 and PI mRNA distribution results from an overproliferation of class B-expressing cells rather than from an ectopic expression of these genes. F, Model of competition between B and C proteins to form MADS-box transcription factors complexes. The balance between class B and C proteins directs which MADS-box protein complexes are formed, and which developmental program subsequently takes place. MADS-box proteins are symbolized by colored circles; red, AG; green, AP3; yellow, PI; gray, SEP. For practical reasons, protein complexes are represented as heterotetramers, according to the quartet model (Theissen and Saedler, 2001), though their exact stoichiometry remains unknown. Quartet size indicates their relative amounts.
Alternatively, SUP was proposed to control the balance of cell proliferation between whorls 3 and 4 (Sakai et al., 1995; Sakai et al., 2000). This second model stipulates that SUP represses cell divisions in the inner third whorl where it is expressed, thus preventing the formation of extra whorls of stamens (compare Fig. 4, C and E). The reduction or disappearance of the gynoecium observed in the sup mutants would then be an indirect effect of an overproliferation in whorl 3, at the expense of whorl 4. There are several arguments in favor of this second model: SUP is not expressed in the fourth whorl, where it is supposed to repress the class B genes; instead, its expression pattern overlaps with that of AP3 and PI in the inner third whorl (compare Fig. 4, A and C; Sakai et al., 1995). Also, AP3 and PI are still excluded from the very center of the FM in sup flowers (Fig. 4B), suggesting that the spatial extension of their expression pattern could be due to an excess of proliferation of AP3- and PI-expressing cells from inner whorl 3, rather than from an ectopic expression in whorl 4 (Sakai et al., 1995). Indeed, SUP's ability to repress cell divisions was confirmed by constitutive expression of SUP in Arabidopsis, tobacco (Nicotiana tabacum), and rice, which results in dwarfed plants with smaller flowers than wild type (Nandi et al., 2000; Bereterbide et al., 2001; Hiratsu et al., 2002), while local overproliferation was found in petunia flowers mutant for the ortholog of SUP (Nakagawa et al., 2004).
Interestingly, these two models are not exclusive. Indeed, constitutive expression of SUP in rice does not only reduce cell proliferation, it sometimes causes replacement of third whorl stamens by stamen-carpel mosaic organs, a phenotype associated with a reduced expression of OsMADS2, a rice PI ortholog (Nandi et al., 2000). Similarly, in Arabidopsis, ectopic expression of SUP under the control of the AP1 promoter (pAP1::SUP) causes both a decrease in overall floral organ number and transformations of petals into sepals and stamens into carpels (Yun et al., 2002). The decrease in floral organ number is likely to be due to repression of cell proliferation in the pAP1::SUP lines, but the changes in petal and stamen identity is symptomatic of a defect in B function. Indeed, it was shown that both AP3 and its activator UFO are down-regulated in pAP1::SUP flowers compared to wild type (Yun et al., 2002). SUP is thus apparently able to repress both cell proliferation and AP3 and PI expression.
However, according to the second, proliferation-based model, the balance of cell divisions between whorls 3 and 4 is affected in sup mutant flowers, inferring that the extra stamens result from an overproliferation of the inner part of whorl 3 at the expense of the FM center, which stops proliferating. Nonetheless, in both sup and wild-type flowers, cells incorporate BrdU on both sides of the boundary between whorl 3 and FM center, indicating that the latter is still proliferating (Breuil-Broyer et al., 2004). Additionally, the delay in FM termination in sup mutant flowers has recently been associated with transient maintenance of WUS expression beyond stage 6 (Prunet et al., 2008), suggesting that the FM keeps functioning normally while the extra whorls of stamens are produced. Also, the occasional fasciation of ag-1 sup-1 double mutant flowers (Bowman et al., 1992) indicates that SUP represses cell divisions in the FM center, and not only in whorl 3. It is therefore unlikely that the extra whorls of stamens in the sup mutant are produced only from an excess of cell proliferation within whorl 3.
Given that SUP does repress the class B genes, and the strong similarities between the sup and p35S::AP3/PI flowers, it is likely that their phenotype results at least partly from the same process, that is, an ectopic expression of AP3 and PI in the center of the flower, which somehow opposes FM termination (Fig. 3).
AP3 and PI May Antagonize AG's Fourth Whorl Functions by Competing with AG and SEP to Form MADS-Box Protein Complexes
With the exception of the occasional fasciation, which may result from SUP's role (independent of B and C genes) in controlling proliferation within the FM, FM termination is not more affected in ag-1 sup-1 than in ag-1 flowers (Bowman et al., 1992). Similarly, ectopic expression of both AP3 and PI delays FM termination in a wild-type AG context, but does not enhance the indeterminacy phenotype of ag mutant flowers (Krizek and Meyerowitz, 1996). This epistasy of ag to sup and p35S::AP3/PI regarding FM termination suggests that the effects of mutations in SUP or the ectopic expression of class B genes on FM termination depends on AG. Indeed, ectopic AP3 and PI in whorl 4 of sup and p35S::AP3/PI flowers may repress AG function in this region. As AG mRNA expression pattern remains unaltered in sup mutant flowers (Bowman et al., 1992), this putative repression is likely to occur at a posttranscriptional level.
Floral organ identity within the third and fourth whorls is thought to rely on two different complexes of MADS-box transcription factors. The former includes AG and SEP together with AP3 and PI, while the latter, which likely promotes FM termination also, is composed of AG and SEP without AP3 and PI (Goto et al., 2001; Theissen, 2001; Theissen and Saedler, 2001; Krizek and Fletcher, 2005). It is therefore tempting to propose that class B genes antagonize AG's fourth whorl functions by competing in the formation of the aforementioned complexes (Fig. 4F). The balance between AP3/PI and AG would then determine whether the FM shifts toward the female program and terminates.
Interestingly, this hypothesis is consistent with the dose-dependent effects of ectopic expression of class B genes, with an increase in stamen number observed between p35S::AP3, sup1, and p35S::AP3 sup1 flowers, respectively (Jack et al., 1994), and between p35S::AP3, sup1, p35S::AP3/PI, and p35S::AP3/PI sup1 flowers (Krizek and Meyerowitz, 1996). Conversely, ag-1/AG sup-2 and ag-4 sup-1 flowers are totally indeterminate male flowers, consisting in an indefinite number of stamens produced inner to whorl 2 by an everlasting FM (Fig. 1H; Schultz et al., 1991; Prunet et al., 2008), suggesting that the defect in FM termination of sup mutant flowers is enhanced by a reduced dose of functional AG. Another strong evidence in favor of a competition for the formation of complexes of MADS-box transcription factors is provided by the phenotype of pAP3::WUS transgenic flowers. As a result of AG activation by WUS, AG expression level is increased in the third whorl, causing stamens to become carpelloid (Lohmann et al., 2001). Thus, increasing the dose of AG within a domain where it is normally already present together with both AP3 and PI is sufficient to promote the female program, at the expense of the male one.
Whatever the exact molecular basis of the inhibition of FM termination by AP3 and PI, it is worth noting that this process is closely linked to the female developmental program: In wild-type flowers, FM termination coincides with female organs initiation. In sup mutant flowers also, FM termination is usually associated with the production of carpelloid organs (Schultz et al., 1991; Bowman et al., 1992). AG is the master gene promoting FM termination, yet it controls both male and female programs within the flower, and appears unable to disrupt stem cell maintenance while the FM is still producing male organs. Exclusion of class B genes from the fourth whorl is responsible for the shift from male to female program, and allows FM termination to occur.
CRC, a Gene Involved in the Female Program, also Controls FM Termination
Other mutants confirm the close link between female program and FM termination. Mutations in CRC and SPATULA (SPT) affect both growth and congenital fusion of carpel primordia (Alvarez and Smyth, 1999). CRC and to a lesser extent SPT also have a role in FM termination, as crc spt double mutant flowers sometimes produce supernumerary whorls of stamens and carpels within the gynoecium. This loss of determinacy is even stronger in crc AG/ag-1 flowers (Alvarez and Smyth, 1999). Thus, CRC, which specifically controls the female developmental program and is expressed from stage 6 on (Bowman and Smyth, 1999), also shares AG's function in FM termination, hence confirming the close link between these two processes. In the case of CRC, both functions seem to be widely conserved in angiosperms, as orthologs of CRC in various species display similar expression patterns in carpel primordia and loss-of-function phenotypes (Nagasawa et al., 2003; Yamaguchi et al., 2004; Fourquin et al., 2005; Lee et al., 2005; Orashakova et al., 2009). In rice, tobacco, petunia, and California poppy (Eschscholzia californica), this loss of function alone is sufficient to cause a strong loss of FM termination, suggesting that CRC orthologs play a more important role in this process in most species compared to Arabidopsis (Yamaguchi et al., 2004; Lee et al., 2005; Orashakova et al., 2009).
CRC is a YABBY transcription factor expressed in developing carpels, but neither in stamens nor in the FM center, suggesting that it modulates stem cell maintenance in a non-cell-autonomous fashion (Bowman and Smyth, 1999). CRC expression is directly activated by AG (Bowman and Smyth, 1999; Gomez-Mena et al., 2005). Therefore, part of AG function in promoting carpel development and FM termination could be mediated by the downstream action of CRC. The possibility that CRC could in turn activate AG in a positive feedback was suggested (Gomez-Mena et al., 2005), but no direct evidence supports it so far. Indeed, CRC is expressed in floral organs in the absence of AG when AP2 is also mutated, and confers on them some carpelloid features, showing that both CRC activation and function are at least partially independent of AG (Alvarez and Smyth, 1999; Bowman and Smyth, 1999).
How could CRC promote FM termination independently of AG? Several members of the YABBY gene family exhibit complex interactions with meristematic genes including KNOX I genes, WUS and CLV3 (Sawa et al., 1999; Siegfried et al., 1999; Kumaran et al., 2002; Goldshmidt et al., 2008). However, whether similar interactions are responsible for CRC's function in FM termination remains unclear, as YAB2, which is not well characterized so far, is the only YABBY gene able to complement crc-1 (Meister et al., 2005; Fourquin et al., 2007).
CONCLUSION
Most angiosperm flowers consist of a determinate number of organs organized in a precise, conserved architecture. In Arabidopsis, the genetic bases of flower determinacy have been progressively unraveled using mutants showing an increase in floral organ number, due to either spatial or temporal defects in the FM. FM termination—the arrest of stem cell maintenance at the appropriate moment in the course of flower development—is of major importance for the generation of determinacy, and relies on a regulatory network centered on the single transcription factor, AG. Consistent with this central role, AG's expression and function are tightly controlled by numerous factors, including activators within the two inner whorls, and inhibitors in the outer whorls. This complex regulation results in a close association between FM termination and the female developmental program: AG is only able to turn off stem cell maintenance after the shift from the male to the female program, when class B genes have been excluded from the center of the flower. This exclusion is likely due to the action of SUP. Despite recent advances in our understanding of the spatial and temporal control of FM termination, one major question remains unanswered. AG promotes FM termination by indirectly repressing WUS (Sieburth et al., 1998; Lenhard et al., 2001; Lohmann et al., 2001) but no intermediate between AG and WUS has been found so far. However, GA4, a gene involved in the biosynthesis of gibberellins, has recently been identified among the targets of AG (Gomez-Mena et al., 2005). Gibberellins oppose meristem activity (for review, see Shani et al., 2006), raising the possibility that AG promotes FM termination by modifying phytohormone levels within the FM (discussed by Sablowski, 2007b). Clarifying the roles of SUP, CRC, and phytohormones appears as the next frontier in a better understanding of this developmental process.
Acknowledgments
We thank John Bowman, Annick Dubois, Michael Frohlich, François Parcy, and Charlie Scutt for their valuable comments on the manuscript, and Pradeep Das for helpful discussions over the months. We also thank the reviewers for their useful comments. We apologize for citing reviews rather than the original literature for points that are not central to the topic of this article.
This work was supported by the Centre National de la Recherche Scientifique, the Institut National de la Recherche Agronomique, the Ecole Normale Supérieure de Lyon, the Université de Lyon, and the Agence Nationale de la Recherche (grant no. ANR–05–BLAN–0280–01).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Christophe Trehin (ctrehin@ens-lyon.fr).
References
- Alvarez J, Smyth DR (1999) CRABS CLAW and SPATULA, two Arabidopsis genes that control carpel development in parallel with AGAMOUS. Development 126 2377–2386 [DOI] [PubMed] [Google Scholar]
- Angenent GC, Franken J, Busscher M, Weiss D, van Tunen AJ (1994) Co-suppression of the petunia homeotic gene fbp2 affects the identity of the generative meristem. Plant J 5 33–44 [DOI] [PubMed] [Google Scholar]
- Aukerman MJ, Sakai H (2003) Regulation of flowering time and floral organ identity by a MicroRNA and its APETALA2-like target genes. Plant Cell 15 2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereterbide A, Hernould M, Castera S, Mouras A (2001) Inhibition of cell proliferation, cell expansion and differentiation by the Arabidopsis SUPERMAN gene in transgenic tobacco plants. Planta 214 22–29 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Sakai H, Jack T, Weigel D, Mayer U, Meyerowitz EM (1992) SUPERMAN, a regulator of floral homeotic genes in Arabidopsis. Development 114 599–615 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR (1999) CRABS CLAW, a gene that regulates carpel and nectary development in Arabidopsis, encodes a novel protein with zinc finger and helix-loop-helix domains. Development 126 2387–2396 [DOI] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR, Meyerowitz EM (1989) Genes directing flower development in Arabidopsis. Plant Cell 1 37–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman JL, Smyth DR, Meyerowitz EM (1991) Genetic interactions among floral homeotic genes of Arabidopsis. Development 112 1–20 [DOI] [PubMed] [Google Scholar]
- Bradley D, Carpenter R, Sommer H, Hartley N, Coen E (1993) Complementary floral homeotic phenotypes result from opposite orientations of a transposon at the plena locus of Antirrhinum. Cell 72 85–95 [DOI] [PubMed] [Google Scholar]
- Breuil-Broyer S, Morel P, de Almeida-Engler J, Coustham V, Negrutiu I, Trehin C (2004) High-resolution boundary analysis during Arabidopsis thaliana flower development. Plant J 38 182–192 [DOI] [PubMed] [Google Scholar]
- Carles CC, Choffnes-Inada D, Reville K, Lertpiriyapong K, Fletcher JC (2005) ULTRAPETALA1 encodes a SAND domain putative transcriptional regulator that controls shoot and floral meristem activity in Arabidopsis. Development 132 897–911 [DOI] [PubMed] [Google Scholar]
- Carles CC, Lertpiriyapong K, Reville K, Fletcher JC (2004) The ULTRAPETALA1 gene functions early in Arabidopsis development to restrict shoot apical meristem activity and acts through WUSCHEL to regulate floral meristem determinacy. Genetics 167 1893–1903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartolano M, Castillo R, Efremova N, Kuckenberg M, Zethof J, Gerats T, Schwarz-Sommer Z, Vandenbussche M (2007) A conserved microRNA module exerts homeotic control over Petunia hybrida and Antirrhinum majus floral organ identity. Nat Genet 39 901–905 [DOI] [PubMed] [Google Scholar]
- Chen X (2004) A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303 2022–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Liu J, Cheng Y, Jia D (2002) HEN1 functions pleiotropically in Arabidopsis development and acts in C function in the flower. Development 129 1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Meyerowitz EM (1999) HUA1 and HUA2 are two members of the floral homeotic AGAMOUS pathway. Mol Cell 3 349–360 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Kato N, Wang W, Li J, Chen X (2003) Two RNA binding proteins, HEN4 and HUA1, act in the processing of AGAMOUS pre-mRNA in Arabidopsis thaliana. Dev Cell 4 53–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang CF, Meyerowitz EM (2000) Specific and heritable genetic interference by double-stranded RNA in Arabidopsis thaliana. Proc Natl Acad Sci USA 97 4985–4990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM (1993) CLAVATA1, a regulator of meristem and flower development in Arabidopsis. Development 119 397–418 [DOI] [PubMed] [Google Scholar]
- Clark SE, Running MP, Meyerowitz EM (1995) CLAVATA3 is a specific regulator of shoot and floral meristem development affecting the same processes as CLAVATA1. Development 121 2057–2067 [Google Scholar]
- Colombo L, Battaglia R, Kater MM (2008) Arabidopsis ovule development and its evolutionary conservation. Trends Plant Sci 13 444–450 [DOI] [PubMed] [Google Scholar]
- Das P, Ito T, Wellmer F, Vernoux T, Dedieu A, Traas J, Meyerowitz EM (2009) Floral stem cell termination involves the direct regulation of AGAMOUS by PERIANTHIA. Development 136 1605–1611 [DOI] [PubMed] [Google Scholar]
- Davies B, Motte P, Keck E, Saedler H, Sommer H, Schwarz-Sommer Z (1999) PLENA and FARINELLI: redundancy and regulatory interactions between two Antirrhinum MADS-box factors controlling flower development. EMBO J 18 4023–4034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditta G, Pinyopich A, Robles P, Pelaz S, Yanofsky MF (2004) The SEP4 gene of Arabidopsis thaliana functions in floral organ and meristem identity. Curr Biol 14 1935–1940 [DOI] [PubMed] [Google Scholar]
- Dreni L, Jacchia S, Fornara F, Fornari M, Ouwerkerk PB, An G, Colombo L, Kater MM (2007) The D-lineage MADS-box gene OsMADS13 controls ovule identity in rice. Plant J 52 690–699 [DOI] [PubMed] [Google Scholar]
- Ferrario S, Immink RG, Angenent GC (2004) Conservation and diversity in flower land. Curr Opin Plant Biol 7 84–91 [DOI] [PubMed] [Google Scholar]
- Ferrario S, Shchennikova AV, Franken J, Immink RG, Angenent GC (2006) Control of floral meristem determinacy in petunia by MADS-box transcription factors. Plant Physiol 140 890–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JC (2001) The ULTRAPETALA gene controls shoot and floral meristem size in Arabidopsis. Development 128 1323–1333 [DOI] [PubMed] [Google Scholar]
- Fourquin C, Vinauger-Douard M, Chambrier P, Berne-Dedieu A, Scutt CP (2007) Functional conservation between CRABS CLAW orthologues from widely diverged angiosperms. Ann Bot (Lond) 100 651–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourquin C, Vinauger-Douard M, Fogliani B, Dumas C, Scutt CP (2005) Evidence that CRABS CLAW and TOUSLED have conserved their roles in carpel development since the ancestor of the extant angiosperms. Proc Natl Acad Sci USA 102 4649–4654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldshmidt A, Alvarez JP, Bowman JL, Eshed Y (2008) Signals derived from YABBY gene activities in organ primordia regulate growth and partitioning of Arabidopsis shoot apical meristems. Plant Cell 20 1217–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Mena C, de Folter S, Costa MM, Angenent GC, Sablowski R (2005) Transcriptional program controlled by the floral homeotic gene AGAMOUS during early organogenesis. Development 132 429–438 [DOI] [PubMed] [Google Scholar]
- Goto K, Kyozuka J, Bowman JL (2001) Turning floral organs into leaves, leaves into floral organs. Curr Opin Genet Dev 11 449–456 [DOI] [PubMed] [Google Scholar]
- Goto K, Meyerowitz EM (1994) Function and regulation of the Arabidopsis floral homeotic gene PISTILLATA. Genes Dev 8 1548–1560 [DOI] [PubMed] [Google Scholar]
- Hiratsu K, Ohta M, Matsui K, Ohme-Takagi M (2002) The SUPERMAN protein is an active repressor whose carboxy-terminal repression domain is required for the development of normal flowers. FEBS Lett 514 351–354 [DOI] [PubMed] [Google Scholar]
- Jack T, Fox GL, Meyerowitz EM (1994) Arabidopsis homeotic gene APETALA3 ectopic expression: transcriptional and posttranscriptional regulation determine floral organ identity. Cell 76 703–716 [DOI] [PubMed] [Google Scholar]
- Jack T, Sieburth L, Meyerowitz E (1997) Targeted misexpression of AGAMOUS in whorl 2 of Arabidopsis flowers. Plant J 11 825–839 [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Meyerowitz EM (1997) Hypermethylated SUPERMAN epigenetic alleles in Arabidopsis. Science 277 1100–1103 [DOI] [PubMed] [Google Scholar]
- Jacobsen SE, Running MP, Meyerowitz EM (1999) Disruption of an RNA helicase/RNAse III gene in Arabidopsis causes unregulated cell division in floral meristems. Development 126 5231–5243 [DOI] [PubMed] [Google Scholar]
- Kayes JM, Clark SE (1998) CLAVATA2, a regulator of meristem and organ development in Arabidopsis. Development 125 3843–3851 [DOI] [PubMed] [Google Scholar]
- Krizek BA, Fletcher JC (2005) Molecular mechanisms of flower development: an armchair guide. Nat Rev Genet 6 688–698 [DOI] [PubMed] [Google Scholar]
- Krizek BA, Meyerowitz EM (1996) The Arabidopsis homeotic genes APETALA3 and PISTILLATA are sufficient to provide the B class organ identity function. Development 122 11–22 [DOI] [PubMed] [Google Scholar]
- Kumaran MK, Bowman JL, Sundaresan V (2002) YABBY polarity genes mediate the repression of KNOX homeobox genes in Arabidopsis. Plant Cell 14 2761–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Wolfe DS, Nilsson O, Weigel D (1997) A LEAFY co-regulator encoded by UNUSUAL FLORAL ORGANS. Curr Biol 7 95–104 [DOI] [PubMed] [Google Scholar]
- Lee JY, Baum SF, Oh SH, Jiang CZ, Chen JC, Bowman JL (2005) Recruitment of CRABS CLAW to promote nectary development within the eudicot clade. Development 132 5021–5032 [DOI] [PubMed] [Google Scholar]
- Lenhard M, Bohnert A, Jurgens G, Laux T (2001) Termination of stem cell maintenance in Arabidopsis floral meristems by interactions between WUSCHEL and AGAMOUS. Cell 105 805–814 [DOI] [PubMed] [Google Scholar]
- Li J, Jia D, Chen X (2001) HUA1, a regulator of stamen and carpel identities in Arabidopsis, codes for a nuclear RNA binding protein. Plant Cell 13 2269–2281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann JU, Hong RL, Hobe M, Busch MA, Parcy F, Simon R, Weigel D (2001) A molecular link between stem cell regulation and floral patterning in Arabidopsis. Cell 105 793–803 [DOI] [PubMed] [Google Scholar]
- Maier AT, Stehling-Sun S, Wollmann H, Demar M, Hong RL, Haubeiss S, Weigel D, Lohmann JU (2009) Dual roles of the bZIP transcription factor PERIANTHIA in the control of floral architecture and homeotic gene expression. Development 136 1613–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer KF, Schoof H, Haecker A, Lenhard M, Jurgens G, Laux T (1998) Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 95 805–815 [DOI] [PubMed] [Google Scholar]
- Meister RJ, Oldenhof H, Bowman JL, Gasser CS (2005) Multiple protein regions contribute to differential activities of YABBY proteins in reproductive development. Plant Physiol 137 651–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y, Ma H (1995) Separation of AG function in floral meristem determinacy from that in reproductive organ identity by expressing antisense AG RNA. Plant Mol Biol 28 767–784 [DOI] [PubMed] [Google Scholar]
- Nagasawa N, Miyoshi M, Sano Y, Satoh H, Hirano H, Sakai H, Nagato Y (2003) SUPERWOMAN1 and DROOPING LEAF genes control floral organ identity in rice. Development 130 705–718 [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Ferrario S, Angenent GC, Kobayashi A, Takatsuji H (2004) The petunia ortholog of Arabidopsis SUPERMAN plays a distinct role in floral organ morphogenesis. Plant Cell 16 920–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandi AK, Kushalappa K, Prasad K, Vijayraghavan U (2000) A conserved function for Arabidopsis SUPERMAN in regulating floral-whorl cell proliferation in rice, a monocotyledonous plant. Curr Biol 10 215–218 [DOI] [PubMed] [Google Scholar]
- Nardmann J, Werr W (2007) The evolution of plant regulatory networks: what Arabidopsis cannot say for itself. Curr Opin Plant Biol 10 653–659 [DOI] [PubMed] [Google Scholar]
- Orashakova S, Lange M, Lange S, Wege S, Becker A (2009) The CRABS CLAW ortholog from California poppy (Eschscholzia californica, Papaveraceae), EcCRC, is involved in floral meristem termination, gynoecium differentiation, and ovule initiation. Plant J 58 682–693 [DOI] [PubMed] [Google Scholar]
- Park W, Li J, Song R, Messing J, Chen X (2002) CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr Biol 12 1484–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelaz S, Ditta GS, Baumann E, Wisman E, Yanofsky MF (2000) B and C floral organ identity functions require SEPALLATA MADS-box genes. Nature 405 200–203 [DOI] [PubMed] [Google Scholar]
- Pnueli L, Hareven D, Broday L, Hurwitz C, Lifschitz E (1994) The TM5 MADS box gene mediates organ differentiation in the three inner whorls of tomato flowers. Plant Cell 6 175–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunet N, Morel P, Thierry AM, Eshed Y, Bowman JL, Negrutiu I, Trehin C (2008) REBELOTE, SQUINT, and ULTRAPETALA1 function redundantly in the temporal regulation of floral meristem termination in Arabidopsis thaliana. Plant Cell 20 901–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde A, Grunau C, De Beck L, Van Montagu M, Rosenthal A, Boerjan W (1999) Carpel, a new Arabidopsis epi-mutant of the SUPERMAN gene: phenotypic analysis and DNA methylation status. Plant Cell Physiol 40 961–972 [DOI] [PubMed] [Google Scholar]
- Sablowski R (2007. a) The dynamic plant stem cell niches. Curr Opin Plant Biol 10 639–644 [DOI] [PubMed] [Google Scholar]
- Sablowski R (2007. b) Flowering and determinacy in Arabidopsis. J Exp Bot 58 899–907 [DOI] [PubMed] [Google Scholar]
- Sakai H, Krizek BA, Jacobsen SE, Meyerowitz EM (2000) Regulation of SUP expression identifies multiple regulators involved in Arabidopsis floral meristem development. Plant Cell 12 1607–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai H, Medrano LJ, Meyerowitz EM (1995) Role of SUPERMAN in maintaining Arabidopsis floral whorl boundaries. Nature 378 199–203 [DOI] [PubMed] [Google Scholar]
- Sawa S, Watanabe K, Goto K, Liu YG, Shibata D, Kanaya E, Morita EH, Okada K (1999) FILAMENTOUS FLOWER, a meristem and organ identity gene of Arabidopsis, encodes a protein with a zinc finger and HMG-related domains. Genes Dev 13 1079–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoof H, Lenhard M, Haecker A, Mayer KF, Jurgens G, Laux T (2000) The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100 635–644 [DOI] [PubMed] [Google Scholar]
- Schultz EA, Pickett FB, Haughn GW (1991) The FLO10 gene product regulates the expression domain of homeotic genes AP3 and PI in Arabidopsis flowers. Plant Cell 3 1221–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani E, Yanai O, Ori N (2006) The role of hormones in shoot apical meristem function. Curr Opin Plant Biol 9 484–489 [DOI] [PubMed] [Google Scholar]
- Sieburth LE, Drews GN, Meyerowitz EM (1998) Non-autonomy of AGAMOUS function in flower development: use of a Cre/loxP method for mosaic analysis in Arabidopsis. Development 125 4303–4312 [DOI] [PubMed] [Google Scholar]
- Sieburth LE, Running MP, Meyerowitz EM (1995) Genetic separation of third and fourth whorl functions of AGAMOUS. Plant Cell 7 1249–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegfried KR, Eshed Y, Baum SF, Otsuga D, Drews GN, Bowman JL (1999) Members of the YABBY gene family specify abaxial cell fate in Arabidopsis. Development 126 4117–4128 [DOI] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM (1990) Early flower development in Arabidopsis. Plant Cell 2 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer H, Beltran JP, Huijser P, Pape H, Lonnig WE, Saedler H, Schwarz-Sommer Z (1990) Deficiens, a homeotic gene involved in the control of flower morphogenesis in Antirrhinum majus: the protein shows homology to transcription factors. EMBO J 9 605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theissen G (2001) Genetics of identity. Nature 414 491. [DOI] [PubMed] [Google Scholar]
- Theissen G, Saedler H (2001) Plant biology: floral quartets. Nature 409 469–471 [DOI] [PubMed] [Google Scholar]
- Trobner W, Ramirez L, Motte P, Hue I, Huijser P, Lonnig WE, Saedler H, Sommer H, Schwarz-Sommer Z (1992) GLOBOSA: a homeotic gene which interacts with DEFICIENS in the control of Antirrhinum floral organogenesis. EMBO J 11 4693–4704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uimari A, Kotilainen M, Elomaa P, Yu D, Albert VA, Teeri TH (2004) Integration of reproductive meristem fates by a SEPALLATA-like MADS-box gene. Proc Natl Acad Sci USA 101 15817–15822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbussche M, Zethof J, Souer E, Koes R, Tornielli GB, Pezzotti M, Ferrario S, Angenent GC, Gerats T (2003) Toward the analysis of the petunia MADS box gene family by reverse and forward transposon insertion mutagenesis approaches: B, C, and D floral organ identity functions require SEPALLATA-like MADS box genes in petunia. Plant Cell 15 2680–2693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L, Fletcher JC (2005) Stem cell regulation in the Arabidopsis shoot apical meristem. Curr Opin Plant Biol 8 582–586 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Nagasawa N, Kawasaki S, Matsuoka M, Nagato Y, Hirano HY (2004) The YABBY gene DROOPING LEAF regulates carpel specification and midrib development in Oryza sativa. Plant Cell 16 500–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky MF, Ma H, Bowman JL, Drews GN, Feldmann KA, Meyerowitz EM (1990) The protein encoded by the Arabidopsis homeotic gene agamous resembles transcription factors. Nature 346 35–39 [DOI] [PubMed] [Google Scholar]
- Yun JY, Weigel D, Lee I (2002) Ectopic expression of SUPERMAN suppresses development of petals and stamens. Plant Cell Physiol 43 52–57 [DOI] [PubMed] [Google Scholar]
- Zhao L, Kim Y, Dinh TT, Chen X (2007) miR172 regulates stem cell fate and defines the inner boundary of APETALA3 and PISTILLATA expression domain in Arabidopsis floral meristems. Plant J 51 840–849 [DOI] [PMC free article] [PubMed] [Google Scholar]