Abstract
Previous reports have suggested that a high serum cyclosporine A (CsA) level could result in a lower incidence of acute-graft-versus-host disease (aGVHD). An elevated serum lactate dehydrogenase (LDH) level has been reported to be an adverse predictor of outcome in stem cell transplantation (SCT) for acute myeloid leukemia. In this study, we retrospectively analyzed the records of 24 patients who received allogeneic SCT from an HLA-matched sibling donor for acute and chronic myelogenous leukemia. Univariate analysis showed that two factors (the serum CsA level at the third week after SCT and the LDH level at the third week after SCT) were significantly associated with the incidence of aGVHD among several variables (age, sex, stem cell source, cell dose, C-reactive protein, absolute lymphocyte count, conditioning regimens, and time to engraftment). A higher serum level of CsA and lower serum LDH level at the third week after SCT were associated with a lower incidence of aGVHD (P=0.015, 0.030). In multivariate analysis, the serum CsA level (hazard ratio [HR], 0.12; 95% confidence interval [CI], 0.022-0.652, P=0.0014) and serum LDH level (HR, 6.59; 95% CI, 1.197-36.316, P=0.030) at the third week after SCT were found to be independent factors that were significantly associated with the development of aGVHD. We conclude that a high CsA level and low LDH level might predict a low cumulative incidence of aGVHD after allogeneic transplantation from a matched sibling donor.
Keywords: Leukemia, Myeloid, Acute; Cyclosporine; Graft vs Host Disease
INTRODUCTION
Allogeneic stem cell transplantation is a potentially curative treatment for patients with acute and chronic leukemia. However, a substantial proportion of transplant recipients experience acute graft-versus-host disease (aGVHD) after transplantation; overall, aGVHD above grade II is significantly associated with treatment-related mortality.
Although supportive care after allogeneic stem cell transplantation (SCT) has improved in recent years, aGVHD remains a life-threatening complication that occurs at transplantation centers and results in significant morbidity and mortality. The combination of methotrexate (MTx) and cyclosporine A (CsA) has significantly reduced the incidence and severity of aGVHD compared to the use of a single agent (1, 2). In a recent study, investigators found that a high serum CsA level after transplantation was associated with a relatively low incidence of aGVHD above grade II (3).
In a previous study, the early absolute lymphocyte count (ALC) and C-reactive protein (CRP) level after transplantation were found to be associated with significant aGVHD (4, 5), while lactate dehydrogenase (LDH) has rarely been studied. One recent study reported that the serum LDH level was a significant adverse predictor of outcome in HLA-matched sibling bone marrow transplants for acute myelogenous leukemia (AML) (6).
Therefore, the present study was performed to evaluate early risk factors for aGVHD after transplantation, including the serum levels of LDH, CsA, ALC, and CRP.
MATERIALS AND METHODS
Patients
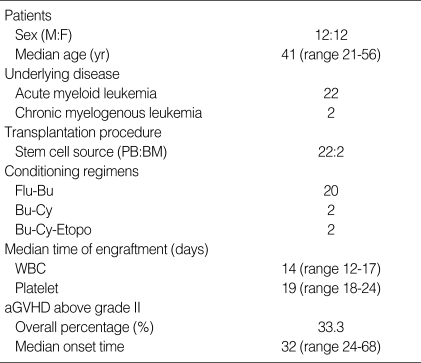
A total of 24 consecutive adult patients who had undergone allogeneic SCT from an HLA-matched sibling donor at Pusan National University Hospital between 2003 and 2006 were included in this retrospective study. The underlying disease was acute myeloid leukemia in 22 patients (91.7%) and chronic myelogenous leukemia in 2 patients (8.3%). The median age of the patients was 41 yr (range 21-56 yr), and the male-to-female ratio was 1:1 (Table 1).
Table 1.
Patient characteristics
PB, peripheral blood; BM, bone marrow; Flu-Bu, fludarabine-busulfan; Bu-Cy, busulfan-cyclophosphamide; Bu-Cy-Etopo, busulfan-cyclophosphamide-etoposide; WBC, white blood cell; aGVHD, acute graft-versus-host disease.
Conditioning regimens
The conditioning regimen included a combination of fludarabine (intravenously, 30 mg/m2 for 6 days) with busulfan (intravenously, 0.8 mg/kg qid for 2 days) in 20 patients (83.3%), or a combination of cyclophosphamide (intravenously, 60 mg/kg for 2 days) and busulfan (0.8 mg/kg qid for 2 days) with or without etoposide (intravenously, 400 mg/m2 for 2 days) in four patients (16.7%) (Table 1).
GVHD prophylaxis
CsA and methotrexate were used for aGVHD prophylaxis. CsA was administered at a dose of 3 mg/kg per day through 24-hr continuous infusion starting on day -1. The dose of CsA was titrated and maintained at a serum level between 200 and 400 ng/mL. The MTx dosage was 5 mg/m2, administered intravenously on days 1, 3, 6, and 11.
Stem cell support and supportive medication
Mononuclear cells (7.25±4.5×108/kg), CD3+ cells (6.0±3.8×108/kg), and CD34+ cells (2.8±1.6×106/kg) were measured in the patients. Granulocyte-colony stimulating factor was administered daily from day +4. All patients received fluconazole for fungal prophylaxis and acyclovir for viral prophylaxis. Trimethoprim-sulfamethoxazole was used as prophylaxis for Pneumocystis pneumonia for at least 6 months.
Engraftment measurement and grading of acute GVHD
Engraftment was defined as an absolute neutrophil count of ≥500/µL and an unsupported platelet count of ≥20,000/µL for two consecutive days or detection of donor DNA by PCR-short tandem repeat (STR). aGVHD was graded uniformly according to the Glucksberg criteria (7).
Statistical considerations
The Mann-Whitney test was used for the comparison of continuous variables. The cumulative incidence of aGVHD was estimated using the Kaplan-Meier method and the log-rank test. In univariate analysis, the serum CsA and LDH level at the third week after allogeneic transplantation were found to significantly affect the incidence of aGVHD above grade II. Cox's proportional regression was used in multivariate models to predict the relative risk of the two factors, the CsA and LDH levels, at the third week after transplantation. All statistical tests were two-sided; P<0.05 was used to indicate statistical significance. Data were analyzed using SPSS 12 for Windows (SPSS Inc., Chicago, IL, U.S.A.) software.
RESULTS
Patient characteristics
Patient characteristics are summarized in Table 1. The median follow-up for the patients was 32 months, with 21 patients surviving with complete chimerism (87.5%); three patients relapsed (12.5%) and two patients died. The cumulative incidence of aGVHD above grade II after transplantation was 33.3% (8 of 24 patients), and the median onset time was 33.5 days (range 24-58 days). Twenty-one patients received a peripheral blood stem cell graft, while two patients received a bone marrow graft. All patients were in their first period of complete remission at the time of transplantation.
Correlation between serum CsA level and cumulative incidence of aGVHD above grade II
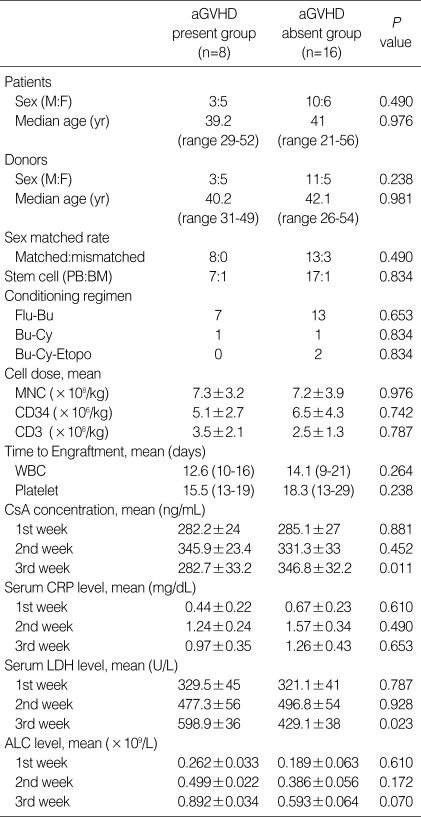
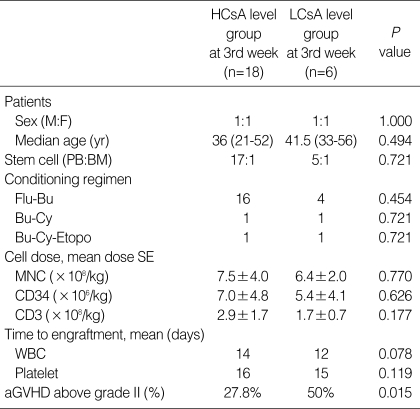
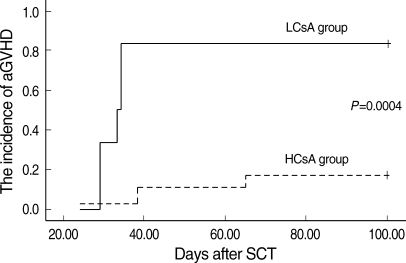
The serum CsA level was measured every other day, and the CsA dose was adjusted to maintain the serum CsA level between 200 and 400 ng/mL. When the patients were grouped according to the mean serum CsA level during each week, into the above 300 ng/mL group (mean CsA level 332.1±15, higher CsA group; HCsA group) and the below 300 ng/mL group (mean CsA level 231.3±28, lower CsA group; LCsA group), serum levels in the first and second week were not correlated with the incidence of aGVHD above grade II (P=0.320) (Table 4). However, in the third week, the HCsA group showed a significantly lower cumulative incidence of aGVHD above grade II compared to the LCsA group (P=0.015) (Table 2, Fig. 1). There were no significant differences in sex, age, stem cell source, conditioning regimen, cell dose, or time to engraftment between the HCsA and the LCsA group in the third week (Table 2).
Table 4.
Comparison between the aGVHD above grade II group and the non-aGVHD group
aGVHD, acute graft-versus-host disease; Flu-Bu, fludarabine-busulfan; Bu-Cy, busulfan-cyclophosphamide; Bu-Cy-Etopo, busulfan-cyclophosphamide-etoposide; MNC, mononuclear cell; WBC, white blood cell; CsA, cyclosporine A; CRP, C-reactive protein; LDH, lactate dehydrogenase; ALC, absolute lymphocyte count.
Table 2.
Comparison between the HCsA group and LCsA group
HCsA, higher cyclosporine A; LCsA, lower cyclosporine A; Flu-Bu, fludarabine-busulfan; Bu-Cy, busulfan-cyclophosphamide; Bu-Cy-Etopo, busulfan-cyclophosphamide-etoposide; MNC, mononuclear cell; WBC, white blood cell; aGVHD, acute graft-versus-host disease.
Fig. 1.
The cumulative incidence of aGVHD of the two serum CsA groups at the third week after transplantation. A higher CsA level was associated with a lower incidence of aGVHD above grade II (P=0.0004).
aGVHD, acute graft-versus-host disease; HCsA, higher cyclosporine A; LCsA, lower cyclosporine A.
The mean CsA level at week three was significantly higher in patients who developed aGVHD above grade II (P=0.011, Table 4). However, there were no significant differences in the mean CsA levels at other weeks between the aGVHD above grade II present and absent groups.
Correlation between serum LDH level and cumulative incidence of aGVHD above grade II
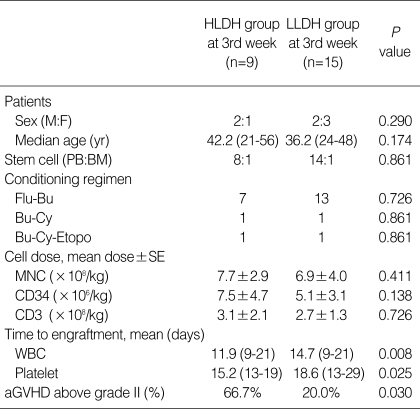
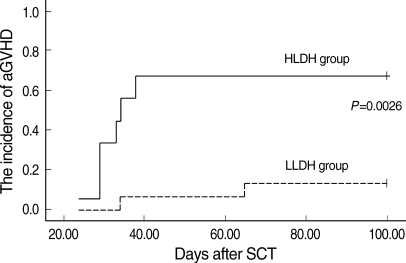
The serum LDH level was measured twice a week after transplantation. When the higher LDH group (HLDH group, mean LDH ≥470 U/L) and lower LDH group (LLDH group, mean LDH <470 U/L) were separated by the upper normal limit (470 U/L), the HLDH group showed a significantly earlier time to engraftment. However, the cumulative incidence of aGVHD above grade II was significantly higher in the HLDH group at the third week (P=0.030, Table 3, Fig. 2). In the group of patients with aGVHD above grade II, the LDH levels at the first and second week were not significantly different (P=0.787, 0.928), but the LDH level at the third week was significantly higher than that in the non-aGVHD group (P=0.023) (Table 4).
Table 3.
Comparison between the HLDH group and LLDH group
HLDH, higher lactate dehydrogenase; LLDH, lower lactate dehydrogenase; Flu-Bu, fludarabine-busulfan; Bu-Cy, busulfan-cyclophosphamide; Bu-Cy-Etopo, busulfan-cyclophosphamide-etoposide; MNC, mononuclear cell; WBC, white blood cell; aGVHD, acute graft-versus-host disease.
Fig. 2.
The cumulative incidence of aGVHD of the two serum LDH groups at the third week after transplantation. A lower LDH level was associated with a lower incidence of aGVHD above grade II (P=0.0026).
aGVHD, acute graft-versus-host disease; HLDH, higher lactate dehydrogenase; LLDH, lower lactate dehydrogenase.
Correlations between ALC, serum CRP level, and cumulative incidence of aGVHD above grade II
ALC was measured at the first, second, and third week after transplantation. ALC levels after transplantation were not significantly associated with the incidence of aGVHD (Table 4). The serum CRP levels did not differ between the aGVHD group and non-aGVHD group in the first, second, or third week (P=0.610, 0.490, 0.653, Table 4).
Risk assessment for aGVHD above grade II using the CsA and LDH levels at the third week after transplantation
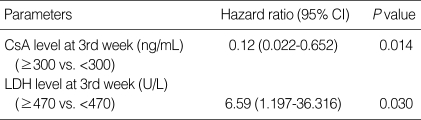
In univariate analysis, the CsA and LDH levels, (not the CRP or ALC level) at the third week after transplantation were significantly associated with aGVHD above grade II (Table 4). In multivariate analysis, the CsA (hazard ratio [HR], 0.12; 95% confidence interval [CI], 0.022-0.652, P=0.014) and LDH (HR, 6.59; 95% CI, 1.197-36.316, P=0.030) levels at the third week were independent risk factors of cumulative incidence of aGVHD above grade II (Table 5).
Table 5.
Results of Cox regression analysis of the cumulative incidence of aGVHD above grade II
aGVHD, acute graft-versus-host disease; CsA, cyclosporine A; LDH, lactate dehydrogenase; CI, confidence interval.
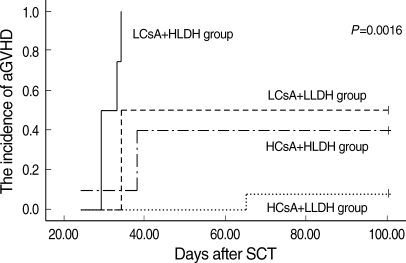
These findings prompted us to measure the predictive value of the combination of these two factors (CsA and LDH) for the cumulative incidence of aGVHD above grade II. The lowest incidence of aGVHD was observed in the HCsA+LLDH group, and the incidence of aGVHD was lower in the HCsA+HLDH group than in the LCsA+LLDH group. The highest incidence of aGVHD was observed in the LCsA+HLDH group (P=0.0016, Fig. 3).
Fig. 3.
The cumulative incidence of aGVHD of four groups (HCsA+LLDH, HCsA+HLDH, LCsA+LLDH, and LCsA+HLDH group) at the third week after transplantation. The HCsA+LLDH group showed significantly better results than the other groups (P=0.0016).
aGVHD, acute graft-versus-host disease; HCsA, higher cyclosporine A; LCsA, lower cyclosporine A; HLDH, higher lactate dehydrogenase; LCsA, lower cyclosporine A.
DISCUSSION
We investigated the relationships between the development of aGVHD after matched sibling allogeneic SCT and several factors, including serum CsA, LDH, CRP, and ALC. CsA is a potent immunosuppressant that is used for aGVHD prophylaxis. One study indicated that the blood CsA concentration, especially at the third week after transplantation, was important in terms of the incidence of aGVHD (3). In this study, the cumulative incidence of aGVHD above grade II was not significantly associated with the serum CsA levels at the first and second week after SCT, but was higher in patients with third week CsA levels lower than 300 ng/mL (LCsA group). This could be explained by the fact that the donor lymphocytes would have proliferated and would have been more activated at the third week compared to the second week after SCT, since the median time to leukocyte engraftment is 14 days. If this is the case, the HCsA group would have a lower incidence of aGVHD at the third week after SCT. Additionally, in this study, an elevated serum CsA level was not associated with renal toxicity, survival, or the incidence of relapse.
In a previous study, ALC after transplantation was found to be associated with aGVHD (5). However, in the present study, despite high-dose conditioning regimens, ALC levels at day 21 following transplantation varied, and were not found to be significantly associated with the incidence of aGVHD. We thought that because ALC included both the host and donor cells, it did not truly reflect overall donor lymphocyte activities.
The serum LDH level is a predictive marker of inflammation or cell turnover. In the present study, only three patients had fever at the first week after SCT. However, the patients did not show any evidence of infection or inflammation after that time. There was also no indication that serum LDH level should be increasing during this time, such as cell turnover, hemolysis, or disease reactivation. The LDH level was also reported to be a prognostic factor in malignancies such as germ cell tumors (8, 9), non-Hodgkin's lymphoma (10, 11), and small cell lung cancer (12). In a recent study, increased LDH levels were shown to adversely affect survival; in another study, a high LDH level was found to be an independent, significantly poor prognostic factor following allogeneic transplantation for acute myeloid leukemia (13). The initiation of aGVHD requires that donor lymphocytes recognize host alloantigens after host tissue damage due to conditioning chemotherapy. Serum LDH is a general indicator of tissue damage (14). In a previous study, elevated LDH was found to result from cell proliferation (15). Therefore, we thought that an elevated LDH level after engraftment may be associated with host organ damage and the repopulation of activated donor lymphocytes; an elevated LDH level may influence the cumulative incidence of aGVHD. In the present study, the LDH levels at the first and second week after transplantation were not found to be associated with the incidence of aGVHD above grade II. When two groups (HLDH group vs. LLDH group) were separated by the upper normal limit of LDH (470 U/L), the HLDH group at the third week had a tendency to have a higher incidence of aGVHD above grade II compared to the LLDH group. At the third week after transplantation, the HLDH group showed a more rapid engraftment rate.
CRP is a well-known marker of inflammation, which is mainly induced by IL-6 (16). IL-6 is produced by many cell types, including monocytes, macrophages, fibroblasts, and endothelial cells, which may survive the conditioning regimen and respond to tissue damage caused by high-dose cytotoxic agents and infections (17). In the present study, CRP levels during the early post-transplantation period were measured, and their associations with the incidence of aGVHD were investigated. However, the impact of CRP on the development of aGVHD was not found to be significant in this study.
In univariate analysis, two factors (serum CsA and LDH levels at the third week after transplantation) were found to be significantly associated with aGVHD above grade II. Four groups were created by combining these two factors (HCsA+HLDH group, HCsA+LLDH group, LCsA+HLDH group, and LCsA+LLDH group). The LCsA+HLDH group had a significantly higher incidence of aGVHD than other groups, while the HCsA+LLDH group had a significantly lower incidence. The HCsA+HLDH group showed a tendency toward a decreased cumulative incidence of aGVHD compared to the LCsA+LLDH group.
Using Cox regression analysis, an elevated CsA level and a lower LDH level were the most important parameters, and were found to be correlated with a lower incidence of aGVHD above grade II. However, due to the small number of patients enrolled in this study, a bias may have affected our analysis. Thus, further, larger-scale studies are needed.
In conclusion, the serum CsA and LDH levels significantly affect the incidence of aGVHD above grade II in the study population. The CsA and LDH levels at the third week after allogeneic stem cell transplantation appear to be the most important factors related to the incidence of aGVHD. aGVHD risk assessment using these two simple predictors is valuable in clinical practice.
Footnotes
This work was supported by two years by Pusan National University Research Grant.
References
- 1.Storb R, Deeg HJ, Whitehead J, Appelbaum F, Beatty P, Bensinger W, Buckner CD, Clift R, Doney K, Farewell V, Hansen J, Hill R, Lum L, Martin P, McGuffin R, Sanders J, Stewart P, Sullivan K, Witherspoon R, Yee G, Thomas ED. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med. 1986;314:729–735. doi: 10.1056/NEJM198603203141201. [DOI] [PubMed] [Google Scholar]
- 2.Storb R, Deeg HJ, Farewell V, Doney K, Appelbaum F, Beatty P, Bensinger W, Buckner CD, Clift R, Hansen J, Hill R, Longton G, Lum L, Martin P, McGuffin R, Sanders J, Singer J, Stewart P, Sullivan K, Witherspoon R, Thomas ED. Marrow transplantation for severe aplastic anemia: methotrexate alone compared with a combination of methotrexate and cyclosporine for prevention of acute graft-versus-host disease. Blood. 1986;68:119–125. [PubMed] [Google Scholar]
- 3.Kanda Y, Hyo R, Yamashita T, Fujimaki K, Oshima K, Onoda M, Mori T, Sakura T, Tanaka M, Sakai M, Taguchi J, Kurakawa M, Maruta A, Okamoto S, Sakamaki H Kanto Study Group of Cell Therapy. Effect of blood cyclosporine concentration on the outcome of hematopoietic stem cell transplantation from an HLA-matched sibling donor. Am J Hematol. 2006;81:838–844. doi: 10.1002/ajh.20710. [DOI] [PubMed] [Google Scholar]
- 4.Schots R, Kaufman L, Van Riet I, Lacor P, Trullemans F, De Waele M, Van Camp B. Monitoring of C-reactive protein after allogeneic bone marrow transplantation identifies patients at risk of severe transplant-related complications and mortality. Bone Marrow Transplant. 1998;22:79–85. doi: 10.1038/sj.bmt.1701286. [DOI] [PubMed] [Google Scholar]
- 5.Lee KH, Choi SJ, Lee JH, Lee JS, Kim WK, Lee KB, Sohn SK, Kim JG, Kim DH, Seol M, Lee YS, Lee JH. Prognostic factors identifiable at the time of onset of acute graft-versus-host disease after allogeneic hematopoietic cell transplantation. Haematologica. 2005;90:939–948. [PubMed] [Google Scholar]
- 6.Kalaycio M, Rybicki L, Pohlman B, Dean R, Sweetenham J, Andresen S, Sobecks R, Sekeres MA, Advani A, Brown S, Bolwell B. Elevated lactate dehydrogenase is an adverse predictor of outcome in HLA-matched sibling bone marrow transplant for acute myelogenous leukemia. Bone Marrow Transplant. 2007;40:753–758. doi: 10.1038/sj.bmt.1705811. [DOI] [PubMed] [Google Scholar]
- 7.Glucksberg H, Storb R, Fefer A, Buckner CD, Neiman PE, Clift RA, Lerner KG, Thomas ED. Clinical manifestations of graft-versus-host disease in human recipients of marrow from HLA matched sibling donors. Transplantation. 1974;18:295–304. doi: 10.1097/00007890-197410000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Von Eyben FE, Blaabjerg O, Hyltoft-Petersen P, Madsen EL, Amato R, Liu F, Fritsche H. Serum lactate dehydrogenase isoenzyme 1 and prediction of death in patients with metastatic testicular germ cell tumors. Clin Chem Lab Med. 2001;39:38–44. doi: 10.1515/CCLM.2001.010. [DOI] [PubMed] [Google Scholar]
- 9.International Germ Cell Cancer Collaborative Group. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- 10.Solal-Céligny P, Roy P, Colombat P, White J, Armitage JO, Arranz-Saez R, Au WY, Bellei M, Brice P, Caballero D, Coiffier B, Conde-Garcia E, Doyen C, Federico M, Fisher RI, Garcia-Conde JF, Guglielmi C, Hagenbeek A, Haioun C, LeBlanc M, Lister AT, Lopez-Guillermo A, McLaughlin P, Milpied N, Morel P, Mounier N, Proctor SJ, Rohatiner A, Smith P, Soubeyran P, Tilly H, Vitolo U, Zinzani PL, Zucca E, Montserrat E. Follicular lymphoma international prognostic index. Blood. 2004;104:1258–1265. doi: 10.1182/blood-2003-12-4434. [DOI] [PubMed] [Google Scholar]
- 11.The International Non-Hodgkin's Lymphoma Prognostic Factors Project. A predictive model for aggressive non-Hodgkin's lymphoma. N Engl J Med. 1993;329:987–994. doi: 10.1056/NEJM199309303291402. [DOI] [PubMed] [Google Scholar]
- 12.Lassen UN, Osterlind K, Hirsch FR, Bergman B, Dombernowsky P, Hansen HH. Early death during chemotherapy in patients with small-cell lung cancer: derivation of a prognostic index for toxic death and progression. Br J Cancer. 1999;79:515–519. doi: 10.1038/sj.bjc.6690080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta J, Gordon LI, Tallman MA, Winter JN, Evens AM, Frankfurt O, Williams SF, Grinblatt D, Kaminer L, Meagher R, Singhal S. Does younger age affect the outcome of reduced-intensity allogeneic hematopoietic stem cell transplantation for hematologic malignancies beneficially? Bone Marrow Transplant. 2006;38:95–100. doi: 10.1038/sj.bmt.1705388. [DOI] [PubMed] [Google Scholar]
- 14.Glick JH., Jr Serum lactate dehydrogenase isoenzyme and total lactate dehydrogenase value in health and disease, and clinical evaluation of these tests by means of discriminant analysis. Am J Clin Pathol. 1969;52:320–328. doi: 10.1093/ajcp/52.3.320. [DOI] [PubMed] [Google Scholar]
- 15.Pan L, Beverley PC, Isaacson PG. Lactate dehydrogenase (LDH) isoenzyme and proliferative activity of lymphoid cells, an immunocytochemical study. Clin Exp Immunol. 1991;86:240–245. doi: 10.1111/j.1365-2249.1991.tb05803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schultz DR, Arnold PI. Properties of four acute phase proteins: C-reactive protein, serum amyloid A protein, alpha 1-acid glycoprotein and fibrinogen. Semin Arthritis Rheum. 1990;20:129–147. doi: 10.1016/0049-0172(90)90055-k. [DOI] [PubMed] [Google Scholar]
- 17.Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]