Abstract
Background
Saethre-Chotzen syndrome (SCS) is a syndromic craniosynostosis defined by a genetic mutation affecting the TWIST1 gene on chromosome 7p21. SCS is typically associated with uni- or bi-coronal synostosis, eyelid ptosis, dysmorphic external ears and other variable facial and limb abnormalities. Surgical management of the craniosynostosis addresses the calvarial deformity and may relieve or reduce risk of intracranial hypertension. The aim of this study was to assess surgical intervention, with particular consideration of reoperation rate for intracranial hypertension, in SCS patients.
Method
A retrospective casenote analysis was performed on all patients with a confirmed TWIST1 gene abnormality, who attended the Oxford Craniofacial Unit over a 15-year period. Each patient’s mutation and clinical features were recorded. Surgical intervention and sequelae were examined in greater detail.
Results
Thirty-four patients with genetically confirmed SCS were identified. All had craniosynostosis (bicoronal 76%, unicoronal 18%, bicoronal and sagittal 6%), and the majority had eyelid ptosis, low frontal hairline and external ear anomalies. Thirty-one cases had received surgical intervention. Nine of 26 patients (35%) with minimum 12 month follow-up after primary intervention, and 8 of 19 patients (42%) with minimum 5 year follow-up, developed intracranial hypertension necessitating secondary calvarial surgery.
Conclusions
Despite standard surgical intervention, patients with SCS have a high rate (35 - 42%) of recurrent intracranial hypertension necessitating further surgical expansion. All patients with either bicoronal synostosis or unicoronal synostosis with syndromic features, should be screened for TWIST1 mutations as this confers a greater risk than non-syndromic synostosis of the same sutures. Regular follow-up of SCS patients throughout the childhood years is essential.
BACKGROUND
Craniosynostosis is a congenital disorder characterised by abnormal craniofacial growth due to premature fusion of calvarial sutures. Saethre-Chotzen syndrome (SCS) is one of the major craniosynostosis syndromes, described by Saethre in 19311 and Chotzen in 19322. It was subsequently delineated in greater detail by Pantke et al. in 19753.
SCS is inherited as an autosomal dominant condition with high penetrance and variable expressivity, although cases do occur due to new mutations. The genetic basis is now well understood: heterozygous intragenic mutations, microdeletions or translocations affecting the TWIST1 gene, located on the short arm of chromosome 7 (7p21.1), lead to the syndrome4-7. SCS has an estimated incidence of 1:25,000 to 1:50,000 live births. Some authors have suggested that this is an underestimate as many cases are mild and do not attend a specialised treatment centre8. However there is equally the possibility of overestimation as many previous clinical studies of SCS are likely to have inadvertently included patients with Muenke syndrome (caused by heterozygous P250R mutation in fibroblast growth factor receptor type 3, encoded by the FGFR3 gene), which has a similar phenotype but a distinct genetic basis9-11.
The clinical features of TWIST1-confirmed SCS have been extensively studied, as there is no single pathognomonic feature associated with the syndrome7,8,10,12-14. Typically, the synostosis affects one or both of the coronal sutures, and usually leads to a pattern of frontal plagiocephaly or brachycephaly. The most consistent facial findings include low set frontal hairline, eyelid ptosis, broad and indented nasal bridge, and external ear anomalies8,10,12,14 (Figure 1). Common limb anomalies include soft tissue syndactyly and broad great toes10,13,14.
Figure 1.
Clinical images of 8 month old girl with asymmetric bicoronal synostosis in Saethre-Chotzen syndrome. She has brachycephaly, right eyelid ptosis, and mild facial asymmetry (slight elevation of left eyebrow and deviation of nose and chin point to the right). The lateral view displays the indented nasal bridge, and dysmorphic external ear with prominent transverse crus.
Surgery for the psychosocial benefits associated with an improved craniofacial appearance is widely accepted. The potential for intracranial hypertension (elevated intracranial pressure, ICP) is also a significant concern in SCS. Intracranial hypertension in SCS is typically due to cranio-cephalic disproportion, as other causes (including hydrocephalus and venous hypertension) are rare15,16. Presently, the frequency of primary intracranial hypertension in SCS is not known. Kress et al.14 described a rate of intracranial hypertension of 35% in their TWIST1-confirmed SCS group of 71 patients, although in most cases the diagnosis was based solely on clinical signs.
Unrelieved intracranial hypertension can lead to neurological impairment, including visual loss or developmental delay. This may progress to seizures and even death. Calvarial surgery addresses cranio-cephalic disproportion to relieve or prevent intracranial hypertension. Despite primary surgical intervention, many SCS patients require re-operation due to symptomatic elevated ICP, due to restenosis. Previous small studies of clinically diagnosed SCS cases suggested that this occurs in 18-20% of patients17-19. Wong et al.20 described 20 clinically diagnosed SCS patients who required reoperation in 65% for supraorbital rim position, but do not comment on ICP. There exists no previous study that has assessed the frequency of intracranial hypertension following surgical intervention in genetically confirmed SCS.
The aim of this study was therefore to assess the morbidity and outcome following surgical treatment of SCS, with particular respect to incidence of subsequent intracranial hypertension and necessity for secondary procedures.
METHOD
A retrospective casenote review of all patients with a diagnosis of TWIST1-positive SCS, attending the Oxford Craniofacial Unit during a 15 year period between July 1993 and June 2008, was performed. Genetic testing of TWIST1 for intragenic mutations, deletions and mutations was performed either at the University of Oxford (until 2003) or by the Oxford Medical Genetics Laboratories (after 2003)7,21-23. Thirty-four patients were identified and the clinical features of these patients assessed. Criteria for clinical suspicion of intracranial hypertension included headache, aggressive behaviour or deteriorating school performance, slowing cranial growth on serial head circumference assessment, and papilloedema on fundoscopy. All patients underwent CT radiography for assessment of calvarial shape, synostosis and intracranial space. Radiological features of intracranial hypertension included copperbeating of the inner calvarial surface, small ventricles with effaced basal cisterns and sulcal spaces, and tonsillar herniation.
The timing and rationale for operative intervention were recorded. This included documentation of all ICP assessments performed for clinical or radiological signs suggestive of intracranial hypertension. ICP measurement was obtained by placing an intraparenchymal Codman Microsensor (Codman; LeLochle, Switzerland) in the right frontal lobe via a small burrhole under general anaesthetic24. The pressure was monitored for a period of 24 hours while the child performed normal activities. It was recorded as elevated if 24 hour monitoring showed a mean pressure equal to, or greater than, 20 mm Hg, or 4 or more B waves during this period. If the initial 24 hour reading was neither clearly elevated nor entirely normal, a further 24 hour period of monitoring was performed.
Primary surgical intervention was performed between 9 and 15 months of age where possible, and comprised two main groups. Most patients were treated with a standard fronto-orbital advancement and remodelling (FOAR) as a single definitive procedure. More recently, severely turribrachycephalic patients have been managed with planned dual-stage intervention: primary posterior calvarial release to increase intracranial volume and prevent progressive deformity, followed by standard FOAR25.
Follow-up was performed in a multidisciplinary clinic at 3 months after surgery, then annually unless the parents requested earlier review. Sequelae of surgery were assessed. Where reoperation was required for raised ICP, clinical and radiological features were noted. Secondary surgery was individualised to address recurrent intracranial hypertension in the optimal aesthetic manner. The frequency of secondary surgery in comparative groups was analysed statistically using Fisher’s exact test, except, in the case of analysis by position within the TWIST1 gene, a runs test was used.
RESULTS
Genetic and clinical features
All patients included in the study had a confirmed heterozygous mutation in the TWIST1 gene. Thirty-four patients (18 male, 16 female) met this criterion during the 15-year study period. The genetic change present in each patient is recorded in Table 1. Our series included four previously unreported mutations of TWIST1. One of these (cases 20,21) was a single nucleotide deletion, and therefore unequivocally pathogenic. Three cases with single nucleotide substitutions encoded missense changes (p.I135M, case 8; p.P139T, case 7; p.I156N, case 31) of the highly conserved helix1-loop-helix2 domain of the TWIST1 protein. One of these (p.P139T) arose de novo, the other two were inherited from the father, who was clinically affected in each case. The naturally occurring amino acids at these positions exhibit 100% sequence conservation in the TWIST1 orthologues of a wide variety of metazoan species, indicating that these novel mutations are highly likely to be pathogenic.
Table 1. Genetic analysis of SCS patients.
| Case | TWIST1 mutation (all patients heterozygous) | Reference |
|---|---|---|
| 1 | Microdeletion | Johnson et al. 7, case SA |
| 2 | Intragenic mutation (c.465C>A, p.Y155X) | Elanko et al.21, case CRS126 |
| 3 | Intragenic mutation (c.368C>G, p.S123W) | Johnson et al.7, case SB |
| 4 | Intragenic mutation (c.[230delA;232T>C], p.K77SfsX48) | Johnson et al.7, case LB |
| 5 | Intragenic mutation (c.[230delA;232T>C], p.K77SfsX48) | Sibling of case 4 |
| 6 | Intragenic mutation (c.376G>T, p.E126X) | Gripp et al.26 described mutation, independent occurrence |
| 7 | Intragenic mutation (c.415C>A, p.P139T) | Novel mutation in loop, de novo in patient |
| 8 | Intragenic mutation (c.405C>G, p.I135M) | Novel mutation in helix 1, inherited from mildly affected father |
| 9 | Intragenic mutation (c.379_381dup, p.A127dup) | Elanko et al.21, case SC25 |
| 10 | Intragenic mutation (c.355delC, p.Q119fsX6) | Elanko et al.21, case C19 |
| 11 | Intragenic mutation (c.421G>T, p.D141Y) | Johnson et al.7, case DE |
| 12 | Intragenic mutation (c.421G>T, p.D141Y) | Sibling of case 11 |
| 13 | Intragenic mutation (c.421G>T, p.D141Y) | Half-sibling of case 11 |
| 14 | Intragenic mutation (c.283delAinsCG, p.S95RfsX143) | Elanko et al.21, case C12 |
| 15 | Microdeletion | Wilkie et al.23, table I |
| 16 | Atypical intragenic mutation (c.115C>G, p.R39G) | Funato et al.22 |
| 17 | Intragenic mutation (c.472T>C, p.F158L) | Elanko et al.21 described mutation, independent occurrence |
| 18 | Intragenic mutation (c.472T>C, p.F158L) | Sibling of case 17 |
| 19 | Intragenic mutation (c.406C>T, p.P136S) | De Heer et al.8 described mutation, independent occurrence |
| 20 | Intragenic mutation (c.331delG, p.V111SfsX14) | Novel mutation |
| 21 | Intragenic mutation (c.331delG, p.V111SfsX14) | Maternal aunt of case 20 |
| 22 | Deletion (karyotype 46,XY, del(7)(p21p21)) | Wilkie et al.23, table I |
| 23 | Intragenic mutation (c.362C>T, p.T121I) | Wilkie et al.23, table I |
| 24 | Intragenic mutation (c.310G>T, p.E104X) | Johnson et al.7, case LM |
| 25 | Intragenic mutation (c.396_416dup, p.K133_P139dup) | Wilkie et al.23, table I |
| 26 | Intragenic mutation (c.396_416dup, p.K133_P139dup) | Sister of case 25 |
| 27 | Intragenic mutation (c.485_488del, p.V162AfsX68) | Elanko et al.21, case SC19 |
| 28 | Intragenic mutation (c.396_416dup, p.K133_P139dup) | Wilkie et al.23, table I |
| 29 | Intragenic mutation (c.329_333del, p.R110HfsX126) | Wilkie et al.23, table I |
| 30 | Intragenic mutation (c.340A>G, p.N114D) | Carbonara et al.27 described mutation, independent occurrence |
| 31 | Intragenic mutation (c.467T>A, p.I156N), also Down syndrome (karyotype 47,XY,+21) | Novel mutation in helix 2, inherited from mildly affected father |
| 32 | Intragenic mutation (c.397_417dup, p.K133_P139dup) | El Ghouzzi et al.5 described mutation, independent occurrence |
| 33 | Miicrodeletion associated with chromosome translocation (karyotype 46,XY, t(7;8)(p21;q13)) | Johnson et al.7, case CP |
| 34 | Large chromosome 7 interstitial deletion (karyotype 46,XX, del(7)(p15.1p21.3)) | Similar to deletions described by Chotai et al.28 |
All 34 patients had craniosynostosis. The affected sutures were bicoronal in 26 (77%), right unicoronal in 4 (12%), left unicoronal in 2 (6%) and combined bicoronal and sagittal in 2 cases (6%). The patient group had variable expression of the other features of SCS, as summarised and compared to previous studies of genetically confirmed SCS in Table 2. The most common clinical features in our group included ear malformation with prominent transverse crus (88%), low set frontal hairline (77%), and eyelid ptosis (62%). Ten patients (29%) had convergent squint, and 2 (6%) had a palatal cleft (one hard palate cleft, one submucous cleft).
Table 2. Clinical features of TWIST1 confirmed SCS.
| Clinical features | Oxford group (n=34) | De Heer8 (n=16) | Paznekas10 (n=39) | El Ghouzzi12 (n=22)a | Trusen13 (n=27) | Kress14 (n=71) |
|---|---|---|---|---|---|---|
| Brachycephaly | 79% | 76% | 59% | 45% | - | 46% |
| Plagiocephaly | 18% | 24% | 23% | 36% | - | 37% |
| Low frontal hairline | 77% | 24% | 36% | 41% | - | 55% |
| Ptosis | 62% | 53% | 59% | 100% | - | 45% |
| Prominent crus helicis | 88% | 47% | - | 91% | - | 51% |
| Syndactyly, hands | 18% | 35% | 33% | 100% | 61% | 52%b |
| Syndactyly, feet | 6% | - | - | - | 52% | 52%b |
| Broad big toe | 9% | - | 54% | 82% | 44% | 55% |
Primary intervention
Twenty-eight patients were cared for primarily at the Oxford Craniofacial Unit. The remaining 6 patients received primary care outside our unit, but had care transferred to the Oxford Craniofacial Unit. Thirty-one patients have had surgical intervention; of the remainder, two are infants and planned for surgery within the next 12 months, and one presented as an adult with mild calvarial abnormality and no features of intracranial hypertension.
Although not a routine part of our protocol, 4 patients had ICP monitoring prior to any surgical intervention. Two of these had acceptable calvarial morphology despite synostosis and were under clinical surveillance, and 2 presented as adults. ICP monitoring was performed due to clinical and radiological (Figure 2) concern of intracranial hypertension, and confirmed its presence in all cases. These individuals were aged 1.2, 4.8, 20.6 and 26.5 years, and included the 3 oldest patients at the time of primary intervention.
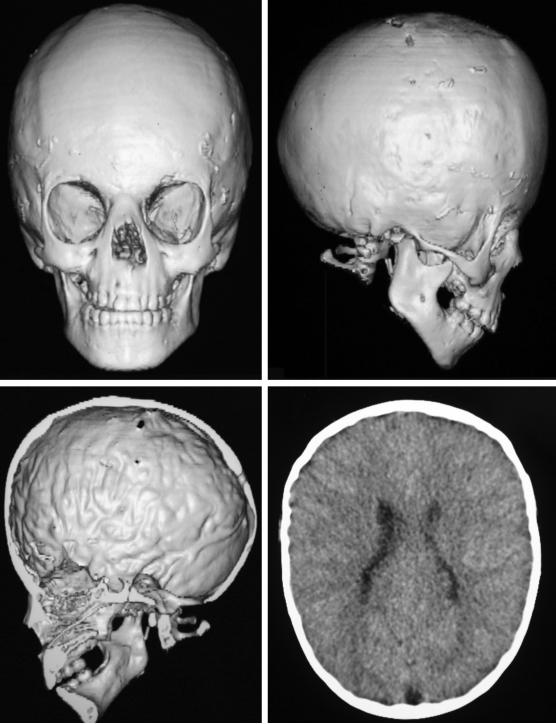
Figure 2.
CT imaging of a 4.8 year old girl with acceptable calvarial morphology despite bicoronal synostosis (upper left, AP reconstruction; upper right, lateral reconstruction). Right frontal bone defect is present from burrhole for ICP monitoring at the same presentation, which confirmed intracranial hypertension. Axial image displaying copperbeating and reduced intracranial space suggestive of intracranial hypertension (bottom).
Of the 31 patients receiving primary surgical intervention, 25 underwent primary FOAR (median age 1.2 years, range 0.5 - 26.5 years), 5 had planned dual-stage procedures (median age 0.6 years, range 0.5 - 0.8 years), and one (managed primarily outside of Oxford) had multiple strip craniectomies prior to FOAR.
Four of the 25 patients (16%) who received primary surgery at the Oxford Craniofacial Unit had complications following FOAR procedures. One patient developed intraoperative cerebral swelling that required the initial procedure to be halted; a CT Scan revealed a small intraventricular haemorrhage that did not require intervention, and the procedure was completed uneventfully one week later. Another patient developed an acute subdural haematoma that was drained intraoperatively, and the procedure completed without further problems. Two patients (8%) required removal of prominent wires. Documentation of surgical complications in cases initially managed at other units is incomplete; one patient required a minor procedure to remove a bony prominence causing discomfort.
Secondary intervention
Five of the 31 patients who had surgery were excluded due to follow-up of less than 12 months (in 3 cases, primary surgical intervention occurred in the final year of the study and the other 2 cases were siblings who moved abroad within 12 months of their surgery). Of the remaining 26 patients, median follow-up after primary surgery was 6.8 years (range 1.3 - 19.3 years). Thirteen underwent ICP monitoring on 16 occasions due to clinical and radiological (Figure 3) suspicion of intracranial hypertension (3 patients underwent monitoring on 2 separate occasions). Nine of these monitoring episodes confirmed elevated ICP.
Figure 3.
CT imaging of a 5.4 year old boy with acceptable calvarial morphology 4.4 years after fronto-orbital advancement and remodelling for bicoronal synostosis (upper left, AP reconstruction; upper right, lateral reconstruction). Inner calvarial reconstruction (lower left) and axial image (lower right) display copperbeating and reduced intracranial space suggestive of intracranial hypertension. ICP monitoring confirmed intracranial hypertension.
Further calvarial surgery to address cranio-cephalic disproportion was thus performed in 9 patients (35%). This included 7 of the 20 cases (35%) with primary surgery performed at the Oxford Craniofacial Unit, and 2 of the 6 cases (33%) with primary surgery elsewhere. Nineteen patients have been followed up for more than 5 years after primary surgery: 8 (42%) had required secondary surgery for elevated ICP. Secondary surgery was performed at median 4.3 years after primary surgery (range: 1.3 - 8.5 years). This comprised calvarial expansion targeting the area of constriction or associated aesthetic deformity: repeat fronto-orbital advancement for frontal constriction, posterior remodelling for posterior constriction and lateral expansion for non-specific space restriction. All procedures were equivalent to grade IV by the Whitaker classification (major craniofacial procedure of same or greater magnitude as the original surgery) 29.
An additional 2 patients (7.7%) without clinical suspicion of intracranial hypertension required secondary calvarial surgery for aesthetic reasons. One patient had surgery for calvarial deformity due to progressive synostosis of the lambdoid sutures, 14 months after FOAR (also equivalent to grade IV by the Whitaker classification), the other required forehead recontouring with bone substitute at growth completion.
Further analysis was carried out on those patients receiving primary surgical intervention at the Oxford Craniofacial Unit. For the single stage (FOAR) group of 16 patients, reoperation rates for elevated ICP were compared in relation to age at primary intervention. Those undergoing intervention before 12 months of age had a significantly higher reoperation rate for intracranial hypertension (4 of 5, 80%) than those operated on after 12 months of age (2 of 11, 18%) [Fisher’s exact test, p=0.04]. All patients requiring secondary operations for elevated ICP had initial intervention at less than 15 months of age.
The clinical and radiological features of all nine patients who had developed confirmed intracranial hypertension following primary intervention are displayed in Table 3. Symptoms of headache and aggression were reported in 5 (56%), slowed cranial growth on serial head circumference measurements in 6 (67%) and papilloedema was present on fundoscopy in 3 patients (33%). CT imaging showed evidence of raised pressure in 8 (88.9%), without features of hydrocephalus.
Table 3. Features of SCS patients requiring reoperation for intracranial hypertension.
| Case | Sex | Suture involved | Primary procedure | Age of primary procedure (years) |
Secondary procedure | Age of secondary procedure (years) |
Headache/Aggression | Slowed cranial growth |
Fundoscopy | CT features of elevated intracranial pressure |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | Left unicoronal | FOAR | 0.9 | CE | 8.9 | + | + | - | + |
| 2 | F | Bicoronal | FOAR | 0.6 | Re-FOAR | 5.4 | - | + | - | + |
| 4 | F | Bicoronal | FOAR | 1.2 | PR | 5.3 | - | + | papilloedema | + |
| 11 | M | Bicoronal | FOAR | 0.8 | CE | 9.1 | + | - | papilloedema | + |
| 17 | M | Bicoronal | Dual stage | 0.5; 1.3 | CE | 5.8 | + | + | - | + |
| 18 | F | Bicoronal | FOAR | 0.9 | PR | 2.1 | - | + | - | + |
| 19 | M | Bicoronal | FOAR | 1 | Re-FOAR | 5.4 | + | + | - | + |
| 24 | M | Bicoronal | FOAR | 0.7 | Re-FOAR | 3.5 | - | - | papilloedema | + |
| 27 | F | Bicoronal | FOAR | 0.8 | CE | 4.3 | + | - | - | - |
FOAR = Fronto-orbital advancement and remodelling, Dual stage = Posterior release followed by FOAR, PR = Posterior remodelling, CE = Calvarial expansion with lateral panel release, Re-FOAR = Repeat FOAR
Finally, we examined whether the type and position of TWIST1 mutation had any influence on the reoperation rate. Dividing the mutations into complete deletions or intragenic disruptions [nonsense mutations, frameshifts, in-frame insertions] (n=17) and missense mutations (n=9), there was no difference in reoperation rate (Fisher’s exact test, p>0.05). For the intragenic mutations (n=21) we also examined whether the position of the mutation (5′→3′) influenced reoperation rate, but again no significant effect was observed (runs test, p>0.08).
DISCUSSION
SCS is a genetically determined condition with a variable clinical phenotype. Genetic testing is indicated to confirm the diagnosis, and to distinguish SCS from clinically similar disorders, especially Muenke syndrome. It allows accurate genetic counselling regarding recurrence risk and enables prenatal testing. All 34 patients in the study group had genetically confirmed abnormalities in the TWIST1 gene.
No previous studies of surgical outcome have comprised only TWIST1-confirmed SCS patients17-20. Hence, previous series may inadvertently have included patients with Muenke syndrome. Indeed, several studies of clinically diagnosed “Saethre-Chotzen syndrome” have identified cases with the FGFR3 P250R mutation in their initial patient groups9,10, and Clauser et al. have reported a case of “Saethre-Chotzen syndrome” with genetic detection of the FGFR3 P250R mutation, confirming an actual diagnosis of Muenke syndrome11. The distinction between these conditions is clinically significant. Kress et al. compared the phenotypes of the two syndromes and found that intracranial hypertension was more common in SCS, while developmental delay and sensorineural hearing loss were more common in Muenke syndrome14.
The clinical features in our SCS group are consistent with previous reports and highlight the wide phenotypic variation in this disorder7,8,10,12-14. While it is common for patients with SCS to exhibit asymmetric bicoronal synostosis, it is notable that 6 of the 34 patients in our group had isolated unicoronal synostosis and 2 had additional sagittal synostosis.
All four patients who had ICP monitoring prior to any calvarial surgery, had evidence of raised ICP. One child presented with intracranial hypertension aged 4.8 years despite acceptable calvarial shape, reinforcing the need to monitor SCS patients who do not receive early operative intervention.
The overall reoperation rate with minimum 12 month follow-up for intracranial hypertension was 35%. When patients with longer follow-up (minimum 5 years) are considered, the rate was 42% suggesting that the lifetime risk may be up to 50%. These results are compared with other studies in Table 4. These other studies offer at best a guide to intracranial hypertension rates following primary surgery in SCS patients, as they have two clear confounding issues. First, none state that identification of a TWIST1 mutation, confirming SCS, was performed in all patients17-20. This may lead to inaccuracy due to inclusion of other syndromes (notably Muenke syndrome). Second, the technique for ICP monitoring was inconsistent in these studies. Intraparenchymal pressure monitoring is the accepted gold standard24. It is more accurate than extra-dural or subdural monitoring, or assessment on clinical or radiological grounds24. Siddiqi et al., Foster et al. and Pollack et al. all used clinical or radiological features for diagnosis and do not describe intraparenchymal pressure monitoring17-19. The group of Wong et al.20, which was the only other large series with long-term follow-up, quotes a 65% reoperation rate for supra-orbital rim position, but describes no assessment for intracranial hypertension. Our study provides the most accurate assessment of intracranial hypertension in the SCS population to date. The oldest patient requiring reoperation for intracranial hypertension was 9.1 years of age, and had received primary intervention 8.3 years earlier. This highlights the need to follow these children in the long term. We believe our protocol of annual reviews until maturity, with prioritized review if new symptoms develop in the interim, is sufficient in this regard and should be encouraged.
Table 4. Reoperation rate for SCS.
| Author | Follow up | SCS casesa | SCS cases undergoing primary surgery |
Reoperations (total) |
Reoperations (intracranial hypertension)b |
|---|---|---|---|---|---|
| Siddiqi17 | 24-61 months | 11 | 11 | 2 (18%) | 2 (18%) |
| Pollack18 | Median 30 months | 5 | 5 | 1 (20%) | 1 (20%) |
| Foster19 | Mean 38 months | 5 | 5 | 1 (20%) | 1 (20%) |
| Wong20 | Minimum 5 years | 25 | 20 | 13 (65%) | unknown |
| Oxford (this work) | Median 6.8 years | 34 | 31 (26)c | 11 (42%) | 9 (35%) |
Oxford -all cases genetically confirmed. All other groups diagnosed clinically with incomplete genetic confirmation.
Intracranial hypertension confirmed by intraparenchymal monitoring in this work. Other groups diagnosed elevated intracranial pressure on clinical and radiological grounds.
Of 31 cases in the Oxford group, 5 had follow-up of less than 12 months and were excluded from statistical analysis.
Patients with SCS, who had primary surgical intervention before 12 months of age, were much more likely to develop subsequent intracranial hypertension necessitating reoperation. This finding may be biased, as often infants with severely abnormal craniofacial morphology will receive early surgery to prevent progression of abnormal head shape. Though clearly the decision for surgical intervention must be based on clinical and radiological findings, in the absence of extreme morphology or intracranial hypertension, the optimal timing appears to be 12 to 15 months.
The need for reoperation due to raised ICP was higher in our SCS group than in other patients with coronal craniosynostosis treated with identical techniques. The lack of genotype-phenotype correlation that we observed for both the type and position of TWIST1 mutation fits with the haploinsufficiency (heterozygous loss of function) mechanism proposed for SCS30. In a previous study from our unit, Thomas et al.31 showed that the reoperation rate for Muenke syndrome was 20.7%, and for mutation-negative non-syndromic coronal synostosis it was 4.3%. When compared to the Muenke group, the SCS group appears to be at increased risk, though this does not reach statistical significance (Fisher’s exact test, p=0.20). The SCS group is at significantly higher risk than the non-syndromic group (Fisher’s exact test, p=0.001). The fact that the same surgical protocol was used in all 3 groups indicates that differences are not due to surgical variation. Our results highlight the importance of genetic assessment and molecular genetic analysis in patients with coronal suture synostosis.
Review of the presenting features in patients with intracranial hypertension after surgical intervention confirmed that screening history and fundoscopy are useful tools in diagnosis. Parental education is also an important adjunct to aid early detection of problems. With the assistance of an experienced neuroradiologist, features on CT imaging can be highly suggestive of raised ICP, and were present in 8 of 9 cases (88.9%). ICP monitoring remains the gold standard to investigate suspected intracranial hypertension. Expertise and modern practice have enabled ICP monitoring to be performed at our unit with extremely low morbidity24.
CONCLUSION
SCS patients are at high risk of developing intracranial hypertension due to cranio-cephalic disproportion. Screening of all patients with either bicoronal craniosynostosis, or unicoronal craniosynostosis with syndromic features for TWIST1 abnormalities will assist in identifying this group. Close monitoring for symptoms and signs of raised ICP is essential in allowing prompt intervention to minimize sequelae. Postoperatively these patients should be closely followed throughout childhood as there is a high risk of recurrent intracranial hypertension.
ACKNOWLEDGEMENTS
We wish to recognise the contributions of Mr M. Poole and Mr M. Briggs, consultant surgeons at the Oxford Craniofacial Unit 1980-1994. We also thank Dr P. Anslow for his expert CT interpretation.
Footnotes
FINANCIAL DISCLOSURE
Wellcome Trust -provision of grant support for Professor AOM Wilkie
None of the authors have a financial interest in the products described in the manuscript.
REFERENCES
- 1.Saethre H. Ein beitrag zum turmschaedelproblem. (pathogenese, erblichkeit und symptomatologie) Dtsch Z Nervenheilk. 1931;117:533–55. [Google Scholar]
- 2.Chotzen F. Eine eigenartige familaere entwicklungsstoerung. (akrocephalosyndaktylie, dysostosis craniofacialis und hypertelorismus) Monatsschr Kinderheilk. 1932;55:97–122. [Google Scholar]
- 3.Pantke O, Cohen M, Witkop C. The Saethre-Chotzen syndrome. Birth defects. 1975;11:190–225. [PubMed] [Google Scholar]
- 4.Howard T, Paznekas W, Green E, et al. Mutations in TWIST, a basic helix-loop-helix transcription factor, in Saethre-Chotzen syndrome. Nat Genet. 1997;15:36–41. doi: 10.1038/ng0197-36. [DOI] [PubMed] [Google Scholar]
- 5.El Ghouzzi V, Le Merrer M, Perrin-Schmitt F, et al. Mutations of the TWIST gene in Saethre-Chotzen syndrome. Nat Genet. 1997;15:42–6. doi: 10.1038/ng0197-42. [DOI] [PubMed] [Google Scholar]
- 6.Rose C, Patel P, Reardon W, et al. The TWIST gene, although not disrupted in Saethre-Chotzen patients with apparently balanced translocations of 7p21, is mutated in familial and sporadic cases. Hum Mol Genet. 1997;6(8):1369–73. doi: 10.1093/hmg/6.8.1369. [DOI] [PubMed] [Google Scholar]
- 7.Johnson D, Horsley SW, Moloney DM, et al. A comprehensive screen for TWIST mutations in patients with craniosynostosis identifies a new microdeletion syndrome of chromosome band 7p21.1. Am J Human Genet. 1998;63:1282–93. doi: 10.1086/302122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Heer I, De Klein A, Van den Ouweland A, et al. Clinical and genetic analysis of patients with Saethre-Chotzen syndrome. Plast Reconstr Surg. 2005;115(7):1894–1902. doi: 10.1097/01.prs.0000165278.72168.51. [DOI] [PubMed] [Google Scholar]
- 9.Chun K, Teebi A, Jung J, et al. Genetic analysis of patients with the Saethre-Chotzen phenotype. Am J Med Genet. 2002;110:136–43. doi: 10.1002/ajmg.10400. [DOI] [PubMed] [Google Scholar]
- 10.Paznekas W, Cunningham M, Howard T, et al. Genetic heterogeneity of Saethre-Chotzen syndrome, due to TWIST and FGFR mutations. Am J Hum Genet. 1998;62:1370–80. doi: 10.1086/301855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clauser L, Galie M, Hassanipour A, et al. Saethre-Chotzen syndrome: review of the literature and report of a case. J Craniofac Surg. 2000;11(5):480–6. doi: 10.1097/00001665-200011050-00007. [DOI] [PubMed] [Google Scholar]
- 12.El Ghouzzi V, Lajeunie E, LeMerrer M, et al. Mutations within or upstream of the basic helix-loop-helix domain of the TWIST gene are specific to Saethre-Chotzen syndrome. Eur J Hum Genet. 1999;7:27–33. doi: 10.1038/sj.ejhg.5200240. [DOI] [PubMed] [Google Scholar]
- 13.Trusen A, Beissert M, Collmann H, et al. The pattern of skeletal anomalies in the cervical spine, hands and feet in patients with Saethre-Chotzen syndrome and Muenke-type mutation. Pediatr Radiol. 2003;33:168–72. doi: 10.1007/s00247-002-0823-3. [DOI] [PubMed] [Google Scholar]
- 14.Kress W, Schropp C, Lieb G, et al. Saethre-Chotzen Syndrome caused by TWIST 1 gene mutations: functional differentiation from Muenke coronal synostosis syndrome. Eur J Hum Genet. 2006;14:39–48. doi: 10.1038/sj.ejhg.5201507. [DOI] [PubMed] [Google Scholar]
- 15.Wiegand C, Richards PG. Measurement of ICP in children: a critical review of current methods. Dev Med Child Neurol. 2007;49:935–41. doi: 10.1111/j.1469-8749.2007.00935.x. [DOI] [PubMed] [Google Scholar]
- 16.Cinalli G, Sainte-Rose C, Kollar EM, et al. Hydrocephalus and craniosynostosis. J Neurosurg. 1998;88:209–14. doi: 10.3171/jns.1998.88.2.0209. [DOI] [PubMed] [Google Scholar]
- 17.Siddiqi S, Posnick J, Buncic R, et al. The detection and management of intracranial hypertension after initial suture release and decompression for craniofacial dysostosis syndromes. Neurosurg. 1995;36(4):703–8. doi: 10.1227/00006123-199504000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Pollack I, Losken H, Biglan A. Incidence of increased ICP after early surgical treatment of syndromic craniosynostosis. Pediatr Neurosurg. 1996;24:202–9. doi: 10.1159/000121038. [DOI] [PubMed] [Google Scholar]
- 19.Foster K, Frim D, McKinnon M. Recurrence of synostosis following surgical repair of craniosynostosis. Plast Reconstr Surg. 2008;121(3):70e–76e. doi: 10.1097/01.prs.0000299393.36063.de. [DOI] [PubMed] [Google Scholar]
- 20.Wong G, Kakulis E, Mulliken J. Analysis of fronto-orbital advancement for Apert, Crouzon, Pfeiffer, and Saethre-Chotzen syndromes. Plast Reconstr Surg. 2000;105(7):2314–23. doi: 10.1097/00006534-200006000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Elanko N, Sibbring JS, Metcalfe KA, et al. A survey of TWIST mutations in craniosynostosis reveals a variable length polyglycine tract in asymptomatic Individuals. Hum Mutat. 2001;18:535–41. doi: 10.1002/humu.1230. [DOI] [PubMed] [Google Scholar]
- 22.Funato N, Twigg SRE, Higashihori N, et al. Functional analysis of natural mutations in two TWIST protein motifs. Hum Mutat. 2005;25:550–6. doi: 10.1002/humu.20176. [DOI] [PubMed] [Google Scholar]
- 23.Wilkie AOM, Bochukova EG, Hansen RMS, et al. Clinical dividends from the molecular genetic diagnosis of craniosynostosis. Am J Med Genet Part A. 2007;143A:1941–9. doi: 10.1002/ajmg.a.31905. [DOI] [PubMed] [Google Scholar]
- 24.Taylor WJ, Hayward RD, Lasjaunias P, et al. Enigma of raised ICP in patients with complex craniosynostosis: the role of abnormal intracranial venous drainage. J Neurosurg. 2001;94:377–85. doi: 10.3171/jns.2001.94.3.0377. [DOI] [PubMed] [Google Scholar]
- 25.Sgouros S, Goldin JH, Hockley AD, et al. Posterior skull surgery in craniosynostosis. Childs Nerv Syst. 1996;12(11):727–33. doi: 10.1007/BF00366158. [DOI] [PubMed] [Google Scholar]
- 26.Gripp KW, Stolle CA, Celle L, et al. TWIST gene mutation in a patient with radial aplasia and craniosynostosis: further evidence for heterogeneity of Baller-Gerold syndrome. Am J Med Genet. 1999;82(2):170–6. doi: 10.1002/(sici)1096-8628(19990115)82:2<170::aid-ajmg14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 27.Carbonara C, Sbaiz L, Genitori L, et al. A novel N114D mutation in a Crouzon-like patient. Am J Human Genet. 1999;65(4):A144. [Google Scholar]
- 28.Chotai KA, Brueton LA, van Herwerden L, et al. Six cases of 7p deletion: clinical, cytogenetic and molecular studies. Am J Med Genet. 1994;51:270–6. doi: 10.1002/ajmg.1320510320. [DOI] [PubMed] [Google Scholar]
- 29.Whitaker LA, Bartlett SP, Schut L, et al. Craniosynostosis: an analysis of the timing, treatment and complications in 164 consecutive patients. Plast Reconstr Surg. 1987;80:195–212. [PubMed] [Google Scholar]
- 30.Jabs EW. TWIST1 and the Saethre-Chotzen syndrome. In: Epstein CJ, Erickson RP, Wynshaw-Boris A, editors. Inborn errors of development. The molecular basis of clinical disorders of morphogenesis. 2nd Ed. Oxford University Press; Oxford: 2008. pp. 474–81. [Google Scholar]
- 31.Thomas GP, Wilkie AOM, Richards PG, et al. FGFR3 P250R mutation increases the risk of reoperation in apparent ‘nonsyndromic’ coronal craniosynostosis. J Craniofac Surg. 2005;16(3):347–52. doi: 10.1097/01.scs.0000157024.56055.f2. [DOI] [PubMed] [Google Scholar]