Abstract
Evidence that programmed cell death contributes to cardiomyocyte loss is substantial for some cardiac pathologies such as myocardial infarction and a variety of cardiomyopathies. For others, such as chronic hibernating and stunned myocardium, its involvement is still debated. Recent studies have indicated that the heart remodels its structure in a rather stereotypical way when subjected to unfavourable conditions such as ischemia and pressure or volume overload. This stereotypical response is characterized by subcellular adaptations in cardiomyocytes whereby the cells switch from an adult (functional) to a fetal (survival) phenotype, a process akin to dedifferentiation. Structural hallmarks of dedifferentiation are reduction of contractile filaments, accumulation of glycogen in the cytosol, dispersion of nuclear heterochromatin, changes in mitochondrial shape and size, and loss of sarcoplasmic reticulum and T-tubules. The changes are accompanied by important alterations in the expression and distribution of structural proteins in these organelles. Today, there is only circumstantial evidence that cardiomyocyte dedifferentiation is an adaptive and reversible phenomenon instead of a degenerative event leading to apoptotic cell death. Indeed, some research groups consider the switch to a fetal phenotype to be a rescue reaction and therefore coined the name ‘programmed cell survival’, whereas others interpret this as an event on the ‘programmed cell death’ pathway. It is obvious that resolving this controversial issue is of direct clinical importance as far as prognosis and therapy are concerned.
Keywords: Apoptosis, Cell survival, Hibernating myocardium
Hibernating myocardium is considered a reversible form of heart failure. It is the response of the heart to impaired blood flow, caused by progressing atherosclerosis of coronary arteries. The heart decreases its contractile function to cope with the lowered blood supply, thereby avoiding infarction. As such, ‘going into hibernation’ is thought to be an act of self-preservation (little blood, little work) and the hibernating heart is often referred to as the ‘smart heart’ (1,2).
IS THE ‘SMART HEART’ ENOUGH?
A number of studies have questioned the viability of the hibernating myocardium and proposed that blood flow should be restored to the myocardium without delay (3,4). Other data favoured the idea that myocardial tissue from hibernating areas is nonfunctioning (sleeping) but healthy, suggesting that cardiac hibernation is an adaptive mechanism capable of preserving myocardial viability for a prolonged period of time (5,6). In this brief survey, I will try to address this issue by exposing the arguments that speak in favour of or against the chances of prolonged survival of hibernating myocardium, paying special attention to aspects of cardiac morphology.
HALLMARKS OF HIBERNATING MYOCARDIUM
The hibernating state of the heart distinguishes itself from other cardiac disease states by the following characteristics: impaired contractility of the cardiac wall (measured by echocardiography or cineangiography); reduced myocardial blood flow at rest or reduced coronary reserve (positron emission tomography [PET], adenosine infusion); metabolic viability (glucose tracer uptake with [PET]); structural viability (light and electron microscopy); and reversibility of function (echocardiography or cineangiography).
HOW DOES HIBERNATING MYOCARDIUM LOOK UNDER A MICROSCOPE?
The structural correlates of hibernating myocardium consist of cells that transformed from a functional state, as present in the normal adult (rich in contractile filaments, poor in glycogen; Figure 1), to a survival state (poor in contractile filaments, rich in glycogen; Figure 2). Interestingly, glycogen occupies about 2% of the cell volume in the adult heart and 30% in the fetal heart. This similarity in glycogen storage suggests that hibernation may induce a reliance on glucose for energy provision, similar to that of the fetal heart. Other changes, which involve the substructural and molecular composition of nuclei, mitochondria, sarcoplasmic reticulum and sarcolemma, make those hibernating cardiomyocytes look like fetal cells. Moreover, fetal proteins become re-expressed or change their distribution pattern in a fetal-like fashion (7). Remarkably, degenerative changes such as vacuolization, mitochondrial swelling, and formation of secondary lysosomes and lipid or myelin droplets are virtually absent from these cells (5).
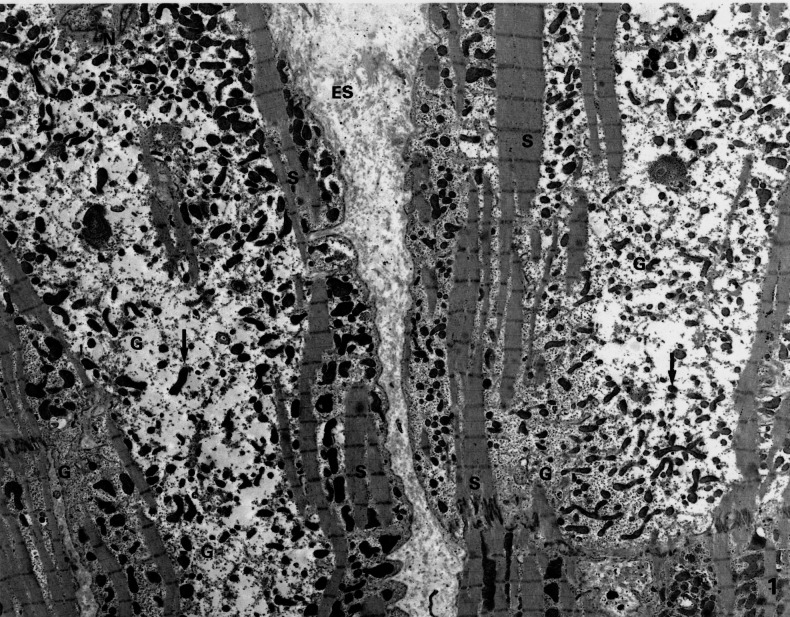
Figure 1).
Low magnification electron microscopic picture of myocardium showing typical changes in the cyosol: depleted number of contractile sarcomeres (S) and the presence of small mitochondria (arrows). The central part of the cell is occupied by glycogen (G). ES Extracellular space; N Nucleus. (Original magnification ×2600)
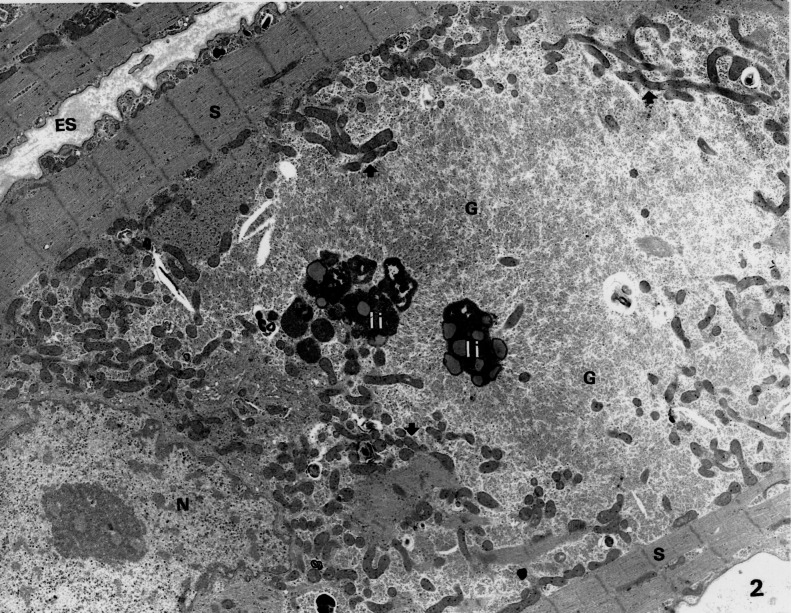
Figure 2).
Electron microscopic picture of a cardiomyocyte from a patient with dilated cardiomyopathy. Note the extreme breakdown of sarcomeres (S), which remain visible only at the cell periphery. The space left by the dissoluted sarcomeres is occupied by glycogen (G) and numerous small mitochondria (arrows). ES Extracellular space; I, li Lipofuscin-containing bodies; N Nucleus. (Original magnification ×7000)
IS THE ADAPTATION TO A FETAL PHENOTYPE A PROGRAM OF CELL SURVIVAL?
There is indirect evidence that cardiomyocytes that adopted the fetal phenotype in hibernating myocardium are more resistant to acute ischemia than their adult (structurally normal) counterparts. Indeed, when biopsies taken from hearts at the end of coronary artery bypass grafting are examined in the electron microscope, classic signs of ischemic damage are observed only in the differentiated (structurally adult) cells, not in the dedifferentiated (structurally fetal) cells (8). It is also well known that the fetal heart resists a lowered oxygen tension much better than the adult heart. Recent work by Depré and co-workers (9), addressing the issue of programmed cell survival after repetitive ischemia leading to chronic stunning or hibernation, has identified that adaptation to ischemic stress is accompnied by the induction of a multitude of genes that promote cell survival. Among them are atrial natriuretic factor, heat shock proteins, cardiac ankyrin repeat protein, activating transcription factor-3, plasminogen activator inhibitor 1 and 2, early growth response gene-1 and H-11kinase.
WHAT TRIGGERS THE CARDIOMYOCYTE TO UNDERGO AND TO MAINTAIN THE PHENOTYPIC CHANGE DURING HIBERNATION?
The mechanisms underlying the phenotypic alteration are far from understood. Oxygen shortage due to chronic or repetitive underperfusion, accompanied by limited coronary flow reserve, may be the initiating but not the intrinsic causal factor. Contractile unloading (quiescence) of the cardiomyocytes shortly after an ischemic event may give rise to an imbalance between synthesis and breakdown of the contractile proteins. The initial ischemia may lead to altered substrate metabolism favouring glucose utilization (10). However, a puzzling observation is that hibernating cells have also been encountered in areas remote from the ischemic zone (11).
Importantly, one intrinsic factor common to all pathologies (see below) and areas in which the fetal phenotype has been found is the elevated physical stretch the cardiomyocytes are subjected to during diastole (12). Indeed, increased dilation of the ventricle is a ubiquitous finding accompanying the cellular change into this phenotype. Other hypotheses (a) altered adrenoceptor density during hibernation (13), which may control myocardial function and hence induction and maintenance of the phenotype; (b) altered cell-cell interaction through defective gap junctions, leading to impaired coordinated contraction and quiescense (14); and (c) increased interstitial tissue, which not only hampers normal contraction but also imposes a different passive stretch on the cardiomyocytes (5).
IS THE FETAL PHENOTYPE SPECIFIC FOR HIBERNATING MYOCARDIUM?
Although this phenotype is consistently found in biopsies from patients with chronic hibernating myocardium, it is not a unique feature for this condition. Very similar cell changes are found in several other cardiac pathologies such as in pressure and volume overload, infarct border zones, end-stage dilated cardiomyopathies (Figure 2) and chronic atrial fibrillation (15). Common to all these pathologies is an increased dilation of the ventricular or atrial wall suggesting that (moderate) increased stretch is involved in triggering or maintaining this very stereotypical rescue behaviour of the cardiomyocyte, namely the adoption of the fetal phenotype.
APOPTOSIS IN HIBERNATING MYOCARDIUM: SLEEPING TO DEATH?
Although there is ample evidence to show that cardiomyocytes from hibernating myocardium are not suffering from ischemia, some doubts remain as to whether the fetal phenotype is a long-lasting viable state that has the potential to reverse to the normal adult phenotype once oxygen supply is restored. The questions are, how long can cardiomyocytes survive in their nonworking (sleeping) state, and what is their fate if oxygen supply falls below a certain treshold? Cell death through apoptosis is suggested in some recent reports (3,4). One observation commonly made in hibernating myocardium is the increase in the extracellular space or connective tissue. Although this can be explained by proliferating interstitial fibroblasts or increased production of connective tissue material such as collagens, another likely explanation is by loss of cardiomyocytes, possibly through apoptosis. However, in patients in whom infarcted areas are meticulously excluded, only a minimal increase in connective tissue was found and there was no evidence of cardiomyocyte apoptosis. So, differences in sampling and all or none inclusion of (micro)infarcts are probably the basis of the discrepant findings related to apoptosis in hibernating myocardium. On the other hand, apoptosis need not necessarily be considered detrimental. Apoptotic control of interstitial cell number, hence limting connective tissue volume, might be essential for timely and complete recovery of function after revascularization of the hibernating segment.
CONCLUSIONS
It looks as if there exists a delicate balance between survival (protective, repair) and apoptotic pathways. Whether the apoptotic pathway becomes activated once protective signals fail or whether these are mechanisms working independently is unknown. The saying “It is better to curse the darkness than to light the wrong candle” might be appropriate here.
The presence or absence of hibernating myocardium has important consequenses for risk stratification and clinical decision making in several of the most common cardiac conditions. Testing for hibernating myocardium (viability testing) which can be performed using PET, nuclear, or echocardiographic techniques, should be performed in selected patients when the potential for reversible myocardial dysfunction is present. This information, when incorporated into the overall clinical database, should lead to improved patient outcomes. (16)
REFERENCES
- 1.Rahimtoola SH. The hibernating myocardium. Am Heart J. 1989;117:211–21. doi: 10.1016/0002-8703(89)90685-6. [DOI] [PubMed] [Google Scholar]
- 2.Rahimtoola SH. Hibernating myocardium: a brief article. Basic Res Cardiol. 1995;90:38–40. doi: 10.1007/BF00795116. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz ER, Schaper J, vom Dahl J, et al. Myocyte degeneration and cell death in hibernating human myocardium. J Am coll Cardiol. 1996;27:1577–85. doi: 10.1016/0735-1097(96)00059-9. [DOI] [PubMed] [Google Scholar]
- 4.Elsässer A, Schlepper M, Klövekorn W, et al. Hibernating myocardium: an incomplete adaptation to ischemia. Circulation. 1997;96:2920–31. doi: 10.1161/01.cir.96.9.2920. [DOI] [PubMed] [Google Scholar]
- 5.Borgers M, Thoné F, Wouters L, Ausma J, Shivalkar B, Flameng W. Structural correlates of regional myocardial dysfunction in patients with critical coronary artery stenosis: chronic hibernation. Cardiovasc Pathol. 1993;2:237–45. [Google Scholar]
- 6.Vanoverschelde JL, Wijns W, Depre C, et al. Mechanism of chronic regional postischemic dysfunction in humans. New insights from the study of noninfarcted collateral-dependent myocardium. Circulation. 1993;87:1513–23. doi: 10.1161/01.cir.87.5.1513. [DOI] [PubMed] [Google Scholar]
- 7.Ausma J, Schaart G, Thone F, et al. Chronic ischemic viable myocardium in man: aspects of dedifferentiation. Cardiovasc Pathol. 1995;4:29–37. doi: 10.1016/1054-8807(94)00028-p. [DOI] [PubMed] [Google Scholar]
- 8.Ausma J, Thoné F, Dispersyn GD, et al. Dedifferentiated cardiomyocytes from chronic hibernating myocardium are not ischemic. Mol Cell Biochem. 1998;186:159–68. [PubMed] [Google Scholar]
- 9.Dupré C, Tomlinson JE, Kudej RK, et al. Ischemia/reperfusion induces the myocardial expression of genes involved in the growth and survival of neoplastic cells. Circulation. 2001;184:II247. (Abstract) [Google Scholar]
- 10.Depre C, Taegtmeyer H. Metabolic aspects of programmed cell survival and cell death in the heart. Cardiovasc Res. 2000;45:538–48. doi: 10.1016/s0008-6363(99)00266-7. [DOI] [PubMed] [Google Scholar]
- 11.Canty JM, Jr, Borgers M, Fallavollita JA, Thomas SA. Myolysis in hibernating myocardium is global and dissociated from regional reductions in coronary flow and SR protein level. Circulation. 2000;102:II136. (Abstract) [Google Scholar]
- 12.Dispersyn GD, Ramaekers FCS, Borgers M. Clinical pathophysiology of hibernating myocardium. Coron Artery Dis. 2001;12:381–5. doi: 10.1097/00019501-200108000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Shan K, Bick RJ, Poindexter BJ, et al. Altered adrenergic receptor density in myocardial hibernation in humans: a possible mechanism of depressed myocardial function. Circulation. 2000;102:2599–606. doi: 10.1161/01.cir.102.21.2599. [DOI] [PubMed] [Google Scholar]
- 14.Kaprielian RR, Gunning M, Dupont E, et al. Downregulation of immunodetectable connexin43 and decreased gap junction size in the pathogenesis of chronic hibernation in the human left ventricle. Circulation. 1998;97:651–60. doi: 10.1161/01.cir.97.7.651. [DOI] [PubMed] [Google Scholar]
- 15.Dispersyn GD.Dedifferentiation in chronic hibernating myocardium: programmed cell survival vs. programmed cell deathPhD Thesis, Maastricht University; June2001 [Google Scholar]
- 16.Cooper HA, Braunwald E. Clinical importance of stunned and hibernating myocardium. Coron Artery Dis. 2001;12:387–92. doi: 10.1097/00019501-200108000-00008. [DOI] [PubMed] [Google Scholar]