Abstract
Background
The current study examines the relationship between maternal depression and infant cortisol concentrations. The potential roles of comorbid maternal anxiety disorders, timing of maternal depression, and maternal treatment with psychotropic medications during pregnancy are addressed.
Methods
Women with 6-month-old infants (105 boys and 84 girls) participated in a laboratory paradigm that included infant saliva collection at six points, noise burst and arm restraint stressor tasks, and a diagnostic interview of the mother.
Results
Lifetime history of maternal depression was associated with increased baseline and mean (average) infant cortisol levels. Comorbidity with anxiety disorder was related to infant cortisol reactivity. Peripartum (prepartum and/or postpartum) maternal depression, rather than a pre-pregnancy history of disorder, was associated with higher infant cortisol reactivity. Prenatal and postnatal exposure to maternal disorder had similar effects, but prenatal maternal psychotropic medication treatment appeared to attenuate infant cortisol increases associated with prenatal maternal disorder exposure.
Conclusions
These data suggest that exposure to maternal depression and anxiety during pregnancy and the postpartum period may increase infant salivary cortisol. This maternal depression–infant cortisol association is independent of the effects of delivery complications, and appears to be modulated by prenatal maternal psychotropic treatment.
Keywords: Anxiety, cortisol, depression, infant, perinatal, prenatal, psychotropic, stress
A recent meta-analysis found that between 8.5% and 11.0% of women meet criteria for depression during pregnancy, while estimates of maternal depression during the postpartum period range from 6.5% to over 12% (Gaynes et al., 2005). Recent data also indicate that a subgroup of women with depression during the postpartum period had symptom onset during pregnancy, resulting in both prenatal and postnatal exposure in their children (Stowe, Hostetter, & Newport, 2005). Depression in mothers translates into a variety of risks for their children. Postnatal depression, for example, has been associated with disrupted mother–infant interactions and infant attachment (Murray, Fiori-Cowley, Hooper,&Cooper, 1996), as well as child behavior problems at age 5 (Murray, Sinclair, Cooper, Ducournau, & Turner, 1999) and anxiety disorders at age 13 (Halligan, Murray, Martins, & Cooper, 2006).
As Goodman and Gotlib (1999) point out, ‘relative to our knowledge of the range of adverse outcomes for children of depressed mothers, we know little about the mechanisms that underlie the risk for these outcomes’ (p. 458). Empirically supported knowledge of these mechanisms is a crucial first step in reducing the disease burden of depression. In part to spur this type of research, Goodman and Gotlib (1999) present a developmental model for risk associated with maternal depression. One of the potential mediators posited in this model is neuroregulatory dysfunction, including alterations in the hypothalamic–pituitary–adrenal (HPA) axis in children of depressed mothers.
Prenatal and postnatal maternal depression have been associated with higher cortisol levels in newborns, children and adolescents (Ashman, Dawson, Panagiotides, Yamada, & Wilkinson, 2002; Diego et al., 2004; Essex, Klein, Cho, & Kalin, 2002; Halligan, Herbert, Goodyer, & Murray, 2004), and HPA axis alterations in turn have been associated with child internalizing problems (Ashman et al., 2002). Elevated levels of cortisol in pregnant women with depression have been found to significantly predict newborns’ level of cortisol (Lundy et al., 1999), implicating maternal prenatal neuroendocrine functioning or genetic factors as possible mechanisms of transmission of psychopathology from mother to child. In order to better understand the mechanism underlying the association between maternal depression and offspring HPA axis functioning, it is important to assess the potential influence of the timing of maternal disorder on these outcomes.
Type of cortisol measure (baseline versus reactivity) might be an important consideration in this area of research. Most studies of maternal depression and offspring cortisol have examined baseline or diurnal measures (e.g., Diego et al., 2004; Essex et al., 2002; Halligan et al., 2004), with at least one of the time points of data collection showing a relationship to maternal depression. In contrast, very few studies have examined cortisol reactivity and its association to maternal depression, and the results of these studies have been mixed (Ashman et al., 2002; Young, Vazquez, Jiang, & Pfeffer, 2006).
There is growing evidence that prenatal maternal anxiety is related to offspring HPA axis function. Self-reports of maternal anxiety during pregnancy have been found to predict children’s cortisol response to novelty (Gutteling, de Weerth, & Buitelaar, 2005), as well as awakening cortisol (O’Connor et al., 2005). Maternal panic disorder has also been associated with elevated salivary cortisol and disturbed sleep patterns in infants (Warren et al., 2003). Although only one of these studies controlled for maternal depressive symptoms, it is notable that this statistical control did not eliminate the association between maternal anxiety and child cortisol (O’Connor et al., 2005). To date, no studies have been published on the relationship between infant HPA axis function and maternal depression controlling for comorbid anxiety disorders. This is problematic because of the notably high rates of comorbidity between depression and anxiety disorders (Brown, Campbell, Lehman, Grisham, & Mancill, 2001). To date it remains unclear whether the apparent relationship between maternal depression and offspring HPA axis function is driven, or perhaps modulated, by comorbid anxiety.
Similarly, the impact of prenatal exposure to psychotropic medication and infant HPA axis activity remains unknown. The use of antidepressant medications during pregnancy has undergone considerable scrutiny with respect to teratogenic and adverse effects. Prior investigations have suggested that any current antidepressant taken during pregnancy crosses the placenta (Hendrick et al., 2003; Loughhead et al., 2006) and that the fetus is exposed to significant quantities of the medications. Detailed neurodevelopmental follow-up studies of fluoxetine and tricyclic antidepressant use during pregnancy have typically failed to demonstrate any adverse sequelae of infant exposure to medication (Nulman et al., 1997). Recent studies, however, have suggested that some infants exposed prenatally to medication demonstrate neuromotor symptoms following delivery (Sanz, De-las-Cuevas, Kiuru, Bate, & Edwards, 2005). It is unknown if such symptoms reflect toxicity, withdrawal, or some form of neonatal antidepressant syndrome (Webster, 1973). In the light of the widespread use of medication for depression and anxiety, as well as the aforementioned findings suggesting lasting symptoms in infants prenatally exposed to such medications, it is imperative that prenatal exposure to psychotropic medications be considered in the assessment of infant HPA axis activity.
In the current study we sought to delineate the impact of maternal depression on infant HPA axis activity while examining a variety of potential confounds to better isolate the independent effects (if any) of maternal depression on infant HPA axis functioning. Our first hypothesis is that infants of mothers with a history of depression will exhibit higher cortisol levels than infants of mothers without a history of depression. Our second hypothesis is that infant cortisol concentrations will be especially associated with maternal depression occurring during the perinatal period (i.e., prenatal or postnatal rather than pre-pregnancy), due to the potential for direct biochemical and environmental influences of the mother’s symptoms on the fetus and infant. Furthermore, we hypothesize that increased cortisol levels in infants of depressed mothers will be apparent even when comorbid anxiety disorders are controlled. Finally, we will explore whether prenatal exposure to maternal disorder and/or psychotropic medications have differential impacts on infant HPA axis functioning. In all analyses, we will examine baseline, mean and reactivity measures of cortisol, and control for demographic, familial, and medical risks as necessary.
Methods
Participants
The sample was derived from two sources: 1) the Emory Women’s Mental Health Program (WMHP), a tertiary referral center for the treatment of mental illness during pregnancy and the postpartum period; and 2) a research subject pool maintained by the Emory University Psychology Department and contacted by community mailing (intended primarily to provide controls). Women recruited from these two sources did not significantly differ in terms of age (t(1,187) = 1.71, p = .09), ethnicity (χ2 (N = 189) = 1.17, p = .28), education level (t(1,187) = .79, p = .43), or employment status (χ2 (N = 189) = .40, p = .53). Similarly their children did not significantly differ in terms of age (t(1,187) = 1.43, p = .16) or gender (χ2 (N = 189) = .30, p = .59).
Lifetime diagnostic information obtained from structured clinical interviews (see below) was used to assign participants to (a) the maternal depression group consisting of women with lifetime Axis I diagnoses of major depression, dysthymia or both (n = 163), or (b) the non-disordered control group consisting of women with no lifetime history of Axis I mood or anxiety disorders (n = 26). Women were excluded from the current study if they qualified for a lifetime diagnosis of schizophrenia (n = 3), a lifetime diagnosis of bipolar disorder (n = 17), or a primary lifetime diagnosis of an anxiety disorder, but no lifetime diagnosis of depression/dysthymia (n = 8).
The infant sample contained 105 boys and 84 girls, with a mean age of 180 ± 17 days. The majority (90%) of the infants were Caucasian, the median mother education level was college graduate, and 92% of the mothers were married or cohabitating at the time of the study. Families were given $20 and a t-shirt for participating in the study.
The Emory University Institutional Review Board approved this study, and all mothers provided written informed consent.
Procedure
All study procedures and sample collection started between 1300 and 1330 hours and were collected within the same day, with completion by 1600 hours. Mothers and their infants were met by a research assistant, escorted to the testing center, and seated together in a quiet room. Following procedural explanation and consent, an initial sample of infant saliva (T0 – baseline) was obtained by swabbing the inside of the infant’s mouth with dental cotton rolls. The saliva was transferred from the cotton roll to a 15cc polypropylene tube via syringe. This procedure for saliva collection was consistent throughout the study.
Next, the mother completed a series of questionnaires, while adjacent to her, the infant was held by the research assistant. After a 20-minute period, the second saliva sample (T1 – post- separation stressor) was obtained. The infant was then placed in a car seat behind an occlusion screen that blocked the mother from view. After being presented with pictures on a TV monitor for several minutes, the TV screen went blank and during a 3-minute trial, a 90 dB sound burst was repeated 3 times. Following the sound burst, an arm restraint stressor task was conducted. In this task, the infant’s arms were held down at his or her sides for a period of up to 2 minutes. During this time, the research assistant was instructed to display an ‘emotionally neutral’ facial expression. If the infant continuously cried for 20 seconds, the arm restraint was stopped. Following restraint, the infant was given a 60-second period to attempt to self-regulate, during which the research assistant continued to display an emotionally neutral face. Saliva samples were taken immediately after the arm restraint stressor task was completed (T2 – post-noise / arm stressor I), and again 20 minutes later (T3 – post-noise / arm stressor II). Our three post-stressor cortisol measures (T1, T2, T3) were taken in post-stressor time windows when cortisol levels typically increase (5 to 40 minutes) (Goldberg et al., 2003; Ramsay & Lewis, 2003).
Following the infant assessment, the mothers completed a clinical interview administered by a Masters-level graduate research assistant. During this time the mother was reunited with the infant and could hold or interact with him / her. Two additional infant saliva samples were obtained during the maternal interview portion of the study (T4 – post clinical interview and T5 – study exit; approximately 80 and 100 minutes after the stressor tasks, respectively).
Research assistants recorded infant food (breast milk, formula, etc.) intake throughout the protocol and this measure was assessed as a potential confound in the analyses.
Measures
Current maternal depressive symptoms
Mothers completed the Beck Depression Inventory-II (BDI), a self-report measure of current depressive symptoms, which has been shown to have excellent reliability and validity (Beck, Steer, & Brown, 1996).
Maternal anxiety and depression diagnoses
Mothers were assessed for lifetime anxiety and depressive disorders using the Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 1995). A reliability analysis based on 10% of our sample, rated by an independent judge, yielded a weighted Kappa of .83 for diagnoses of major depressive episode, .75 for diagnoses of dysthymia, .83 for diagnoses of panic disorder, .86 for diagnoses of generalized anxiety disorder, 1.0 for diagnoses of obsessive compulsive disorder, 1.0 for diagnosis of social phobia, and 1.0 for diagnoses of post-traumatic stress disorder. In this study mothers were considered to have comorbid anxiety disorders if they qualified for both a depressive disorder and an anxiety disorder during their lifetime.
Mothers who qualified for a lifetime diagnosis of anxiety or depressive disorder were further queried about the timing of this disorder relative to the developmental age of their infant (i.e., pre-pregnancy, pre-partum, postpartum). In this study, the use of the term prepartum is equivalent to prenatal, and the use of the term postpartum is equivalent to postnatal. We used the terms postpartum and postnatal to reflect the presence or absence of a depressive or anxiety episode during the time between the birth of the child, and the day of the SCID interview. We did not employ the strict ICD or DSM criteria that limit the diagnoses of postpartum or postnatal depression on the basis of the timing of onset and / or the absence of symptoms during pregnancy.
Many of the mothers in this study were not seen prospectively throughout pregnancy and had no WMHP clinic records; therefore all analyses on exposure to maternal depression relied on maternal retrospective reports. It should be noted, however, that retrospective reports of the presence or absence of maternal depression during pregnancy were found to be significantly related to prospectively obtained BDI scores in a sub-sample of the women referred from the Emory WMHP (mean BDI t(101) = 3.73, p < .001, maximum BDI t(101) = 4.35, p < .001).
Obstetrical health history
Mothers completed an obstetrical history questionnaire detailing medical illnesses, medication exposure, exposure to toxins (e.g. nicotine, illicit drugs), method of delivery, and obstetrical complications associated with the infant’s birth. Women who took antidepressants, antipsychotics or anxiolytics of any type during pregnancy were coded as taking psychotropic medications. Use of these medications during pregnancy was confirmed through chart review in the subsample of women (n = 101) who attended the WMHP clinic. There were no discrepancies between mother report and chart record on the global report (yes or no) of psychotropic medication use during pregnancy.
Delivery complications were coded on a frequency scale and included items such as bleeding during delivery, abnormal fetal position, use of forceps, umbilical cord complications, and abnormal fetal heart rate at delivery.
Salivary cortisol concentrations
Six infant saliva samples were collected over the course of the study and frozen at −20°C, within 15 minutes of collection. All saliva was assayed for cortisol concentration using a commercially available radioimmunoassay kit (DiaSorin GammaCoat, Stillwater, Minnesota). Sensitivity of this kit for saliva cortisol is .05 µg/dL and inter and intraassay coefficients of variation are 6.0% and 3.5% respectively. All standards and samples were run in duplicate by a research assistant blind to both maternal diagnosis and the time point at which the sample was obtained.
Infant salivary cortisol measures were assessed along three dimensions: 1) baseline (T0 – study entry); 2) an overall measure of HPA axis activity consisting of the mean cortisol level for all samples obtained; and 3) HPA axis reactivity calculated as the area under the curve (AUC; linear trapezoid method) for T1, T2, and T3 cortisol samples, as measured from baseline (T0). Reactivity is positively correlated with mean cortisol (r = .25), and negatively correlated with baseline (r = −.47). This latter correlation confirmed the law of initial value (the commonly noted negative correlation between baseline and reactivity in physiological responses: Wilder, 1958); all analyses examining cortisol reactivity therefore included baseline cortisol as a covariate.
Results
A total of 171 infants (91% of the sample) were included in the final data analysis (95 males and 76 females; mean age of 180 ± 17 days). The remaining 18 infants were tested but excluded from the analyses secondary to the following reasons: 1) Saliva volume for three or more samples was insufficient for assay (n = 8); 2) The infant became too fussy to complete the protocol (n = 6); 3) Cortisol concentrations for three or more samples represented statistical outlier values (i.e., >3 SDs above the mean; n = 4). Infants excluded from the sample did not differ significantly from those included in terms of rates of either maternal depression (χ2(1,189) = 1.13, p = .29) or maternal anxiety (χ2(1,189) = 0.01, p = 1.0).
Preliminary analyses examined the relationship between potential confounds and each of the dependent variables of interest (see Table 1). Three of the potential confounds were positively related to one or more of the cortisol measures, and were therefore statistically controlled in analyses of those variables (i.e., number of siblings, r = .16, p = .04 with baseline; mother’s use of psychotropic drugs during pregnancy t = −3.08, p < .01 for baseline, t = −2.04, p = .04 for mean; and delivery complications, r = .18, p = .02 for baseline, r = .23, p < .01 for mean). As noted above, we also included the baseline cortisol measure (pre-stressor tasks) as a statistical control in our analyses of reactivity.
Table 1.
Relationship between potential confounds and infant cortisol measures
| Infant cortisol measure | |||
|---|---|---|---|
| Potential confound | Baseline (N = 171) | Mean (N = 171) | Reactivity (N = 155) |
| Gender | t= .50, p= .62 | t= 1.75, p= .08 | t= .86, p= .39 |
| Mother age | r= .11, p= .16 | r= .00, p= .95 | r= −.14, p= .09 |
| Mother marital status | t= .42, p= .68 | t= .25, p= .80 | t= −1.68, p= .10 |
| Mother education | r= −.05, p= .54 | r= −.01, p= .86 | r= −.02, p= .84 |
| Number siblings | r= .16, p= .04 | r= .07, p= .36 | r= −.07, p= .39 |
| Prenatal smoking | t= −.16, p= .87 | t= .41, p= .68 | t= 1.09, p= .27 |
| Prenatal psychotropics | t= −3.08, p < .01 | t= −2.04, p= .04 | t= −1.03, p= .31 |
| Days premature | r= .01, p= .88 | r= .03, p= .70 | r= .13, p= .12 |
| Birthweight | r= .05, p= .52 | r= .06, p= .41 | r= .01, p= .88 |
| Delivery complications | r= .18, p= .02 | r= .23, p < .01 | r= .10, p= .20 |
| Cesarean | t= .12, p= .90 | t= −.50, p= .96 | t= .54, p= .59 |
| Breast feeding | t= .29, p= .78 | t= −.44, p= .66 | t= −.76, p= .45 |
| Infant food intake | t= .07, p= .95 | t= −.18, p= .85 | t= −.56, p= .58 |
| Infant current medication | t= −.07, p= .94 | t= −.28, p= .78 | t= −.84, p= .40 |
Note: Significant findings highlighted in bold.
Cortisol values were log-transformed prior to analyses (NB: non-transformed values are presented in the figures for ease of interpretation). Regression analyses or analyses of covariance were completed to test each hypothesis as specified below, and an alpha of .05 was used throughout.
Maternal depression and infant cortisol
Table 2 presents the results from separate regression analyses predicting infant baseline, mean, and reactivity cortisol from maternal lifetime history of depression (with statistical controls as outlined above). Lifetime maternal depression significantly predicted both baseline (ΔF(1,165) = 4.64, p = .03) and mean infant cortisol (ΔF(1,167) = 7.45, p < .01), but not cortisol reactivity (ΔF(1,151) = 2.52, p = .12).
Table 2.
Maternal depression and infant cortisol concentrations
| Outcome | Beta | ΔF | ΔR2 | p |
|---|---|---|---|---|
| Lifetime maternal depression | ||||
| Baseline | .17 | 4.64 | .03 | .03 |
| Mean | .21 | 7.45 | .04 | .01 |
| Reactivity | .12 | 2.52 | .01 | .12 |
| Peripartum maternal depression controlling for lifetime maternal depression | ||||
| Baseline | −.05 | .18 | .00 | .68 |
| Mean | .05 | .14 | .00 | .71 |
| Reactivity | .25 | 4.91 | .02 | .03 |
Note: statistical controls entered in Block One of each regression analysis.
A second set of regression analyses examining the contribution of maternal peripartum depression, above and beyond the effect of maternal lifetime depression (see Table 2), revealed that maternal depression occurring in the peripartum phase predicted to infant cortisol reactivity (ΔF(1,150) = 4.91, p = .03).
We further broke down the peripartum exposure group to examine whether infant cortisol levels would differ according to pregnancy exposure only (n = 32), postpartum exposure only (n = 36), or both (n = 36). ANCOVAs revealed no significant differences in infant cortisol in these three groups (baseline: F(2, 103) = .14, p = .87, eta-squared = .00; mean: F(2, 104) = 1.07, p = .35, eta-squared = .02; reactivity: F(2, 94) = 2.57, p = .08, eta-squared = .06).
We also examined the contribution of current maternal depressive state to infant cortisol responses. Multiple regression analyses revealed that current maternal BDI scores were not significant predictors of infant cortisol measures (baseline: ΔF(1,159) = .36, p = .55, beta = −.05; mean: ΔF(1,161) = .01, p = .94, beta = −.01; reactivity: ΔF(1,146) = 3.77, p = .06, beta = .14). It should be noted that the addition of the variable representing exposure to maternal depression in the postpartum period (as assessed by the SCID) into the regression analysis substantially reduced the BDI-reactivity association (reactivity: ΔF(1,145) = .26, p = .61, beta = .04).
Maternal depression, maternal anxiety and infant cortisol
In order to examine the relationship between maternal depression, comorbid anxiety and infant cortisol, we completed a series of linear regression analyses where statistical controls were entered in Block 1, comorbid anxiety disorder was added in Block 2, and maternal depression was added in Block 3. Results from these analyses are reported in Table 3. As can be seen, depression comorbid with anxiety predicted infant cortisol reactivity (ΔF(1,151) = 5.25, p = .02)., but depression separate from comorbid anxiety did not (ΔF(1,150) = .57, p = .45). The opposite pattern was observed for infant baseline and mean cortisol levels with comorbid anxiety having no significant effect on these outcomes (baseline ΔF(1,165) = .03, p = .86; mean ΔF(1,167) = 2.27, p = .13), and maternal depression predicting each of them even when comorbid anxiety was controlled (baseline ΔF(1,164) = 5.51, p = .03; mean ΔF(1,166) = 5.46, p = .02).
Table 3.
Maternal depression and anxiety and infant cortisol concentrations
| Outcome | Beta | ΔF | ΔR2 | p |
|---|---|---|---|---|
| Comorbid maternal anxiety disorder | ||||
| Baseline | −.01 | .03 | .00 | .86 |
| Mean | .05 | 2.27 | .01 | .13 |
| Reactivity | .16 | 5.25 | .03 | .02 |
| Lifetime maternal depression controlling for comorbid maternal anxiety disorder | ||||
| Baseline | .20 | 5.51 | .03 | .02 |
| Mean | .20 | 5.46 | .03 | .02 |
| Reactivity | .06 | .57 | .00 | .45 |
Note: statistical controls entered in Block One of each regression analysis.
Disorder versus medication exposure in pregnancy
Finally, we examined whether exposure to psychotropic medication during pregnancy might have independent effects on infant cortisol concentrations, above and beyond the effects of exposure to maternal anxiety or depression. We also explored whether maternal psychotropic medication use during pregnancy moderated the effects of prenatal exposure to anxiety or depression on infant cortisol outcomes. Specifically, we completed a series of linear regressions predicting to infant cortisol outcomes with statistical controls and lifetime and prenatal exposure to anxious / depressive disorders entered in Block 1, use of psychotropic medications in pregnancy entered into Block 2, and the maternal disorder during pregnancy X maternal medication during pregnancy interaction term entered into Block 3. As can be seen in Table 4, psychotropic medication use during pregnancy interacted with maternal disorder during pregnancy to predict infant mean cortisol (ΔF(1,164) = 6.88, p = .01) and infant cortisol reactivity (ΔF(1,148) = 4.23, p = .04).
Table 4.
Maternal depression and anxiety, psychotropic medications during pregnancy, and infant cortisol concentrations
| Predictor | Beta | ΔF | ΔR2 | p |
|---|---|---|---|---|
| Infant baseline cortisol | ||||
| Maternal medication in pregnancy | .15 | 3.27 | .02 | .07 |
| Maternal disorder × medication | −.05 | .06 | .00 | .80 |
| Infant mean cortisol | ||||
| Maternal medication in pregnancy | .04 | .24 | .00 | .63 |
| Maternal disorder × medication | −.46 | 6.88 | .04 | .01 |
| Infant cortisol reactivity | ||||
| Maternal medication in pregnancy | −.02 | .04 | .00 | .84 |
| Maternal disorder × medication | −.34 | 4.23 | .02 | .04 |
Note: statistical controls and maternal anxiety and depression were entered in Block One of each regression analysis.
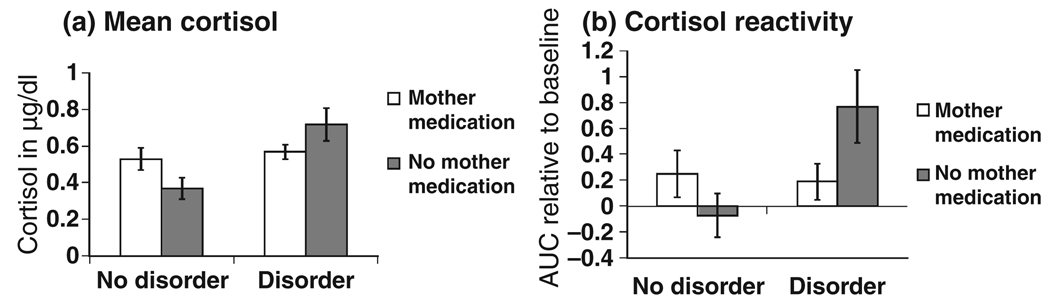
In order to interpret these interaction effects, we examined infant mean cortisol and cortisol reactivity in groups with or without exposure to maternal disorder and maternal psychotropic medication during pregnancy. These results are presented in Figure 1. As can be seen, maternal medication treatment during pregnancy appeared to offset the effects of maternal disorder on infant cortisol outcomes. Notably, infants who were exposed to maternal depression or anxiety, and whose mothers were not medicated during pregnancy, display the highest cortisol levels.
Figure 1.
Mother prenatal disorder and medication use and infant cortisol
Discussion
This detailed investigation of the relationship between maternal depression, maternal anxiety, and infant cortisol confirmed recent research in this area, and extended such data with novel findings. As per our primary hypothesis, we replicated the finding that maternal depression is associated with increased baseline and mean cortisol concentrations in infants (Diego et al., 2004). We further noted that peripartum exposure to maternal depression and maternal depression comorbid with anxiety disorder were more strongly associated with cortisol reactivity than a history of maternal depression alone. Our most novel findings suggest that prenatal psychotropic medication treatment may moderate the relationship between prenatal maternal disorder and infant cortisol levels.
We made a concerted effort to tease out the independent effects of maternal depression and anxiety on infant HPA axis activity. We examined a large number of potential confounds and included statistical controls as indicated. Of particular note, we found that delivery complications were positively related to infant cortisol concentrations in this sample. This delivery complications–cortisol relationship has been noted previously (Taylor, Fisk, & Glover, 2000), but we are not aware of any other studies of maternal depression and infant / child cortisol levels that have controlled for this potential confound. Future research might also examine whether maternal depression and delivery complications have additive or interactive effects on infant HPA axis activity, and whether particular types of delivery complications might be specifically related to infant cortisol levels or might mediate the relationship between maternal depression and infant HPA axis functioning.
The role of comorbid anxiety
Maternal depression was found to be associated with baseline and mean cortisol concentrations in 6-month-old infants, even when comorbid anxiety disorder was controlled. This result is consistent with previous findings that depressive disorders are associated with alterations in the functioning of the HPA axis (Plotsky, Owens, & Nemeroff, 1998), as well as findings that maternal depression is related to infant cortisol levels (Diego et al., 2004; Lundy et al., 1999). Our findings concerning anxiety disorder also suggest that comorbidity of this type might be specifically related to increased cortisol reactivity in offspring in response to stress. It is interesting to note that Warren et al. (2003) also found maternal panic disorder associations with cortisol measures taken in the context of stressful research procedures, but not with those taken by the mothers at home in the normal course of the day. Because different subtypes of anxiety (e.g., panic disorder versus post traumatic stress disorder) have been differentially related to HPA axis function, a finer grain analysis of maternal comorbidity may be an important focus of future research.
Timing of maternal depression
Our findings shed some initial light on the differential effect of maternal depression history versus exposure to perinatal maternal depression. Although we cannot make definitive statements about genetic influences (ours is not a genetically sensitive design), one speculative interpretation of our results is that baseline differences in infant cortisol may originate from genetic influences (because they are associated with maternal depression history rather than exposure to maternal depression), whereas changes in infant cortisol reactivity may result from exposure to maternal depression, and / or a combination of genetic influences and exposure.
Of note, the immediate, and possibly transient, ‘state’ effects of maternal depressive symptoms did not appear to be related to infant cortisol baseline or reactivity. An apparent relationship between BDI scores and infant cortisol reactivity was better explained (statistically) by postpartum exposure to maternal depression (as measured by the SCID). This finding suggests that chronicity or severity of postpartum maternal depression might play an important role in infant cortisol reactivity.
We did not find differences in infant cortisol levels based on exposure to maternal depression during pregnancy versus the postpartum period. This finding is consistent with a recent report indicating no differences in infant urinary cortisol across similar comparison groups (Diego et al., 2004). It is possible that exposure to maternal depression during pregnancy may affect infant HPA axis activity through direct physiological effects of maternal cortisol (Gitau, Cameron, Fisk, & Glover, 1998), whereas exposure during postpartum may affect infant HPA axis activity through the environmental stressors associated with maternal depression (Essex et al., 2002). If this is the case, we still may have expected a potentiating effect of exposure during both of these time periods; our data reveal no such additive or interactive effect.
Our findings suggest that multiple measures of cortisol may be useful in terms of teasing out differential effects of maternal depression. Our baseline cortisol measure (like baseline and diurnal measures used in previous studies) was related to maternal depression, even when important confounds were controlled. Our reactivity cortisol measure was associated with maternal depression only under certain conditions (i.e., when comorbid maternal anxiety or exposure to symptoms was present). This finding makes sense in the context of mixed results from previous studies where moderators were not assessed or controlled.
Clinical implications
Our results concerning medication use in pregnancy, although preliminary, are germane to the development of treatment guidelines for maternal depression and anxiety. We found that prenatal exposure to maternal psychotropic medications was associated with lower infant mean cortisol levels and infant cortisol reactivity than prenatal exposure to maternal anxiety or depression. This finding was unexpected, given concerns about the potentially toxic effects of these medications on infant neurodevelopment. Perhaps our results reflect the psychotropic effects on mother’s cortisol (i.e., a dampening of her cortisol), which then translates to lower cortisol concentrations in the infant. It is feasible that medication exposure has a direct effect on the infant HPA axis and/or stress reactivity.
At this juncture, our finding that medication use during pregnancy decreases infant cortisol reactivity cannot be interpreted as a ‘benefit’ associated with treatment; long-term implications of higher infant cortisol reactivity are largely unknown. These results also need to be considered in the context of the literature on other developmental outcomes associated with psychotropic medications taken during pregnancy. Two well-controlled child follow-up studies failed to find adverse effects of maternal antidepressants on child cognition and behavior (e.g., Nulman et al., 1997, 2002). In contrast, two recent studies have noted that maternal selective serotonin reuptake inhibitor (SSRI) use during pregnancy is associated with an increase in infant persistent pulmonary hypertension (Chambers et al., 2006) and neonatal withdrawal syndrome (Sanz et al., 2005).
Limitations
The prenatal medication measure used was relatively crude – yes or no retrospective maternal reports of any type of psychotropic medication use. For these results to be more useful in terms of treatment guidelines, it would be necessary to confirm the accuracy of the maternal reports, to replicate the findings in a larger sample where Type II errors would be a lesser concern, and to assess particular types and dosages of medication and their differential effects on a variety of infant behavioral and physiological outcomes. For example, post-hoc analyses revealed a similar pattern of results for prenatal exposure to maternal symptoms versus exposure to SSRIs (the most commonly used psychotropic medication in this sample), but the effects of antipsychotics or anxiolytics likely differ from those that have been noted here.
This study has other methodological limitations as well. For example, our findings concerning anxiety disorders were limited by sample restrictions. Specifically, we did not have a large enough comparison group of women who had anxiety disorder without depression to assess their infant cortisol levels. A further complication in this issue is the fact that our comorbid depression and anxiety disorder mothers may have suffered from more severe depression than the non-comorbid group, and the increased severity of depression may have impacted infant cortisol levels. Notably, post-hoc analyses revealed that the comorbid anxiety/depression mothers were not significantly different from mothers with depression alone in terms of age of onset of depression or likelihood of having been hospitalized for a psychiatric disorder. It is possible, however, that there are other severity or chronicity characteristics of maternal depression that account for the differences in cortisol levels between our comorbid and non-comorbid groups.
In this study, we relied on retrospective maternal reports for the timing of disorders, and recall bias may have impacted our findings. Prospectively collected data on symptom levels throughout the peripartum period might better be able to tease apart the differential versus combined effects of exposure to maternal depression during pregnancy and / or the postpartum.
This study is focused primarily on the association between maternal depression and infant cortisol levels, with only maternal medication use in pregnancy assessed as a potential moderator. It is likely that other biological and environmental factors that were not tested here (including the mother–child relationship) interact with maternal psychopathology to predict to infant HPA axis functioning.
Finally, it is important to note that the current study is limited by its cross-sectional nature, as well as its sole focus on infant cortisol outcomes. The contribution of infant HPA axis alterations at 6 months of age (secondary to maternal depression and anxiety) to the development of later psychopathology in the offspring warrants further investigation. It is not clear whether these differential responses persist later in the child’s life or have any long-term negative implications. A study by Essex et al. (2002) found that high cortisol levels noted during preschool were associated with children’s behavioral problems, especially social withdrawal. A separate study found that internalizing problems assessed in kindergarten were associated with higher baseline cortisol levels 1.5 years earlier (Goldsmith & Lemery, 2000). The cohort from this study remains under investigation to assess whether long-term behavioral and temperament differences might also be evidenced in these children as they mature.
Acknowledgment
Patricia Brennan, Rebecca Pargas, Elaine Walker, Department of Psychology, and Paula Green, D. Jeffrey Newport, and Zachary Stowe, Department of Psychiatry and Behavioral Sciences, Emory University. The authors thank research staff and Eric Vanman for their assistance with data collection and coding, and Paul Plotsky for his comments on an earlier draft of the manuscript. This study was supported by a NARSAD Young Investigator Grant awarded to Brennan, the Emory University Silvio O. Conte Center for the Neurobiology of Mental Disease (MH58922), and a Specialized Center of Research (SCOR) on Sex and Gender Effects (MH68036).
Footnotes
Conflict of interest statement: Dr Newport has received research support from Eli Lilly, GlaxoSmithKline (GSK), Janssen, and Wyeth as well as NARSAD and NIH, and speaker’s honoraria from Astra-Zeneca, Eli Lilly, GSK, and Pfizer. Dr Stowe has received research support from GSK, NIH, and Wyeth, served on advisory boards for Wyeth, BMS and GSK, and received speaker’s honoraria from Eli Lilly, GSK, Pfizer, and Wyeth. All of the remaining authors have no current financial ties to for-profit enterprises.
References
- Ashman SB, Dawson G, Panagiotides H, Yamada E, Wilkinson CW. Stress hormone levels of children of depressed mothers. Development and Psychopathology. 2002;14:333–349. doi: 10.1017/s0954579402002080. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. The Beck Depression Inventory. 2nd edn. San Antonio, TX: The Psychological Corporation; 1996. [Google Scholar]
- Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. Journal of Abnormal Psychology. 2001;110:585–599. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- Chambers C, Hernandez-Diaz S, Van Marter LJ, Werler MM, Louik C, Jones KL, Mitchell AA. Selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. New England Journal of Medicine. 2006;354:579–587. doi: 10.1056/NEJMoa052744. [DOI] [PubMed] [Google Scholar]
- Diego MA, Field T, Hernandez-Reif M, Cullen C, Schanberg S, Kuhn C. Prepartum, postpartum, and chronic depression effects on newborns. Psychiatry. 2004;67:63–80. doi: 10.1521/psyc.67.1.63.31251. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: Effects on cortisol and behavior. Biological Psychiatry. 2002;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I disorders. Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC. Perinatal depression: Prevalence, screening accuracy, and screening outcomes. Rockville, MD: AHRQ; Evidence Report/Technology Assessment No 119 AHRQ Publication No. 05-E006-2. 2005 doi: 10.1037/e439372005-001. [DOI] [PMC free article] [PubMed]
- Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet. 1998;352:707–708. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- Goldberg S, Levitan R, Leung E, Masellis M, Basile VS, Nemeroff CB, Atkinson L. Cortisol concentrations in 12- to 18-month-old infants: Stability over time, location and stressor. Biological Psychiatry. 2003;54:719–726. doi: 10.1016/s0006-3223(03)00010-6. [DOI] [PubMed] [Google Scholar]
- Goldsmith HH, Lemery KS. Linking temperamental fearfulness and anxiety symptoms: A behavior-genetic perspective. Biological Psychiatry. 2000;48:1199–1209. doi: 10.1016/s0006-3223(00)01003-9. [DOI] [PubMed] [Google Scholar]
- Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: A developmental model for understanding mechanisms of transmission. Psychological Review. 1999;106:458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- Gutteling BM, de Weerth C, Buitelaar JK. Prenatal stress and children’s cortisol reaction to the first day of school. Psychoneuroendocrinology. 2005;30:541–549. doi: 10.1016/j.psyneuen.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Herbert J, Goodyer IM, Murray L. Exposure to postnatal depression predicts elevated cortisol in adolescent offspring. Biological Psychiatry. 2004;55:376–381. doi: 10.1016/j.biopsych.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Halligan SL, Murray L, Martins C, Cooper PJ. Maternal depression and psychiatric outcomes in adolescent offspring: A 13 year longitudinal study. Journal of Affective Disorders. 2006;97:145–154. doi: 10.1016/j.jad.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Hendrick V, Stowe ZN, Altshuler LL, Hwang S, Lee E, Haynes D. Placental passage of antidepressant medications. American Journal of Psychiatry. 2003;190:993–997. doi: 10.1176/appi.ajp.160.5.993. [DOI] [PubMed] [Google Scholar]
- Loughhead AM, Stowe ZN, Newport DJ, Ritchie JC, De Vane CL, Owens MJ. Placental passage of trycyclic antidepressants. Biological Psychiatry. 2006;59:287–290. doi: 10.1016/j.biopsych.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Lundy BL, Jones NA, Field T, Nearing G, Davalos M, Pietro PA, Schanberg S, Kuhn C. Prenatal depression effects on neonates. Infant Behavior and Development. 1999;22:119–129. [Google Scholar]
- Murray L, Fiori-Cowley A, Hooper R, Cooper P. The impact of postnatal depression and associated adversity on early mother–infant interactions and later infant outcome. Child Development. 1996;67:2512–2526. [PubMed] [Google Scholar]
- Murray L, Sinclair D, Cooper P, Ducournau P, Turner P. The socioemotional development of 5-year-old children of postnatally depressed mothers. Journal of Child Psychology and Psychiatry. 1999;40:1259–1271. [PubMed] [Google Scholar]
- Nulman I, Laslo D, Fried S, Uleryk E, Lishner M, Koren G. Neurodevelopment of children exposed in utero to antidepressant drugs. New England Journal of Medicine. 1997;336:258–262. doi: 10.1056/NEJM199701233360404. [DOI] [PubMed] [Google Scholar]
- Nulman I, Rovet J, Stewart DE, Wolpin J, Pace-Asciak P, Shuhaiber S, Koren G. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: A prospective, controlled study. American Journal of Psychiatry. 2002;159:1889–1995. doi: 10.1176/appi.ajp.159.11.1889. [DOI] [PubMed] [Google Scholar]
- O’Connor TG, Ben-Shlomo Y, Heron J, Golding J, Adams D, Glover V. Prenatal anxiety predicts individual differences in cortisol in pre-adolescent children. Biological Psychiatry. 2005;58:211–217. doi: 10.1016/j.biopsych.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Owens MJ, Nemeroff CB. Psychoneuroendocrinology of depression. Psychiatric Clinics of North America. 1998;21:293–307. doi: 10.1016/s0193-953x(05)70006-x. [DOI] [PubMed] [Google Scholar]
- Ramsay D, Lewis M. Reactivity and regulation in cortisol and behavioral responses to stress. Child Development. 2003;74:456–464. doi: 10.1111/1467-8624.7402009. [DOI] [PubMed] [Google Scholar]
- Sanz EJ, De-las-Cuevas C, Kiuru A, Bate A, Edwards R. Selective serotonin reuptake inhibitors in pregnant women and neonatal withdrawal syndrome: A database analysis. Lancet. 2005;365:482–487. doi: 10.1016/S0140-6736(05)17865-9. [DOI] [PubMed] [Google Scholar]
- Stowe ZN, Hostetter AL, Newport DJ. The onset of postpartum depression: Implications for clinical screening in obstetrical and primary care. American Journal of Obstetrics and Gynecology. 2005;192:522–526. doi: 10.1016/j.ajog.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Taylor A, Fisk NM, Glover V. Mode of delivery and subsequent stress response. Lancet. 2000;355:120. doi: 10.1016/S0140-6736(99)02549-0. [DOI] [PubMed] [Google Scholar]
- Warren SL, Gunnar MR, Kagan J, Anders TF, Simmens SJ, Rones M, Wease S, Aron E, Dahl RE, Sroufe LA. Maternal panic disorder: Infant temperament, neurophysiology, and parenting behaviors. Journal of the American Academy of Child and Adolescent Psychiatry. 2003;42:814–825. doi: 10.1097/01.CHI.0000046872.56865.02. [DOI] [PubMed] [Google Scholar]
- Webster PA. Withdrawal symptoms in neonates associated with maternal antidepressant therapy. Lancet. 1973;2:318–319. doi: 10.1016/s0140-6736(73)90815-5. [DOI] [PubMed] [Google Scholar]
- Wilder J. Modern psychophysiology and the Law of Initial Value. American Journal of Psychotherapy. 1958;12:199–221. doi: 10.1176/appi.psychotherapy.1958.12.2.199. [DOI] [PubMed] [Google Scholar]
- Young EA, Vazquez D, Jiang H, Pfeffer CR. Salivary cortisol and response to dexamethasone in children of depressed parents. Biological Psychiatry. 2006;60:831–836. doi: 10.1016/j.biopsych.2006.03.077. [DOI] [PubMed] [Google Scholar]