Abstract
Late complications of diabetes mellitus (DM) are the leading cause of adult blindness and end-stage renal disease in the western world, and a major contributor to cardiovascular, cerebrovascular and peripheral vascular disease. The etiology of the development of chronic complications of DM is unclear, and several theories have been proposed to explain the mechanisms involved. Interest in the role of genetic factors predisposing individuals to the vascular complications of DM has grown enormously in recent years. The authors recently published evidence that haptoglobin phenotype may serve as a predictor of the relative risk of diabetes-related vascular disorders. Several mechanisms whereby haptoglobin phenotype may determine diabetic vascular complications are presented. First, the haptoglobin protein products of the different alleles differ in their antioxidant capacity. Second, the haptoglobin polymers present in individuals with 1-1, 2-1 or 2-2 phenotype appear to have differential sieving properties. Third, the haptoglobin types appear to differ in their immunomodulatory functions. These studies point towards haptoglobin phenotype as a new risk factor for vascular disease in diabetes. In addition to providing insight into the pathogenesis of diabetic vascular complications, these studies suggest a new therapeutic target for prevention of these diseases.
Keywords: Complications, Diabetes, Haptoglobin, Polymorphism
There are both acute and chronic complications of diabetes mellitus (DM). However, it is the late complications of DM that cause the great majority of morbidity and mortality in these patients, and they are the focus of this review.
Late complications of DM are the leading cause of adult blindness and end-stage renal disease in the western world, and a major contributor to cardiovascular, cerebrovascular and peripheral vascular disease (PVD). These complications are mainly vascular in nature and may be loosely classified as either microvascular or macrovascular. The microvascular complications include diabetic retinopathy (DR), nephropathy (DN) and neuropathy, while macrovascular complications are represented by coronary artery disease (CAD), PVD and cerebrovascular disease. There are also chronic complications such as skin changes, gastroparesis and sexual dysfunction, which are considered to be nonvascular although the pathogenesis appears to be multifactorial (1).
The etiology of the development of chronic complications of DM is unclear, and several theories have been proposed to explain the mechanisms involved. However, the common denominator in all of these hypotheses is the role of the hyperglycemic state. Moreover, several large multicentre studies have demonstrated the benefit of strict glycemic control to normo- or near-normoglycemic levels in the prevention of the onset of complications and further progression of the disease process (2–4).
Interest in the role of genetic factors predisposing individuals to the vascular complications of DM has grown enormously in recent years as the result of evidence that certain individuals appear to be resistant to these complications, although they do not differ significantly with regard to known risk factors from many other patients with severe clinical manifestations of the disease.
PROPOSED MECHANISMS FOR THE DEVELOPMENT OF COMPLICATIONS
Effects of advanced glycosylation end products:
Advanced glycosylation end products (AGEs) are formed as the result of non-enzymatic glycosylation of proteins, and their level increases with increasing blood glucose levels. AGEs result in an augmented rate of atherosclerosis, a decrease in synthesis of nitric oxide, and endothelial and glomerular dysfunction.
Activation of protein kinase C:
Protein kinase C is activated via increased rates of diacylglycerol formation in the hyperglycemic state, whereas this activation then leads to several alterations in intracellular processes, including changes in transcription rates of genes for fibronectin and extracellular matrix proteins in endothelial and neuronal cells. Increasing evidence for the role of growth factors in the pathogenesis of diabetic vascular disease has accumulated, and these growth factors also appear to be influenced by the activation of protein kinase C. Vascular endothelial growth factor expression has been found to be increased in the retinas of patients suffering from DR, while transforming growth factor-beta levels are increased in patients with DN. There may also be a role for insulin-like growth factor 1, platelet-derived growth factor and several other factors.
Increases in glucose metabolism via the polyol pathway:
This also occurs in the presence of increased levels of intracellular glucose and leads to the production of the toxic metabolite sorbitol. This theory has been partially discredited by the lack of clinical benefit to diabetic patients treated with aldose reductase inhibitors (1).
It has recently been demonstrated that increased AGE levels, PKC activation and glucose metabolism via the aldose reductase pathway are linked by a common pathway, leading to increased production of reactive oxygen species in the setting of increased oxidative stress (5).
PATHOPHYSIOLOGY AND GENETIC INFLUENCES
The development of the vascular complications of DM is related to age at onset of the hyperglycemic state, the duration of the disease and the degree of glycemic control. As previously mentioned, strict control of glucose levels has clearly been shown to delay the onset of diabetic complications and to significantly decrease the progression of the disease (2–4). However, a significant percentage of the diabetic population has been shown to be resistant to the development of these complications in spite of glycemic control levels, which are similar to those in patients who rapidly progress to severe disease. Hyperglycemia is therefore a necessary but not sufficient condition for the development of diabetic complications, which appear to develop only in those patients who are genetically susceptible.
Support for this hypothesis of genetic susceptibility may be found in family studies, which have indicated a familial clustering of DN and CAD (6–10). Analysis of population groups, such as the Pima Indians, has shown significantly higher rates of DR and DN than would be expected based on the known risk factors (11).
Studies of these genetic risk factors have been based primarily on two approaches: sib-pair linkage analysis and association studies using polymorphisms in suspected genes. On this basis, four chromosomal regions have been identified with some evidence linkage to complication on chromosomes 3, 7, 9 and 20 (11). Many candidate genes with known polymorphisms have been identified, and these may mediate pathways involved in the suspected pathogenesis of diabetic complications. In this review, we focus on the polymorphism in the human haptoglobin (Hp) gene.
Haptoglobin
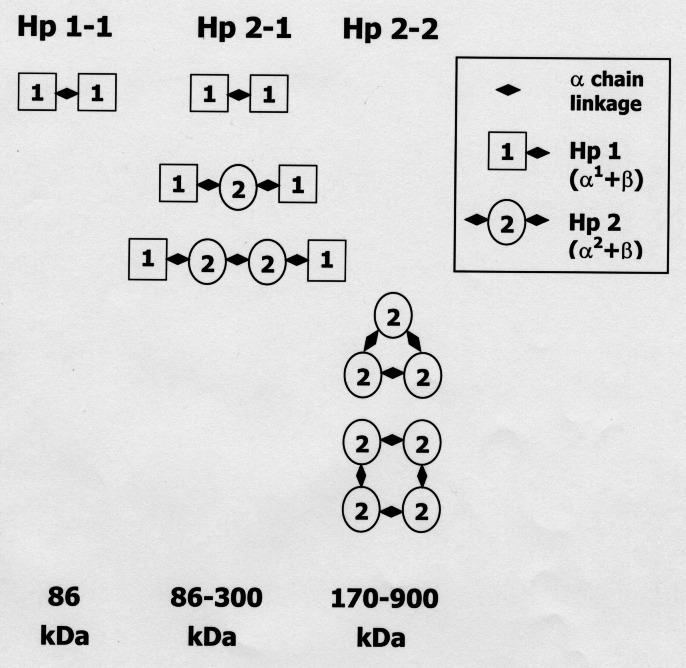
Hp is a hemoglobin (Hb)-binding acute phase protein. It is synthesized primarily in the liver and appears in the plasma at 0.2 to 2.0 g/L. In humans, Hp is encoded by two alleles Hp1 and Hp2, giving rise to three major phenotypes, which seem to differ in their biochemical and biophysical properties in various aspects (12,13). The Hp protein is composed of two types of polypeptide chains, alpha and beta. The 40 kDa beta-chain is identical in both Hp alleles, but the alpha-chain differs. The alpha-chain of Hp2 (α2) is 16 to 20 kDa, and the alpha-chain of Hp1 (α1) is 9 kDa. Additional allelic variation exists among Hp1 alleles due to the existence of α1F and α1S, which differ by one amino acid at position 54. The α2 allele, a fusion of partial α1F and α1S sequences, is a result of intragenic duplication, presumably by a nonhomologous DNA crossing-over event (14,15). Figure 1 (16) portrays the various structures and sizes of the Hp proteins. When complexed to Hb, Hp protein phenotypes are easily distinguishable by non-denaturing gel eletrophoresis due to these size and shape differences. The classical method for Hp phenotyping is based on starch gels and benzidine staining (17). We use a modification of previously published techniques, combining the advantages of acrylamide gels (18) with the reduced toxicity of 3,3′,5,5′-tetramethylbenzidine, originally published as a stain for agarose gels (19). This highly sensitive and reproducible method enables rapid phenotyping of Hp from merely 10 μL of plasma. A representative gel, shown in Figure 2, demonstrates the characteristic pattern of the different Hp phenotypes. The α2 allele, which is unique to humans, is thought to have originated in India and to have spread from there all over the world. Great variation exists in the distribution of Hp gene frequencies among various populations. The lowest incidence of the Hp1 allele is in Southeast Asia (minimum 0.07 in Indian Irulas) and the highest in Africa and South America (up to 0.92 in Mexican Lacandon). In most European populations the incidence is 0.35 to 0.43, and in Israeli Jews 0.28 to 0.3. The gradual displacement of the monopoly of Hp1 by the Hp2 allele and its current domination in some populations must have occurred under strong genetic pressure, suggesting that Hp2 provides some selective advantage (12).
Figure 1).
Schematic structural illustration of the different haptoglobin (Hp) phenotypes. The α1 chain forms disulphide bonds with one alpha-chain and one beta-chain. Thus, in subjects with Hp 1-1, a linear 86 kDa (α1β)2 homodimer is found. The α2 chain can bind one beta-chain, and either one α1 or two α2 chains. Therefore, Hp 2-2 is expressed as cyclic (α2β)n multimers with a molecular weight ranging between 170 and 900 kDa. Individuals with Hp 2-1 have (α1β)2 homodimers and linear (α2β)n multimers with a molecular weight of 86 to 300 kDa (16)
Figure 2).
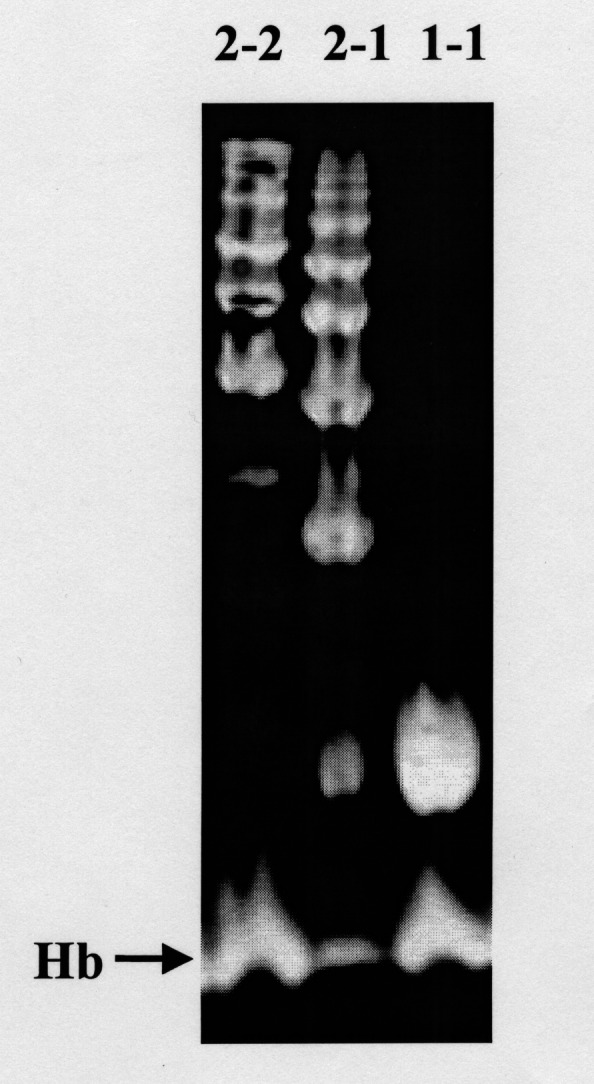
Representative patterns of the different haptoglobin (Hp) phenotypes following polyacrylamide gel electrophoresis and benzidine staining of hemoglobin (Hb)-enriched serum. The upper bands correspond to Hp-Hb complexes. A band at the bottom of each lane corresponds to free Hb, and is indicated with an arrow. Hp 2-2 polymers form a series of slowly migrating bands. Hp 1-1 homodimers appear as a single rapidly migrating band. Hp 2-1 displays a mixture of slowly migrating bands and a weak band that migrates similar to the Hp 1-1 band
HP PHENOTYPE AND VASCULAR DISEASES
Hp phenotype and atherosclerotic disorders
Atherosclerotic vascular diseases, often regarded as a form of chronic inflammation, were found to be associated with Hp. For example, Hp and other plasma proteins were detected in aortic fatty streaks and lesions but were not present in normal aortic intima (20). Myocardial infarction is preceded and followed by an acute phase response, involving an increase in plasma Hp levels (21). The extent of this increase is quantitatively related to infarct size (22).
There are also many examples of associations between Hp phenotypes and atherosclerotic vascular diseases. According to one report, Hp1 allele frequency is increased among patients with essential hypertension (23), while others demonstrate that the Hp 2-2 phenotype is more frequent in patients with essential hypertension (24). In any case, Hp 2-2 hypertensives have higher therapeutic needs. They tend to develop more atherosclerotic lesions in the coronary and peripheral arteries, and suffer from more treatment-refractory hypertension than the other Hp phenotypes (25,26). Patients with Hp 2-2 have a significantly higher release of cardiac enzymes and a higher incidence of severe left ventricular failure following a myocardial infarction, suggesting predisposition of Hp 2-2 carriers to greater damage (27). Among patients who underwent coronary artery bypass surgery, patients with Hp 2-2 were more likely to be younger, to have had a previous myocardial infarction and to have more diseased vessels. Hp 2-2 patients had more bypass grafts and a shorter graft survival time (28). In contrast to these findings from cross-sectional studies, a prospective study demonstrated that Hp 1-1 individuals are at doubled risk for CAD mortality in comparison with the other Hp phenotype individuals. The authors suggest that Hp 2-2 may be involved in the earlier stage of the atherogenic process, while Hp 1-1 may play a role in more advanced and life-threatening stages of the disease (29).
The Hp2 allele is significantly more frequent in patients with PVD, another severe atherosclerotic condition, mostly affecting the lower limbs (30).
Hp phenotype and diabetic vascular disorders
Two previous studies reported a lack of association between Hp phenotype and diabetic microvascular complications. Ratzmann et al (31) compared patients with type I diabetes with or without DR, decreased creatinine clearance or proteinuria, and found no difference in Hp phenotype distribution between the two groups. Chandra et al (32) compared patients with types I and II diabetes with or without DR and also found no significant correlation between Hp phenotype and DR.
However, a battery of reports from our laboratory established a strong association between Hp phenotypes and vascular complications in diabetics. In the following section, we summarize these studies.
Diabetic nephropathy:
Levy et al (33) and Nakhoul et al (34) studied 110 consecutive normotensive type I and type II diabetics. The difference in the incidence of DN between patients with the three Hp phenotypes was statistically significant for type I diabetic patients and for all diabetic patients combined. Of the 54 type I diabetics, 13 demonstrated DN. None (0 of 13) of the patients with Hp 1-1 phenotype had DN, whereas 5 of 22 (23%) with Hp 2-1 and eight of 21 (38%) with Hp 2-2 had evidence of DN (P<0.04). For both types of diabetes combined, none (0 of 18) of the Hp 1-1 patients showed any sign of DN, as compared with 10 of 37 (27%) with Hp 2-1 and 19 of 55 (34%) with Hp 2-2 (P<0.02).
There were 15 patients with macroalbuminuria in this study. The risk of developing macroalbuminuria was found to be significantly correlated with Hp phenotype for both types of diabetes combined. None (0 of 18) of the Hp 1-1 patients had macroalbuminuria, whereas three of 37 (8%) patients with the Hp 2-1 and 12 of 55 (22%) patients with Hp 2-2 had macroalbuminuria (P<0.03).
This study demonstrates a graded risk correlation between presence, as well as severity, of DN and Hp phenotype.
Diabetic retinopathy:
Nakhoul et al (35) studied 52 consecutive patients with type I diabetes, of whom 25 had evidence of DR. There was significantly lower prevalence of DR in patients with the Hp 1-1 than in patients with the Hp 2-1 and 2-2 phenotypes. Only one of 12 patients (8%) with Hp 1-1 phenotype had DR, whereas 12 of 20 (60%) with Hp 2-1 and 12 of 20 patients (60%) with Hp 2-2 had evidence of DR (P<0.002). Thus, this study clearly demonstrates that Hp 1-1 diabetics are provided with increased protection against the development of DR in comparison with the other Hp phenotypes.
Restenosis after percutaneous transluminal coronary angioplasty:
Levy et al (33) and Roguin et al (36) studied 218 consecutive patients who had a prior percutaneous transluminal coronary angioplasty (PTCA) and follow-up angiography. Restenosis was found in 152 of these patients. Frequency of restenosis was significantly different between the three phenotypes in both diabetic and nondiabetic patients. In diabetic patients only one of six (16%) patients with Hp 1-1 phenotype had restenosis, whereas 26 of 34 (76%) with Hp 2-1 and 22 of 28 (78%) with Hp 2-2 had evidence of restenosis (P<0.004). In nondiabetic patients 10 of 19 (52%) patients with Hp 1-1 phenotype had restenosis, whereas 37 of 60 (61%) with Hp 2-1 and 55 of 71 (77%) with Hp 2-2 had evidence of restenosis (P<0.05).
There seems to be a graded risk related to Hp phenotype in the development of restenosis in all patients combined. Hp 2-2 patients were more likely to develop restenosis than Hp 2-1 patients (79% versus 67%, P=0.06), and Hp 2-1 patients were more likely to develop restenosis than Hp 1-1 patients (67% versus 44%, P=0.03).
This study suggests that Hp phenotype may serve as a predictor of the risk of restenosis after PTCA.
Coronary collaterals in diabetic patients:
Due to the known importance of coronary collaterals in the setting of CAD, we examined the possible relationship between Hp phenotype and development of coronary collaterals. Levy et al (37) studied 82 consecutive diabetic patients and 138 consecutive nondiabetic patients. We found that diabetic patients with the Hp 2-1 phenotype were more likely to have collaterals than the Hp 2-2 patients. Among patients with the Hp 2-1 phenotype 81% had collaterals, while only 52% of the patients with Hp 2-2 phenotype had collaterals (P=0.007). Patients with collaterals were 3.4 times (95% CI 1.2 to 9.7) more likely to have the 2-1 phenotype than the 2-2 phenotype. The paucity of diabetic patients with the Hp 1-1 phenotype (3) did not allow us to reach any conclusions regarding the relationship of this phenotype to coronary collaterals. Furthermore, segregation of diabetic patients according to the number of diseased vessels demonstrated a trend for higher prevalence of collaterals in diabetic patients with Hp 2-1 than in diabetic patients with Hp 2-2. There was no difference in the presence of collaterals between the different phenotypes in the nondiabetic population (P=0.84).
Once again, this study demonstrates a graded risk correlation between presence, as well as severity, of CAD and Hp phenotype.
In summary, our studies demonstrate that Hp phenotype is a predictor of the relative risk of diabetes-related vascular disorders. Hp 1-1 confers significant protection from these disorders, Hp 2-1 confers partial protection, and Hp 2-2 can be regarded as a major risk factor for vascular complications in diabetics.
THEORIES REGARDING THE RELATION BETWEEN Hp AND DIABETIC VASCULAR DISEASES
Several roles of Hp may influence its relation with diabetic vascular complications.
The role of oxidative stress in diabetic complications and restenosis
Considerable evidence has been reported regarding the involvement of oxidative stress in the development of diabetic vascular complications (38) and restenosis (39). Many studies show decreased endogenous antioxidants or increased oxidative stress in the blood of diabetic patients (40,41).
Hyperglycemia increases oxidative stress by several mechanisms, including glucose auto-oxidation forming free radicals, which enhance formation of AGEs; AGEs themselves supply more free radicals; and the polyol pathway by which hyperglycemia activates the enzymes aldose reductase and sorbitol dehydrogenase, thus depleting cellular NADPH (38). Poor glycemic control is correlated with increased free radical production (42–44). Many studies demonstrate a correlation between oxidative stress and diabetic microvascular damage, and there are indications that inhibition of oxidative stress may prevent these complications.
Free oxy-Hb is a potent oxidant, generating reactive oxygen species as it transforms to met-Hb, as well as catalyzing oxidation of many other compounds (45). Hp functions as an antioxidant by virtue of its ability to bind Hb (46,47). Hp knockout mice are more prone to oxidative tissue damage and mortality following hemolysis, demonstrating the importance of Hp in the physiological defense against Hb toxicity (48).
Our group recently reported a significant difference in the in vitro antioxidant capabilities of the different Hp phenotypes, Hp 1-1 being a superior antioxidant than Hp 2-2. As was demonstrated in this study, this finding is not related to lower Hb-binding capacities of Hp 2-2, but to a mechanistic reason, perhaps a difference in the ability to prevent release of heme or a different potency of peroxidase activity (49). The extremely strong affinity between Hp and Hb (10 to 15 M) makes it unlikely that varied affinity of the Hp phenotypes to Hb contributes to the observed differences (50).
Our data are consistent with earlier in vivo findings of a correlation between Hp and serum vitamin C. Langlois et al (51) found significantly lower vitamin C concentrations in Hp 2-2 subjects, suggesting less efficient clearance of Hb from the plasma. In vitro, they measured rapid consumption of vitamin C in plasma from Hp 2-2 individuals compared with plasma from Hp 1-1 individuals. The rate of vitamin C depletion was inversely related to the Hp concentrations.
The reported differences in the antioxidant capacity of the different Hp phenotypes may be dramatically amplified in vivo due to the different sizes of the Hp polymers. This would differentially affect the ability of the different Hp proteins to sieve into the vessel wall, where Hp is needed in order to neutralize the harmful oxidizing effect of Hb. In diabetic individuals, already burdened with increased oxidative stress, this would be of even greater importance.
The acute phase reaction and diabetes
A current theory proposes that one of the factors contributing to the pathogenesis of diabetes and its complications is chronic inflammation through the innate immune system (52). This theory is based on the observed elevation of several acute phase proteins in type I and type II diabetics. Even higher increases are measured in patients with DN (53–56).
Acute phase markers have been recognized as prognostic factors in CAD (57–60). Hp, along with other acute phase proteins, significantly increases before and after myocardial infarction (21,22). Given that the acute phase response is involved in diabetes, DN, CAD and restenosis, and that Hp is an acute phase protein, there is a possibility that the effect of Hp on diabetic complications may be through its involvement in the acute phase response. This activity and its difference between Hp phenotypes are not completely understood. Hp 1-1 is apparently a stronger inhibitor of inflammation, while Hp 2-2 confers better immune activity, but it is difficult to speculate how these differences may affect diabetes complications.
Immunomodulation by Hp
Another important aspect that may contribute to the biochemical basis of the different influence of the Hp phenotypes is their effect on various types of leukocytes. Hp has been reported to bind to CD11b/CD18 on monocytes, granulocytes and T-lymphocytes and to CD22 on B-lymphocytes, but with relatively low affinity and specificity (61,62). On the other hand, Hp-Hb complexes bind with high affinity to CD163 on monocytes and macrophages (63). There is some evidence that CD163 activation results in an anti-inflammatory effect (64,65), and that CD163 contributes to the adhesion of monocytes to activated endothelial cells (66). Hp 2-2 exhibits a 10-fold higher affinity to CD163 and a higher rate of uptake than Hp 1-1 (63). The consequences of this binding and internalization have not been characterized yet, but a possibly important aspect is the intracellular oxidation status caused by the internalization of the Hp-Hb complexes. The combination of more uptake of Hb-Hp 2-2 along with the poorer antioxidant capabilities of Hp 2-2 is bound to influence the redox environment within the cell, affecting its activities in general.
As we recognize the major role played by leukocytes in physiological and pathological vascular processes, it is not unreasonable to speculate that differential affinity and cellular activation of the different Hp phenotypes may be the key to understanding the strong association between Hp and vascular disease.
Hp and angiogenesis
Cid et al (67) demonstrated in vitro and in vivo that sera from patients with systemic vasculitis manifest angiogenic properties, and they identified Hp as one of the angiogenic factors. Anti-Hp antibodies partially repressed the angiogenic activity. They also demonstrated that Hp 2-2 was the most angiogenic, Hp 2-1 had intermediate activity, and Hp 1-1 possessed only weak angiogenic activity. Interestingly, while Hp 2-2 has been identified as an independent risk factor for developing PVD, it is also associated with a longer maximal walking distance, suggesting the existence of a better developed collateral circulation in the affected limb (30).
SUMMARY
It has been recognized for a long time that genetic factors influence diabetic complications. We have recognized Hp as a genetic marker strongly associated with individual predisposition to microvascular and macrovascular complications in diabetes. Previous studies have reported association of several genes with diabetic complications, but the association with Hp is unique in several aspects: Hp is relevant to nearly all important vascular complications in diabetes, including DN, DR, restenosis, CAD and development of coronary collaterals. For all these conditions, the Hp1 allele appears to confer a significant protection.
Other genes, which have been suggested to correlate with diabetic vascular complications, are usually part of an established pathway in the pathophysiology of diabetic complications. However, most studies found no association between Hp phenotype and diabetes (31,68–70). The finding that Hp is a genetic modulator of diabetic complications suggests either the existence of an unrecognized important mechanism involved in the development of vascular damage in diabetes, or an unrecognized important aspect of Hp function. Extensive research in these two directions may reveal a mechanistic connection between Hp and diabetic vascular complications, as well as more general insights pertaining to the pathophysiology of diabetic complications.
Further research in large patient populations is being carried out in order to confirm our results and accurately estimate the predictive value of Hp phenotype for complications in diabetes. These large-scale studies should also provide solid information regarding the interrelation of Hp phenotype with other risk factors for vascular complications in diabetes, especially modifiable factors such as control of blood glucose, hypertension and hyperlipidemia.
The fact that patients with Hp 1-1 develop remarkably less DN, DR, restenosis and CAD could have practical applications in treatment and follow-up, suggesting Hp phenotyping of all diabetic patients as valuable for risk assessment and care planning. Patients with Hp 1-1 may require less frequent retinal and renal follow-ups, and angioplasty can be recommended for them in more indications than in other diabetic patients. In light of our findings regarding the superior antioxidant capacity of Hp 1-1, patients with Hp 2-2 may benefit from antioxidant therapy.
Although the Hp phenotype of a subject is a fixed genetic property, future investigation of the mechanism of Hp1 protection in diabetes may enable development of novel therapeutic approaches, specifically interfering with the damage or providing protection similar to that conferred by Hp1.
REFERENCES
- 1.Powers AC. Diabetes mellitus. In: Braunwald E, Fauci A, Kasper D, et al., editors. Harrison’s Principles of Internal Medicine. New York: McGraw-Hill; 2001. pp. 2119–21. [Google Scholar]
- 2.The diabetes Control and Complications Trial Research Group The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.The diabetes Control and Complications Trial Research Group The relationship of glycemic exposure (HbA1c) to the risk of development and progression of retinopathy. Diabetes. 1995;44:968–983. [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) Group Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 5.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 6.Chowdhury TA, Kumar S, Barnett AH, Bain SC. Nephropathy in type 1 diabetes: the role of genetic factors. Diabet Med. 1995;12:1059–67. doi: 10.1111/j.1464-5491.1995.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 7.Parving HH, Tarnow L, Rossing P. Genetics of diabetic nephropathy. J Am Soc Nephrol. 1996;7:2509–17. doi: 10.1681/ASN.V7122509. [DOI] [PubMed] [Google Scholar]
- 8.Ruiz J. Diabetes mellitus and the late complications: influence of the genetic factors. Diabetes Metab. 1997;23(Suppl 2):57–63. [PubMed] [Google Scholar]
- 9.Chowdhury TA, Dyer PH, Kumar S, et al. Genetic determinants of diabetic nephropathy. Clin Sci. 1999;96:221–30. [PubMed] [Google Scholar]
- 10.Marre M. Genetics and the prediction of complications in type 1 diabetes. Diabetes Care. 1999;22(Suppl 2):B53–8. [PubMed] [Google Scholar]
- 11.Imperatore G, Hanson RL, Pettitt DJ, et al. Sib-pair linkage analysis for susceptibility genes for microvascular complications among Pima Indians with type 2 diabetes. Pima Diabetes Genes Group. Diabetes. 1998;47:821–30. doi: 10.2337/diabetes.47.5.821. [DOI] [PubMed] [Google Scholar]
- 12.Giblett ER. Haptoglobin. In: Daris FA, editor. Genetic Markers in Human Blood. Oxford: Blackwell; 1969. [Google Scholar]
- 13.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem. 1996;42:1589–600. [PubMed] [Google Scholar]
- 14.Black JA, Dixon GH. Amino-acid sequence of alpha chains of human haptoglobins. Nature. 1968;218:736–41. doi: 10.1038/218736a0. [DOI] [PubMed] [Google Scholar]
- 15.Maeda N, Yang F, Barnett DR, Bowman BH, Smithies O. Duplication within the haptoglobin Hp2 gene. Nature. 1984;309:131–5. doi: 10.1038/309131a0. [DOI] [PubMed] [Google Scholar]
- 16.Wejman JC, Hoversusepian D, Wall JS, Hainfeld JF, Greer J. Structure and assembly of haptoglobin polymers by electron microscopy. J Mol Biol. 1984;174:343–68. doi: 10.1016/0022-2836(84)90342-5. [DOI] [PubMed] [Google Scholar]
- 17.Smithies O. Zone electrophoresis in starch gels: Group variations in the serum proteins of normal individuals. Biochem J. 1955;61:629–41. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linke RP. Typing and subtyping of haptoglobin from native serum using disc gel electrophoresis in alkaline buffer: application to routine screening. Anal Biochem. 1984;141:55–61. doi: 10.1016/0003-2697(84)90424-x. [DOI] [PubMed] [Google Scholar]
- 19.Wassell J, Keevil B. A new method for haptoglobin phenotyping. Ann Clin Biochem. 1999;36:609–12. doi: 10.1177/000456329903600507. [DOI] [PubMed] [Google Scholar]
- 20.Stastny JJ, Fosslien E. Quantitative alteration of some aortic intima proteins in fatty streaks and fibro-fatty lesions. Exp Mol Pathol. 1992;57:205–14. doi: 10.1016/0014-4800(92)90011-y. [DOI] [PubMed] [Google Scholar]
- 21.Bernard DR, Langlois MR, Delanghe JR, De Buyzere ML. Evolution of haptoglobin concentration in serum during the early phase of acute myocardial infarction. Eur J Clin Chem Clin Biochem. 1997;35:85–8. doi: 10.1515/cclm.1997.35.2.85. [DOI] [PubMed] [Google Scholar]
- 22.Smith SJ, Bos G, Esseveld MR, Van Ejik HG. Acute-phase proteins from the liver and enzymes from myocardial infarction; a quantitative relationship. Clin Chim Acta. 1977;81:75–85. doi: 10.1016/0009-8981(77)90415-6. [DOI] [PubMed] [Google Scholar]
- 23.John A, Henke J, Morich FJ. Identification of a so-far not characterized human serum protein associated with essential hypertension. Electrophoresis. 1985;6:292–5. [Google Scholar]
- 24.Surya PP, Padma T, Ramaswamy M. Haptoglobin patterns in essential hypertension and associated conditions-increased risk for Hp 2-2. Hum Hered. 1987;37:345–8. doi: 10.1159/000153732. [DOI] [PubMed] [Google Scholar]
- 25.Delanghe JR, Duprez DA, De Buyzere ML, et al. Haptoglobin polymorphism and complications in established essential arterial hypertension. J Hypertens. 1993;11:861–7. doi: 10.1097/00004872-199308000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Delanghe JR, Duprez DA, De Buyzere ML, et al. Refractory hypertension is associated with the haptoglobin 2-2 phenotype. J Cardiovasc Risk. 1995;2:131–6. [PubMed] [Google Scholar]
- 27.Chapelle JP, Albert A, Smeets JP, Heusghem C, Kulbertus HE. Effect of the haptoglobin phenotype on the size of a myocardial infarct. N Engl J Med. 1982;307:457–63. doi: 10.1056/NEJM198208193070801. [DOI] [PubMed] [Google Scholar]
- 28.Delanghe J, Cambier B, Langlois M, et al. Haptoglobin polymorphism, a genetic risk factor in coronary artery bypass surgery. Atherosclerosis. 1997;132:215–9. doi: 10.1016/s0021-9150(97)00089-0. [DOI] [PubMed] [Google Scholar]
- 29.De Bacquer D, De Backer G, Langlois M, Delanghe J, Kestellot H, Kornitzer M. Haptoglobin polymorphism as a risk factor for coronary heart disease mortality. Atherosclerosis. 2001;157:161–6. doi: 10.1016/s0021-9150(00)00690-0. [DOI] [PubMed] [Google Scholar]
- 30.Delanghe J, Langlois M, Duprez D, De Buyzere M, Clement D. Haptoglobin polymorphism and peripheral arterial occlusive disease. Atherosclerosis. 1999;145:287–92. doi: 10.1016/s0021-9150(99)00079-9. [DOI] [PubMed] [Google Scholar]
- 31.Ratzmann KP, Strese J, Keilacker H, Gielbelmann R, Scheibe F, Witt S. Is there a relationship between genetically determined haptoglobin phenotype and insulin-dependent diabetes mellitus (IDDM)? Exp Clin Endocrinol. 1984;83:207–15. doi: 10.1055/s-0029-1210333. [DOI] [PubMed] [Google Scholar]
- 32.Chandra T, Lakshmi CN, Padma T, Vidyavathi M, Satapathy M. Haptoglobin phenotypes in diabetes mellitus and diabetic retinopathy. Hum Hered. 1991;41:347–50. doi: 10.1159/000154023. [DOI] [PubMed] [Google Scholar]
- 33.Levy AP, Roguin A, Hochberg I, et al. Haptoglobin phenotype and vascular complications in patients with diabetes. N Engl J Med. 2000;343:969–70. doi: 10.1056/NEJM200009283431313. [DOI] [PubMed] [Google Scholar]
- 34.Nakhoul FM, Zoabi R, Kanter Y, et al. Haptoglobin phenotype and diabetic nephropathy. Diabetologia. 2001;44:602–4. doi: 10.1007/s001250051666. [DOI] [PubMed] [Google Scholar]
- 35.Nakhoul FM, Marsh S, Hochberg I, Leibu R, Miller BP, Levy AP. Haptoglobin genotype as a risk factor for diabetic retinopathy. JAMA. 2000;284:1244–5. doi: 10.1001/jama.284.10.1244-a. [DOI] [PubMed] [Google Scholar]
- 36.Roguin A, Hochberg I, Nikolsky E, et al. Haptoglobin phenotype as a predictor of restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol. 2001;87:330–2. A9. doi: 10.1016/s0002-9149(00)01368-0. [DOI] [PubMed] [Google Scholar]
- 37.Hochberg I, Roguin A, Nikolsky E, Chandershekhar PV, Cohen S, Levy AP. Haptoglobin phenotype and coronary artery collaterals in diabetic patients. Atherosclerosis. 2002;161:441–6. doi: 10.1016/s0021-9150(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 38.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–67. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 39.Godfried SL, Deckelbaum LI. Natural antioxidants and restenosis after percutaneous transluminal coronary angioplasty. Am Heart J. 1995;129:203–10. doi: 10.1016/0002-8703(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 40.Asayama K, Uchida N, Nakane T, et al. Antioxidants in the serum of children with insulin-dependent diabetes mellitus. Free Radic Biol Med. 1993;15:597–602. doi: 10.1016/0891-5849(93)90162-n. [DOI] [PubMed] [Google Scholar]
- 41.Gurler B, Vural H, Yilmaz N, Oguz H, Satici A, Aksoy N. The role of oxidative stress in diabetic retinopathy. Eye. 2000;14:730–5. doi: 10.1038/eye.2000.193. [DOI] [PubMed] [Google Scholar]
- 42.Altomare E, Vendemiale G, Chicco D, Procacci V, Cirelli F. Increased lipid peroxidation in type 2 poorly controlled diabetic patients. Diabete Metab. 1992;18:264–71. [PubMed] [Google Scholar]
- 43.Ceriello A, Giugliano D, Quatraro A, Dello RP, Lefebvre PJ. Metabolic control may influence the increased superoxide generation in diabetic serum. Diabet Med. 1991;8:540–2. doi: 10.1111/j.1464-5491.1991.tb01647.x. [DOI] [PubMed] [Google Scholar]
- 44.Wierusz-Wysocka B, Wysocki H, Byks H, Zozulinska D, Wykretowicz A, Kazmierczak M. Metabolic control quality and free radical activity in diabetic patients. Diabetes Res Clin Pract. 1995;27:193–7. doi: 10.1016/0168-8227(95)01043-d. [DOI] [PubMed] [Google Scholar]
- 45.Everse J, Hsia N. The toxicities of native and modified hemoglobins. Free Radic Biol Med. 1997;22:1075–99. doi: 10.1016/s0891-5849(96)00499-6. [DOI] [PubMed] [Google Scholar]
- 46.Gutteridge JM. The antioxidant activity of haptoglobin towards haemoglobin-stimulated lipid peroxidation. Biochim Biophys Acta. 1987;917:219–23. doi: 10.1016/0005-2760(87)90125-1. [DOI] [PubMed] [Google Scholar]
- 47.Miller YI, Altamentova SM, Shaklai N. Oxidation of low-density lipoprotein by hemoglobin stems from a heme-initiated globin radical: antioxidant role of haptoglobin. Biochemistry. 1997;36:12189–98. doi: 10.1021/bi970258a. [DOI] [PubMed] [Google Scholar]
- 48.Lim SK, Kim H, Bin AA, et al. Increased susceptibility in Hp knockout mice during acute hemolysis. Blood. 1998;92:1870–7. [PubMed] [Google Scholar]
- 49.Melamed-Frank M, Lache O, Enav BI, et al. Structure-function analysis of the antioxidant properties of haptoglobin. Blood. 2001;98:3693–8. doi: 10.1182/blood.v98.13.3693. [DOI] [PubMed] [Google Scholar]
- 50.Dobryszycka W. Biological functions of haptoglobin – new pieces to an old puzzle. Eur J Clin Chem Clin Biochem. 1997;35:647–54. [PubMed] [Google Scholar]
- 51.Langlois MR, Delanghe JR, De Buyzere ML, Bernard DR, Ouyang J. Effect of haptoglobin on the metabolism of vitamin C. Am J Clin Nutr. 1997;66:606–10. doi: 10.1093/ajcn/66.3.606. [DOI] [PubMed] [Google Scholar]
- 52.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–8. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 53.McMillan DE. Increased levels of acute-phase serum proteins in diabetes. Metabolism. 1989;38:1042–6. doi: 10.1016/0026-0495(89)90038-3. [DOI] [PubMed] [Google Scholar]
- 54.Zimmermann J, Schramm L, Wanner C, et al. Hemorheology, plasma protein composition and von Willebrand factor in type I diabetic nephropathy. Clin Nephrol. 1996;46:230–6. [PubMed] [Google Scholar]
- 55.Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286–92. doi: 10.1007/s001250050822. [DOI] [PubMed] [Google Scholar]
- 56.Festa A, D’Agostino RJ, Howard G, Mykkanen L, Tracy RP, Haffner SM. Chronic subclinical inflammation as part of the insulin resistance syndrome: the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2000;102:42–7. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 57.Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. N Engl J Med. 1995;332:635–41. doi: 10.1056/NEJM199503093321003. [DOI] [PubMed] [Google Scholar]
- 58.Haverkate F, Thompson SG, Pyke SD, Gallimore JR, Pepys MB. Production of C-reactive protein and risk of coronary events in stable and unstable angina. European Concerted Action on Thrombosis and Disabilities Angina Pectoris Study Group. Lancet. 1997;349:462–6. doi: 10.1016/s0140-6736(96)07591-5. [DOI] [PubMed] [Google Scholar]
- 59.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 60.Danesh J, Whincup P, Walker M, et al. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.El Ghmati SM, Van Hoeyveld EM, Van Strijp JG, Ceuppens JL, Stevens EA. Identification of haptoglobin as an alternative ligand for CD11b/CD18. J Immunol. 1996;156:2542–52. [PubMed] [Google Scholar]
- 62.Hanasaki K, Powell LD, Varki A. Binding of human plasma sialoglycoproteins by the B cell-specific lectin CD22. Selective recognition of immunoglobulin M and haptoglobin. J Biol Chem. 1995;270:7543–50. doi: 10.1074/jbc.270.13.7543. [DOI] [PubMed] [Google Scholar]
- 63.Kristiansen M, Graversen JH, Jacobsen C, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 64.Zwadlo G, Voegeli R, Osthoff KS, Sorg C. A monoclonal antibody to a novel differentiation antigen on human macrophages associated with the down-regulatory phase of the inflammatory process. Exp Cell Biol. 1987;55:295–304. doi: 10.1159/000163432. [DOI] [PubMed] [Google Scholar]
- 65.Hamann W, Floter A, Schmutzler W, Zwadlo-Klarwasser G. Characterization of a novel anti-inflammatory factor produced by RM3/1 macrophages derived from glucocorticoid treated human monocytes. Inflamm Res. 1995;44:535–40. doi: 10.1007/BF01757358. [DOI] [PubMed] [Google Scholar]
- 66.Wenzel I, Roth J, Sorg C. Identification of a novel surface molecule, RM3/1, that contributes to the adhesion of glucocorticoid-induced human monocytes to endothelial cells. Eur J Immunol. 1996;26:2758–63. doi: 10.1002/eji.1830261131. [DOI] [PubMed] [Google Scholar]
- 67.Cid MC, Grant DS, Hoffman GS, Auerbach R, Fauci AS, Kleinman HK. Identification of haptoglobin as an angiogenic factor in sera from patients with systemic vasculitis. J Clin Invest. 1993;91:977–85. doi: 10.1172/JCI116319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Iyengar S, Hamman RF, Marshall JA, Baxter J, Majumder PP, Ferrell RE. Genetic studies of type 2 (non-insulin-dependent) diabetes mellitus: lack of association with seven genetic markers. Diabetologia. 1989;32:690–3. doi: 10.1007/BF00274258. [DOI] [PubMed] [Google Scholar]
- 69.Rich SS, Panter SS, Goetz FC, Hedlund B, Barbosa J. Shared genetic susceptibility of type 1 (insulin-dependent) and type 2 (non-insulin-dependent) diabetes mellitus: contributions of HLA and haptoglobin. Diabetologia. 1991;34:350–5. doi: 10.1007/BF00405008. [DOI] [PubMed] [Google Scholar]
- 70.Awadallah S, Hamad M. The prevalence of type II diabetes mellitus is haptoglobin phenotype-independent. Cytobios. 2000;101:145–50. [PubMed] [Google Scholar]