Abstract
Biliary cyst tumors (cystadenoma and cystadeno-carcinoma) are an indication for liver resection. They account for only 5% of all solitary cystic lesions of the liver, but differential diagnosis with multiloculated or complicated biliary cysts, atypical hemangiomas, hamartomas and lymphangiomas may be difficult. The most frequent challenge is to differentiate biliary cyst tumors from hemorrhagic cysts. Computerized tomography (CT) and magnetic resonance imaging (MRI) are often not diagnostic and in these cases fine needle aspiration (FNA) is used to confirm the presence of atypical biliary cells. FNA, however, lacks adequate sensitivity and specificity and should always be used in conjunction with imaging. Pre-operative differentiation of cystadenoma from cystadenocarcinoma is impossible and surgery must be performed if a biliary cyst tumor is suspected. When multiple cystic lesions are observed throughout the liver parenchyma, it is important to exclude liver metastasis, of which colonic cancer is the most common primary site. Multiple biliary hamartomas (von Meyenburg complex) can appear as a mixture of solid and cystic lesions and can be confused with cystic metastasis. Strong and uniform T2 hyperintensity on MRI is usually diagnostic, but occasionally a percutaneous biopsy may be required.
Keywords: Biliary cyst tumor, Liver cystic neoplasia, Cystadenoma, Cystadenocarcinoma, Atypical hepatic cysts
INTRODUCTION
Although rare, cystic neoplasms of the liver may represent a diagnostic challenge in everyday practice. Cystic tumors may be solitary or multiple and vary from the most benign (e.g. simple cysts, hamartomas) to potentially malignant (cystadenoma) or overtly malignant (cystadenocarcinoma). There are also atypical cystic presentations of normally non-cystic tumors, like cystic hemangiomas or cystic hepatocarcinomas and congenital diseases presenting as diffuse cyst-like involvement of the liver (Caroli’s disease and von Meyenburg complex) that must be differentiated from cystic metastasis. The most important issue in the case of solitary cystic tumors is to distinguish biliary cystadenoma and cystadenocarcinoma from other benign conditions that require only observation of the patient.
SOLITARY CYSTIC TUMORS
Cystadenoma is a biliary cyst tumor arising from biliary epithelium. With its malignant counterpart (cystadenocarcinoma), it accounts for less than 5% of all cystic lesions of the liver; but it is dangerous for its propensity toward local recurrence and malignant change[1,2]. These tumors usually present in middle aged women with a mean age of 50 years and have a great variability in size, ranging from 1.5 cm to 30 cm. The majority of patients are asymptomatic, but in the case of large tumors they may present with a palpable mass and cause symptoms[3]. Cystadenocarcinoma can arise de novo or from a pre-existent cystadenoma, from which it is difficult to differentiate since both have a multiloculated appearance at ultrasound (US), computerized tomography (CT) and magnetic resonance imaging (MRI). Cystadenoma has thinner septa and a less thick and more regular walls than cystadenocarcinoma[4]. However, internal papillary projections and foldings with arterial enhancement of the external wall at dynamic CT scan and MRI may be present in both tumors, so that imaging itself cannot reliably differentiate these neoplasms. Internal septa and papillary projections are more often hypovascular in the case of cystadenoma, and the demonstration of vascular signals at color Doppler can be a sign of its malignant transformation (Figure 1). The diagnosis of cystadenocarcinoma is straightforward only when ultrasound, CT scan or MRI shows nodular septa and thick, irregular walls with strong contrast enhancement, but in many cases there are overlapping features. Intracystic hemorrhage and fine punctuate calcifications may present in both conditions and can also be observed in complicated hemorrhagic cysts, their role as diagnostic criterion are, therefore, doubtful[5]. Fine needle aspiration can be used to differentiate a biliary cyst from other benign conditions and from a single metastasis, but is totally unreliable in differentiating cystadenoma from cystadenocarcinoma, since small neoplastic foci are undetectable by fine needle aspiration and may be revealed only in the surgical specimen[6].
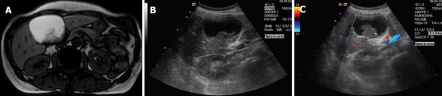
Figure 1.
T1 weighted MR image (A) and ultrasound examination (B) showing a large cystic lesion of the left liver lobe with a thick and irregular septum, raising the suspicion of a biliary cystadenoma, and Doppler ultrasound (C) showing vascular signals within the septum disclosing a focus of cystadenocarcinoma confirmed in the surgical specimen.
In conclusion, there is no definite, reliable criterion for differentiating cystadenoma from cystadeno-carcinoma and the correct diagnosis is often made only in the surgical specimen.
The majority of biliary cyst tumors do not usually communicate with the bile ducts, although direct luminal communication is occasionally observed. In these cases, dysplastic mucinous epithelium itself may proliferate within the bile ducts and cause obstruction[7]. This variant is considered an intraductal papillary neoplasm with prominent cystic dilatation rather than a true biliary cyst neoplasm and must be differentiated from biliary papillomatosis and cholangiocarcinoma of intraductal growth type. CT and MRI are insufficient to show the luminal communication, probably because the communication is too narrow, while intraoperative cholangiography can establish the correct diagnosis. Intraductal spreading of neoplastic cells into the bile duct portends a poorer prognosis of this variant of biliary cyst tumor.
If it is practically impossible to distinguish pre-operatively cystadenoma from cystadenocarcinoma, every effort should be made to differentiate these neoplasms from biliary cysts and other benign tumors without malignant potential. In case of simple cysts, without internal septa or papillary projections, cystadenoma can be reliably excluded and the patient can only be observed. In case of multiloculated biliary or complicated biliary cyst (hemorrhagic cyst), imaging is often not reliable in ruling out cystadenoma. The most challenging differential diagnosis is hemorrhagic cysts where ultrasound can visualize internal clots as papillary excrescences or nodular and irregular septal images. CT scan is less sensitive than ultrasound in visualizing intracystic blood clots and at times it can only depict homogeneous low density areas inside a huge cyst. In these cases, a discrepancy between US and CT may suggest the correct diagnosis of hemorrhagic cysts. Recently MRI has been shown to be helpful. It was reported that high signals from clot formation could be detected both in T1 and in T2 weighted sequences, and are useful in differentiating it from cystadenocarcinoma, which usually exhibits low signals[8]. It should be noted that if a hemorrhagic cyst is suspected, MRI should not be delayed because hemorrhagic signal intensity becomes rapidly low when clots are liquefied[9]. History, on the contrary, is totally unreliable to suspect a hemorrhagic cyst since intracystic hemorrhage can occur in the absence of any symptom[10]. In the presence of a complicated or multiloculated hepatic cyst, a fine needle aspiration (FNA) of intracystic fluid can be performed to rule out a biliary cyst neoplasm. The presence of atypical cells, mucinous material and elevated levels of CEA and CA19-9 in the cystic fluid has been typically observed both in cystadenoma and in cystadenocarcinoma, but not in hepatic cysts[11,12] although CA19-9 is equally expressed in paraffin-embedded tissues from both hepatic cysts and biliary cyst neoplasms[13]. To complicate things further, high CA19-9 cyst-fluid levels have been occasionally found in complicated hepatic cysts[14] and data on CA19-9 serum levels are scanty and inconclusive[13,15]. The macroscopic appearance of the intracystic fluid is likewise useless since in biliary cyst tumors it can be mucinous, but also bile stained or clear, as it occurs when there is abundance of ovary stroma[16]. On the whole, FNA alone does not have adequate sensitivity and specificity to confirm or exclude biliary cyst tumors and should be always evaluated together with imaging.
Similar to intracystic bleeding, non suppurative, granulomatous infection of a biliary cyst may simulate cystadenoma and cystadenocarcinoma by the presence of a thickened wall and a solid component infiltrating the peri-cystic surrounding tissue, thus mimicking a neoplastic lesion[17]. On the other hand, suppurated hepatic cysts and echinococcal cysts do not generally pose diagnostic problems. Sonography can easily differentiate the mobile internal debris typical of abscess formation or the multilayered appearance of the echinococcal cystic wall, and only an unexperienced sonographer can confuse the multiloculated appearance of cystadenoma with multiple daughter cysts of echinococcus[18]. When in doubt, the presence of anti-echinococcal antibodies is diagnostic.
Mesenchymal hamartoma is an uncommon benign lesion composed of bile ducts, immature mesenchymal cells and hepatocytes and may appear as a multiseptated cyst, causing confusion with biliary cystadenoma. Isolated septal calcification can be observed in both lesions and does not aid in the correct diagnosis[19]. Most of the cases are diagnosed in childhood when it presents as a large cystic mass[20] and very few cases have been reported in adults[21]. FNA can be diagnostic by showing clusters of both epithelial and mesenchymal spindle-shaped cells with pieces of loose connective tissue[22].
Ciliated hepatic foregut cyst is a very rare benign lesion arising from an abnormal budding of the primitive foregut and lined by stratified ciliated columnar cells, similar to the bronchial epithelium. These cysts are often anechogenic, but at times they may show internal echoes and can reach considerable dimensions posing differential diagnostic problems of cystadenoma. The typical subcapsular location in segment IV and the presence of a strong T2 hyper-intensity with T1 signal variability on MRI are quite characteristic. Another helpful clue may be the presence of scattered hyperecogenic spots within the cystic wall with no acoustic shadowing, which are related to cartilaginous remnants[23].
Abdominal lymphangioma may be occasionally located in the liver and appear as a single multiseptated lesion[24]. If this diagnosis is suspected, fine needle aspiration is not recommended due to the risk of massive lymphorrhea[25] and complete surgical resection should be accomplished, even without a precise pre-operative diagnosis.
Differential diagnosis with cystic hemangioma may also be a problem, especially in the case of a giant hemangioma, with large hypoechogenic central areas simulating the giant cystadenoma. CT and magnetic resonance imaging point out to the correct diagnosis by showing an enhancing rim with globular vessels and centripetal filling, with sparing of the large central lacunar areas[26].
Hepatocellular carcinoma and cholangiocarcinoma may occasionally present as a large hypodense and multiseptated mass at CT scan, simulating a cystic lesion or a biliary cyst tumor. In these cases, arterial phase enhancement on dynamic CT and washing out of the contrast material in the portal phase, are diagnostic[27].
Isolated mucin producing metastasis from melanoma or colon adenocarcinoma may at times simulate biliary cyst tumors and even benign hepatic cysts. These metastases may be associated to segmental dilatation of the peripheral bile ducts, caused by the presence of mucin casts occluding the bile ducts themselves[28].
Once a diagnosis of cystadenoma is made, surgery should be performed in any case, because differential diagnosis with cystadenocarcinoma is unreliable and cystadenoma itself has a malignant potential. If the pre-operative diagnostic work up, including cytologic aspiration of the lesion, has not produced a definitive diagnosis and surgery is performed because of a suspected bile cyst tumor, an intraoperative biopsy of the lesion is recommended since an extensive lymph node resection would be required if the tumor is proved to be a cholangiocarcinoma[14]. It is important that final decisions regarding indications and type of intervention are jointly discussed by the surgeon and a radiologist expert in liver tumor imaging, particularly in the case of cystic liver tumors, the most frequent mistake is the resection of a complicated biliary cyst incorrectly diagnosed as a cystadenoma.
MULTIPLE CYSTIC TUMORS
When multiple cystic lesions are observed throughout the liver parenchyma, the most important diagnostic problem is to exclude cystic liver metastases. The primary tumor is usually colonic adenocarcinoma, melanoma, carcinoid, breast or renal or ovarian cancer. Colonic cancer is the common, accounting for about 50% of all hepatic metastases[27]. The presence of intra- or peri-tumoral calcifications may suggest a specific diagnosis, being more frequent in the case of gastrointestinal, ovarian, breast and renal metastases compared to other types of tumors[29]. The cystic nature of the metastasis is secondary to the rapid growth and insufficient hepatic arterial supply of the lesion, leading to a large central necrosis simulating a cyst[30].
The differential diagnosis with polycystic liver disease and multiple liver abscesses is usually an easy task on US and CT scan. In case of cystic metastases, the borders of the cystic lesions are heterogeneous and ill-defined, the cystic wall is irregular and the vessels are amputated, but not displaced as in polycystic liver disease. In addition, cystic metastasis has a peripheral enhancing rim on the arterial phase of CT scan and MRI[27], while polycystic liver disease does not show any type of enhancement[31]. Another helpful sign may be the presence of peribiliary cysts, such as small cysts with a diameter of less than 10 mm, located within the hilum and adjacent to the hepatic ducts, more frequently on the left. These small cysts are typically observed only in polycystic liver disease and should not be confused with the segmental biliary dilatations occasionally observed in the case of metastasis or macronodular cirrhosis, which are less regular and never adjacent to the main ducts[32].
Multiple hepatic pyogenic microabscesses are easy to differentiate from metastasis by clinical symptoms and the presence of a late faint enhancing peripheral rim on CT scan and MRI[33]. This rim is quite different from the early arterial enhancing ring observed in the case of metastasis. An additional sign pointing to liver abscesses is the presence of air densities inside the lesions, which is almost never observed in the case of cystic metastasis.
The differential diagnosis with other types of ductal plate malformations, such as Caroli’s disease, Caroli’s syndrome and von Meyenburg complex, may at times be more difficult. Caroli’s disease is a congenital autosomal recessive malformation characterized by diffuse or segmental cystic dilation of the intrahepatic biliary system. In Caroli’s syndrome, periportal congenital fibrosis or multicystic renal diseases are observed in addition to biliary dilations. In both cases, these dilations are less regular than in polycystic liver disease and have intraluminal protrusions or may be associated to segmental dilation of the intrahepatic bile ducts, thus simulating cholangiocarcinoma or multiple cystic metastases. The presence of the “dot signs”, such as an intracystic portal branch, surrounded by the dilated bile duct and the demonstration of a communication of the cysts with the biliary system on MR cholangiography, is diagnostic and excludes liver metastasis[34].
Von Meyenburg complex and bile duct hamartoma are small focal developmental lesions composed of innumerable dilated bile ducts mixed with a dense collagenous stroma. The dilated bile duct foci, contrary to those observed in Caroli’s disease and Caroli’s syndrome do not communicate with the biliary system[16]. Depending on the prevalence of fibrous stroma or biliary dilation, these lesions can appear as predominantly solid and cystic or intermediate and may be easily confused with metastases, microabscesses and even biliary cystadenocarcinoma[35]. Occasionally, the differential diagnosis may be a real dilemma. Although biliary hamartomas are more uniform in size and measure less than 1 cm in diameter, CT scan and ultrasound are often not specific. MRI, on the contrary, can be very helpful by identifying strong hyper-intensity in biliary hamartomas on heavily T2 weighted images (Figure 2), often with a signal intensity similar to that of the spleen[36]. These features, however, are not always sufficient to make a precise diagnosis, especially in intermediate or predominantly solid von Meyenburg complex and a percutaneous or even surgical biopsy is occasionally required[37]. The issue is further complicated by very rare reports on malignant transformation of these hamartomas to cholangiocarcinoma, making this type of cystic malformation the most challenging to differentiate from cystic neoplastic lesions.
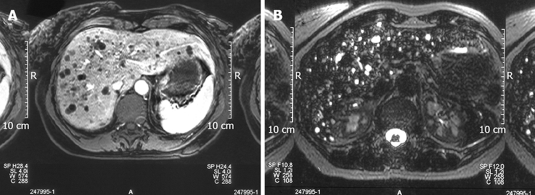
Figure 2.
T1 weighted images showing the left multiple hypointense focal lesions of the liver parenchyma simulating metastases (A) and T2 weighted images showing a strong hyper-intensity in Von Meyenburg complex disclosing its cystic nature (B).
Acknowledgments
The authors are indebted to Dr. Luigi Grazioli for kindly providing iconographic material.
Peer reviewer: Dr. Nahum Méndez-Sánchez, Departments of Gastroenterology and Liver Unit, Medica Sur Clinic and Foundation, Puente de Piedra 150, Col. Toriello Guerra, Mexico City 14050, Mexico
S- Editor Zhong XY L- Editor Wang XL E- Editor Lu W
References
- 1.Wheeler DA, Edmondson HA. Cystadenoma with mesenchymal stroma (CMS) in the liver and bile ducts. A clinicopathologic study of 17 cases, 4 with malignant change. Cancer. 1985;56:1434–1445. doi: 10.1002/1097-0142(19850915)56:6<1434::aid-cncr2820560635>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 2.Devaney K, Goodman ZD, Ishak KG. Hepatobiliary cystadenoma and cystadenocarcinoma. A light microscopic and immunohistochemical study of 70 patients. Am J Surg Pathol. 1994;18:1078–1091. [PubMed] [Google Scholar]
- 3.Kim HG. [Biliary cystic neoplasm: biliary cystadenoma and biliary cystadenocarcinoma] Korean J Gastroenterol. 2006;47:5–14. [PubMed] [Google Scholar]
- 4.Teoh AY, Ng SS, Lee KF, Lai PB. Biliary cystadenoma and other complicated cystic lesions of the liver: diagnostic and therapeutic challenges. World J Surg. 2006;30:1560–1566. doi: 10.1007/s00268-005-0461-7. [DOI] [PubMed] [Google Scholar]
- 5.Choi BI, Lim JH, Han MC, Lee DH, Kim SH, Kim YI, Kim CW. Biliary cystadenoma and cystadenocarcinoma: CT and sonographic findings. Radiology. 1989;171:57–61. doi: 10.1148/radiology.171.1.2648477. [DOI] [PubMed] [Google Scholar]
- 6.Del Poggio P, Jamoletti C, Forloni B, De Benedictis R, Mattiello M, Corti D, Pezzica E. Malignant transformation of biliary cystadenoma: a difficult diagnosis. Dig Liver Dis. 2000;32:733–736. doi: 10.1016/s1590-8658(00)80339-4. [DOI] [PubMed] [Google Scholar]
- 7.Zen Y, Fujii T, Itatsu K, Nakamura K, Konishi F, Masuda S, Mitsui T, Asada Y, Miura S, Miyayama S, et al. Biliary cystic tumors with bile duct communication: a cystic variant of intraductal papillary neoplasm of the bile duct. Mod Pathol. 2006;19:1243–1254. doi: 10.1038/modpathol.3800643. [DOI] [PubMed] [Google Scholar]
- 8.Vilgrain V, Silbermann O, Benhamou JP, Nahum H. MR imaging in intracystic hemorrhage of simple hepatic cysts. Abdom Imaging. 1993;18:164–167. doi: 10.1007/BF00198056. [DOI] [PubMed] [Google Scholar]
- 9.Gomori JM. Head and neck hemorrhage in Kressel HY editor. Magnetic Resonance annual 1987. New York: Raven; 1987. pp. 71–112. [PubMed] [Google Scholar]
- 10.Kitajima Y, Okayama Y, Hirai M, Hayashi K, Imai H, Okamoto T, Aoki S, Akita S, Gotoh K, Ohara H, et al. Intracystic hemorrhage of a simple liver cyst mimicking a biliary cystadenocarcinoma. J Gastroenterol. 2003;38:190–193. doi: 10.1007/s005350300032. [DOI] [PubMed] [Google Scholar]
- 11.Pinto MM, Kaye AD. Fine needle aspiration of cystic liver lesions. Cytologic examination and carcinoembryonic antigen assay of cyst contents. Acta Cytol. 1989;33:852–856. [PubMed] [Google Scholar]
- 12.Horsmans Y, Laka A, Gigot JF, Geubel AP. Serum and cystic fluid CA 19-9 determinations as a diagnostic help in liver cysts of uncertain nature. Liver. 1996;16:255–257. doi: 10.1111/j.1600-0676.1996.tb00738.x. [DOI] [PubMed] [Google Scholar]
- 13.Park KH, Kim JS, Lee JH, Kim HJ, Kim JY, Yeon JE, Park JJ, Byun KS, Bak YT, Lee CH. [Significances of serum level and immunohistochemical stain of CA19-9 in simple hepatic cysts and intrahepatic biliary cystic neoplasms] Korean J Gastroenterol. 2006;47:52–58. [PubMed] [Google Scholar]
- 14.Shimada M, Takenaka K, Gion T, Fujiwara Y, Taguchi K, Kajiyama K, Shirabe K, Sugimachi K. Treatment strategy for patients with cystic lesions mimicking a liver tumor: a recent 10-year surgical experience in Japan. Arch Surg. 1998;133:643–646. doi: 10.1001/archsurg.133.6.643. [DOI] [PubMed] [Google Scholar]
- 15.Kim K, Choi J, Park Y, Lee W, Kim B. Biliary cystadenoma of the liver. J Hepatobiliary Pancreat Surg. 1998;5:348–352. doi: 10.1007/s005340050058. [DOI] [PubMed] [Google Scholar]
- 16.Precetti S, Gandon Y, Vilgrain V. [Imaging of cystic liver diseases] J Radiol. 2007;88:1061–1072. doi: 10.1016/s0221-0363(07)89919-7. [DOI] [PubMed] [Google Scholar]
- 17.Kawashita Y, Kamohara Y, Furui J, Fujita F, Miyamoto S, Takatsuki M, Abe K, Hayashi T, Ohno Y, Kanematsu T. Destructive granuloma derived from a liver cyst: a case report. World J Gastroenterol. 2006;12:1798–1801. doi: 10.3748/wjg.v12.i11.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewall DB, McCorkell SJ. Hepatic echinococcal cysts: sonographic appearance and classification. Radiology. 1985;155:773–775. doi: 10.1148/radiology.155.3.3890008. [DOI] [PubMed] [Google Scholar]
- 19.Konez O, Goyal M, Vyas PK, Boinapally SB. Mesenchymal hamartoma of the liver. Comput Med Imaging Graph. 2001;25:61–65. doi: 10.1016/s0895-6111(00)00053-7. [DOI] [PubMed] [Google Scholar]
- 20.Papastratis G, Margaris H, Zografos GN, Korkolis D, Mannika Z. Mesenchymal hamartoma of the liver in an adult: a review of the literature. Int J Clin Pract. 2000;54:552–554. [PubMed] [Google Scholar]
- 21.Brkic T, Hrstic I, Vucelic B, Jakic-Razumovic J, Skegro M, Romic B, Cukovic-Cavka S, Pulanic R, Ostojic R. Benign mesenchymal liver hamartoma in an adult male: a case report and review of the literature. Acta Med Austriaca. 2003;30:134–137. [PubMed] [Google Scholar]
- 22.al-Rikabi AC, Buckai A, al-Sumayer S, al-Damegh S, al-Bassam AR. Fine needle aspiration cytology of mesenchymal hamartoma of the liver. A case report. Acta Cytol. 2000;44:449–453. doi: 10.1159/000328497. [DOI] [PubMed] [Google Scholar]
- 23.Del Poggio P, Jamoletti C, Mattiello M, Corti D, Pezzica E. Images in Hepatology. Ciliated hepatic foregut cyst. J Hepatol. 2003;39:1090. doi: 10.1016/s0168-8278(03)00462-8. [DOI] [PubMed] [Google Scholar]
- 24.Allen JG, Riall TS, Cameron JL, Askin FB, Hruban RH, Campbell KA. Abdominal lymphangiomas in adults. J Gastrointest Surg. 2006;10:746–751. doi: 10.1016/j.gassur.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Damascelli B, Spagnoli I, Garbagnati F, Ceglia E, Milella M, Masciadri N. Massive lymphorrhoea after fine needle biopsy of the cystic haemolymphangioma of the liver. Eur J Radiol. 1984;4:107–109. [PubMed] [Google Scholar]
- 26.Coumbaras M, Wendum D, Monnier-Cholley L, Dahan H, Tubiana JM, Arrive L. CT and MR imaging features of pathologically proven atypical giant hemangiomas of the liver. AJR Am J Roentgenol. 2002;179:1457–1463. doi: 10.2214/ajr.179.6.1791457. [DOI] [PubMed] [Google Scholar]
- 27.Alobaidi M, Shirkhoda A. Malignant cystic and necrotic liver lesions: a pattern approach to discrimination. Curr Probl Diagn Radiol. 2004;33:254–268. doi: 10.1067/j.cpradiol.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Tokai H, Kawashita Y, Eguchi S, Kamohara Y, Takatsuki M, Okudaira S, Tajima Y, Hayashi T, Kanematsu T. A case of mucin producing liver metastases with intrabiliary extension. World J Gastroenterol. 2006;12:4918–4921. doi: 10.3748/wjg.v12.i30.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sica GT, Ji H, Ros PR. CT and MR imaging of hepatic metastases. AJR Am J Roentgenol. 2000;174:691–698. doi: 10.2214/ajr.174.3.1740691. [DOI] [PubMed] [Google Scholar]
- 30.Mortele KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics. 2001;21:895–910. doi: 10.1148/radiographics.21.4.g01jl16895. [DOI] [PubMed] [Google Scholar]
- 31.Gupta S, Seith A, Dhiman RK, Chawla YK, Sud K, Kohli HS, Sakhuja V, Suri S. CT of liver cysts in patients with autosomal dominant polycystic kidney disease. Acta Radiol. 1999;40:444–448. doi: 10.3109/02841859909177762. [DOI] [PubMed] [Google Scholar]
- 32.Terayama N, Matsui O, Hoshiba K, Kadoya M, Yoshikawa J, Gabata T, Takashima T, Terada T, Nakanuma Y, Shinozaki K. Peribiliary cysts in liver cirrhosis: US, CT, and MR findings. J Comput Assist Tomogr. 1995;19:419–423. doi: 10.1097/00004728-199505000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Mortele KJ, Segatto E, Ros PR. The infected liver: radiologic-pathologic correlation. Radiographics. 2004;24:937–955. doi: 10.1148/rg.244035719. [DOI] [PubMed] [Google Scholar]
- 34.Brancatelli G, Federle MP, Vilgrain V, Vullierme MP, Marin D, Lagalla R. Fibropolycystic liver disease: CT and MR imaging findings. Radiographics. 2005;25:659–670. doi: 10.1148/rg.253045114. [DOI] [PubMed] [Google Scholar]
- 35.Karahan OI, Kahriman G, Soyuer I, Ok E. Hepatic von Meyenburg complex simulating biliary cystadenocarcinoma. Clin Imaging. 2007;31:50–53. doi: 10.1016/j.clinimag.2006.08.022. [DOI] [PubMed] [Google Scholar]
- 36.Maher MM, Dervan P, Keogh B, Murray JG. Bile duct hamartomas (von Meyenburg complexes): value of MR imaging in diagnosis. Abdom Imaging. 1999;24:171–173. doi: 10.1007/s002619900469. [DOI] [PubMed] [Google Scholar]
- 37.Eisenberg D, Hurwitz L, Yu AC. CT and sonography of multiple bile-duct hamartomas simulating malignant liver disease (case report) AJR Am J Roentgenol. 1986;147:279–280. doi: 10.2214/ajr.147.2.279. [DOI] [PubMed] [Google Scholar]