Abstract
Dietary polyunsaturated fatty acids (PUFAs) affect a wide variety of physiological processes. Much attention has been given to the n-3 PUFAs and their role in the prevention and treatment of cardiovascular disease, stemming from evidence obtained through a number of epidemiological studies and clinical trials. Investigators are now focused on elucidating the pathways and mechanisms for the biological action of n-3 PUFAs. Dietary intervention is recognized as a key measure in patient therapy and in the maintenance of human health in general. This review provides a summary of several important clinical trials, and while the exact modes of action of n-3 PUFA are not known, current viewpoints regarding the mechanisms of these fatty acids on atherosclerosis, circulating lipid profile, cell membranes, cell proliferation, platelet aggregation and cardiac arrhythmias are discussed.
Keywords: Arrhythmias, Atherosclerosis, Cardiovascular disease, Diet, Polyunsaturated fatty acids
INTRODUCTION TO POLYUNSATURATED FATTY ACIDS
Fats are essential for living organisms. Fatty acid (FA) molecules have a variable length carbon chain with a methyl terminus and a carboxylic acid head group (1). They can be categorized based on the degree of saturation of their carbon chains. Saturated FAs possess the maximal number of hydrogen atoms, while monounsaturated FAs and polyunsaturated FAs (PUFAs) have one, or two or more, double bonds, respectively. PUFAs can be further subdivided on the basis of the location of the first double bond relative to the methyl terminus of the chain. For example, n-3 and n-6 FAs are two of the most biologically significant PUFA classes, and have their first double bond on either the third or sixth carbon from the chain terminus, respectively. The final carbon in the FA chain is also known as the omega carbon, hence the common reference to these FAs as omega-3 or omega-6 PUFAs.
Long-chain n-3 and n-6 PUFAs are synthesized from the essential FAs (EFAs) alpha-linolenic acid (ALA) and linoleic acid, respectively. Basic structures of these two parent PUFAs are shown in Figure 1. An EFA cannot be made by the body and must be obtained through dietary sources. Animals and humans have the capacity to metabolize EFAs to long-chain derivatives. Figure 2 shows the conversion of EFAs to their downstream products through multiple elongation and desaturation steps. Because the n-6 and n-3 pathways compete with one another for enzyme activity, the ratio of n-6 to n-3 PUFAs is very important to human health. An overabundance of FAs from one family will limit the metabolic production of the longer chain products of the other. The typical Western diet provides n-6 and n-3 PUFAs in a ratio ranging from 8:1 to 25:1 (1), values in severe contrast with the recommendations from national health agencies of approximately 4:1 (2). Lowering the n-6:n-3 ratio would reduce competition for the enzymes and facilitate the metabolism of more downstream products of ALA.
Figure 1).
The structure of two essential fatty acids – linoleic acid and alpha-linolenic acid
Figure 2).
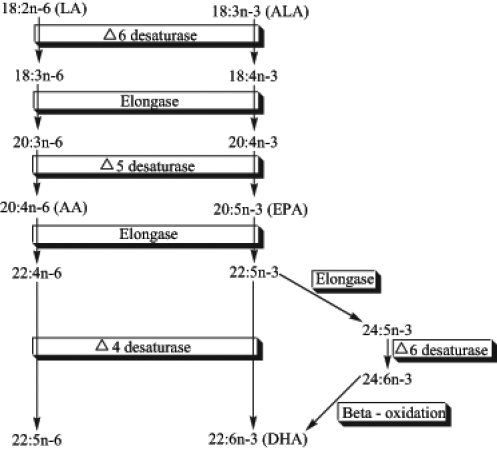
The metabolic pathways of n-6 and n-3 polyunsaturated fatty acids. Metabolism of the two fatty acid families requires competition for the same elongation and desaturation enzymes. Note that the desaturation steps tend to be slow and rate-limiting compared with the more rapid elongation steps. Eicosanoids, such as prostaglandins, leukotrienes and thromboxanes, can be derived from arachidonic acid (AA) and eicosapentaenoic acid (EPA), and can mediate different physiological actions. ALA Alpha-linolenic acid; DHA Docosahexaenoic acid; LA Linoleic acid
Because most diets are already very rich in n-6 PUFAs, greater focus needs to be placed on incorporating n-3 PUFAs into the diet. Dietary sources of n-3 PUFAs are readily available but in limited quantities. Many foods contain ALA, including certain vegetable oils, dairy products, flaxseed, walnuts and vegetables (3). Fatty fish, such as mackerel, herring and salmon, provide an excellent source of the long-chain derivatives of ALA, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (2).
EFFECTS OF PUFAs ON CLINICAL END POINTS ASSOCIATED WITH CARDIOVASCULAR DISEASE
Omega-3 PUFAs are perceived as a beneficial dietary intervention for the prevention and treatment of cardiovascular disease (CVD). Clinical studies have evaluated the possible benefits using either n-3 PUFA supplements or the consumption of fish. The findings of trials using n-3 PUFA supplements will be summarized first.
In the Multiple Risk Factor Intervention Trial (MRFIT [4]), the diets of individuals at high risk for coronary artery disease (CAD) were monitored for 10.5 years. A reduction in mortality from CAD, CVD and all other causes was observed in those consuming 665 mg/day of fish-derived n-3 PUFAs (4). The Indian Experiment of Infarct Survival (5) provided patients with suspected myocardial infarction (MI) with a dietary supplement of 2 g/day of n-3 PUFAs (EPA and DHA), mustard seed oil (containing 2.9 g/day of ALA) or a placebo. After one year, the number of cardiac events in the groups receiving the fish oil and the mustard seed oil was lower, and the incidence of nonfatal MI was significantly reduced (5). The Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico-Prevenzione trial (6) did not find any decrease in the incidence of nonfatal MI with a 1 g/day n-3 PUFA treatment over 3.5 years, but an early, significant reduction of sudden cardiac death was recorded.
Not all trials with n-3 PUFA supplements have demonstrated positive effects. In patients who recently survived an MI, capsules providing approximately 575 mg/day of EPA and 1150 mg/day DHA had no beneficial effect on the incidence of cardiac events when compared with a corn oil (n-6 PUFA-rich) control (7). However, this may have been the result of a relatively small sample size or the possibility that the n-3-rich Norwegian diet did not allow for a true ‘control’ population (8).
Studies using dietary fish consumption have found beneficial effects similar to those observed in studies with n-3 PUFA supplements. The United States Physicians’ Health Study recruited male physicians with no prior history of MI, cerebrovascular disease or cancer to maintain a dietary record and allow follow-up for 11 years (9). Sudden cardiac death was significantly reduced by consumption of at least one meal of fish weekly, while nonfatal MI levels were unchanged (10). The Nurses’ Health Study (11), which followed female nurses with no prior CVD or cancer for 16 years, identified a significant inverse relationship between fish consumption and CAD. Dietary ALA appeared to be the most protective PUFA (10). Yuan et al (12) examined fish and shellfish consumption in Chinese men and found that the ingestion of over 200 g/week reduced fatal MI risk by 59% compared with those consuming less than 50 g/week. Secondary cardiovascular complications were greatly reduced by adopting a ‘Mediterranean-style’ diet in the Lyon Heart Study (13). Analysis of the Finnish, Dutch and Italian cohorts of the Seven Countries Study (14) revealed an inverse relationship between fatty fish consumption and 20-year CAD mortality (15).
While many trials have found positive effects of n-3 PUFA supplementation and fish consumption on CVD, others have found less of a correlation. The Health Professionals Follow-up Study (16) found no reduction in risk for CAD in men without established CVD when the number of fish meals per week was increased beyond one or two meals. In addition, adverse effects related to fish intake and n-3 PUFA supplementation have been found. A grossly elevated intake of fish oil can cause an increased risk of bleeding (2,16,17). Encapsulated fish oil supplements can also cause minor gastrointestinal disturbances and eructation (2). A recent study (17) also reported that fish may contain methyl mercury (and other contaminants) that may negatively affect CAD. Finally, unsaturated FAs, including n-3 PUFAs, are prone to oxidation. An antioxidant may need to be added to concentrated n-3 PUFA supplements to prevent the production of damaging lipid peroxides (18).
Ultimately, n-3 PUFAs may protect against CVD through several mechanisms, including acting as an antiatherogenic agent (1,17); lowering serum triglycerides (1); slightly lowering blood pressure (17,19); improving endothelial function (17); reducing inflammatory responses (17); inhibiting platelet aggregation and thombosis (1,17); and decreasing the incidence of arrhythmias (1,17).
MECHANISM FOR THE EFFECTS OF n-3 PUFAs ON CVD
Antiatherogenic effects of n-3 FAs
Atherosclerosis is an inflammatory disease of the vascular system. Dietary factors play a significant role in the development of atherosclerosis. Consumption of long-chain n-3 PUFAs demonstrated antiatherogenic effects in experimental and epidemiological studies (20–22). The recent Study on Prevention of Coronary Atherosclerosis by Intervention with Marine Omega-3 FAs (SCIMO [22]) demonstrated that consumption of 1.65 g/day of a fish oil supplement by patients with CAD resulted in less progression and more regression of coronary atherosclerotic plaques. However, this effect was not observed in the carotid arteries, suggesting that n-3 PUFAs may have different effects in different vascular beds (22). Results from the Seven Countries Study (14) showed an inverse relationship between fish consumption and CAD, while the Health Professionals Study (16) found no relationship. Dietary ALA demonstrated a beneficial effect on CAD in the Health Professionals Study (16), but this result was not observed in the Dutch cohort from the Seven Countries Study (2). The only study to show a negative effect of fish consumption on CAD was conducted in Finland, but these results may have been influenced by mercury contamination of the fish (16).
n-3 PUFAs provide their antiatherogenic effects through one or a combination of several potential mechanisms. They may exert their effect on atherogenesis by altering the circulating lipid profile; changing the physicochemical function of cell membranes, thereby affecting eicosanoid biosynthesis, cell signalling and gene expression; and modulating vascular smooth muscle cell proliferation and migration.
Effects of n-3 PUFAs on circulating lipid profile:
Many epidemiological and dietary interventions have shown that consumption of n-3 PUFAs significantly alters the serum lipid profile. A strong inverse relationship exists between n-3 PUFA consumption and circulating plasma triacylglycerol (TG) concentrations (23). Dutch men who consumed approximately 30 g/day of fish over a long period of time had lowered serum TG concentrations compared with a control group (15). A health survey of the Inuit of Nunavik, Canada, who traditionally consume large amounts of marine foods rich in n-3 PUFAs, revealed abnormally high plasma phospholipid concentrations of n-3 PUFAs (24). A negative correlation between n-3 PUFAs and plasma TG levels, and a positive relationship with high density lipoprotein (HDL) cholesterol levels may account for the low mortality rate due to CVD observed in this population (24). Interestingly, an increase in low density lipoprotein (LDL) cholesterol and total cholesterol levels was also discovered (24). Elevated plasma cholesterol levels have long been associated with an increased risk of atherosclerosis. This may help to explain the cardioprotective effects of PUFAs. However, the antiatherogenic effect of n-3 PUFA supplementation is not always linked to a change in total plasma cholesterol levels (23,25,26). Dietary intervention trials suggest that LDL cholesterol levels increase with n-3 PUFA supplementation in a dose-dependent manner (24,25,27,28). HDL levels may also be altered by fish oil. Nilsen et al (7) showed a significant decrease in total cholesterol and a significant increase in HDL cholesterol after fish oil supplementation. The concomitant increase in HDL cholesterol levels relative to increasing LDL cholesterol often leaves the total cholesterol to HDL cholesterol ratio, a common measure of atherogenic risk, unchanged (16,24,25). This evidence suggests that n-3 PUFAs reduce atherosclerotic development through mechanisms other than lowering LDL cholesterol.
The increase in LDL cholesterol levels from n-3 PUFA supplementation appears to be due to an increase in LDL particle size rather than to the number of LDL molecules. n-3 PUFAs modify the composition of LDL cholesterol by increasing apolipoprotein B and decreasing lipoprotein levels, resulting in a less atherogenic molecule (28). The hypotriacylglycerolemic effect and the consequent increase in LDL cholesterol observed with n-3 PUFA supplementation may be due to altered very low density lipoprotein (VLDL) metabolism. A recent dietary intervention trial confirmed that n-3 PUFAs decreased plasma TG and VLDL apolipoprotein B levels (26). The VLDL pool size decreased due to a reduction in hepatic secretion of VLDL and increased conversion of VLDL to LDL (26).
Effects of n-3 PUFAs on cell membranes:
Increased intake of EPA and DHA inevitably results in greater incorporation of these FAs into circulating lipids and into tissues. n-3 PUFAs may replace n-6 PUFAs in cell membrane phospholipids, thus altering the physicochemical properties of the membrane (2). The physicochemical alterations in membrane properties may directly or indirectly influence the function of membrane-bound receptors, ion channels and enzymes, and affect downstream signalling pathways that will have a direct effect on vascular endothelial and smooth muscle cell function (29). Eicosanoid production is also affected by the FA composition of the membrane.
Alteration of the eicosanoid profile may have important effects on inflammation (30). Eicosanoids, 20-carbon FAs derived from n-6 and n-3 EFAs through the addition of oxygen atoms into the FA chains, confer a wide variety of potent, hormone-like actions on various tissues. The eicosanoid families, including the prostaglandins, thromboxanes and leukotrienes, influence many biological activities, such as platelet aggregation, smooth muscle contraction and inflammatory responses. The 20-carbon n-3 and n-6 PUFAs compete for the cyclooxygenase (COX) and lipooxygenase enzymes. The 2- and 4-series eicosanoids derived from n-6 PUFAs are more biologically active than the 3- and 5-series eicosanoids derived from n-3 PUFA. Thromboxane A2 (TxA2), a metabolite of arachidonic acid (AA), is a potent vasoconstrictor and platelet aggregator. Fish oils inhibit TxA2 in vitro and in vivo (31). n-3 derivatives also decrease the affinity of the TxA2 receptor for TxA2, thus further inhibiting TxA2-induced platelet aggregation (32). Increased consumption of n-3 PUFAs results in greater incorporation of n-3 PUFAs into cell membrane phospholipids, ultimately leading to the generation of more n-3-derived eicosanoids. This results in simultaneous reduction of n-6 PUFA-derived proinflammatory eicosanoids because n-3 PUFAs can competitively inhibit the conversion of AA to proinflammatory eicosanoids. n-3 PUFAs act as potential COX substrates, decreasing the affinity of the COX enzyme for n-6 PUFAs and suppressing the production of n-6 eicosanoid inflammatory mediators (2,33).
The vascular endothelium is also modified by the ingestion of n-3 PUFAs. Vasoactive substances and growth factors are released by the vascular endothelium that activate immune cells, gene transcription, and functions involved in the regulation of monocyte adhesion, inflammation, vascular cell growth, cell migration and vascular tone (34). In response to stress or injury, the endothelium can become dysfunctional and susceptible to atherogenesis. The endothelium becomes proadhesive through cytokine-induced endothelial activation, which is important for the initiation and progression of atherosclerosis because it enables surface expression of endothelial leukocyte adhesion molecules and secretion of soluble proinflammatory products, such as interleukins-1 and -4, tumour necrosis factor, vascular cell adhesion molecule-1, platelet-derived growth factors (PDGFs) and monocyte chemoattractant proteins (35). Because most adhesion molecules are not expressed under basal conditions, cytokine-induced endothelial activation requires the initiation of gene transcription. Nuclear factor kappa B (NFκB), a gene regulatory protein implicated in the development of atherosclerosis, can activate gene transcription and expression of adhesion molecules (35,36).
n-3 PUFAs may modulate atherogenesis by inhibiting signalling events related to endothelial activation. Expression of endothelial leukocyte adhesion molecules and soluble proinflammatory proteins is inhibited when n-3 PUFAs alter the expression and production of macrophage cytokines (21,35–39). This modulatory effect of n-3 PUFAs on gene expression is associated with a parallel reduction in steady-state messenger RNA (mRNA) levels of proatherogenic molecules (35,38). The magnitude of the inhibitory effect of n-3 PUFAs on endothelial activation is related to the extent to which n-3 PUFAs are incorporated into cellular lipids. Nutritional supplementation can produce large enough elevations in DHA concentration to observe noticeable effects in in vitro studies (33,36). The exact mechanisms of the inhibitory effects of n-3 PUFAs on endothelial activation are unknown. However, the reduction of steady-state levels of adhesion molecule and growth factor mRNA by n-3 PUFAs persists after adhesion is activated. Furthermore, the effects of n-3 PUFAs occur before the translation of mRNA into proteins and are independent of receptor activation (37,38).
The multiple double bonds of n-3 PUFAs can have a direct physical effect on NFκB-induced expression of proinflammatory substances. The double bonds may inactivate superoxide anions that are generated early in cytokine-induced intracellular signal transduction, thus inhibiting hydrogen peroxide formation, which is directly responsible for the activation of NFκB and the induction of adhesion molecule expression (35).
n-3 PUFAs also display antiatherogenic effects through direct modulation of nitric oxide (NO) production and release (31,40). NO is synthesized from L-arginine by NO synthases, specifically endothelial (eNOS) and inducible (iNOS) NO synthases. NO regulates vascular relaxation and inhibits key atherosclerotic processes such as platelet aggregation, monocyte adhesion and vascular smooth muscle cell (VSMC) proliferation and migration. The cellular mechanisms by which n-3 PUFAs improve endothelial function remain unclear. However, recent reports suggest that n-3 PUFAs enhance eNOS and iNOS production of NO. Translocation and activation of eNOS induced by EPA result in endothelium-dependent vasorelaxation (41). DHA increases NO production by potentiating interleukin-1β-induced iNOS mRNA expression in VSMC through activation of the p44/42 mitogen-activated protein kinase signalling cascade (29,42).
Effects of PUFAs on VSMC proliferation and migration:
Migration and proliferation of VSMCs cause intimal hyperplasia, which contributes to the development of atherogenic lesions. Activated platelets aggregate at sites of endothelial dysfunction and release peptide growth factors, such as PDGF, and nonpeptide growth factors, such as serotonin (5-HT) and adenosine diphosphate (ADP). Previous studies indicate that PDGF, 5-HT, ADP and TxA2 can stimulate VSMC to proliferate (39). Although epidemiological and clinical evidence suggests that n-3 PUFAs may reduce both VSMC proliferation and VSMC excitability, very few studies have demonstrated the cellular mechanisms by which n-3 PUFAs modulate VSMCs. n-3 PUFAs may inhibit VSMC proliferation through multiple signal transduction pathways that modulate growth factors. Alternatively, EPA and DHA block 5-HT-induced VSMC proliferation due to increases in mRNA levels of the 5-HT2 receptor (39). n-3 PUFAs also inhibit receptor binding of PDGF, reducing mRNA expression of early genes involved in the development of atherosclerosis (2).
Antiaggregatory effects of n-3 PUFAs
The narrowing of blood vessels due to an atherosclerotic plaque can provide a setting in which a thrombus can more readily block blood flow and cause an MI. As described previously, n-3 PUFAs compete for the same elongation and desaturation enzymes as n-6 PUFAs. While eicosanoids derived from both parent FAs are proaggregatory, those derived from AA (2- and 4-series) have 100-fold greater activity than those derived from EPA (3- and 5-series). Consequently, the EPA metabolites are generally considered antiaggregatory. An improved balance between AA and EPA could reduce the likelihood of clot formation.
A variety of models have been used to observe the effects of enriching the diet with n-3 PUFAs. Experiments in which either saturated fats or PUFAs (either n-3 or n-6) were added to the diet found striking results. In response to ADP, platelet aggregation was significantly increased in plasma obtained from rabbits consuming the n-6-rich diet compared with the n-3-fed groups consuming fish oil (EPA and DHA) or flaxseed oil (ALA) (43). This increased aggregation was even greater than that observed in the coconut oil group (saturated fat). The same trends applied when platelet activation was initiated by collagen but not thrombin (43). The inhibitory effects of platelet aggregation were directly related to the FA composition of platelet lipids. It was also hypothesized that a PUFA effect on membrane viscosity could affect the activity of the proteins in the platelet membranes that are involved in aggregation as receptors or enzymes.
Although beneficial in preventing the potential blocking of a blood vessel by a thrombus, extreme inhibition of clotting mechanisms could have side effects. Increases in bleeding times have been reported in humans with increased intake of fish oils (44,45) but not flaxseed oil (46). There is some anecdotal evidence of individuals taking acetylsalicylic acid and n-3 supplements experiencing hematuria and spontaneous nosebleeds, likely resulting from severe effects on platelet aggregation (47).
Antiarrhythmic effects of n-3 PUFAs
Initial experiments performed on isolated hearts in the early 1980s found that PUFAs antagonized the depressed ventricular arrhythmia threshold in hypoxia (48). Animals consuming n-3 PUFAs exhibited significant reductions, or even abolition, of arrhythmias compared with control groups (49,50). Direct intravenous injection of an emulsion of concentrated fish oil proved effective at preventing fatal ventricular fibrillation in dogs subjected to exercise stress tests following coronary artery ligation (51). Follow-up studies showed that injections of purified EPA and DHA, as well as the parent n-3, ALA, were each equally protective against ventricular fibrillation (52). The antiarrhythmic effects of n-3 fish oils have also been demonstrated in nonhuman primates (53). Some studies report a reduced incidence of cardiac arrhythmias in groups receiving n-6 PUFA supplementation (50,54), whereas others have shown no protective effect (49). The difference in findings may be due to the duration of the feeding trials because no effect was observed after four weeks of feeding compared with 10 or 12 weeks of feeding. However, the protective effects from n-3 PUFAs are consistently greater than those from n-6 PUFAs. Subsequently, much work has focused on the n-3 PUFAs found in fish oils, EPA and DHA, due to the overwhelming epidemiological evidence relating fatty fish intake to the decrease in CVD.
The sarcolemmal membrane contains a variety of ion channels, exchangers and pumps important to the conduction of action potentials and the maintenance of ion gradients. Voltage-gated sodium channels (VGSCs), potassium channels and calcium channels are responsible for the initiation, duration and propagation of the action potential. Effects of n-3 PUFAs on these proteins are summarized in Table 1. Generally, in cultured neonatal cardiomyocytes, perfusion with n-3 and n-6 PUFAs raises the threshold potential required for action potential stimulation, decreases resting membrane potential and shortens action potential duration (55). All of these effects could help the heart maintain electrical stability during ischemia and reduce the likelihood of arrhythmogenesis.
TABLE 1.
Summary of effects of omega-3 polyunsaturated fatty acids (PUFAs) on ion channels and transporters
| Channel/transporter | Omega-3 PUFA tested | Effect on conductance/activity | Reference(s) |
|---|---|---|---|
| SL | |||
| VGSC | ALA | ↓ | (56,57,83) |
| EPA | ↓ | (56,57,77,83) | |
| DHA | ↓ | (57,82,83) | |
| Ca2+L-type | ALA | ↑*↓† | (59)† (84)* |
| EPA | None*, ↓† | (57)† (61,62)* | |
| DHA | None*, ↓† | (57)† (61,62)* | |
| Ito | ALA | ↓ | (65) |
| EPA | ↓ | (57,65) | |
| DHA | ↓ | (57,65) | |
| IK | ALA | ↓ | (65) |
| EPA | ↓ | (65) | |
| DHA | ↓ | (65) | |
| IK1 | ALA | None | (65) |
| EPA | None*, ↓† | (57)† (65,66)* | |
| DHA | None*, ↓† | (57)† (65,66)* | |
| Isus | EPA | ↓ | (66) |
| DHA | ↓ | (66) | |
| KATP | ALA | ↓ | (67) |
| TRAAK | EPA | ↑ | (85) |
| DHA | ↑ | (85) | |
| TREK | EPA | ↑ | (85) |
| DHA | ↑ | (85) | |
| NHE | EPA | ↓ | (69) |
| DHA | ↓ | (69) | |
| NCX | ALA | ↑ | (70) |
| SR | |||
| RyR | EPA | ↓ open state | (73,74) |
| SERCA | EPA | ↓ | (86) |
| DHA | ↓ | (86) | |
Corresponds to reference(s) indicated;
Corresponds to reference(s) indicated. ALA Alpha-linolenic acid; DHA Docosahexaenoic acid; EPA Eicosapentaenoic acid; IK Delayed rectifier channels; IK1 Inward rectifier K+ channel; Isus K+ channel; Ito Transient outward K+ channel; KATP ATP-sensitive K+ channel; NCX Na+/Ca2+ exchanger; NHE Na+/H+ exchanger; RyR Ryanodine receptor; SERCA Sarcoplasmic endoplasmic reticulum calcium ATPase; SL Sarcolemmal; SR Sarcoplasmic reticulum; VGSC Voltage gated sodium channel
Na+ channels:
Treatment of neonatal cardiomyocytes with PUFAs increases the voltage threshold required for opening of the Na+ channel (55). Raising the threshold of Na+ channel activation would make the cell less prone to spontaneous stimulations that could induce arrhythmias. The peak Na+ current is also significantly decreased by n-3 PUFAs (56,57).
Acute in vitro experiments have found inhibitory effects of n-3 PUFAs on cardiac Na+ currents, which would slow conduction velocities and allow arrhythmogenic re-entrant circuits to develop. This, of course, is potentially dangerous. In theory, the targeting and inhibition of VGSCs was a viable strategy in the design of antiarrhythmic drugs. However, the Cardiac Arrhythmia Suppresion Trial demonstrated a greater incidence of death in patients treated with class I drugs compared with placebo (58). Despite this, it is readily apparent that n-3 PUFAs are safe. Similar to amiodarone chloride, currently the most effective drug for treatment of cardiac arrhythmias, the n-3 PUFAs have blocking effects not only on cardiac Na+ channels but also on Ca2+ and K+ channels, which are discussed below. This broad effect may be part of the reason why n-3 PUFAs have such a potent antiarrhythmic effect.
Ca2+ channels:
L-type Ca2+ current plays a very important role in the plateau of the cardiac action potential (phase 2) and greatly affects its duration. Typically, Ca2+ enters the cell through voltage-gated Ca2+ channels and triggers the release of Ca2+ from the sarcoplasmic reticulum (SR), which is required for contraction of the heart. The reported effects of n-3 PUFAs on the L-type Ca2+ channel are varied but show either a decreasing effect or no effect on Ca2+ currents. For example, some evidence suggests n-3 PUFAs directly inhibit the Ca2+ current through L-type Ca2+ channels (59,60). This inhibition could reduce the incidence of Ca2+ release from the SR and act to limit Ca2+ overload. Other studies that observed no direct effects of n-3 PUFAs on L-type Ca2+ current have still discovered an interaction with the channel, related to the maintenance of normal function in the presence of channel agonists or antagonists. In neonatal cardiomyocytes, DHA was able to block the effects of the L-type Ca2+ channel agonist Bay K8664 and the antagonist nitrendipine (61,62). However, DHA did not block the effects of the L-type Ca2+ channel antagonists verapamil chloride {confirm} and diltiazem chloride (61). Because the latter two drugs block the channel at sites other than the dihydropyridine binding site (63), this appears to be strong evidence for n-3 PUFA interaction with the L-type Ca2+ channel at a functionally associated but different site, which can affect binding to the dihydropyridine site. Similarly, in adult cells, DHA almost completely prevented the effects of isoproterenol hydrochloride (64). Interestingly, in this study, administration of DHA alone did not result in a blockade of Ca2+ current. Rather, the channels are regulated to provide the proper influx of Ca2+ required for normal release of Ca2+ from the SR.
K+ channels:
K+ channels are largely responsible for determining the duration of the cardiac action potential and maintaining the cellular resting potential. The delayed rectifier channels (IK) are primarily responsible for repolarization of the cells in the later phases of the cardiac action potential. PUFAs can inhibit IK channels, which would result in a prolongation of the action potential and increase the refractoriness of the heart. This would aid in the prevention of re-entry mechanisms of arrhythmia because re-entrant circuits cannot act in refractory tissue. However, the PUFA concentration required to have similar inhibitory effects on IK as for are about four times greater than for the sodium current and 20 times greater than for Ca2+ (59,65). This would suggest that PUFAs exert the greatest antiarrhythmic effect through an action on Ca2+ channels, followed by Na+ channels, and remotely by K+ channels.
Transient outward K+ channels are also blocked by PUFAs (57,65,66). These K+ channels are responsible for the very rapid and large outward K+ current that opposes the inward flow of Ca2+ and Na+ that depolarizes the cell. The notch observed at phase 1 of the action potential characterizes the current through this channel. Another K+ channel more sustained than transient outward K+ channels, also activated upon depolarization, is known as Isus. It is also inhibited by DHA (66). Both of these outward currents play a key role in the repolarization of the cell, and their blockade results in a prolongation of the action potential. As mentioned previously, the increased refractoriness of the tissue would inhibit the creation of reentrant circuits.
The ATP-sensitive K+ channel is normally inactive, but under conditions that reduce the cytosolic ATP concentration, such as ischemia, the channels are opened and pass an outward current. These channels are also blocked by n-3 PUFAs (67). However, not all of the K+ channels are affected by n-3 PUFAs. The inward rectifier K+ channel does not appear to be influenced by n-3 PUFAs (65,66). Because these channels are activated by hyperpolarization, the normal or slightly depolarized membrane potential is maintained even though other K+ channels are blocked.
The two-pore domain K+ channels are a class of channels that have drawn recent attention because of several unique characteristics. One of the first discovered was the TRAAK channel. This outward current K+ channel is stimulated by AA, as well as by an acidic pH (68), which would be of aid in an ischemic environment. A more recently discovered member of this two-pore family is TREK-1. It also is stimulated by PUFAs and decreased pH but appears to be activated by an even wider range of stimuli. The net effect of activation of these channels is shortening of the action potential or hyper-polarization. This may help to reduce the excitability of the heart and limit the release of Ca2+.
Sarcolemmal ion pumps and transporters:
During ischemia, intracellular H+ accumulates under anaerobic metabolism. This stimulates the Na+/H+ exchanger (NHE) to remove H+ from the cell in exchange for Na+. The concomitant rise in intracellular Na+ stimulates the reverse mode operation of the Na+/Ca2+ exchanger (NCX) whereby three Na+ ions are removed from the cell in exchange for the entry of a single Ca2+ ion. This results in arrhythmias and/or cell death (Figure 3). Based on the stoichiometry of the NCX, its forward motion (Ca2+ outward) results in a net flux of inward charge. When the intracellular Na+ concentration decreases and the intracellular Ca2+ concentration increases, the exchanger will function in forward mode. The electrogenic operation of the NCX can result in transient depolarizations, which are a mechanism for delayed after-depolarizations, a substrate for torsade de pointes.
Figure 3).
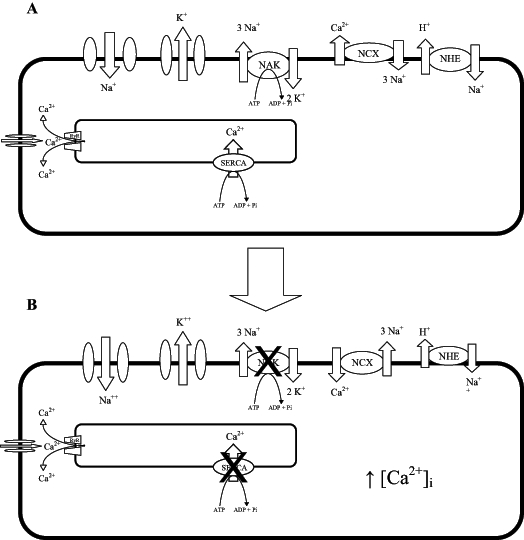
The major ion channels and transporters in a myocardial cell. The Na+/Ca2+ exchanger (NCX) and sarcoplasmic endoplasmic reticulum calcium ATPase (SERCA) are normally responsible for Ca2+ extrusion following each contraction (A), but during ischemia (B), lack of O2 limits ATP production and, thus, the function of the Na+/K+ ATPase (NAK) and SERCA. Uptake of Ca2+ by SERCA is inhibited and the NCX functions in reverse to compensate and removes Na+, but brings Ca2+ into the cell, resulting in Ca2+ overload and subsequent contracture and/or potentially lethal arrhythmias. [Ca2+]i intracellular Ca2+ concentration; NAK Na+, K+ ATPase; RyR ryanodine receptor
Although studies are limited, n-3 PUFAs appear to inhibit the NHE. However, this inhibitory effect is limited to the longer n-3 FAs because ALA does not affect the exchanger (69). In contrast to the NHE, the NCX is significantly stimulated by ALA (70). The authors speculated that the negative charge of the free FAs facilitates binding of Ca2+ to the exchanger and increases exchange rates. However, the effects of the longer n-3 PUFAs on the NCX remain unclear.
PUFAs inhibit Na+/K+ ATPase activity (71). This effect would not be beneficial. Intracellular Na+ levels would rise with Na+ pump inhibition and this would stimulate reverse Na+/Ca2+ exchange and elevate intracellular Ca2+ concentration with potentially damaging effects.
Cardiac SR:
Many types of arrhythmias occur due to abnormal Ca2+ handling and not due to changes in electrical excitability. Because the SR is a critical site within the cardiomyocyte for the regulation of intracellular Ca2+, it is also important to consider the effects of n-3 PUFAs on the SR function. The SR stores calcium that is released during contraction. In response to trigger Ca2+ passed via VGSC, Ca2+ is released into the cytosol through the ryanodine-sensitive channels in the SR. Calcium is responsible for activation of many intracellular enzymes and signalling cascades, along with playing a pivotal role in muscle contraction. To maintain proper rhythm, the Ca2+ must be cleared from the cytosol after contraction. Most Ca2+ is returned to the SR via the sarcoplasmic endoplasmic reticulum calcium ATPase (SERCA), and the rest is cleared via the NCX. The SERCA pump is regulated by phospholamban. Phospholamban must be phosphorylated to allow Ca2+ uptake into the SR via SERCA.
During ischemia, damage to cardiomyocytes can result in cellular instability due to a change in the regulation of Ca2+-induced Ca2+ release from the SR. Increases in cytosolic Ca2+ can activate phospholipases that cleave phospholipids from the cell membrane (72). Depending on the FA content of the membrane, n-3 PUFAs could be released to exert their effects on nearby channels, potentially regaining some electrical stability in the cell. PUFAs also directly inhibit the Ca2+ release channel of the SR, the ryanodine receptor (RyR) (73). This is important, especially in postischemic settings in which the SR is overloaded with Ca2+. In a model of elevated intracellular Ca2+, addition of EPA to cardiomyocytes reduced the frequency of spontaneous Ca2+ waves from the SR but slightly increased the total Ca2+ that was released with each wave (73,74). The net effect was a decrease in intracellular Ca2+ released from the SR over the same time period compared with controls. Inhibition of the RyR by EPA was apparent when the n-3 PUFAs were removed from solution and the frequency of spontaneous Ca2+ waves increased above control levels (74). This indicates that the SR contained an elevated amount of Ca2+ and that EPA was directly inhibiting its release via the RyR. The reason for the higher than normal stores of Ca2+ could be either an increased uptake of Ca2+ through the SERCA and/or a reduced leak of Ca2+ from the SR.
The molecular mechanism by which n-3 PUFAs and other PUFAs exert their antiarrhythmic effects within the membrane is not known. However, the mode of action is likely via one of two ways. First, the PUFA may be interacting directly with a site on the protein responsible for transporting ions, affecting its ability to function. Alternatively, the function of the protein embedded in the membrane is indirectly affected through the lipid bilayer. The relative contribution of the direct actions of PUFAs on the membrane protein versus the indirect effects achieved through a general membrane bilayer disordering effect is still unclear. However, several lines of evidence would suggest that the former option is more likely to produce the greatest effect:
The application of PUFAs to the internal or external side of the cell membrane can determine the degree of its effects (75). This would suggest that the FAs are acting directly on a specific site of the channel that is present only on one surface.
The administration of PUFAs can block specific radioligand binding to the sodium channel, suggesting a competition for a specific site of interaction (76).
A single point mutation induced in the sodium channel expressed in human embryonic kidney cells significantly diminished the effect of EPA on the inhibition of the sodium current (77). This is perhaps the best support for a pharmacological site of interaction for PUFAs.
PUFA-mediated effects are reversed after addition of delipidated bovine serum albumin to the membrane (55,62,73,76,78). The reversibility of the PUFA-mediated effects suggests the FAs are acting directly on the protein because FAs that are incorporated into the membrane would not be expected to be so easily scavenged by the addition of delipidated bovine serum albumin (76).
Because different PUFAs have distinctly different effects on ion channels and exchangers, their simple incorporation into the membrane and the change of membrane fluidity is not the primary mechanism of action. If it were simply a question of membrane fluidity, AA, a highly ‘bent’ n-6 PUFA, should contribute toward membrane fluidity to a significant extent and result in the same effects as long-chain n-3 PUFAs. In many cases it does, but in several instances, AA does not produce the same effects (61,66). Possibly, AA is converted to other eicosanoid metabolites, whereas DHA, for example, is not always converted to other products and has more potential to exert its effects as a free PUFA. Use of the nonmetabolizable AA analogue eicosatetraynoic acid may help determine the direct effects of AA (66).
The concentrations of PUFAs that significantly alter ionic currents are normally too low to create a change in the overall fluidity of the membrane by altering the packing of the membrane phospholipids (78).
Despite the lines of evidence identified above, there is still reason to believe that the actions of PUFAs are achieved through an indirect membrane disordering effect. For example, although the experimental PUFA concentrations are generally considered too low to induce membrane fluidity changes throughout the whole membrane (78), they could potentially alter the composition of the microdomains immediately surrounding the ion channels. Changes in the packing of phospholipids in the regions immediately surrounding a protein could have direct bearing on its conformation and, consequently, function (79–81). Studies in which membrane fluidity was altered produced results that are strikingly similar to the effects of PUFAs on ion channels (82). In addition, in the same study where cardiac sodium currents were inhibited by n-3 PUFAs, addition of a membrane fluidizing agent (benzyl alcohol) produced nearly identical inhibition in peak Na+ current and increases in channel activation thresholds (82). Similarly, L-type Ca2+ channel inhibition by DHA was matched by this same agent (64). A great deal of work has investigated the effects of membrane stiffness on channel function (80). There does appear to be a direct relation. Generally, the degree of membrane fluidity is associated with the degree of unsaturation in the FA.
CONCLUSIONS
There is substantial evidence that PUFAs induce significant beneficial cardiovascular effects. One of the interesting aspects concerning this beneficial action is that it is not achieved through one mechanism of action, but appears to be achieved via different effects on the heart, vasculature and blood. This makes PUFAs even more important as a therapeutic modality. Despite our knowledge, much remains to be investigated about the health-related benefits of PUFAs. Perhaps our greatest challenge continues to be discovering a way to introduce these compounds into the human diet in safe, therapeutic doses.
Footnotes
FUNDING: BP Ander is a trainee of the Heart and Stroke Foundation of Canada. CMC Dupasquier holds a National Sciences and Engineering Research Council of Canada Postgraduate Scholarship. Dr GN Pierce ia a Canadian Institutes of Health Research Senior Investigator.
REFERENCES
- 1.Salem N., Jr Introduction to polyunsaturated fatty acids. Backgrounder. 1999;3:1–8. [Google Scholar]
- 2.Holub BJ. Clinical nutrition: 4. Omega-3 fatty acids in cardiovascular care. CMAJ. 2002;166:608–15. [PMC free article] [PubMed] [Google Scholar]
- 3.Lanzmann-Petithory D. Alpha-linolenic acid and cardiovascular diseases. J Nutr Health Aging. 2001;5:179–83. [PubMed] [Google Scholar]
- 4.Dolecek TA, Granditis G. Dietary polyunsaturated fatty acids and mortality in the Multiple Risk Factor Intervention Trial (MRFIT) World Rev Nutr Diet. 1991;66:205–16. doi: 10.1159/000419291. [DOI] [PubMed] [Google Scholar]
- 5.Singh RB, Niaz MA, Sharma JP, Kumar R, Rastogi V, Moshiri M. Randomized, double-blind, placebo-controlled trial of fish oil and mustard oil in patients with suspected acute myocardial infarction: The Indian experiment of infarct survival – 4. Cardiovasc Drugs Ther. 1997;11:485–91. doi: 10.1023/a:1007757724505. [DOI] [PubMed] [Google Scholar]
- 6.Marchioli R, Barzi F, Bomba E, et al. Early protection against sudden death by n-3 polyunsaturated fatty acids after myocardial infarction: Time-course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico (GISSI)-Prevenzione. Circulation. 2002;105:1897–903. doi: 10.1161/01.cir.0000014682.14181.f2. [DOI] [PubMed] [Google Scholar]
- 7.Nilsen DW, Albrektsen G, Landmark K, Moen S, Aarsland T, Woie L. Effects of a high-dose concentrate of n-3 fatty acids or corn oil introduced early after an acute myocardial infarction on serum triacylglycerol and HDL cholesterol. Am J Clin Nutr. 2001;74:50–6. doi: 10.1093/ajcn/74.1.50. [DOI] [PubMed] [Google Scholar]
- 8.Harris WS, Park Y, Isley WL. Cardiovascular disease and long-chain omega-3 fatty acids. Curr Opin Lipidol. 2003;14:9–14. doi: 10.1097/00041433-200302000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Albert CM, Hennekens CH, O’Donnell CJ, et al. Fish consumption and risk of sudden cardiac death. JAMA. 1998;279:23–8. doi: 10.1001/jama.279.1.23. [DOI] [PubMed] [Google Scholar]
- 10.Nestel P. Effects of fish oils and fish on cardiovascular disease. Curr Atheroscler Rep. 2001;3:68–73. doi: 10.1007/s11883-001-0013-z. [DOI] [PubMed] [Google Scholar]
- 11.Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA. 2002;287:1815–21. doi: 10.1001/jama.287.14.1815. [DOI] [PubMed] [Google Scholar]
- 12.Yuan JM, Ross RK, Gao YT, Yu MC. Fish and shellfish consumption in relation to death from myocardial infarction among men in Shanghai, China. Am J Epidemiol. 2001;154:809–16. doi: 10.1093/aje/154.9.809. [DOI] [PubMed] [Google Scholar]
- 13.de Lorgeril M, Salen P. Mediterranean type of diet for the prevention of coronary heart disease. A global perspective from the seven countries study to the most recent dietary trials. Int J Vitam Nutr Res. 2001;71:166–72. doi: 10.1024/0300-9831.71.3.166. [DOI] [PubMed] [Google Scholar]
- 14.Keys A, Aravanis C, Blackburn HW, et al. Epidemiological studies related to coronary heart disease: Characteristics of men aged 40–59 in seven countries. Acta Med Scand Suppl. 1966;460:1–392. [PubMed] [Google Scholar]
- 15.Oomen CM, Feskens EJ, Rasanen L, et al. Fish consumption and coronary heart disease mortality in Finland, Italy, and The Netherlands. Am J Epidemiol. 2000;151:999–1006. doi: 10.1093/oxfordjournals.aje.a010144. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt EB, Christensen JH, Aardestrup I, et al. Marine n-3 fatty acids: Basic features and background. Lipids. 2001;36(Suppl):S65–8. doi: 10.1007/s11745-001-0684-x. [DOI] [PubMed] [Google Scholar]
- 17.Kris-Etherton PM, Harris WS, Appel LJ. Omega-3 fatty acids and cardiovascular disease: New recommendations from the American Heart Association. Arterioscler Thromb Vasc Biol. 2003;23:151–2. doi: 10.1161/01.atv.0000057393.97337.ae. [DOI] [PubMed] [Google Scholar]
- 18.Nordoy A, Marchioli R, Arnesen H, Videbaek J. n-3 polyunsaturated fatty acids and cardiovascular diseases. Lipids. 2001;36(Suppl):S127–9. doi: 10.1007/s11745-001-0695-7. [DOI] [PubMed] [Google Scholar]
- 19.von Schacky C. The role of omega-3 fatty acids in cardiovascular disease. Curr Atheroscler Rep. 2003;5:139–45. doi: 10.1007/s11883-003-0086-y. [DOI] [PubMed] [Google Scholar]
- 20.Thies F, Garry JM, Yaqoob P, et al. Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: A randomised controlled trial. Lancet. 2003;361:477–85. doi: 10.1016/S0140-6736(03)12468-3. [DOI] [PubMed] [Google Scholar]
- 21.Renier G, Skamene E, DeSanctis J, Radzioch D. Dietary n-3 polyunsaturated fatty acids prevent the development of atherosclerotic lesions in mice. Modulation of macrophage secretory activities. Arterioscler Thromb. 1993;13:1515–24. doi: 10.1161/01.atv.13.10.1515. [DOI] [PubMed] [Google Scholar]
- 22.von Schacky C, Angerer P, Kothny W, Theisen K, Mudra H. The effect of dietary omega-3 fatty acids on coronary atherosclerosis. A randomized, double-blind, placebo-controlled trial. Ann Intern Med. 1999;130:554–62. doi: 10.7326/0003-4819-130-7-199904060-00003. [DOI] [PubMed] [Google Scholar]
- 23.Harris WS. n-3 fatty acids and serum lipoproteins: Human studies. Am J Clin Nutr. 1997;65(5 Suppl):1645S–54S. doi: 10.1093/ajcn/65.5.1645S. [DOI] [PubMed] [Google Scholar]
- 24.Dewailly E, Blanchet C, Lemieux S, et al. n-3 Fatty acids and cardiovascular disease risk factors among the Inuit of Nunavik. Am J Clin Nutr. 2001;74:464–73. doi: 10.1093/ajcn/74.4.464. [DOI] [PubMed] [Google Scholar]
- 25.Adler AJ, Holub BJ. Effect of garlic and fish-oil supplementation on serum lipid and lipoprotein concentrations in hypercholesterolemic men. Am J Clin Nutr. 1997;65:445–50. doi: 10.1093/ajcn/65.2.445. [DOI] [PubMed] [Google Scholar]
- 26.Chan DC, Watts GF, Mori TA, Barrett PH, Redgrave TG, Beilin LJ. Randomized controlled trial of the effect of n-3 fatty acid supplementation on the metabolism of apolipoprotein B-100 and chylomicron remnants in men with visceral obesity. Am J Clin Nutr. 2003;77:300–7. doi: 10.1093/ajcn/77.2.300. [DOI] [PubMed] [Google Scholar]
- 27.Angerer P, von Schacky C. n-3 polyunsaturated fatty acids and the cardiovascular system. Curr Opin Clin Nutr Metab Care. 2000;3:439–45. doi: 10.1097/00075197-200011000-00005. [DOI] [PubMed] [Google Scholar]
- 28.von Schacky C. n-3 fatty acids and the prevention of coronary atherosclerosis. Am J Clin Nutr. 2000;71(1 Suppl):224S–7S. doi: 10.1093/ajcn/71.1.224s. [DOI] [PubMed] [Google Scholar]
- 29.Hirafuji M, Machida T, Hamaue N, Minami M. Cardiovascular protective effects of n-3 polyunsaturated fatty acids with special emphasis on docosahexaenoic acid. J Pharmacol Sci. 2003;92:308–16. doi: 10.1254/jphs.92.308. [DOI] [PubMed] [Google Scholar]
- 30.Pischon T, Hankinson SE, Hotamisligil GS, Rifai N, Willett WC, Rimm EB. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108:155–60. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 31.Abeywardena MY, Head RJ. Longchain n-3 polyunsaturated fatty acids and blood vessel function. Cardiovasc Res. 2001;52:361–71. doi: 10.1016/s0008-6363(01)00406-0. [DOI] [PubMed] [Google Scholar]
- 32.Karanian JW, Kim HY, Salem N., Jr The structure-activity relationship of lipoxygenase products of long-chain polyunsaturated fatty acids: Effects on human platelet aggregation. Lipids. 1996;31(Suppl):S305–8. doi: 10.1007/BF02637097. [DOI] [PubMed] [Google Scholar]
- 33.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71(1 Suppl):343S–8S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 34.Celermajer DS. Endothelial dysfunction: Does it matter? Is it reversible? J Am Coll Cardiol. 1997;30:325–33. doi: 10.1016/s0735-1097(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 35.De Caterina R, Massaro M. Effects of diet and of dietary components on endothelial leukocyte adhesion molecules. Curr Atheroscler Rep. 1999;1:188–95. doi: 10.1007/s11883-999-0031-9. [DOI] [PubMed] [Google Scholar]
- 36.De Caterina R, Liao JK, Libby P. Fatty acid modulation of endothelial activation. Am J Clin Nutr. 2000;71(1 Suppl):213S–23S. doi: 10.1093/ajcn/71.1.213S. [DOI] [PubMed] [Google Scholar]
- 37.Baumann KH, Hessel F, Larass I, et al. Dietary omega-3, omega-6, and omega-9 unsaturated fatty acids and growth factor and cytokine gene expression in unstimulated and stimulated monocytes. A randomized volunteer study. Arterioscler Thromb Vasc Biol. 1999;19:59–66. doi: 10.1161/01.atv.19.1.59. [DOI] [PubMed] [Google Scholar]
- 38.De Caterina R, Bernini W, Carluccio MA, Liao JK, Libby P. Structural requirements for inhibition of cytokine-induced endothelial activation by unsaturated fatty acids. J Lipid Res. 1998;39:1062–70. [PubMed] [Google Scholar]
- 39.Pakala R, Sheng WL, Benedict CR. Eicosapentaenoic acid and docosahexaenoic acid block serotonin-induced smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol. 1999;19:2316–22. doi: 10.1161/01.atv.19.10.2316. [DOI] [PubMed] [Google Scholar]
- 40.Bhatnagar D, Durrington PN. Omega-3 fatty acids: Their role in the prevention and treatment of atherosclerosis related risk factors and complications. Int J Clin Pract. 2003;57:305–14. [PubMed] [Google Scholar]
- 41.Omura M, Kobayashi S, Mizukami Y, et al. Eicosapentaenoic acid (EPA) induces Ca(2+)-independent activation and translocation of endothelial nitric oxide synthase and endothelium-dependent vasorelaxation. FEBS Lett. 2001;487:361–6. doi: 10.1016/s0014-5793(00)02351-6. [DOI] [PubMed] [Google Scholar]
- 42.Hirafuji M, Machida T, Tsunoda M, Miyamoto A, Minami M. Docosahexaenoic acid potentiates interleukin-1beta induction of nitric oxide synthase through mechanism involving p44/42 MAPK activation in rat vascular smooth muscle cells. Br J Pharmacol. 2002;136:613–9. doi: 10.1038/sj.bjp.0704768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vas Dias FW, Gibney MJ, Taylor TG. The effect of polyunsaturated fatty acids on the n-3 and n-6 series on platelet aggregation and platelet and aortic fatty acid composition in rabbits. Atherosclerosis. 1982;43:245–57. doi: 10.1016/0021-9150(82)90026-0. [DOI] [PubMed] [Google Scholar]
- 44.Hansen JB, Lyngmo V, Svensson B, Nordoy A. Inhibition of exercise-induced shortening of bleeding time by fish oil in familial hypercholesterolemia (type IIa) Arterioscler Thromb. 1993;13:98–104. doi: 10.1161/01.atv.13.1.98. [DOI] [PubMed] [Google Scholar]
- 45.Sanders TA, Roshanai F. The influence of different types of omega 3 polyunsaturated fatty acids on blood lipids and platelet function in healthy volunteers. Clin Sci (Lond) 1983;64:91–9. doi: 10.1042/cs0640091. [DOI] [PubMed] [Google Scholar]
- 46.Kelley DS, Nelson GJ, Love JE, et al. Dietary alpha-linolenic acid alters tissue fatty acid composition, but not blood lipids, lipoproteins or coagulation status in humans. Lipids. 1993;28:533–7. doi: 10.1007/BF02536085. [DOI] [PubMed] [Google Scholar]
- 47.Gruver DI.Does flaxseed interfere with the clotting system? Plast Reconstr Surg 2003112934(Lett) [DOI] [PubMed] [Google Scholar]
- 48.Murnaghan MF. Effect of fatty acids on the ventricular arrhythmia threshold in the isolated heart of the rabbit. Br J Pharmacol. 1981;73:909–15. doi: 10.1111/j.1476-5381.1981.tb08745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hock CE, Beck LD, Bodine RC, Reibel DK. Influence of dietary n-3 fatty acids on myocardial ischemia and reperfusion. Am J Physiol. 1990;259:H1518–26. doi: 10.1152/ajpheart.1990.259.5.H1518. [DOI] [PubMed] [Google Scholar]
- 50.Isensee H, Jacob R. Differential effects of various oil diets on the risk of cardiac arrhythmias in rats. J Cardiovasc Risk. 1994;1:353–9. [PubMed] [Google Scholar]
- 51.Billman GE, Hallaq H, Leaf A. Prevention of ischemia-induced ventricular fibrillation by omega 3 fatty acids. Proc Natl Acad Sci USA. 1994;91:4427–30. doi: 10.1073/pnas.91.10.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Billman GE, Kang JX, Leaf A. Prevention of sudden cardiac death by dietary pure omega-3 polyunsaturated fatty acids in dogs. Circulation. 1999;99:2452–7. doi: 10.1161/01.cir.99.18.2452. [DOI] [PubMed] [Google Scholar]
- 53.McLennan PL, Bridle TM, Abeywardena MY, Charnock JS. Comparative efficacy of n-3 and n-6 polyunsaturated fatty acids in modulating ventricular fibrillation threshold in marmoset monkeys. Am J Clin Nutr. 1993;58:666–9. doi: 10.1093/ajcn/58.5.666. [DOI] [PubMed] [Google Scholar]
- 54.McLennan PL. Relative effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on cardiac arrhythmias in rats. Am J Clin Nutr. 1993;57:207–12. doi: 10.1093/ajcn/57.2.207. [DOI] [PubMed] [Google Scholar]
- 55.Kang JX, Xiao YF, Leaf A. Free, long-chain, polyunsaturated fatty acids reduce membrane electrical excitability in neonatal rat cardiac myocytes. Proc Natl Acad Sci USA. 1995;92:3997–4001. doi: 10.1073/pnas.92.9.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xiao YF, Kang JX, Morgan JP, Leaf A. Blocking effects of polyunsaturated fatty acids on Na+ channels of neonatal rat ventricular myocytes. Proc Natl Acad Sci USA. 1995;92:11000–4. doi: 10.1073/pnas.92.24.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Macleod JC, Macknight AD, Rodrigo GC. The electrical and mechanical response of adult guinea pig and rat ventricular myocytes to omega3 polyunsaturated fatty acids. Eur J Pharmacol. 1998;356:261–70. doi: 10.1016/s0014-2999(98)00528-7. [DOI] [PubMed] [Google Scholar]
- 58.Preliminary report: Effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST) Investigators. N Engl J Med. 1989;321:406–12. doi: 10.1056/NEJM198908103210629. [DOI] [PubMed] [Google Scholar]
- 59.Xiao YF, Gomez AM, Morgan JP, Lederer WJ, Leaf A. Suppression of voltage-gated L-type Ca2+ currents by polyunsaturated fatty acids in adult and neonatal rat ventricular myocytes. Proc Natl Acad Sci USA. 1997;94:4182–7. doi: 10.1073/pnas.94.8.4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrier GR, Redondo I, Zhu J, Murphy MG. Differential effects of docosahexaenoic acid on contractions and L-type Ca2+ current in adult cardiac myocytes. Cardiovasc Res. 2002;54:601–10. doi: 10.1016/s0008-6363(02)00275-4. [DOI] [PubMed] [Google Scholar]
- 61.Hallaq H, Smith TW, Leaf A. Modulation of dihydropyridine-sensitive calcium channels in heart cells by fish oil fatty acids. Proc Natl Acad Sci USA. 1992;89:1760–4. doi: 10.1073/pnas.89.5.1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pepe S, Bogdanov K, Hallaq H, Spurgeon H, Leaf A, Lakatta E. Omega 3 polyunsaturated fatty acid modulates dihydropyridine effects on L-type Ca2+ channels, cytosolic Ca2+, and contraction in adult rat cardiac myocytes. Proc Natl Acad Sci USA. 1994;91:8832–6. doi: 10.1073/pnas.91.19.8832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Renaud JF, Kazazoglou T, Schmid A, Romey G, Lazdunski M. Differentiation of receptor sites for [3H]nitrendipine in chick hearts and physiological relation to the slow Ca2+ channel and to excitation-contraction coupling. Eur J Biochem. 1984;139:673–81. doi: 10.1111/j.1432-1033.1984.tb08056.x. [DOI] [PubMed] [Google Scholar]
- 64.Leifert WRJ, Jahangiri A, McMurchie EJ. Membrane fluidity changes are associated with the antiarrhythmic effects of docosahexaenoic acid in adult rat cardiomyocytes. J Nutr Biochem. 2000;11:38–44. doi: 10.1016/s0955-2863(99)00069-8. [DOI] [PubMed] [Google Scholar]
- 65.Xiao YF, Morgan JP, Leaf A. Effects of polyunsaturated fatty acids on cardiac voltage-activated K+ currents in adult ferret cardiomyocytes. Sheng Li Xue Bao. 2002;54:271–81. [PubMed] [Google Scholar]
- 66.Bogdanov KY, Spurgeon HA, Vinogradova TM, Lakatta EG. Modulation of the transient outward current in adult rat ventricular myocytes by polyunsaturated fatty acids. Am J Physiol. 1998;274:H571–9. doi: 10.1152/ajpheart.1998.274.2.H571. [DOI] [PubMed] [Google Scholar]
- 67.Kim D, Duff RA. Regulation of K+ channels in cardiac myocytes by free fatty acids. Circ Res. 1990;67:1040–6. doi: 10.1161/01.res.67.4.1040. [DOI] [PubMed] [Google Scholar]
- 68.Kim D, Clapham DE. Potassium channels in cardiac cells activated by arachidonic acid and phospholipids. Science. 1989;244:1174–6. doi: 10.1126/science.2727703. [DOI] [PubMed] [Google Scholar]
- 69.Goel DP, Maddaford TG, Pierce GN. Effects of omega-3 polyunsaturated fatty acids on cardiac sarcolemmal (Na+)/(H+) exchange. Am J Physiol Heart Circ Physiol. 2002;283:H1688–94. doi: 10.1152/ajpheart.00664.2001. [DOI] [PubMed] [Google Scholar]
- 70.Philipson KD, Ward R. Effects of fatty acids on Na+-Ca2+ exchange and Ca2+ permeability of cardiac sarcolemmal vesicles. J Biol Chem. 1985;260:9666–71. [PubMed] [Google Scholar]
- 71.Miller HM, Woodhouse SP. Long chain fatty acid inhibition of sodium plus potassium-activated adenosine triphosphatase from rat heart. Aust J Exp Biol Med Sci. 1977;55:741–52. doi: 10.1038/icb.1977.69. [DOI] [PubMed] [Google Scholar]
- 72.Van der Vusse GJ, Reneman RS, van Bilsen M. Accumulation of arachidonic acid in ischemic/reperfused cardiac tissue: Possible causes and consequences. Prostaglandins Leukot Essent Fatty Acids. 1997;57:85–93. doi: 10.1016/s0952-3278(97)90497-x. [DOI] [PubMed] [Google Scholar]
- 73.Negretti N, Perez MR, Walker D, O’Neill SC. Inhibition of sarcoplasmic reticulum function by polyunsaturated fatty acids in intact, isolated myocytes from rat ventricular muscle. J Physiol. 2000;523:367–75. doi: 10.1111/j.1469-7793.2000.t01-1-00367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Neill SC, Perez MR, Hammond KE, Sheader EA, Negretti N. Direct and indirect modulation of rat cardiac sarcoplasmic reticulum function by n-3 polyunsaturated fatty acids. J Physiol. 2002;538:179–84. doi: 10.1113/j.1469-7793.2001.00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Honore E, Barhanin J, Attali B, Lesage F, Lazdunski M. External blockade of the major cardiac delayed-rectifier K+ channel (Kv1.5) by polyunsaturated fatty acids. Proc Natl Acad Sci USA. 1994;91:1937–41. doi: 10.1073/pnas.91.5.1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kang JX, Leaf A. Evidence that free polyunsaturated fatty acids modify Na+ channels by directly binding to the channel proteins. Proc Natl Acad Sci USA. 1996;93:3542–6. doi: 10.1073/pnas.93.8.3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiao YF, Ke Q, Wang SY, et al. Single point mutations affect fatty acid block of human myocardial sodium channel alpha subunit Na+ channels. Proc Natl Acad Sci USA. 2001;98:3606–11. doi: 10.1073/pnas.061003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pound EM, Kang JX, Leaf A. Partitioning of polyunsaturated fatty acids, which prevent cardiac arrhythmias, into phospholipid cell membranes. J Lipid Res. 2001;42:346–51. [PubMed] [Google Scholar]
- 79.Andersen OS, Nielsen C, Maer AM, Lundbaek JA, Goulian M, Koeppe RE., II Ion channels as tools to monitor lipid bilayer-membrane protein interactions: Gramicidin channels as molecular force transducers. Methods Enzymol. 1999;294:208–24. doi: 10.1016/s0076-6879(99)94013-2. [DOI] [PubMed] [Google Scholar]
- 80.Lundbaek JA, Birn P, Girshman J, Hansen AJ, Andersen OS. Membrane stiffness and channel function. Biochemistry. 1996;35:3825–30. doi: 10.1021/bi952250b. [DOI] [PubMed] [Google Scholar]
- 81.Leaf A, Xiao Y, Kang J. Interactions of n-3 fatty acids with ion channels in excitable tissues. Prostaglandins Leukot Essent Fatty Acids. 2002;67:113–20. doi: 10.1054/plef.2002.0407. [DOI] [PubMed] [Google Scholar]
- 82.Leifert WR, McMurchie EJ, Saint DA. Inhibition of cardiac sodium currents in adult rat myocytes by n-3 polyunsaturated fatty acids. J Physiol. 1999;520:671–9. doi: 10.1111/j.1469-7793.1999.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xiao YF, Wright SN, Wang GK, Morgan JP, Leaf A. Coexpression with beta(1)-subunit modifies the kinetics and fatty acid block of hH1(alpha) Na(+) channels. Am J Physiol Heart Circ Physiol. 2000;279:H35–46. doi: 10.1152/ajpheart.2000.279.1.H35. [DOI] [PubMed] [Google Scholar]
- 84.Huang JM, Xian H, Bacaner M. Long-chain fatty acids activate calcium channels in ventricular myocytes. Proc Natl Acad Sci USA. 1992;89:6452–6. doi: 10.1073/pnas.89.14.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lesage F, Lazdunski M. Molecular and functional properties of two-pore-domain potassium channels. Am J Physiol Renal Physiol. 2000;279:F793–801. doi: 10.1152/ajprenal.2000.279.5.F793. [DOI] [PubMed] [Google Scholar]
- 86.Taffet GE, Pham TT, Bick DL, Entman ML, Pownall HJ, Bick RJ. The calcium uptake of the rat heart sarcoplasmic reticulum is altered by dietary lipid. J Membr Biol. 1993;131:35–42. doi: 10.1007/BF02258532. [DOI] [PubMed] [Google Scholar]


