Abstract
Rationale
The development and validation of animal models of the cognitive impairments of schizophrenia have remained challenging subjects.
Objective
We review evidence from a series of experiments concerning an animal model that dissociates between the disruption of attentional capacities during acute illness periods and the cognitive load-dependent impairments that characterize periods of remission. The model focuses on the long-term attentional consequences of an escalating-dosing pretreatment regimen with amphetamine (AMPH).
Results
Acute illness periods are modeled by the administration of AMPH challenges. Such challenges result in extensive impairments in attentional performance and the “freezing” of performance-associated cortical acetylcholine (ACh) release at pretask levels. During periods of remission (in the absence of AMPH challenges), AMPH-pretreated animals’ attentional performance is associated with abnormally high levels of performance-associated cortical ACh release, indicative of the elevated attentional effort required to maintain performance. Furthermore, and corresponding with clinical evidence, attentional performance during remission periods is exquisitely vulnerable to distractors, reflecting impaired top-down control and abnormalities in fronto–mesolimbic–basal forebrain circuitry. Finally, this animal model detects the moderately beneficial cognitive effects of low-dose treatment with haloperidol and clozapine that were observed in clinical studies.
Conclusions
The usefulness and limitations of this model for research on the neuronal mechanisms underlying the cognitive impairments in schizophrenia and for drug-finding efforts are discussed.
Keywords: Schizophrenia, Cognition, Amphetamine, Clozapine, Haloperidol
Introduction
Despite the often reflexive attribution of the initial conceptualization of schizophrenia as a cognitive disorder to Kraepelin and Bleuler, or Morel (“démence-precoce”; Morel 1860), the “cognitive revolution”, beginning with the 1950s, and the subsequent birth of cognitive science during 1960s and 1970s (Miller 2003) have fostered a wider acceptance of theories that consider abnormal cognitive, specifically attentional, processes as central, disease-defining characteristics of schizophrenia (McGhie and Chapman 1961; Venables 1964; Nuechterlein and Dawson 1984). However, it is noteworthy that Kraepelin already embraced a relatively dynamic conceptualization of the central role of attentional impairments in schizophrenia (“In dementia praecox a striking disorder of the attention is present from almost the inception of the disease”; p. 19; Kraepelin 1907). Furthermore, Kraepelin dissociated between levels of severity of attentional impairments (blunting, suppression, blockade, retardation of attention) and stressed the long-term consequences of such impairments in terms of the general weakening of the perceptual process and the accessibility and accuracy of memories. Kraepelin also conceptualized the effects of impairments in the ability to sustain attention (“Unsteadiness of attention”; p. 6; Kraepelin 1919), specifically in the presence of distractors, and the long-term consequences of such impairments for reality monitoring (“The more distractable a man is, the less perception is controlled by inner motives arising from experience, and the less coherent and uniform is the conception of the external world.”; p. 22; Kraepelin 1907). Likewise, Bleuler attributed the distractibility of schizophrenic patients to their general inability to select stimuli for attentional processing (“Thus, the facilitating as well as the inhibiting properties of attention are equally disturbed”; p. 68; Bleuler 1950).
Both Bleuler and Kraepelin understood that similar attentional processes govern the detection, selection, and extended processing of exogenous stimuli, as well as the selection and maintenance of internal stimuli (or associations). Consequently, they were able to consider the profound, escalating consequences of attentional impairments for the general cognitive capacity of patients.
Contemporary research has confirmed that attentional impairments represent an essential component of the cognitive symptomatology of schizophrenics and perhaps even a vulnerability factor for schizophrenia (Braff 1993; Nuechterlein et al. 1994; Green 1996; Cohen et al. 1998, 1999; Kapur 2003; Braff and Light 2004; Silver and Feldman 2005; Filbey et al. 2008; Gur et al. 2007). Although the view that the diverse cognitive symptoms of schizophrenia reflect, and escalate from, a single fundamental cognitive dysfunction remains debated (Goldstein and Shemansky 1995; Lieh-Mak and Lee 1997), such a reductionistic perspective underlies our focus on the modeling of attentional, particularly sustained attentional impairments of schizophrenia in animals. Studies that attempted to dissociate the attentional impairments present during active disease periods (disease periods characterized by the presence of florid psychotic symptoms and severe disability) versus the persistent deficits observed in patients experiencing symptomatic remission (i.e., disease periods during which patients experience attenuated symptoms below diagnostic threshold) have remained unexpectedly scarce (Andreasen et al. 2005; Remington and Kapur 2005). However, the available evidence is indicative of severe impairments present during active periods, manifesting in the absence of special demands on cognitive processes (Nuechterlein et al. 1994). In contrast, heightened demands on attentional effort (for definition of attentional effort, see Sarter et al. 2006) as a result, for example, of the presentation of distractors, expose the limited attentional capacities that persist outside active disease periods (Grillon et al. 1990, 1991; Nuechterlein et al. 1994; Servan-Schreiber et al. 1996; Robert et al. 1997; Goldberg et al. 1998; Seidman et al. 1998; Smith et al. 1998; Gorissen et al. 2005; Fuller et al. 2006; Gold et al. 2007; Jazbec et al. 2007). As will be pointed out below, different forms of dysregulation in telencephalic–limbic–basal forebrain circuitry are hypothesized to underlie the severe attentional impairments in baseline attentional performance of animals modeling active disease periods versus the impairments in attention that persist during periods of remission and that become apparent in response to increased demands on attentional effort. Treatment with low doses of antipsychotic drugs benefits, albeit to a limited degree, the attentional performance during both disease periods. Different neuro-psychopharmacological mechanisms may mediate these beneficial drug effects during periods of psychosis and remission (below).
As the measurement of attentional performance in laboratory rodents represents a central component of the evaluation of this model, we begin this review by describing the task, measures of performance, and the effects of distractors that are thought to recruit top-down mechanisms. The role of the cholinergic system for attentional performance will be briefly reviewed. We then introduce and discuss the parameters and validity of the pharmacological model, followed by separate discussion of the modeling of the two disease states, including predictive validity and underlying neurobiological mechanisms.
Measurement of top-down control of sustained attention in rodents and cholinergic mediation
The operant task employed in our experiments for the measurement of sustained attention and a set of data exemplifying the measures of performance generated by healthy rats, in the presence and absence of a distractor, are illustrated in Fig. 1.
Fig. 1.
Illustration of the main events (a), response categories and outcome (b) of the sustained attention task, and illustration of performance data from sessions involving the presence and absence of a distractor (c–h). The task consists of a random sequence of signal and non-signal events that occur unpredictably following a variable ITI. Following a signal (center panel light illumination for 500, 50, or 25 ms) or a non-signal event, levers are extended into the chambers 2 s later and remained active for 4 s. In the version illustrated in a, following a signal, a left lever press is counted as a hit and rewarded (water port situated between the levers; not shown). Following a non-signal event, a right lever press indicated a “correct rejection” and is also rewarded. Incorrect responses are misses and false alarms, respectively, and trigger an ITI. An error of omission is defined as the failure to operate a lever within 4 s. Note that arrows indicating the four responses are color-coded to match the arrows in the outcome matrix in b. Top-down control of attention is tested by the presentation of a distractor, requiring the recruitment of top-down effects designed to stabilize residual attentional performance (see text). The performance data shown in c–h depict performance over blocks of trials as, in this example, the distractor (chamber houselights flashing on/off at 0.5 Hz) was presented during the second block of trials only (standard task: black lines, squares; distractor condition: orange lines, triangles). As illustrated in c and d, the distractor reduced the relative number of hits to 500 and 50 ms signals in block 3, suggesting that as a result of distractor presentation, animals were not able to sustain levels of signal detection during the remainder of the task. For hits to shortest signals (e), floor effects prohibited the demonstration of the detrimental effects of the distractor. In contrast to the effects of the distractor on hits, the ability to respond correctly in non-signal trials was acutely disrupted by the distractor but completely recovered during block 3 (f). Errors of omission were not robustly affected by the distractor and generally remained below 10% of all trials, substantiating the persistent motivation of the animals to stay-on-task and to regain performance in response to distractor challenges (Sarter et al. 2006). The performance in signal and non-signal trials is collapsed into one measure of performance, the VI (h). VI values range from −1 to + 1. Values of 0 indicate randomized lever section (scores close to zero were seen in AMPH-pretreated animals challenged with AMPH; for formula and data, see Kozak et al. 2007), +1 indicates perfect response accuracy, and −1 indicates perfect inaccuracy. VI can be calculated for each signal duration (500, 50, 25 ms; VI500,50,25) or averaged over all signal durations as shown in h. The VI scores shown in h illustrate that the overall performance of intact animals in the standard task declines over blocks of trials, further supporting the construct validity of this task (McGaughy and Sarter 1995). Moreover, this particular distractor robustly impaired overall performance and continued to impair performance during the third block of trials (no distractor)
The task consists of trials involving the presentation of signals or the absence of signal events, followed by the extension of the levers, triggering a 4-s response period. Subjects report the presence of a signal by pressing one lever and the absence of a signal by pressing the opposite lever. Correct responses are hits and correct rejections, respectively, and are rewarded. Incorrect responses are misses and false alarms, respectively, and trigger the next intertrial interval (ITI). Signal duration (Parasuraman and Mouloua 1987; Koelega et al. 1990) and other task parameters vary by species to ensure construct validity of the performance measures (for details on construct validity in animals and humans, see Demeter et al. 2008; McGaughy and Sarter 1995; Mar et al. 1996; Bushnell et al. 2003).
Figure 1(c–h) depicts the major measures of performance generated by this task and the performance of intact rats. The analysis of performance from a test session (162 trials total) typically divides data into three blocks of 54 trials, primarily in order to allow comparisons with data from sessions during which a distractor was presented during the second (Fig. 1) or the second and third blocks of trials. Standard-task performance is characterized by signal duration-dependent hit-rates (c–e), relatively high levels of correct rejections (>80%), and low numbers of errors of omission (<10% of all trials). As detailed in the legend of Fig. 1, hits and correct rejections are collapsed into a single measure of performance [vigilance index (VI); Fig. 1h; for formula, see Kozak et al. 2007]. This measure indicates that intact animals typically exhibit a small but robust decline in performance across the three blocks of trials, consistent with the classification of the standard version of the task as a sustained attention task. Disruption of neuronal processing usually augments this decrement in performance.
The standard version of this task has been interpreted as based largely on bottom-up, signal-driven processes (Sarter et al. 2005a). In other words, during well-practiced standard-task performance, cognitive processes are not required to supervise and influence the detection of signals, the discrimination of non-signal events, or the processing of response rules for each trial type. Following extensive practice, such rules are executed on the basis of a relatively automatic level of processing (Fisk and Scerbo 1987), as indicated by their insensitivity to reversal learning (e.g., Sarter 1990).
In order to recruit top-down mechanisms, distractors are presented during one or several blocks of trials, depending on species (Nobre et al. 2002; Weissman et al. 2002; Gilbert and Sigman 2007). A substantial amount of evidence from human imaging studies, as well as animal experiments, indicates that such mechanisms are mediated via prefrontal efferent circuitries, which act to optimize receptive field properties and suppress distractor representation in sensory and sensory-associational cortical regions (see references and Table 1 in Sarter et al. 2006). These mechanisms act to stabilize and recover attentional performance depending on the specific task version and distractor parameters. Figure 1(c–h) illustrates the effects of a distractor on performance. The distractor consisted of operant chamber houselights flashing on–off at 0.5 Hz during the second (middle) block of trials. For hits to longest and medium-duration signals, the effects of the distractor manifest primarily in the third block of trials. This effect of the distractor in rats corresponds with effects in humans (Demeter et al. 2008) and is hypothesized to indicate the exhaustion of the subjects’ detection capacity in response to the distractor presence. For shortest signals, “floor effects” likely prevented the manifestation of distractor effects (Fig. 1e). With respect to the performance on non-signal trials, the distractor acutely leads to an increase in the number of false detections (1f). However, following the termination of a distractor, the animals’ correct rejection rate immediately recovers. The contrast between the lasting effects on hits and the immediate recovery of non-signal trial performance is consistent with the assumption that non-signal trial performance does not depend on attentional resources and resource management (see below). Importantly, presentation of the distractor does not robustly increase errors of omission (1 g), reflecting the close relationships between motivational processes and increases in attentional effort (Maunsell 2004; Small et al. 2005; Sarter et al. 2006; Watanabe 2007). The effects of the distractor will be addressed again further below in the context of the modeling of remission states.1
Table 1.
Cognitive effects of repeated AMPH administration
| Species | Pretreatment regimen |
Test time following pretreatment | Test | Challenge dose? | Results | Study | ||
|---|---|---|---|---|---|---|---|---|
| Dose | Frequency | Duration | ||||||
| Rhesus monkeys | 0.1–1.0 ;mg/kg; i.m.; escalating | Twice daily; weekends off | 6 or 12 ;weeks | >6 ;months | Delayed response; delayed non-match to sample | In selected cases; 0.4 ;mg/kg | Impaired acquisition of delayed response and DMNS; challenges disrupt DR performance | Castner et al. (2005) |
| Rat | 4.0 ;mg/kg; s.c. | Once daily | 5 ;days | Immediately after | Associative blocking/ selective attention | Testing took place on days 4 and 5 of the pretreatment | Blocking effect absent | Crider et al. (1982) |
| Rat | 1.0–5.0 ;mg/kg; i.p. | 3× daily | 6 ;days | Immediately after | Operant sustained attention | On day 7; 1.0 ;mg/kg | Increased false alarm rate; no effects of challenge | Kondrad and Burk (2004) |
| Rat | 1.0–3.0 ;mg/kg; i.p. | 3× per week | 3 or 5 ;weeks | 22 ;days | Prepulse inhibition | No | PPI disruption | Tenn et al. (2003) |
| Rat | 1.0–5.0 ;mg/kg; i.p. | 3× per week | 5 ;weeks | 4 ;weeks | Attentional set shifting | No | Impaired on the extra-dimensional shift and on reversal discriminations | Fletcher et al. (2005) |
| Rat | 1.0–5.0 ;mg/kg; i.p. | 3× per week | 5 ;weeks | Testing took place during 5 ;weeks withdrawal | 5-Choice serial reaction time | No | Persistent increase in omissions reduced response accuracy under reduced stimulus duration | Fletcher et al. (2007) |
| Rat | 1.0– 10.0 ;mg/kg; i.p. escalating | Twice daily; weekends off | 40 ;days | 20 ;days | Operant sustained attention | None, 0.5 or 1.0 ;mg/kg | Decreased hit rate after challenge | Martinez et al. (2005) |
| Rat | 1.0– 10.0 ;mg/kg; i.p. escalating | Twice daily; weekends off | 40 ;days | >50 ;days | Operant sustained attention | None or 1.0 ;mg/kg | Decreased hit and correct rejection rates after challenge | Kozak et al. (2007) |
| Rat | 1.0– 10.0 ;mg/kg; i.p. escalating | Twice daily; weekends off | 40 ;days | 35–55 ;days | Operant sustained attention | None or 1.0 ;mg/kg | Decreased hit and correct rejection rates after challenge; distractor-induced disruption in the absence of challenges | Young et al. (2007) |
| Rat | 1.0– 10.0 ;mg/kg; i.p. escalating | Twice daily; weekends off | 40 ;days | 10–20 ;days | Operant sustained attention | None or 1.0 ;mg/kg | Decreased hit rat after challenge | Martinez and Sarter (2008) |
Data from studies involving neurotoxicity-inducing pretreatment regimen are not listed. Likewise, studies that explicitly addressed the cognitive correlates of psychostimulant withdrawal were not considered for this synopsis (except of studies that generated measures at >4 weeks since completion of the pretreatment regimen).
Cholinergic mediation of attentional performance
Studies assessing the effects of selective lesions of the cortical cholinergic input system and the effects of cholinergic receptor antagonists consistently indicated that the cholinergic system in general and the cortical cholinergic input system in particular is necessary for the performance of a wide range of attentional functions and capacities and attention-dependent learning (Everitt and Robbins 1997; Hasselmo and McGaughy 2004; Sarter et al. 2005a; McGaughy et al. 2000). Cholinergic lesions selectively disrupt the detection of signals in the task described above, sparing non-signal trial performance (McGaughy et al. 1996). This finding indicates that the lesion does not affect the processing of response rules and corresponds with the general hypothesis that the cortical cholinergic input system mediates the detection, selection, and processing of stimuli but is less involved in the associational (or intrinsic) processing underlying trials not involving stimulus detection (Sarter et al. 2005a). Moreover, cholinergic lesions of the right but not left hemisphere reproduce the effects of bilateral lesions on performance (Martinez and Sarter 2004), corresponding with results from human imaging studies that collectively suggest that sustained attention is mediated primarily via right-hemispheric circuitry (e.g., Pardo et al. 1991; Cohen et al. 1992).
Studies measuring the release of acetylcholine (ACh) in task-performing animals demonstrated that the increases in cortical ACh release that are reliably observed in attention task-performing animals are not reproduced in animals performing tasks controlling for operant responding, reward rate, motor activity, or non-contingent stimulus presentation (Himmelheber et al. 1997, 2000; Passetti et al. 2000; Dalley et al. 2001, 2005; Arnold et al. 2002). More recent experiments made use of a new electrochemical technique that allows the monitoring of second-to-second changes in ACh release in task-performing animals. Evidence from initial experiments suggest that phasic (on the scale of seconds) cholinergic signals in the medial prefrontal cortex selectively mediate the incorporation of signals into ongoing cognitive and behavioral processes, allowing such a signal to control behavior (Parikh et al. 2007). This finding corroborates the conclusion that was deduced from the lesion studies. In the absence of cholinergic transients, the probability and efficacy of the detection process is decreased, and, thus, a large proportion of signals are missed. Non-signal trial performance does not require signal detection and thus remains unaffected by such lesions.
Levels of prefrontal ACh release in attentional task-performing animals do not seem to be correlated with levels of performance but rather with the maintenance of (residual) performance under challenging conditions (Kozak et al. 2006). Although more evidence is required to substantiate this hypothesis, it is consistent with the general view that cholinergic activity in prefrontal regions specifically contributes to the recruitment of top-down mechanisms to combat further performance decline and support performance stabilization and recovery in the presence of a distractor (Sarter et al. 2006).
As the activity of cholinergic inputs to the prefrontal cortex influences the activity of cholinergic inputs to other cortical regions (Nelson et al. 2005), the basal forebrain cholinergic input system has been hypothesized to contribute functionally to the mediation of top-down effects in two different ways. First, cholinergic inputs to the prefrontal cortex are particularly active during challenges on attention, thereby assisting in the activation and orchestration of the prefrontal efferent circuitry that mediates top-down effects (see also the distractor-induced neurophysiological effects in the prefrontal cortex described in Gill et al. 2000). Second, cholinergic inputs to other cortical regions contribute to the mediation of top-down effects including receptive field optimization and amplification of thalamic input processing (McKenna et al. 1989; Oldford and Castro-Alamancos 2003; Puckett et al. 2007). Cholinergic projections to other cortical regions are regulated in part by direct prefrontal projections to the basal forebrain, as well as multi-synaptic circuits involving prefrontal projections to the nucleus accumbens (NAC) and other limbic regions, which in turn project to the basal forebrain (e.g., Zaborszky et al. 1997; for an illustration of this model, see Fig. 3 in Sarter et al. 2006).
Fig. 3.
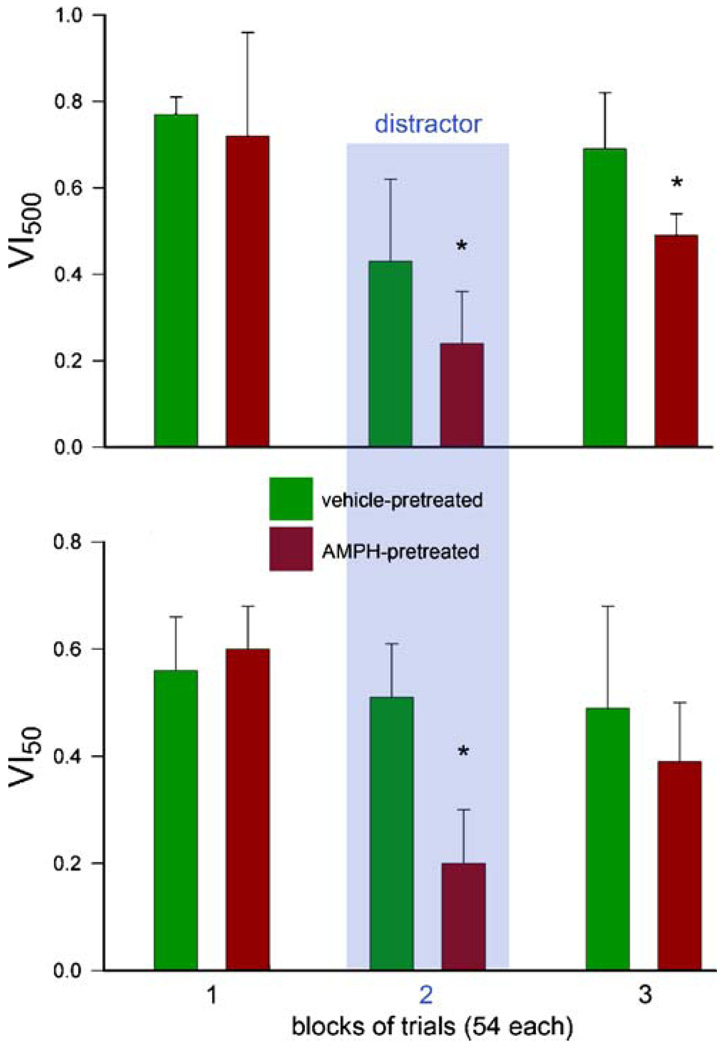
Effects of distractor challenges on the performance of AMPH-pretreated animals (Young et al. 2007) as indicated by VI500,50 (see legend of Fig. 1 for an explanation of this overall measure of attentional performance). AMPH-pretreated animals performed equally to control animals during block 1. The distractor impaired the performance of all animals, with AMPH-pretreated animals exhibiting significantly greater impairments and incomplete performance recovery in block 3. Importantly, omissions do not enter the calculation of VI and remained generally low, suggesting the animals’ continued motivation to stabilize and recover performance and achieve normal rates of reward delivery (see also Fig. 1g). Thus, in the absence of AMPH challenges, demands on attentional effort and associated recruitment of top-down mechanisms (see text) reveal robust impairments in attentional performance
Taken together, accumulating evidence indicates the necessity of the cortical cholinergic input system for attentional performance and specifies that cholinergic activity selectively mediates the detection of signals. Furthermore, prefrontal cholinergic activity is particularly high during performance challenges, indicating the special role of prefrontal cholinergic inputs in the recruitment of top-down mechanisms. The available evidence about the dynamic changes in cholinergic activity in task-performing animals that underwent the amphetamine (AMPH)-pretreatment regimen will be discussed below in the sections on the modeling of the two major stages of schizophrenia.
Cholinergic mechanisms of attention and cholinergic neurotransmission in schizophrenia
As discussed above, extensive evidence supports the general hypothesis that the cortical cholinergic input system is necessary for, and the cortical cholinergic activity mediates, a wide range of attentional processes and capacity. Therefore, abnormalities in the temporal and spatial orchestration of cholinergic activity in the cortex can be predicted to cause robust impairments in the ability to detect and select stimuli for extended processing and to perform cognitive tasks involving demands on attentional processes. Given the crucial role of the cortical cholinergic system in fundamental aspects of cognition, it seems likely that abnormalities in cholinergic neurotransmission are a necessary component of the abnormally regulated circuitry that mediates the cognitive symptoms of schizophrenia (Heimer 2000).
For several reasons, our knowledge about the role of cholinergic dysregulation in schizophrenia has remained limited. It is presently not possible to monitor in vivo dynamic abnormalities in cholinergic neurotransmission in humans. Furthermore, the post-mortem determination of levels of choline acetyltransferase or acetylcholinesterase is unlikely to indicate regulatory abnormalities (Powchik et al. 1998), largely because these enzymes are present at relatively high concentrations that do not limit the synthesis and hydrolysis, respectively, of ACh. Moreover, the evidence from the model (see below) suggests that the demonstration of such abnormalities would require recruitment of the cholinergic system and thus cognitive performance in patients (Sarter et al. 2007). However, postmortem and in vivo imaging studies consistently indicated abnormally low levels of muscarinic M1 and M4 receptor densities, as well as elevated levels of M2 receptors in the cortex (Crook et al. 2000, 2001; Lai et al. 2001; Dean et al. 2002, 2003; Mancama et al. 2003; Raedler et al. 2003; Deng and Huang 2005; Newell et al. 2007; Scarr et al. 2008). Based in part on our evidence concerning the mesolimbic regulation of basal forebrain cholinergic neurons (e.g., Zmarowski et al. 2005, 2007; Neigh et al. 2004), these changes in muscarinic receptor density were suggested to reflect abnormal interactions between mesolimbic dopaminergic and basal forebrain cholinergic systems (Hyde and Crook 2001; Raedler et al. 2007). Furthermore, a potential role of dysregulated nicotinic acetylcholine receptors has been suggested (De Luca et al. 2006).
Circumstantial evidence often cited in support of a role of the cholinergic system in the development of the symptoms of schizophrenia includes the potency of clozapine to increase basal ACh release (Ichikawa et al. 2002), the considerable affinity of clozapine and other second-generation antipsychotic drugs to muscarinic receptors (Raedler et al. 2000), and the muscarinic receptor agonist-like properties of a major metabolite of clozapine, N-desmethylclozapine (Weiner et al. 2004; Li et al. 2005; Lameh et al. 2007). Finally, it should be noted that the use of acetylcholine esterase inhibitors as adjunctive treatment produced little or no beneficial cognitive effects (MacEwan et al. 2001; Friedman et al. 2002; Friedman 2004; Aasen et al. 2005; Erickson et al. 2005; Keefe et al. 2008). Similar to the limited efficacy of these drugs in senile dementia, this lack of therapeutic efficacy has been hypothesized to reflect the limited capacity of these drugs to reinstate or amplify the second-based, phasic cholinergic activity that mediates signal detection in attention tasks (above; Sarter and Bruno 1999). Further below, we will discuss attentional performance-associated cholinergic activity in the animal model that is in the central subject of this review.
Repeated amphetamine exposure as an animal model
The psychotogenic effects of repeated exposure to psychostimulants, primarily but not exclusively the AMPHs, have been extensively documented (Wallis et al. 1949; O’Flanagan and Taylor 1950; Weiner 1964; Bell 1965; Snyder et al. 1972, Snyder 1973; Bell 1973; Janowsky and Risch 1979; Kokkinidis and Anisman 1981; LeDuc and Mittleman 1995; Bartlett et al. 1997). Generally, the effects of repeated exposure to AMPH reproduce the main features of paranoid schizophrenia (hallucinations in all modalities, paranoid delusions, thought disorder, obsessive and compulsive cognitive activity and behaviors). Following discontinuation of drug use, subjects remain more sensitive to the psychotogenic effects of AMPH (Segal and Janowski 1978; Robinson and Becker 1986).
In addition to this important face validity of the model, ample evidence indicates that repeated psychostimulant exposure reproduces important neurobiological and cognitive hallmarks of schizophrenia (Segal and Mandell 1974; Segal and Janowski 1978; Lieberman et al. 1997; Strakowski et al. 1997; Castner and Goldman-Rakic 1999, 2003; Yui et al. 1999; Kapur 2003). Perhaps the most important feature of the model is the increased sensitivity of the mesolimbic dopamine system to the effects of psychostimulants. Such a hyper-responsive mesolimbic system appears to be present in schizophrenic patients, including never-medicated schizophrenic patients (Strakowski et al. 1997), and has been associated specifically with acute psychotic disease periods (Breier et al. 1997; Abi-Dargham et al. 1998; Laruelle and Abi-Dargham 1999; Laruelle et al. 1999, Laruelle 2000). Repeated psychostimulant exposure was demonstrated to produce lasting impairments in cognitive information processing and disruption of prefrontal circuit function (e.g., Homayoun and Moghaddam 2006; see below for effects on attention).
Below, we will review important aspects of experimental paradigms used to reproduce the cognitive and non-cognitive consequences of repeated AMPH exposure in laboratory animals. The bewildering heterogeneity of pretreatment regimens used in these experiments and the resulting variability of measures of the cognitive and associated neuronal consequences of such regimens together have rendered a rather confusing literature that requires clarification.
How much and how often? AMPH-pretreatment regimens
The heterogeneity of psychostimulant pretreatment regimens (number, frequency, and doses of AMPH administration) used in experiments focusing on cognitive effects has yielded a complex body of evidence (see Table 1 for a partial list; the issue of neurotoxicity will be addressed separately below). As discussed by Segal and Janowski (1978), anecdotal evidence indicates highly variable self-administration pattern by AMPH users who experienced AMPH-induced psychosis. However, this evidence does not support the widespread view that AMPH-induced psychosis requires the administration of high doses over long periods of time. Indeed, AMPH users more typically begin by administering relatively small doses and incrementally increase doses as tolerance to the autonomic and perhaps also the psychotomimetic effects of AMPH develops (Ellinwood 1972).
Extensive experimental work in animals indicated that compared to continuous AMPH exposure, repeated intermittent AMPH administration produces more effective behavioral and mesolimbic sensitization (Robinson and Becker 1986). Moreover, effective AMPH regimen should model the escalating increases in dose that are administered over several days (termed “runs” in the context of AMPH addiction), as well as the short breaks that separate runs (“crashes”). Ideally, an AMPH administration regimen should be devoid of neurotoxic consequences (below) while producing lasting abnormalities in the animals’ behavioral, cognitive, and neuronal (particularly dopaminergic) responses to stressors and drug challenges.
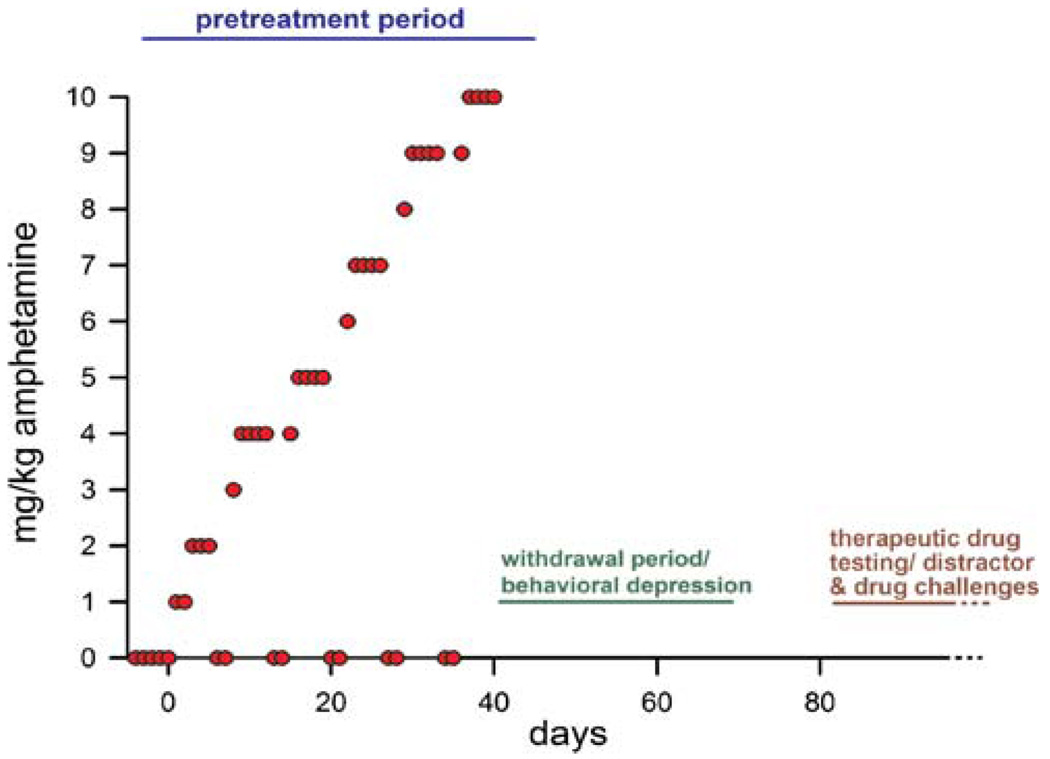
Numerous administration regimens have been characterized in different species (Segal and Mandell 1974; Paulson and Robinson 1995; Segal and Kuczenski 1997; Castner and Goldman-Rakic 2003; O’Neil et al. 2006). Robinson and colleagues characterized a regimen that involves the administration of escalating doses over 40 days and regular “crash” periods (Robinson and Camp 1987; Robinson et al. 1988; Paulson et al. 1991; Paulson and Robinson 1995). This regimen was employed in our recent experiments on the role of the cholinergic system in the attentional consequences of repeated AMPH exposure (Kozak et al. 2007; Martinez et al. 2005; below), as well as to study the pro-cognitive effects of antipsychotic treatments (Martinez and Sarter 2008; below). This AMPH-pretreatment regimen is illustrated in Fig. 2.
Fig. 2.
Illustration of the AMPH-pretreatment regimen developed by Robinson et al. (Robinson and Camp 1987; Robinson et al. 1988; Paulson et al. 1991; Paulson and Robinson 1995) and employed in our experiments on the cognitive and neuronal consequences of AMPH pretreatment and on the attenuation of these effects by low-dose treatment with antipsychotic drugs (Martinez et al. 2005; Kozak et al. 2007; Martinez and Sarter 2008). The pretreatment regimen consists of twice daily administration of AMPH in accordance with an escalating-dosing regimen over 40 days. While not producing neurotoxic effects, this AMPH-pretreatment regimen results in long-term sensitization of the mesolimbic dopamine system, impairments in behavioral and cognitive functions (see text), and in a hypersensitivity of these functions to AMPH challenges. Animals are on-task during the pretreatment regimen, although they cease to perform after doses >2 mg/kg. During weekends, vehicle is administered to model the crashes that are typically observed in AMPH abusers and are known to foster AMPH-induced psychosis (see text). Additionally, attentional performance recovers partially during the drug-off days. Our evidence indicates that the long-term consequences of repeated, escalating AMPH treatment on cortical cholinergic neurotransmission cannot be reproduced in animals that underwent such pretreatment but did not perform the task. Attentional performance recovers during a 10–15-day post-pretreatment period, after which the effects of distractors, AMPH challenges, and putative therapeutic treatments are assessed
Over a 40-day period, rats are administered successively increasing doses of D-amphetamine sulfate (1–10 mg/kg; i.p.; concentrations include salt weight) twice per day, with approximately 8 h separating the two injections. During weekends, animals are treated with saline to model crashes. Following completion of the pretreatment period, animals undergo a withdrawal period that lasts a minimum of 10 days. This period is characterized by behavioral depression and transient decreases in the concentration of norepinephrine in the hypothalamus and transient attenuation of AMPH-induced stimulation of dorsal and ventral striatal dopamine release (Paulson et al. 1991). Cognitive performance ascertained during the withdrawal period is likely to be modulated by a wide range of sensorimotor, motivational, and behavioral correlates of withdrawal and thus is not the primary focus of models of the cognitive symptoms of schizophrenia (Russig et al. 2003; Dalley et al. 2005). Indeed, the lasting effects of the pretreatment do not emerge, or are difficult to demonstrate, until the cessation of withdrawal effects (Paulson et al. 1991). Thus, it becomes important to identify this period of depression (or withdrawal) and to sufficiently postpone assessing the lasting cognitive effects of prior AMPH exposure and/or the effects of putative therapeutics until after this period has subsided. As a result of this pretreatment regimen, aspects of the animals’ behavior, including their attentional performance (below), remain hypersensitive to the detrimental effects of doses of psychostimulants that are ineffective in control animals (Paulson et al. 1991; below).
As experiments involving psychostimulant pretreatment regimens have formed a rather complex literature, it will be crucial that future experiments provide an explicit justification for the selection of doses, the frequency of administration, and the duration of the treatment regimen. Moreover, and as will be discussed further below, the behavioral and cognitive state of the animals during the pretreatment period (passive versus task performing) may determine the long-term consequences of repeated AMPH exposure and therefore requires justification.
Neurotoxicity
Potential neurotoxic effects of repeated AMPH exposure would limit the validity of this animal model for research on schizophrenia. Although repeated AMPH exposure has often been stated to cause neurotoxicity, the neurotoxic efficacy of AMPH is a strict function of dose (high) and administration regimen (continuous), with the methylated form of AMPH generally exhibiting greater neurotoxic efficacy (Robinson and Becker 1986). Concerning the regimen illustrated in Fig. 2, considerable evidence indicates that this pretreatment regimen does not produce neurotoxicity. For example, this regimen does not result in the depletion of dopamine and serotonin in the dorsal and ventral striatum (Robinson and Camp 1987; Robinson et al. 1988). The absence of neurotoxic effects resulting from intermittent, escalating-dosing administration regimen contrasts with the neurotoxic effects resulting from continuous AMPH infusion, administration of high doses of AMPH that are not preceded by lower doses and incremental increases in dose, or the effects of continuously high blood-AMPH levels maintained by implants or mini-pumps (Ellison et al. 1978; Morgan and Gibb 1980; Ridley et al. 1982; Richards et al. 1993; Wallace et al. 2001; Belcher et al. 2006; O’Neil et al. 2006).
Locomotor sensitization, stereotyped behavior, and anorexic effects as confounding variables?
A frequently expressed interpretational concern regarding the behavioral /cognitive effects of repeated AMPH administration stems from the misconception that increases in locomotor activity, stereotypic behavior, and anorexia are compulsive consequences of repeated psychostimulant exposure that therefore necessarily confound the behavioral measures of cognition. However, sensitized locomotor activity and stereotyped behaviors not only depend on the dose of AMPH but also manifest primarily in situations in which the animals’ behavior remains unconstrained, such as an open field test. In contrast, if administered in the context of a stable environment, persistent behavioral contingencies and cognitive activity, repeated AMPH exposure, and AMPH challenges do not necessarily produce such behavioral effects (Crombag et al. 1996, 2000, 2001). For example, if stereotyped behaviors and locomotor hyperactivity were compulsive consequences of AMPH pretreatment and challenge, performance in the operant sustained attention task (described above) would be characterized by high omission rates and side and lever biases.
Although such effects were observed during the AMPH-pretreatment period, particularly following the administration of higher doses, they did not manifest following the withdrawal period or following low-dose challenges that revealed specific performance impairments. Rather, the performance impairments observed during AMPH challenges were specific in nature, as indicated by signal duration-dependent performance and the absence of high levels of errors of omission or abnormal response latencies. Collectively, task performance was not confounded by the presence of overt motor stereotypies or response biases.
Likewise, there is strong evidence indicating that the anorexic effects of AMPH undergo contingent tolerance. In simplified terms, this means that the dissipation of such effects is not merely the result of the repeated presence of the drug but of adaptive appetitive and consummatory behavior that develops in response to the repeated presence of the drug while reward is retrieved and consumed (Hughes et al. 1998; Wolgin 2002; Wolgin and Jakubow 2004). Thus, locomotor hyperactivity, stereotyped behavior, and the anorexic effects of repeated AMPH exposure are not observed in animals that learned to perform tasks in the presence of AMPH. Although these findings do not detract from the importance of analyzing the potential role of such behaviors in the long-term effects of AMPH, they permit the conclusion that such behaviors do not compulsively confound the cognitive consequences of AMPH exposure.
Modeling the severe attentional impairments during active illness periods, neurobiological mechanisms, and predictive validity
Severe attentional impairments
The demonstration of lasting behavioral, cognitive, and neuronal consequences of AMPH pretreatment typically, but not necessarily (below), requires the administration of a challenge dose of AMPH and the demonstration of unusually high potency and/or efficacy of such challenges. The need for such challenges has occasionally been considered to represent a weakness or even a limitation of this pharmacological disease model. However, there are several arguments that suggest that such challenges in fact model an essential symptom-provoking characteristic of the disorder. Recurrence of AMPH psychosis in humans is often triggered by flashbacks, stress, and psychostimulant administration (e.g., Yui et al. 1997, 1999, 2000). Thus, low-dose AMPH challenges in animals are considered to model the ability of such events to elicit acute disease periods (prodromal, initial, and relapse periods) and reveal the underlying neuronal dysfunction (Antelman et al. 1980).
Furthermore, psychostimulant challenges reveal the hyper-responsive mesolimbic dopamine system that is present during active illness periods (Lieberman et al. 1990; Laruelle and Abi-Dargham 1999; Laruelle 2000; Kapur 2003; Tenn et al. 2003). Therefore, the cognitive impairments observed in response to challenges may model primarily the impairments that are associated with acute disease periods (Featherstone et al. 2007; see further below for a discussion of the relationship between such impairments and the presence of positive symptoms). We administered the pretreatment regimen described in Fig. 2 to animals during daily practice of the previously acquired sustained attention task (Fig. 1). Following completion of this pretreatment regimen, animals regained baseline performance over 8–10 days (Martinez et al. 2005; Kozak et al. 2007; Martinez and Sarter 2008). Although the severity and nature of the performance effects of AMPH challenges varied slightly among experiments due primarily to the presence or absence of tethering and intracranial microdialysis probes, the results from four separate studies (see Table 1) uniformly indicated that AMPH challenges resulted in a profound disruption of performance. As indicated by random selection of levers in both trial types (signal, non-signal trials) that is nearly 50% hits for all signal durations and 50% correct rejections, the selection of response levers was no longer governed by the presence or absence of a cue. As animals continued to perform with minimal increases in omissions and without exhibiting overt lever or side biases, their performance reflected a complete loss of stimulus control and/or the inability to process the response rules that are a function of the presence of absence of signals and/or the execution of the response (Kozak et al. 2007). Because of the interpretation of challenges as triggers for acute disease periods and because of the severity of the disruption of attentional performance evoked by challenges, the effects of AMPH challenges in this model on performance are hypothesized to model the cognitive symptoms of acute illness periods.
Neurobiological mechanisms underlying the disruption of attentional performance during active illness periods
We monitored prefrontal ACh release in animals that were pretreated and challenged with AMPH during performance of the sustained attention task. Moreover, we also monitored ACh release in AMPH-pretreated animals that were challenged with AMPH but never acquired or performed the sustained attention task (Kozak et al. 2007). The results from this experiment were straightforward. In animals pretreated with vehicle and “challenged” with a low dose of AMPH (1 mg/kg), performance was not affected and performance-associated increases in prefrontal ACh release did not differ from animals that never received AMPH. In contrast, ACh release in AMPH-pretreated and AMPH-challenged animals remained unchanged relative to pretask baseline levels. Specifically, ACh release in AMPH-pretreated and AMPH-challenged animals did not display any degree of performance-associated increase in ACh release.
Importantly, when the attentional performance of AMPH-pretreated animals was assessed in the absence of a challenge dose, performance was comparable to control animals. However, performance-associated increases in ACh release reached, and in fact exceeded, the increases observed in vehicle-pretreated control animals (below). This result clearly rejects the possibility that the AMPH-pretreatment regimen per se attenuated the reactivity of the cortical cholinergic input system. This conclusion was also supported by the finding that in non-performing animals that underwent the AMPH-pretreatment procedure, AMPH challenges resulted in increases in ACh release that were similar to those seen in drug-naïve animals. Collectively, these results indicate that the “frozen” cholinergic system in AMPH-pretreated and task-performing animals was not merely a result of AMPH preexposure alone but a result of interactions between task performance and the AMPH pretreatment. This point deserves emphasis: AMPH pretreatment and challenge per se did not disrupt cholinergic neurotransmission. Rather, interactions between task performance and AMPH challenge are necessary to reveal cholinergic dysregulation. This conclusion corresponds with the general view that disease-related, dynamic dysregulation of neuronal system is a function of the recruitment of this system, and thus of cognitive activity (see also Sarter et al. 2007).
Causal relationships between ACh release and performance
Because of the severe disruption of attentional performance in AMPH-pretreated and AMPH-challenged animals, the demonstration of a frozen cholinergic system may be considered a mere correlate of disrupted performance. However, the available evidence supports an alternative view.
Following the administration of the AMPH challenges and before the task was initiated, ACh release levels in vehicle-pretreated animals robustly increased. Presumably, exposure to the chambers and the expectation of task onset alone were sufficient to initiate such preperformance increases in ACh release. In contrast, in AMPH-pretreated animals, this preperformance increase in ACh release did not take place (Kozak et al. 2007). Because ACh levels failed to increase before task onset, it can be concluded that such failure during task performance was not merely a correlate of the failure to perform above chance levels. Rather, the disruption of performance in AMPH-pretreated and AMPH-challenged animals was a result of the failure of the cholinergic system to activate and subsequently mediate attention performance. To reiterate, such “freezing” of ACh release at baseline levels was not observed in non-performing animals, excluding the possibility the AMPH preexposure per se resulted in the failure of the cortical cholinergic input system to activate. Thus, interactions between task performance and pretreatment resulted in a major dysregulation of the cortical cholinergic input system that is revealed in the presence of low doses of AMPH. This cholinergic dysregulation likely caused the disruption of attentional performance (Kozak et al. 2007).
Hypothetical neuronal mechanisms
Neuronal activity in the NAC is necessary for the demonstration of performance-associated increases in cortical ACh release (Neigh et al. 2004). This NAC-basal forebrain interplay does not take place in situations not involving motivated cognitive activity (Neigh et al. 2001). While there is no direct evidence to support the hypothesis that a hyper-responsive mesolimbic dopamine system inhibits the basal forebrain to the point that cortical cholinergic projections can no longer be recruited, circumstantial evidence is consistent with this hypothesis. For example, reduced dopaminergic neurotransmission in the NAC was shown to benefit cognitive performance following cholinergic lesions (Grigoryan et al. 1996). Furthermore, evidence indicates that manipulations of neurotransmission in the NAC on cortical ACh release affect cortical ACh release exclusively in prefrontal regions (Zmarowski et al. 2005, 2007). Collectively, this evidence is consistent with the general hypothesis that neuronal dysregulation, manifesting at multiple sites within prefrontal–mesolimbic–basal forebrain–prefrontal circuits, is essential for the expression of the cognitive symptoms of schizophrenia (Moore et al. 1999; Sarter and Bruno 1999; Floresco et al. 2005; Goto and Grace 2005; Sarter et al. 2005b; see Fig. 4). The exact neuronal mechanisms that underlie the interactions between the effects of repeated AMPH on NAC-basal forebrain interactions, and the recruitment of these circuits by attentional performance, remain poorly understood.
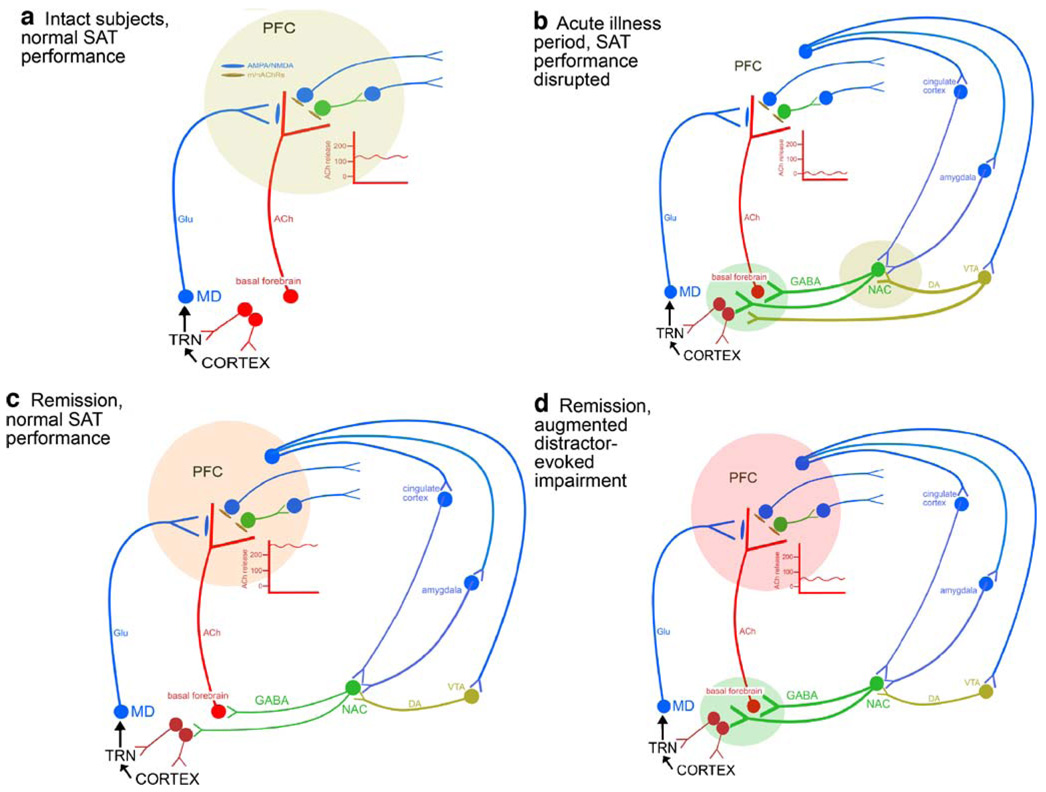
Fig. 4.
Circuitry models describing the regulation of the prefrontal (PFC) cholinergic input system mediating the performance of the standard sustained attention task (SAT) by healthy subjects (a), the disrupted SAT performance during acute illness periods (b), and the “normal” SAT performance (c) and the augmented detrimental effects of distractors during remission periods (d). The models explain key findings discussed in the main text and incorporate hypotheses concerning the mesolimbic regulation of prefrontal acetylcholine (ACh) release, as well as the abnormal top-down control of the basal forebrain and afferent mesolimbic systems during different disease states. a In intact subjects, standard SAT performance requires increases in PFC ACh release by about 100–140% over baseline (see insert); such increases in cholinergic activity specifically are required for the detection of signals (e.g., Parikh et al. 2007). As top-down control of the basal forebrain and its afferent mesolimbic systems [projections from prefrontal regions to the basal forebrain and to mesolimbic regions (nucleus accumbens, NAC, and ventral tegmentum, VTA), which in turn innervate the basal forebrain] is assumed to play an only minimal and perhaps negligible role for SAT performance by healthy subjects, standard-task performance-associated increases in PFC ACh release is thought to be based primarily on bottom-up mechanisms, meaning that the signal per se evokes cholinergic activity via local mechanisms in the PFC. We demonstrated that in the PFC, increases in cholinergic activity require glutamate (Glu) release and glutamatergic ionotropic receptor stimulation (Parikh et al. 2008). Such glutamate release originates from thalamic, mediodorsal (MD) afferents and is thought to “import” information about the signal to the PFC. This information does not concern the primary sensory representation of the signal but rather a signal-evoked “attentional searchlight”, a term that refers to a preattentional narrowing of the “place demanding attention”. The topographic projections from sensory cortical regions to the thalamic reticular thalamic nucleus (TRN), that in turn contacts MD neurons, underlie this preattentional processing of signals. The finding that attentional orienting is impaired following lesions of the TRN corresponds with this model (Weese et al. 1999). The TRN also receives cholinergic and non-cholinergic (not illustrated) inputs from the basal forebrain. The positive glutamatergic modulation of prefrontal cholinergic activity is necessary for normal signal detection and thus attentional performance. b As discussed in the main text, acute illness periods are associated with a severe disruption of attentional performance. The freezing of PFC ACh release at baseline causes such disruption. As the collective evidence is consistent with the hypothesis that mesolimbic dopaminergic (DA) activity is abnormally reactive during acute disease periods, and as NAC manipulations preferably affect PFC ACh release (e.g., Zmarowski et al. 2005, 2007), we hypothesize that basal forebrain cholinergic activity is robustly attenuated in part as a result of abnormally active dopaminergic afferents from the VTA and, indirectly, GABAergic afferents from the NAC. The basal forebrain may be further dysregulated by abnormal prefrontal output to mesolimbic regions and directly to the basal forebrain (not shown; Zaborszky et al. 1997). As a result of such freezing of basal forebrain activity, prefrontal ACh release remains at baseline and fails to support attentional performance. c During remission periods, standard (unchallenged) SAT performance is normal but mediated via abnormally high levels of PFC ACh release. The mechanisms underlying this finding remain unclear but are likely a result of abnormal levels of top-down control of the basal forebrain and its afferent mesolimbic systems (note that in contrast to the scenario described in b, in c such abnormal top-down activity does not interact with a hyper-responsive mesolimbic dopamine system). As discussed in the text, levels of prefrontal ACh release correlate with demands for top-down control, consistent with the hypothesis that during periods of remission, standard SAT performance requires abnormally high levels of top-down control. d During remission periods, attentional performance is extremely vulnerable to additional demands for top-down control, such as the presentation of distractors. The severity of the distractor effect allows the prediction that performance-associated ACh release levels will be close to baseline, similar to the scenario in b; however, this aspect of the model requires substantiation. As even standard SAT performance requires top-down control during remission periods, the additional challenge by distractors is speculated to reveal the abnormal interplay between dysregulated prefrontal output systems and abnormally reactive mesolimbic systems, yielding a result that may approach the freezing of the basal forebrain similar to the scenario described in b. The severity of the distractor-induced impairment in attentional performance, in patients and animals modeling remission periods (see main text), is consistent with an attenuated responsivity of the prefrontal cholinergic input system
Predictive validity: effects of treatment with low doses of haloperidol and clozapine on AMPH-challenge-evoked disruption of attention
The administration of relatively low doses of typical and atypical antipsychotic drugs has been repeatedly shown to produce moderately beneficial cognitive effects in schizophrenic patients (Green et al. 2002; Keefe et al. 2004, 2006; Mishara and Goldberg 2004). This evidence contrasts with the frequently expressed view that first generation antipsychotic drugs lack pro-cognitive therapeutic efficacy, whereas second-generation antipsychotic drugs possess a more promising pro-cognitive profile. Thus, given the absence of more potent, true cognition enhancers in schizophrenia, the demonstration of moderately beneficial effects of antipsychotic drugs provides an opportunity for a test of the predictive validity of animal models (Hagan and Jones 2005).
In animals pretreated with AMPH as described in Fig. 2, semi-chronic treatment with low-dose haloperidol (0.025 mg/kg) or clozapine (2.5 mg/kg) attenuated the attentional disruption produced by AMPH challenges (1 mg/kg; Martinez and Sarter 2008). Low doses were defined, in accordance with Kapur (2003), as resulting in <50% D2 receptor occupancy. Although the effects of clozapine statistically were more robust, the efficacy of the two compounds did not differ, consistent with clinical evidence (references above). Thus, and although more pharmacological evidence is necessary, these results indicate that this aspect of the model, concerning the disruption of attentional performance during active disease periods, is capable of detecting the moderately efficacious, beneficial effects of low-dose antipsychotic treatment.
Antipsychotic or true pro-cognitive effects?
Repeated exposure to AMPH causes positive symptoms in psychostimulant abusers, and AMPH triggers acute disease periods in schizophrenics (references above). Thus, it is important to address whether the component of this model that is thought to reproduce acute disease periods, as a result of AMPH challenges, merely indicates the collapse of attentional performance in association with, and perhaps even due to, the expression of “positive symptoms” in the animal model. Likewise, we need to discuss further the possibility that the beneficial attentional effects of low-dose antipsychotic-drug treatment were merely secondary to their antipsychotic efficacy. This discussion does not concern the attentional impairments observed during periods of remission and in the absence of AMPH challenges (below).
In the animal model, AMPH challenges resulted in a disruption of performance that in essence reflected random lever selection in signal and non-signal trials. However, the number of errors of omissions did not robustly increase. Thus, it was not the case that animals simply disengaged from task and engaged in competing behaviors that interfered with operations of the levers. Rather, they maintained orientation toward the intelligence panel and retrieved the rewards earned during approximately 50% of all trials. Thus, AMPH challenges in AMPH-pretreated animals did not seem to generate repetitive or perseverative behaviors as such behaviors would likely cause cessation of responding or at least strong side or lever biases. As repetitive perseverative behaviors were suggested to represent animal analogues of positive symptoms (Segal et al. 1981), our evidence does not provide support for the possibility that symptoms analogous to positive symptoms in humans were present in AMPH-pretreated and AMPH-challenged animals. Consequently, it seems highly unlikely that the disruption of performance was secondary to the presence of positive symptoms. Likewise, the evidence does not support the possibility that the beneficial effects of the treatments on attentional performance were merely due and secondary to the attenuation of behavioral analogues of positive symptoms in animals.
Although interactions between repeated AMPH exposure and attentional performance may primarily model the cognitive symptoms of the disease, the potential relationships between antipsychotic effects and pro-cognitive effects of treatments in patients remain a conceptually challenging subject. The general assumption that positive symptoms and cognitive deficits are poorly correlated is not universally shared (Kay 1990; Mortimer et al. 1990; Strauss 1993; Berman et al. 1997; Brebion et al. 1999). Moreover, numerous studies indicated that the treatment-induced improvement of positive symptoms is associated with alleviation of cognitive, specifically attentional symptoms (Gruzelier and Hammond 1978; Oltmanns et al. 1978; Wahba et al. 1981; Braff and Saccuzzo 1982; Marder et al. 1984; Cassens et al. 1990; King 1990; Addington et al. 1991; Dollfus and Petit 1995). The present animal model does not appear to be capable of addressing these potential interactions between positive and cognitive symptoms. However, this limitation could also be considered an advantage as the model therefore allows a more exclusive assessment of the therapeutic efficacy of potential pro-cognitive treatments.
Neuronal mechanisms underlying beneficial attentional effects of haloperidol and clozapine during active illness periods
Evidence concerning the performance-associated activity of the prefrontal cholinergic input system in AMPH-challenged and antipsychotic-drug-treated animals is not available. However, for beneficial attentional effects of treatment to take place, the available evidence suggests that the frozen status of the cortical cholinergic input system (above) needs to be reversed, restoring the capacity of this system to activate in response to task onset and attentional performance. In support of this hypothesis, results indicate that the beneficial attentional effects of clozapine cannot be demonstrated following restricted removal of medial prefrontal cholinergic inputs (Young et al. 2007). The finding that clozapine increases basal cortical ACh release (Parada et al. 1997; Ichikawa et al. 2002) corresponds with the general view that the beneficial effects of this drug is directly related to its effects on cholinergic neurotransmission. However, the exact neuronal mechanisms mediating the beneficial effects of clozapine and, even more so of low-dose treatment with haloperidol, remain unclear.
Modeling the cognitive load-dependent impairments during remission, neurobiological mechanisms, and predictive validity
Increased demands on attentional effort reveal impairments
Cognitive impairments in schizophrenia persist during periods of remission, long after the initial induction of the disease, and outside active illness periods (references above). Prior efforts using pharmacological models to reproduce this aspect of the disease have been frustrated by the absence of robust, residual cognitive impairments. This failure resulted, at least in part, from focusing on the variation of pharmacological pretreatment parameters while ignoring the more likely possibility that increases in cognitive load and top-down control reveal such persistent impairments. Clinically stable, medicated outpatients do not reliably exhibit impairments in attention tasks if such tasks are characterized by salient, fixed, spatially invariant targets and can be performed primarily via bottom-up processes [Mar et al. 1996; Mori et al. 1996; Gold et al. 2007; see Sarter et al. (2001) for a definition and discussion of bottom-up versus top-down mechanisms contributing to attentional performance]. In contrast, tasks involving top-down control evoked by, for example, the presentation of distractors, demands on switching between stimulus attributes, or high event rates, revealed persistent impairments (Oltmanns and Neale 1975; Oltmanns 1978; Grillon et al. 1990; Nuechterlein et al. 1994; Seidman et al. 1998; Smith et al. 1998; Birkett et al. 2006; Fuller et al. 2006; Gold et al. 2007; Gur et al. 2007; Jazbec et al. 2007; Luck and Gold 2008).
The attentional performance of AMPH-pretreated animals likewise remains sensitive to demands on top-down control. Following a 10-day drug-free period during which performance recovers from the effects of the pretreatment regimen, AMPH-pretreated rats (using the regimen shown in Fig. 2) exhibit normal performance in the sustained attention task (Martinez et al. 2005; Kozak et al. 2007; Martinez and Sarter 2008). When presented with a distractor, and compared with vehicle-pretreated controls, AMPH-pretreated animals exhibited greater impairments in performance and delayed performance recovery following distractor termination (Fig. 3; Young et al. 2007). Thus, similar to the performance of schizophrenic patients during periods of remission, baseline attention performance in AMPH-pretreated rats is stable in the absence of AMPH challenges. However, demands on top-down control reveal robust limitations in the capacity to maintain and recover attentional performance. The demonstration of such impairments in an animal model arguably is highly relevant for modeling disease-associated cognitive symptoms.
Abnormally high levels of prefrontal cholinergic activity mediate unimpaired attentional functioning during standard-task performance throughout periods of symptomatic remission. AMPH-pretreated animals exhibit normal attentional performance in the absence of AMPH challenges. However, performance-associated prefrontal ACh release in AMPH-pretreated animals is significantly higher than in normal animals (Kozak et al. 2007). Indeed, levels of ACh release in these animals resembled those observed in response to performance challenges that presumably evoke top-down mechanisms (Kozak et al. 2006). Therefore, we hypothesize that the unimpaired performance of the standard attention task by AMPH-pretreated animals is mediated via elevated levels of attentional effort and top-down control. Again, we know that these abnormally high levels of ACh release are not a consequence of prior AMPH exposure per se as they are not observed in non-performing animals pretreated with AMPH (Kozak et al. 2007).
In AMPH-pretreated animals, the specific aspects of the performance of the standard attention task that necessitates top-down control are not clear. In control animals, cognitive mechanisms such as trial-type-specific processing of the task rules and the shifting of processing modes between trial types (intrinsic processing of task rules in non-signal trials versus sensory stimulus-evoked rule processing) may be executed rather automatically, akin to habits. In AMPH-pretreated animals, the determination of the signal status may involve more elaborate and less automatic processes and/or the capacity for the processing of the competing response rules may be limited. Forthcoming research designed to measure phasic cholinergic activity in AMPH-pretreated and task-performing animals will inform these speculations.
Cortical cholinergic neurotransmission during distractor-induced disruption of attentional performance
In AMPH-pretreated animals, augmented levels of performance-associated ACh release during standard-task performance are taken to indicate that such performance comes at high costs for attentional effort; the exquisite vulnerability of these animals’ performance to distractors is consistent with this hypothesis. In vehicle-pretreated subjects, the distractor transiently impairs performance, reflecting the efficacy of top-down mechanisms. In contrast, in AMPH-pretreated animals, such additional demands more severely impair these animals’ performance (as shown in Fig. 3).
The absence of evidence describing cortical cholinergic activity during distractor-induced disruption of attentional performance represents a significant empirical gap. However, the present framework allows the prediction of results with some confidence. As the distractors resulted in a severe impairment in performance in AMPH-pretreated animals that approached the level of impairment observed following AMPH challenges in these animals, and as a frozen cholinergic system is hypothesized to have caused the performance disruption that resulted from AMPH challenges (above), distractors likewise are expected to attenuate or “freeze” ACh release in AMPH-pretreated animals at baseline levels (Kozak et al. 2007; see Fig. 4). Furthermore, distractor effects on performance-associated ACh release in AMPH-pretreated animals are hypothesized to manifest on the basis of recruiting abnormally regulated prefrontal–mesolimbic–basal forebrain circuitry. Clearly, however, these mechanisms remain poorly understood.
Predictive validity: effects of low-dose haloperidol and clozapine during periods of remission
Evidence concerning the efficacy of treatments in reversing the distractor-induced disruption of performance is not available. With respect to standard attention task performance, semi-chronic treatment with low doses of haloperidol and clozapine did not affect the performance of AMPH-pretreated animals. However, AMPH-pretreated animals’ performance was protected from the detrimental performance effects of clozapine that manifested in controls (Martinez and Sarter 2008). Detrimental effects of clozapine on cognitive performance of control animals have been rarely documented, in part because control groups treated with antipsychotic drugs were often not included in experiments in this field. However, several studies did report clozapine-induced impairments in attentional performance (Cheal 1984; Amitai et al. 2007). Likewise, there is little evidence describing the cognitive effects of clozapine in healthy volunteers. Olanzapine, however, was reported to disrupt attention in healthy subjects (Beuzen et al. 1999). We may speculate that the antimuscarinic properties of clozapine contributed to the detrimental performance effects of this drug in control animals (Bymaster et al. 1996; Minzenberg et al. 2004), also because these effects resemble those observed following muscarinic receptor blockade (Bushnell et al. 1997). In attentional task performing, AMPH-pretreated animals, the augmented levels of cortical cholinergic activity required to maintain standard-task performance may be speculated to counteract these antimuscarinic effects of clozapine, thereby preventing clozapine-induced impairments in performance.
Conclusions
Table 2 summarizes the available evidence in support of the usefulness of the present animal model in terms of dissociating between the cognitive status during active disease periods and periods of remission.
Table 2.
Modeling the cognitive status during acute illness versus remission: attentional performance, performance-associated prefrontal ACh release, distractor challenges, and effects of low-dose treatment with clozapine or haloperidol
| Measure/manipulation | AMPH challenge/acute illness period | Remission period |
|---|---|---|
| Level of attentional performance | Complete loss of cognitive control (responding not controlled by presence or absence of cues)a,b,c | Normal performance at baseline levela,b,c |
| Performance-associated ACh release in prefrontal cortex | Remains “frozen” at pretask baselineb | Abnormally high; augmented release levels mirror those in controls coping with distractorsb |
| Effects of semi-chronic low-dose haloperidol on standard-task performance | Partial restorationc | No effects (irrespective of pretreatment condition)c |
| Effects of semi-chronic low-dose haloperidol on standard-task performance-associated ACh release | Performance effects predict partial restoration of performance-evoked increase in cortical ACh released | No effects on performance; therefore, Ach release remains abnormally high to support normal performanced |
| Effects of semi-chronic low-dose clozapine on standard-task performance | Robust restoration of performancec | No effects (in contrast to impairments in vehicle-pretreated animals)c |
| Effects of semi-chronic low-dose clozapine on standard-task performance-associated ACh release | Predicted to restore performance- associated increases in cortical ACh released | As (normal) performance remains unaffected in AMPH-pretreated animals, no effects on (abnormally high) levels of ACh released |
| Distractor manipulation: effects on performance | No further impairment possible | Robust disruption of performance, comparable to distractor effects in animals with prefrontal cholinergic deafferentatione |
| Distractor manipulation: effects on performance-associated ACh release | No further attenuation possible; Ach release remains at pretask baseline | Performance effects predict attenuation of Ach release to levels close to pretask baselined |
| Performance and cholinergic effects of semi-chronic low-dose antipsychotic treatment on distractor-induced challenges | No data, no basis for hypothesis | Partially attenuate the performance effects of distractor challenges by restoring recruitment of the cortical cholinergic input systemd |
Hypothesis (no evidence)
Acute disease periods are modeled by AMPH challenges in AMPH-pretreated animals and are characterized by the loss of stimulus control as a result of, at least in part, a frozen cortical cholinergic input system (see Fig. 4). Treatment with low-dose antipsychotic drugs partly ameliorates this impairment. During periods of remission, normal attentional performance is mediated via abnormally high levels of ACh release that are hypothesized to indicate the abnormally high demands on attentional effort required to maintain normal performance. Consequently, the attentional capacities of AMPH-pretreated animals are extremely vulnerable to manipulations, such as distractors, that further tax prefrontal circuitry and the (top-down) processes required to support unimpaired levels of performance in these animals. The standard-task performance of AMPH-pretreated animals is not affected by antipsychotic-drug treatment; evidence concerning antipsychotic-drug effects on distractor-induced performance deficits and associated ACh release in AMPH-pretreated but not AMPH-challenged animals currently is not available.
Given the crucial role of cognitive load in revealing cognitive deficits in patients, particularly during periods of remission, it is perhaps to be expected that simpler behavioral tasks which, by definition, involve little cognitive control and little recruitment of prefrontal efferent circuits for top-down modulation of input functions do not effectively reveal the cognitive deficits of schizophrenia. Although there may be less effortful experimental means to produce abnormalities in the mesolimbic–telencephalic circuits that mediate the expression of cognitive symptoms, it may be of critical importance that repeated AMPH exposure consistently interacts with task-associated neuronal activity. Our evidence clearly indicates that, with regards to the regulation of cortical cholinergic inputs, the pathophysiological manipulation (i.e., AMPH pretreatment) remains ineffective if these circuits are inactive (in non-performing animals) during exposure to AMPH. As the manifestation of the cognitive symptoms of schizophrenia is likely to involve, perhaps necessarily, long-term interactions between dysregulated forebrain systems and cognitive activity, this aspect of the model may be considered additional support for its validity, rather than an aspect that complicates or even limits the usefulness of this model.
This review is guided by the general view that animal models of the cognitive symptoms of schizophrenia ideally dissociate between the cognitive correlates of acute versus chronic illness states. The available evidence suggests substantial dissociations between neurobiological mechanisms mediating the symptoms of the two major disease states. Animal models that dissociate between the symptoms of major disease states allow the test of differential psycho-pharmacological treatment approaches for the cognitive and non-cognitive symptoms of acute versus remitted states. The present model may assist in discovering and characterizing non-antipsychotic compounds that could be administered as adjunctive treatments to benefit the patients’ cognitive abilities specifically during periods of remission.
The evidence and hypotheses summarized in Table 2 also reinforce the general view that non-dynamic, global hypotheses concerning the status of individual neurotransmitter systems in schizophrenia are of limited usefulness (Sarter et al. 2007). As stressed throughout this review, it is crucial that neurobiological hypotheses differentiate not only between disease states but more importantly the impact of demands on cognitive processes. In other words, it is plausible that conventional, global hypotheses concerning the role of a neurotransmitter system in schizophrenia (e.g., “too much dopamine”) will have to be replaced by hypotheses that not only specify the state of the neurotransmitter system during a particular disease state but also with respect to the specific cognitive target function and, finally, the demands on cognitive control.
Acknowledgments
The authors’ research that is reviewed in this paper was supported by PHS Grants MH063114, NS37026, MH080426, MH073600, and KO2 MH01072. Vicente Martinez is now at the University of Washington, Dept. of Psychiatry and Behavioral Sciences.
Footnotes
The task described in this manuscript was recently selected by the CNTRICS initiative (Cognitive Neuroscience Treatment Research to Improve Cognition in Schizophrenia) for development for assessing the domain “control of attention” (http://cntrics.ucdavis.edu/meetings.shtml).
Disclosure of biomedical financial interests and potential conflicts of interests Dr. Martin Sarter has received honoraria for speaking at Abbott Laboratories and Pfizer, Inc. He conducted a research that has been supported by grants from Abbott Laboratories and Pfizer Pharmaceuticals and served as a consultant for Sonexa Therapeutics Inc. in 2007.
Dr. Vicente Martinez indicated that he has no conflict of interest.
Dr. Rouba Kozak is employed by Pfizer, Inc.
Contributor Information
Martin Sarter, Email: msarter@umich.edu, Department of Psychology, University of Michigan, 530 Church Street, 4032 East Hall, Ann Arbor, MI 48109-8862, USA.
Vicente Martinez, Department of Psychology, University of Michigan, 530 Church Street, 4032 East Hall, Ann Arbor, MI 48109-8862, USA.
Rouba Kozak, CNS Biology, Pfizer, Inc., Groton, CT, USA.
References
- Aasen I, Kumari V, Sharma T. Effects of rivastigmine on sustained attention in schizophrenia: an FMRI study. J Clin Psychopharmacol. 2005;25:311–317. doi: 10.1097/01.jcp.0000169267.36797.76. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Gil R, Krystal J, Baldwin RM, Seibyl JP, Bowers M, van Dyck CH, Charney DS, Innis RB, Laruelle M. Increased striatal dopamine transmission in schizophrenia: confirmation in a second cohort. Am J Psychiatry. 1998;155:761–767. doi: 10.1176/ajp.155.6.761. [DOI] [PubMed] [Google Scholar]
- Addington J, Addington D, Maticka-Tyndale E. Cognitive functioning and positive and negative symptoms in schizophrenia. Schizophr Res. 1991;5:123–134. doi: 10.1016/0920-9964(91)90039-t. [DOI] [PubMed] [Google Scholar]
- Amitai N, Semenova S, Markou A. Cognitive-disruptive effects of the psychotomimetic phencyclidine and attenuation by atypical antipsychotic medications in rats. Psychopharmacology. 2007;193:521–537. doi: 10.1007/s00213-007-0808-x. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Carpenter WT, Jr, Kane JM, Lasser RA, Marder SR, Weinberger DR. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162:441–449. doi: 10.1176/appi.ajp.162.3.441. [DOI] [PubMed] [Google Scholar]
- Antelman SM, Eichler AJ, Black CA, Kocan D. Interchange-ability of stress and amphetamine in sensitization. Science. 1980;207:329–331. doi: 10.1126/science.7188649. [DOI] [PubMed] [Google Scholar]
- Arnold HM, Burk JA, Hodgson EM, Sarter M, Bruno JP. Differential cortical acetylcholine release in rats performing a sustained attention task versus behavioral control tasks that do not explicitly tax attention. Neuroscience. 2002;114:451–460. doi: 10.1016/s0306-4522(02)00292-0. [DOI] [PubMed] [Google Scholar]
- Bartlett E, Hallin A, Chapman B, Angrist B. Selective sensitization to the psychosis-inducing effects of cocaine: a possible marker for addiction relapse vulnerability? Neuropsychopharmacology. 1997;16:77–82. doi: 10.1016/S0893-133X(96)00164-9. [DOI] [PubMed] [Google Scholar]
- Belcher AM, O’Dell SJ, Marshall JF. A sensitizing regimen of methamphetamine causes impairments in a novelty preference task of object recognition. Behav Brain Res. 2006;170:167–172. doi: 10.1016/j.bbr.2006.02.025. [DOI] [PubMed] [Google Scholar]
- Bell DS. Comparison of amphetamine psychosis and schizophrenia. Br J Psychiatry. 1965;111:701–707. doi: 10.1192/bjp.111.477.701. [DOI] [PubMed] [Google Scholar]
- Bell DS. The experimental reproduction of amphetamine psychosis. Arch Gen Psychiatry. 1973;29:35–40. doi: 10.1001/archpsyc.1973.04200010020003. [DOI] [PubMed] [Google Scholar]
- Berman I, Viegner B, Merson A, Allan E, Pappas D, Green AI. Differential relationships between positive and negative symptoms and neuropsychological deficits in schizophrenia. Schizophr Res. 1997;25:1–10. doi: 10.1016/S0920-9964(96)00098-9. [DOI] [PubMed] [Google Scholar]
- Beuzen JN, Taylor N, Wesnes K, Wood A. A comparison of the effects of olanzapine, haloperidol and placebo on cognitive and psychomotor functions in healthy elderly volunteers. J Psycho-pharmacol. 1999;13:152–158. doi: 10.1177/026988119901300207. [DOI] [PubMed] [Google Scholar]
- Birkett P, Brindley A, Norman P, Harrison G, Baddeley A. Control of attention in schizophrenia. J Psychiatr Res. 2006;40:579–588. doi: 10.1016/j.jpsychires.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Bleuler E. Dementia praecox or the group of schizophrenias. International Universities Press; New York: 1950. [Google Scholar]
- Braff DL. Information processing and attention dysfunctions in schizophrenia. Schizophr Bull. 1993;19:233–259. doi: 10.1093/schbul/19.2.233. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA. Preattentional and attentional cognitive deficits as targets for treating schizophrenia. Psychopharmacology. 2004;174:75–85. doi: 10.1007/s00213-004-1848-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Saccuzzo DP. Effect of antipsychotic medication on speed of information processing in schizophrenic patients. Am J Psychiatry. 1982;139:1127–1130. doi: 10.1176/ajp.139.9.1127. [DOI] [PubMed] [Google Scholar]
- Brebion G, Amador X, Smith MJ, Malaspina D, Sharif Z, Gorman JM. Opposite links of positive and negative symptomatology with memory errors in schizophrenia. Psychiatry Res. 1999;88:15–24. doi: 10.1016/s0165-1781(99)00076-1. [DOI] [PubMed] [Google Scholar]
- Breier A, Su TP, Saunders R, Carson RE, Kolachana BS, de Bartolomeis A, Weinberger DR, Weisenfeld N, Malhotra AK, Eckelman WC, Pickar D. Schizophrenia is associated with elevated amphetamine-induced synaptic dopamine concentrations: evidence from a novel positron emission tomography method. Proc Natl Acad Sci U S A. 1997;94:2569–2574. doi: 10.1073/pnas.94.6.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell PJ, Oshiro WM, Padnos BK. Detection of visual signals by rats: effects of chlordiazepoxide and cholinergic and adrenergic drugs on sustained attention. Psychopharmacology. 1997;134:230–241. doi: 10.1007/s002130050446. [DOI] [PubMed] [Google Scholar]
- Bushnell PJ, Benignus VA, Case MW. Signal detection behavior in humans and rats: a comparison with matched tasks. Behav Processes. 2003;64:121–129. doi: 10.1016/s0376-6357(03)00146-3. [DOI] [PubMed] [Google Scholar]
- Bymaster FP, Calligaro DO, Falcone JF, Marsh RD, Moore NA, Tye NC, Seeman P, Wong DT. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996;14:87–96. doi: 10.1016/0893-133X(94)00129-N. [DOI] [PubMed] [Google Scholar]
- Cassens G, Inglis AK, Appelbaum PS, Gutheil TG. Neuroleptics: effects on neuropsychological function in chronic schizophrenic patients. Schizophr Bull. 1990;16:477–499. doi: 10.1093/schbul/16.3.477. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS. Long-lasting psychotomimetic consequences of repeated low-dose amphetamine exposure in rhesus monkeys. Neuropsychopharmacology. 1999;20:10–28. doi: 10.1016/S0893-133X(98)00050-5. [DOI] [PubMed] [Google Scholar]
- Castner SA, Goldman-Rakic PS. Amphetamine sensitization of hallucinatory-like behaviors is dependent on prefrontal cortex in nonhuman primates. Biol Psychiatry. 2003;54:105–110. doi: 10.1016/s0006-3223(03)00292-0. [DOI] [PubMed] [Google Scholar]
- Castner SA, Vosler PS, Goldman-Rakic PS. Amphetamine sensitization impairs cognition and reduces dopamine turnover in primate prefrontal cortex. Biol Psychiatry. 2005;57:743–751. doi: 10.1016/j.biopsych.2004.12.019. [DOI] [PubMed] [Google Scholar]
- Cheal M. Differential effects of haloperidol and clozapine on attention. Psychopharmacology. 1984;84:268–273. doi: 10.1007/BF00427457. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Semple WE, Gross M, King AC, Nordahl TE. Metabolic brain pattern of sustained auditory discrimination. Exp Brain Res. 1992;92:165–172. doi: 10.1007/BF00230392. [DOI] [PubMed] [Google Scholar]
- Cohen RM, Nordahl TE, Semple WE, Andreason P, Pickar D. Abnormalities in the distributed network of sustained attention predict neuroleptic treatment response in schizophrenia. Neuro-psychopharmacology. 1998;19:36–47. doi: 10.1016/S0893-133X(97)00201-7. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Barch DM, Carter C, Servan-Schreiber D. Context-processing deficits in schizophrenia: converging evidence from three theoretically motivated cognitive tasks. J Abnorm Psychol. 1999;108:120–133. doi: 10.1037//0021-843x.108.1.120. [DOI] [PubMed] [Google Scholar]
- Crider A, Solomon PR, McMahon MA. Disruption of selective attention in the rat following chronic d-amphetamine administration: relationship to schizophrenic attention disorder. Biol Psychiatry. 1982;17:351–361. [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Robinson TE. Signalled versus unsignalled intravenous amphetamine: large differences in the acute psychomotor response and sensitization. Brain Res. 1996;722:227–231. doi: 10.1016/0006-8993(96)00066-2. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Maren S, Robinson TE. The role of contextual versus discrete drug-associated cues in promoting the induction of psychomotor sensitization to intravenous amphetamine. Behav Brain Res. 2000;116:1–22. doi: 10.1016/s0166-4328(00)00243-6. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Badiani A, Chan J, Dell’Orco J, Dineen SP, Robinson TE. The ability of environmental context to facilitate psychomotor sensitization to amphetamine can be dissociated from its effect on acute drug responsiveness and on conditioned responding. Neuropsychopharmacology. 2001;24:680–690. doi: 10.1016/S0893-133X(00)00238-4. [DOI] [PubMed] [Google Scholar]
- Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Decreased muscarinic receptor binding in subjects with schizophrenia: a study of the human hippocampal formation. Biol Psychiatry. 2000;48:381–388. doi: 10.1016/s0006-3223(00)00918-5. [DOI] [PubMed] [Google Scholar]
- Crook JM, Tomaskovic-Crook E, Copolov DL, Dean B. Low muscarinic receptor binding in prefrontal cortex from subjects with schizophrenia: a study of Brodmann’s areas 8, 9, 10, and 46 and the effects of neuroleptic drug treatment. Am J Psychiatry. 2001;158:918–925. doi: 10.1176/appi.ajp.158.6.918. [DOI] [PubMed] [Google Scholar]
- Dalley JW, McGaughy J, O’Connell MT, Cardinal RN, Levita L, Robbins TW. Distinct changes in cortical acetylcholine and noradrenaline efflux during contingent and noncontingent performance of a visual attentional task. J Neurosci. 2001;21:4908–4914. doi: 10.1523/JNEUROSCI.21-13-04908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Berry D, Milstein JA, Laane K, Everitt BJ, Robbins TW. Cognitive sequelae of intravenous amphetamine self-administration in rats: evidence for selective effects on attentional performance. Neuropsychopharmacology. 2005;30:525–537. doi: 10.1038/sj.npp.1300590. [DOI] [PubMed] [Google Scholar]
- De Luca V, Voineskos S, Wong G, Kennedy JL. Genetic interaction between alpha4 and beta2 subunits of high affinity nicotinic receptor: analysis in schizophrenia. Exp Brain Res. 2006;174:292–296. doi: 10.1007/s00221-006-0458-y. [DOI] [PubMed] [Google Scholar]
- Dean B, McLeod M, Keriakous D, McKenzie J, Scarr E. Decreased muscarinic1 receptors in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2002;7:1083–1091. doi: 10.1038/sj.mp.4001199. [DOI] [PubMed] [Google Scholar]
- Dean B, Bymaster FP, Scarr E. Muscarinic receptors in schizophrenia. Curr Mol Med. 2003;3:419–426. doi: 10.2174/1566524033479654. [DOI] [PubMed] [Google Scholar]
- Demeter E, Sarter M, Lustig C. Rats and humans paying attention: cross-species task development for translational research. Neuropsychology. 2008 doi: 10.1037/a0013712. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng C, Huang XF. Decreased density of muscarinic receptors in the superior temporal gyrusin schizophrenia. J Neurosci Res. 2005;81:883–890. doi: 10.1002/jnr.20600. [DOI] [PubMed] [Google Scholar]
- Dollfus S, Petit M. Negative symptoms in schizophrenia: their evolution during an acute phase. Schizophr Res. 1995;17:187–194. doi: 10.1016/0920-9964(94)00087-o. [DOI] [PubMed] [Google Scholar]
- Ellinwood EH. Amphetamine psychosis: individuals, settings, and sequences. In: Ellinwood EH, Cohen S, editors. Current concepts on amphetamine abuse. US Government Printing Office; Washington, D.C: 1972. pp. 143–157. [Google Scholar]
- Ellison G, Eison MS, Huberman HS, Daniel F. Long-term changes in dopaminergic innervation of caudate nucleus after continuous amphetamine administration. Science. 1978;201:276–278. doi: 10.1126/science.26975. [DOI] [PubMed] [Google Scholar]
- Erickson SK, Schwarzkopf SB, Palumbo D, Badgley-Fleeman J, Smirnow AM, Light GA. Efficacy and tolerability of low-dose donepezil in schizophrenia. Clin Neuropharmacol. 2005;28:179–184. doi: 10.1097/01.wnf.0000173714.61744.e6. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–684. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Kapur S, Fletcher PJ. The amphetamine-induced sensitized state as a model of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1556–1571. doi: 10.1016/j.pnpbp.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Toulopoulou T, Morris RG, McDonald C, Bramon E, Walshe M, Murray RM. Selective attention deficits reflect increased genetic vulnerability to schizophrenia. Schizophr Res. 2008;101:169–175. doi: 10.1016/j.schres.2008.01.019. [DOI] [PubMed] [Google Scholar]
- Fisk AD, Scerbo MW. Automatic and control processing approach to interpreting vigilance performance: a review and reevaluation. Hum Factors. 1987;29:653–660. doi: 10.1177/001872088702900605. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tenn CC, Rizos Z, Lovic V, Kapur S. Sensitization to amphetamine, but not PCP, impairs attentional set shifting: reversal by a D1 receptor agonist injected into the medial prefrontal cortex. Psychopharmacology. 2005;183:190–200. doi: 10.1007/s00213-005-0157-6. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Tenn CC, Sinyard J, Rizos Z, Kapur S. A sensitizing regimen of amphetamine impairs visual attention in the 5-choice serial reaction time test: reversal by a D1 receptor agonist injected into the medical prefrontal cortex. Neuropsycho-pharmacology. 2007;32:1122–1132. doi: 10.1038/sj.npp.1301221. [DOI] [PubMed] [Google Scholar]
- Floresco SB, Geyer MA, Gold LH, Grace AA. Developing predictive animal models and establishing a preclinical trials network for assessing treatment effects on cognition in schizophrenia. Schizophr Bull. 2005;31:888–894. doi: 10.1093/schbul/sbi041. [DOI] [PubMed] [Google Scholar]
- Friedman JI. Cholinergic targets for cognitive enhancement in schizophrenia: focus on cholinesterase inhibitors and muscarinic agonists. Psychopharmacology. 2004;174:45–53. doi: 10.1007/s00213-004-1794-x. [DOI] [PubMed] [Google Scholar]
- Friedman JI, Adler DN, Howanitz E, Harvey PD, Brenner G, Temporini H, White L, Parrella M, Davis KL. A double blind placebo controlled trial of donepezil adjunctive treatment to risperidone for the cognitive impairment of schizophrenia. Biol Psychiatry. 2002;51:349–357. doi: 10.1016/s0006-3223(01)01342-7. [DOI] [PubMed] [Google Scholar]
- Fuller RL, Luck SJ, Braun EL, Robinson BM, McMahon RP, Gold JM. Impaired control of visual attention in schizophrenia. J Abnorm Psychol. 2006;115:266–275. doi: 10.1037/0021-843X.115.2.266. [DOI] [PubMed] [Google Scholar]
- Gilbert CD, Sigman M. Brain states: top-down influences in sensory processing. Neuron. 2007;54:677–696. doi: 10.1016/j.neuron.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Gill TM, Sarter M, Givens B. Sustained visual attention performance-associated prefrontal neuronal activity: evidence for cholinergic modulation. J Neurosci. 2000;20:4745–4757. doi: 10.1523/JNEUROSCI.20-12-04745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Fuller RL, Robinson BM, Braun EL, Luck SJ. Impaired top-down control of visual search in schizophrenia. Schizophr Res. 2007;94:148–155. doi: 10.1016/j.schres.2007.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg TE, Patterson KJ, Taqqu Y, Wilder K. Capacity limitations in short-term memory in schizophrenia: tests of competing hypotheses. Psychol Med. 1998;28:665–673. doi: 10.1017/s0033291797006429. [DOI] [PubMed] [Google Scholar]
- Goldstein G, Shemansky WJ. Influences on cognitive heterogeneity in schizophrenia. Schizophr Res. 1995;18:59–69. doi: 10.1016/0920-9964(95)00040-2. [DOI] [PubMed] [Google Scholar]
- Gorissen M, Sanz JC, Schmand B. Effort and cognition in schizophrenia patients. Schizophr Res. 2005;78:199–208. doi: 10.1016/j.schres.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Goto Y, Grace AA. Dopamine-dependent interactions between limbic and prefrontal cortical plasticity in the nucleus accumbens: disruption by cocaine sensitization. Neuron. 2005;47:255–266. doi: 10.1016/j.neuron.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Green MF. What are the functional consequences of neuro-cognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Green MF, Marder SR, Glynn SM, McGurk SR, Wirshing WC, Wirshing DA, Liberman RP, Mintz J. The neurocognitive effects of low-dose haloperidol: a two-year comparison with risperidone. Biol Psychiatry. 2002;51:972–978. doi: 10.1016/s0006-3223(02)01370-7. [DOI] [PubMed] [Google Scholar]
- Grigoryan G, Hodges H, Mitchell S, Sinden JD, Gray JA. 6-OHDA lesions of the nucleus accumbens accentuate memory deficits in animals with lesions to the forebrain cholinergic projection system: effects of nicotine administration on learning and memory in the water maze. Neurobiol Learn Mem. 1996;65:135–153. doi: 10.1006/nlme.1996.0016. [DOI] [PubMed] [Google Scholar]
- Grillon C, Courchesne E, Ameli R, Geyer MA, Braff DL. Increased distractibility in schizophrenic patients. Electrophysiologic and behavioral evidence. Arch Gen Psychiatry. 1990;47:171–179. doi: 10.1001/archpsyc.1990.01810140071010. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R, Courchesne E, Braff DL. Effects of task relevance and attention on P3 in schizophrenic patients. Schizophr Res. 1991;4:11–21. doi: 10.1016/0920-9964(91)90005-c. [DOI] [PubMed] [Google Scholar]
- Gruzelier JH, Hammond NV. The effect of chlorpromazine upon psychophysiological, endocrine and information processing measures in schizophrenia. J Psychiatr Res. 1978;14:167–182. doi: 10.1016/0022-3956(78)90019-5. [DOI] [PubMed] [Google Scholar]
- Gur RE, Turetsky BI, Loughead J, Snyder W, Kohler C, Elliott M, Pratiwadi R, Ragland JD, Bilker WB, Siegel SJ, Kanes SJ, Arnold SE, Gur RC. Visual attention circuitry in schizophrenia investigated with oddball event-related functional magnetic resonance imaging. Am J Psychiatry. 2007;164:442–449. doi: 10.1176/ajp.2007.164.3.442. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Jones DN. Predicting drug efficacy for cognitive deficits in schizophrenia. Schizophr Bull. 2005;31:830–853. doi: 10.1093/schbul/sbi058. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, McGaughy J. High acetylcholine levels set circuit dynamics for attention and encoding and low acetylcholine levels set dynamics for consolidation. Progr Brain Res. 2004;145:201–231. doi: 10.1016/S0079-6123(03)45015-2. [DOI] [PubMed] [Google Scholar]
- Heimer L. Basal forebrain in the context of schizophrenia. Brain Res Rev. 2000;31:205–235. doi: 10.1016/s0165-0173(99)00039-9. [DOI] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP. Operant performance and cortical acetylcholine release: role of response rate, reward density, and non-contingent stimuli. Cogn Brain Res. 1997;6:23–36. doi: 10.1016/s0926-6410(97)00014-1. [DOI] [PubMed] [Google Scholar]
- Himmelheber AM, Sarter M, Bruno JP. Increases in cortical acetylcholine release during sustained attention performance in rats. Cogn Brain Res. 2000;9:313–325. doi: 10.1016/s0926-6410(00)00012-4. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Moghaddam B. Progression of cellular adaptations in medial prefrontal and orbitofrontal cortex in response to repeated amphetamine. J Neurosci. 2006;26:8025–8039. doi: 10.1523/JNEUROSCI.0842-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes KM, Popi L, Wolgin DL. Experiential constraints on the development of tolerance to amphetamine hypophagia following sensitization of stereotypy: instrumental contingencies regulate the expression of sensitization. Psychopharmacology. 1998;140:445–449. doi: 10.1007/s002130050788. [DOI] [PubMed] [Google Scholar]
- Hyde TM, Crook JM. Cholinergic systems and schizophrenia: primary pathology or epiphenomena? J Chem Neuroanat. 2001;22:53–63. doi: 10.1016/s0891-0618(01)00101-6. [DOI] [PubMed] [Google Scholar]
- Ichikawa J, Dai J, O’Laughlin IA, Fowler WL, Meltzer HY. Atypical, but not typical, antipsychotic drugs increase cortical acetylcholine release without an effect in the nucleus accumbens or striatum. Neuropsychopharmacology. 2002;26:325–339. doi: 10.1016/S0893-133X(01)00312-8. [DOI] [PubMed] [Google Scholar]
- Janowsky DS, Risch C. Amphetamine psychosis and psychotic symptoms. Psychopharmacology. 1979;65:73–77. doi: 10.1007/BF00491982. [DOI] [PubMed] [Google Scholar]
- Jazbec S, Pantelis C, Robbins T, Weickert T, Weinberger DR, Goldberg TE. Intra-dimensional/extra-dimensional set-shifting performance in schizophrenia: impact of distractors. Schizophr Res. 2007;89:339–349. doi: 10.1016/j.schres.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13–23. doi: 10.1176/appi.ajp.160.1.13. [DOI] [PubMed] [Google Scholar]
- Kay SR. Significance of the positive-negative distinction in schizophrenia. Schizophr Bull. 1990;16:635–652. doi: 10.1093/schbul/16.4.635. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Seidman LJ, Christensen BK, Hamer RM, Sharma T, Sitskoorn MM, Lewine RR, Yurgelun-Todd DA, Gur RC, Tohen M, Tollefson GD, Sanger TM, Lieberman JA. Comparative effect of atypical and conventional antipsychotic drugs on neurocognition in first-episode psychosis: a randomized, double-blind trial of olanzapine versus low doses of haloperidol. Am J Psychiatry. 2004;161:985–995. doi: 10.1176/appi.ajp.161.6.985. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Bilder RM, Harvey PD, Davis SM, Palmer BW, Gold JM, Meltzer HY, Green MF, Miller del D, Canive JM, Adler LW, Manschreck TC, Swartz M, Rosenheck R, Perkins DO, Walker TM, Stroup TS, McEvoy JP, Lieberman JA. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuro-psychopharmacology. 2006;31:2033–2046. doi: 10.1038/sj.npp.1301072. [DOI] [PubMed] [Google Scholar]
- Keefe RS, Malhotra AK, Meltzer HY, Kane JM, Buchanan RW, Murthy A, Sovel M, Chunming L, Goldman R. Efficacy and safety of donepezil in patients with schizophrenia or schizoaffective disorder: significant placebo/practice effects in a 12-week, randomized, double-blind, placebo-controlled trial. Neuropsychopharmacology. 2008;33:1217–1228. doi: 10.1038/sj.npp.1301499. [DOI] [PubMed] [Google Scholar]
- King DJ. The effect of neuroleptics on cognitive and psychomotor function. Br J Psychiatry. 1990;157:799–811. doi: 10.1192/bjp.157.6.799. [DOI] [PubMed] [Google Scholar]
- Koelega HS, Brinkman JA, Zwep B, Verbaten MN. Dynamic vs static stimuli in their effect on visual vigilance performance. Percept Mot Skills. 1990;70:823–831. doi: 10.2466/pms.1990.70.3.823. [DOI] [PubMed] [Google Scholar]
- Kokkinidis L, Anisman H. Amphetamine psychosis and schizophrenia: a dual model. Neurosci Biobehav Rev. 1981;5:449–461. doi: 10.1016/0149-7634(81)90015-4. [DOI] [PubMed] [Google Scholar]
- Kondrad RL, Burk JA. Transient disruption of attentional performance following escalating amphetamine administration in rats. Psychopharmacology. 2004;175:436–442. doi: 10.1007/s00213-004-1857-z. [DOI] [PubMed] [Google Scholar]
- Kozak R, Bruno JP, Sarter M. Augmented prefrontal acetylcholine release during challenged attentional performance. Cereb Cortex. 2006;16:9–17. doi: 10.1093/cercor/bhi079. [DOI] [PubMed] [Google Scholar]
- Kozak R, Martinez V, Young D, Brown H, Bruno JP, Sarter M. Toward a neuro-cognitive animal model of the cognitive symptoms of schizophrenia: disruption of cortical cholinergic neurotransmission following repeated amphetamine exposure in attentional task-performing, but not non-performing, rats. Neuropsychopharmacology. 2007;32:2074–2086. doi: 10.1038/sj.npp.1301352. [DOI] [PubMed] [Google Scholar]
- Kraepelin E. Scholars’ facsimiles & reprints. 1981 edition. Delmar; 1907. Clinical psychiatry. [Google Scholar]
- Kraepelin E. Dementia praecox and paraphrenia. Facsimile 1971 Edition. Robert E. Krieger; Huntington: 1919. [Google Scholar]
- Lai MK, Lai OF, Keene J, Esiri MM, Francis PT, Hope T, Chen CP. Psychosis of Alzheimer’s disease is associated with elevated muscarinic M2 binding in the cortex. Neurology. 2001;57:805–811. doi: 10.1212/wnl.57.5.805. [DOI] [PubMed] [Google Scholar]
- Lameh J, Burstein ES, Taylor E, Weiner DM, Vanover KE, Bonhaus DW. Pharmacology of N-desmethylclozapine. Pharmacol Ther. 2007;115:223–231. doi: 10.1016/j.pharmthera.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Laruelle M. The role of endogenous sensitization in the pathophysiology of schizophrenia: implications from recent brain imaging studies. Brain Res Rev. 2000;31:371–384. doi: 10.1016/s0165-0173(99)00054-5. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A. Dopamine as the wind of the psychotic fire: new evidence from brain imaging studies. J Psychopharmacol. 1999;13:358–371. doi: 10.1177/026988119901300405. [DOI] [PubMed] [Google Scholar]
- Laruelle M, Abi-Dargham A, Gil R, Kegeles L, Innis R. Increased dopamine transmission in schizophrenia: relationship to illness phases. Biol Psychiatry. 1999;46:56–72. doi: 10.1016/s0006-3223(99)00067-0. [DOI] [PubMed] [Google Scholar]
- LeDuc PA, Mittleman G. Schizophrenia and psychostimulant abuse: a review and re-analysis of clinical evidence. Psycho-pharmacology. 1995;121:407–427. doi: 10.1007/BF02246489. [DOI] [PubMed] [Google Scholar]
- Li Z, Huang M, Ichikawa J, Dai J, Meltzer HY. N-desmethylclozapine, a major metabolite of clozapine, increases cortical acetylcholine and dopamine release in vivo via stimulation of M1 muscarinic receptors. Neuropsychopharmacology. 2005;30:1986–1995. doi: 10.1038/sj.npp.1300768. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Kinon BJ, Loebel AD. Dopaminergic mechanisms in idiopathic and drug-induced psychoses. Schizophr Bull. 1990;16:97–110. doi: 10.1093/schbul/16.1.97. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Sheitman BB, Kinon BJ. Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacology. 1997;17:205–229. doi: 10.1016/S0893-133X(97)00045-6. [DOI] [PubMed] [Google Scholar]
- Lieh-Mak F, Lee PW. Cognitive deficit measures in schizophrenia: factor structure and clinical correlates. Am J Psychiatry. 1997;154:39–46. doi: 10.1176/ajp.154.6.39. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Gold JM. The construct of attention in schizophrenia. Biol Psychiatry. 2008;64:34–39. doi: 10.1016/j.biopsych.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacEwan GW, Ehmann TS, Khanbhai I, Wrixon C. Donepezil in schizophrenia—is it helpful? An experimental design case study. Acta Psychiatr Scand. 2001;104:469–472. doi: 10.1046/j.0001-690x.2001.acp1c008d.x. [DOI] [PubMed] [Google Scholar]
- Mancama D, Arranz MJ, Landau S, Kerwin R. Reduced expression of the muscarinic 1 receptor cortical subtype in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2003;119:2–6. doi: 10.1002/ajmg.b.20020. [DOI] [PubMed] [Google Scholar]
- Mar CM, Smith DA, Sarter M. Behavioural vigilance in schizophrenia. Evidence for hyperattentional processing. Br J Psychiatry. 1996;169:781–789. doi: 10.1192/bjp.169.6.781. [DOI] [PubMed] [Google Scholar]
- Marder SR, Asarnow RF, Van Putten T. Information processing and neuroleptic response in acute and stabilized schizophrenic patients. Psychiatry Res. 1984;13:41–49. doi: 10.1016/0165-1781(84)90117-3. [DOI] [PubMed] [Google Scholar]
- Martinez V, Sarter M. Lateralized attentional functions of cortical cholinergic inputs. Behav Neurosci. 2004;118:984–991. doi: 10.1037/0735-7044.118.5.984. [DOI] [PubMed] [Google Scholar]
- Martinez V, Sarter M. Detection of the moderately beneficial cognitive effects of low-dose treatment with haloperidol or clozapine in an animal model of the attentional impairments of schizophrenia. Neuropsychopharmacology. 2008 doi: 10.1038/sj.npp.1301661. in press. [DOI] [PubMed] [Google Scholar]
- Martinez V, Parikh V, Sarter M. Sensitized attentional performance and Fos-immunoreactive cholinergic neurons in the basal forebrain of amphetamine-pretreated rats. Biol Psychiatry. 2005;57:1138–1146. doi: 10.1016/j.biopsych.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Maunsell JH. Neuronal representations of cognitive state: reward or attention? Trends Cogn Sci. 2004;8:261–265. doi: 10.1016/j.tics.2004.04.003. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Sarter M. Behavioral vigilance in rats: task validation and effects of age, amphetamine, and benzodiazepine receptor ligands. Psychopharmacology. 1995;117:340–357. doi: 10.1007/BF02246109. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Kaiser T, Sarter M. Behavioral vigilance following infusions of 192 IgG-saporin into the basal forebrain: selectivity of the behavioral impairment and relation to cortical AChE-positive fiber density. Behav Neurosci. 1996;110:247–265. doi: 10.1037//0735-7044.110.2.247. [DOI] [PubMed] [Google Scholar]
- McGaughy J, Everitt BJ, Robbins TW, Sarter M. The role of cortical cholinergic afferent projections in cognition: impact of new selective immunotoxins. Behav Brain Res. 2000;115:251–263. doi: 10.1016/s0166-4328(00)00262-x. [DOI] [PubMed] [Google Scholar]
- McGhie A, Chapman J. Disorders of attention and perception in early schizophrenia. Brit J Med Psychol. 1961;34:103–117. doi: 10.1111/j.2044-8341.1961.tb00936.x. [DOI] [PubMed] [Google Scholar]
- McKenna TM, Ashe JH, Weinberger NM. Cholinergic modulation of frequency receptive fields in auditory cortex: I. Frequency-specific effects of muscarinic agonists. Synapse. 1989;4:30–43. doi: 10.1002/syn.890040105. [DOI] [PubMed] [Google Scholar]
- Miller GA. The cognitive revolution: a historical perspective. Trends Cogn Sci. 2003;7:141–144. doi: 10.1016/s1364-6613(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161:116–124. doi: 10.1176/appi.ajp.161.1.116. [DOI] [PubMed] [Google Scholar]
- Mishara AL, Goldberg TE. A meta-analysis and critical review of the effects of conventional neuroleptic treatment on cognition in schizophrenia: opening a closed book. Biol Psychiatry. 2004;55:1013–1022. doi: 10.1016/j.biopsych.2004.01.027. [DOI] [PubMed] [Google Scholar]
- Moore H, West AR, Grace AA. The regulation of forebrain dopamine transmission: relevance to the pathophysiology and psychopathology of schizophrenia. Biol Psychiatry. 1999;46:40–55. doi: 10.1016/s0006-3223(99)00078-5. [DOI] [PubMed] [Google Scholar]
- Morel BA. Traité des maladies mentales. Masson; Paris: 1860. [Google Scholar]
- Morgan ME, Gibb JW. Short-term and long-term effects of methamphetamine on biogenic amine metabolism in extra-striatal dopaminergic nuclei. Neuropharmacology. 1980;19:989–995. doi: 10.1016/0028-3908(80)90010-6. [DOI] [PubMed] [Google Scholar]
- Mori S, Tanaka G, Ayaka Y, Michitsuji S, Niwa H, Uemura M, Ohta Y. Preattentive and focal attentional processes in schizophrenia: a visual search study. Schizophr Res. 1996;22:69–76. doi: 10.1016/0920-9964(96)00049-7. [DOI] [PubMed] [Google Scholar]
- Mortimer AM, Lund CE, McKenna PJ. The positive:negative dichotomy in schizophrenia. Br J Psychiatry. 1990;157:41–49. doi: 10.1192/bjp.157.1.41. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Arnold HM, Sarter M, Bruno JP. Dissociations between the effects of intra-accumbens administration of amphetamine and exposure to a novel environment on accumbens do-pamine and cortical acetylcholine release. Brain Res. 2001;894:354–358. doi: 10.1016/s0006-8993(01)02059-5. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Arnold HM, Rabenstein RL, Sarter M, Bruno JP. Neuronal activity in the nucleus accumbens is necessary for performance-related increases in cortical acetylcholine release. Neuroscience. 2004;123:635–645. doi: 10.1016/j.neuroscience.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Nelson CL, Sarter M, Bruno JP. Prefrontal cortical modulation of acetylcholine release in posterior parietal cortex. Neuroscience. 2005;132:347–359. doi: 10.1016/j.neuroscience.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Newell KA, Zavitsanou K, Jew SK, Huang XF. Alterations of muscarinic and GABA receptor binding in the posterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:225–233. doi: 10.1016/j.pnpbp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Nobre AC, Sebestyen GN, Gitelman DR, Frith CD, Mesulam MM. Filtering of distractors during visual search studied by positron emission tomography. Neuroimage. 2002;16:968–976. doi: 10.1006/nimg.2002.1137. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME. Information processing and attentional functioning in the developmental course of schizophrenic disorders. Schizophr Bull. 1984;10:160–203. doi: 10.1093/schbul/10.2.160. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Dawson ME, Green MF. Information-processing abnormalities as neuropsychological vulnerability indicators for schizophrenia. Acta Psychiatr Scand (Suppl) 1994;384:71–79. doi: 10.1111/j.1600-0447.1994.tb05894.x. [DOI] [PubMed] [Google Scholar]
- O’Flanagan PM, Taylor RB. A case of recurrent psychosis associated with amphetamine addiction. J Ment Sci. 1950;96:1033–1036. doi: 10.1192/bjp.96.405.1033. [DOI] [PubMed] [Google Scholar]
- O’Neil ML, Kuczenski R, Segal DS, Cho AK, Lacan G, Melega WP. Escalating dose pretreatment induces pharmacodynamic and not pharmacokinetic tolerance to a subsequent high-dose methamphetamine binge. Synapse. 2006;60:465–473. doi: 10.1002/syn.20320. [DOI] [PubMed] [Google Scholar]
- Oldford E, Castro-Alamancos MA. Input-specific effects of acetylcholine on sensory and intracortical evoked responses in the “barrel cortex” in vivo. Neuroscience. 2003;117:769–778. doi: 10.1016/s0306-4522(02)00663-2. [DOI] [PubMed] [Google Scholar]
- Oltmanns TF. Selective attention in schizophrenic and manic psychoses: the effect of distraction on information processing. J Abnorm Psychol. 1978;87:212–225. doi: 10.1037//0021-843x.87.2.212. [DOI] [PubMed] [Google Scholar]
- Oltmanns TF, Neale JM. Schizophrenic performance when distractors are present: attentional deficit or differential task difficulty? J Abnorm Psychol. 1975;84:205–209. doi: 10.1037/h0076721. [DOI] [PubMed] [Google Scholar]
- Oltmanns TF, Ohayon J, Neale JM. The effect of anti-psychotic medication and diagnostic criteria on distractibility in schizophrenia. J Psychiatr Res. 1978;14:81–91. doi: 10.1016/0022-3956(78)90010-9. [DOI] [PubMed] [Google Scholar]
- Parada MA, Hernandez L, Puig de Parada M, Rada P, Murzi E. Selective action of acute systemic clozapine on acetylcholine release in the rat prefrontal cortex by reference to the nucleus accumbens and striatum. J Pharmacol Exp Ther. 1997;281:582–588. [PubMed] [Google Scholar]
- Parasuraman R, Mouloua M. Interaction of signal discriminability and task type in vigilance decrement. Percept Psychophys. 1987;41:17–22. doi: 10.3758/bf03208208. [DOI] [PubMed] [Google Scholar]
- Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Parikh V, Kozak R, Martinez V, Sarter M. Prefrontal acetylcholine release controls cue detection on multiple time-scales. Neuron. 2007;56:141–154. doi: 10.1016/j.neuron.2007.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh V, Man K, Decker MW, Sarter M. Glutamatergic contributions to nAChR agonist-evoked cholinergic transients in the prefrontal cortex. J Neurosci. 2008;28:3769–3780. doi: 10.1523/JNEUROSCI.5251-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passetti F, Dalley JW, O’Connell MT, Everitt BJ, Robbins TW. Increased acetylcholine release in the rat medial prefrontal cortex during performance of a visual attentional task. Eur J Neurosci. 2000;12:3051–3058. doi: 10.1046/j.1460-9568.2000.00183.x. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Amphetamine-induced time-dependent sensitization of dopamine neurotransmission in the dorsal and ventral striatum: a microdialysis study in behaving rats. Synapse. 1995;19:56–65. doi: 10.1002/syn.890190108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson PE, Camp DM, Robinson TE. Time course of transient behavioral depression and persistent behavioral sensitization in relation to regional brain monoamine concentrations during amphetamine withdrawal in rats. Psychopharmacology. 1991;103:480–492. doi: 10.1007/BF02244248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powchik P, Davidson M, Haroutunian V, Gabriel SM, Purohit DP, Perl DP, Harvey PD, Davis KL. Postmortem studies in schizophrenia. Schizophr Bull. 1998;24:325–341. doi: 10.1093/oxfordjournals.schbul.a033330. [DOI] [PubMed] [Google Scholar]
- Puckett AC, Pandya PK, Moucha R, Dai W, Kilgard MP. Plasticity in the rat posterior auditory field following nucleus basalis stimulation. J Neurophysiol. 2007;98:253–265. doi: 10.1152/jn.01309.2006. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Knable MB, Jones DW, Lafargue T, Urbina RA, Egan MF, Pickar D, Weinberger DR. In vivo olanzapine occupancy of muscarinic acetylcholine receptors in patients with schizophrenia. Neuropsychopharmacology. 2000;23:56–68. doi: 10.1016/S0893-133X(99)00162-1. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Knable MB, Jones DW, Urbina RA, Gorey JG, Lee KS, Egan MF, Coppola R, Weinberger DR. In vivo determination of muscarinic acetylcholine receptor availability in schizophrenia. Am J Psychiatry. 2003;160:118–127. doi: 10.1176/appi.ajp.160.1.118. [DOI] [PubMed] [Google Scholar]
- Raedler TJ, Bymaster FP, Tandon R, Copolov D, Dean B. Towards a muscarinic hypothesis of schizophrenia. Mol Psychiatry. 2007;12:232–246. doi: 10.1038/sj.mp.4001924. [DOI] [PubMed] [Google Scholar]
- Remington G, Kapur S. Remission: what’s in a name? Am J Psychiatry. 2005;162:2393–2394. doi: 10.1176/appi.ajp.162.12.2393-a. [DOI] [PubMed] [Google Scholar]
- Richards JB, Baggott MJ, Sabol KE, Seiden LS. A high-dose methamphetamine regimen results in long-lasting deficits on performance of a reaction-time task. Brain Res. 1993;627:254–260. doi: 10.1016/0006-8993(93)90328-k. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Baker HF, Owen F, Cross AJ, Crow TJ. Behavioural and biochemical effects of chronic amphetamine treatment in the vervet monkey. Psychopharmacology. 1982;78:245–251. doi: 10.1007/BF00428159. [DOI] [PubMed] [Google Scholar]
- Robert PH, Migneco V, Chaix I, Berthet L, Kazes M, Danion JM, Baudu C, Darcourt G. Use of a sequencing task designed to stress the supervisory system in schizophrenic subjects. Psychol Med. 1997;27:1287–1294. doi: 10.1017/s0033291797005515. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396:157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Camp DM. Long-lasting effects of escalating doses of d-amphetamine on brain monoamines, amphetamine-induced stereotyped behavior and spontaneous nocturnal locomotion. Pharmacol Biochem Behav. 1987;26:821–827. doi: 10.1016/0091-3057(87)90616-2. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Jurson PA, Bennett JA, Bentgen KM. Persistent sensitization of dopamine neurotransmission in ventral striatum (nucleus accumbens) produced by prior experience with (+)-amphetamine: a microdialysis study in freely moving rats. Brain Res. 1988;462:211–222. doi: 10.1016/0006-8993(88)90549-5. [DOI] [PubMed] [Google Scholar]
- Russig H, Durrer A, Yee BK, Murphy CA, Feldon J. The acquisition, retention and reversal of spatial learning in the morris water maze task following withdrawal from an escalating dosage schedule of amphetamine in wistar rats. Neuroscience. 2003;119:167–179. doi: 10.1016/s0306-4522(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Sarter M. Retrieval of well-learned propositional rules: insensitive to changes in activity of individual neurotransmitter systems? Psychobiology. 1990;18:451–459. [Google Scholar]
- Sarter M, Bruno JP. Abnormal regulation of corticopetal cholinergic neurons and impaired information processing in neuropsychiatric disorders. Trends Neurosci. 1999;22:67–74. doi: 10.1016/s0166-2236(98)01289-2. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: where top-down meets bottom-up. Brain Res Rev. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Res Rev. 2005a;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Sarter M, Nelson CL, Bruno JP. Cortical cholinergic transmission and cortical information processing following psychostimulant-sensitization: implications for models of schizophrenia. Schizophren Bull. 2005b;31:117–138. doi: 10.1093/schbul/sbi006. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: the neurobiology of attentional effort. Brain Res Rev. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Bruno JP, Parikh V. Abnormal neurotransmitter release underlying behavioral and cognitive disorders: toward concepts of dynamic and function-specific dysregulation. Neuro-psychopharmacology. 2007;32:1452–1461. doi: 10.1038/sj.npp.1301285. [DOI] [PubMed] [Google Scholar]
- Scarr E, Cowie TF, Kanellakis S, Sundram S, Pantelis C, Dean B. Decreased cortical muscarinic receptors define a subgroup of subjects with schizophrenia. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.28. in press. [DOI] [PubMed] [Google Scholar]
- Segal DS, Janowski DS. Psychostimulant-induced behavioral effects: possible models of schizophrenia. In: Lipton MA, DiMascio A, Killam KF, editors. Psychopharmacology: a generation of progress. Raven; New York: 1978. pp. 1113–1124. [Google Scholar]
- Segal DS, Kuczenski R. An escalating dose “binge” model of amphetamine psychosis: behavioral and neurochemical characteristics. J Neurosci. 1997;17:2551–2566. doi: 10.1523/JNEUROSCI.17-07-02551.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS, Mandell AJ. Long-term administration of d-amphetamine: progressive augmentation of motor activity and stereotypy. Pharmacol Biochem Behav. 1974;2:249–255. doi: 10.1016/0091-3057(74)90060-4. [DOI] [PubMed] [Google Scholar]
- Segal DS, Geyer MA, Schuckit MA. Stimulant-induced psychosis: an evaluation of animal models. In: Youdim MBH, Lovenberg W, Sharman DF, Lagnado JR, editors. Essays in neurochemistry and neuropharmacology. Wiley; New York: 1981. pp. 95–129. [PubMed] [Google Scholar]
- Seidman LJ, Van Manen KJ, Turner WM, Gamser DM, Faraone SV, Goldstein JM, Tsuang MT. The effects of increasing resource demand on vigilance performance in adults with schizophrenia or developmental attentional/learning disorders: a preliminary study. Schizophr Res. 1998;34:101–112. doi: 10.1016/s0920-9964(98)00097-8. [DOI] [PubMed] [Google Scholar]
- Servan-Schreiber D, Cohen JD, Steingard S. Schizophrenic deficits in the processing of context. A test of a theoretical model. Arch Gen Psychiatry. 1996;53:1105–1112. doi: 10.1001/archpsyc.1996.01830120037008. [DOI] [PubMed] [Google Scholar]
- Silver H, Feldman P. Evidence for sustained attention and working memory in schizophrenia sharing a common mechanism. J Neuropsychiatry Clin Neurosci. 2005;17:391–398. doi: 10.1176/jnp.17.3.391. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman D, Simmons K, Bloise SM, Parrish T, Mesulam MM. Monetary incentives enhance processing in brain regions mediating top-down control of attention. Cereb Cortex. 2005;15:1855–1865. doi: 10.1093/cercor/bhi063. [DOI] [PubMed] [Google Scholar]
- Smith GL, Large MM, Kavanagh DJ, Karayanidis F, Barrett NA, Michie PT, O’Sullivan BT. Further evidence for a deficit in switching attention in schizophrenia. J Abnorm Psychol. 1998;107:390–398. doi: 10.1037//0021-843x.107.3.390. [DOI] [PubMed] [Google Scholar]
- Snyder SH. Amphetamine psychosis: a “model” schizophrenia mediated by catecholamines. Am J Psychiatry. 1973;130:61–67. doi: 10.1176/ajp.130.1.61. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Aghajanian GK, Matthysse S. Prospects for research on schizophrenia. V. Pharmacological observations, drug-induced psychoses. Neurosci Res Program Bull. 1972;10:430–445. [PubMed] [Google Scholar]
- Strakowski SM, Sax KW, Setters MJ, Stanton SP, Keck PE., Jr Lack of enhanced response to repeated d-amphetamine challenge in first-episode psychosis: implications for a sensitization model of psychosis in humans. Biol Psychiatry. 1997;42:749–755. doi: 10.1016/s0006-3223(97)00052-8. [DOI] [PubMed] [Google Scholar]
- Strauss ME. Relations of symptoms to cognitive deficits in schizophrenia. Schizophr Bull. 1993;19:215–231. doi: 10.1093/schbul/19.2.215. [DOI] [PubMed] [Google Scholar]
- Tenn CC, Fletcher PJ, Kapur S. Amphetamine-sensitized animals show a sensorimotor gating and neurochemical abnormality similar to that of schizophrenia. Schizophr Res. 2003;64:103–114. doi: 10.1016/s0920-9964(03)00009-4. [DOI] [PubMed] [Google Scholar]
- Venables PH. Input dysfunction in schizophrenia. In: Maher BA, editor. Progress in experimental personality research. Academic; New York: 1964. pp. 1–47. [PubMed] [Google Scholar]
- Wahba M, Donlon PT, Meadow A. Cognitive changes in acute schizophrenia with brief neuroleptic treatment. Am J Psychiatry. 1981;138:1307–1310. doi: 10.1176/ajp.138.10.1307. [DOI] [PubMed] [Google Scholar]
- Wallace TL, Gudelsky GA, Vorhees CV. Neurotoxic regimen of methamphetamine produces evidence of behavioral sensitization in the rat. Synapse. 2001;39:1–7. doi: 10.1002/1098-2396(20010101)39:1<1::AID-SYN1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Wallis GG, Mc HJ, Scott OC. Acute psychosis caused by dextro-amphetamine. Br Med J. 1949;2:1394. doi: 10.1136/bmj.2.4641.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M. Role of anticipated reward in cognitive behavioral control. Curr Opin Neurobiol. 2007;17:213–219. doi: 10.1016/j.conb.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Weese GD, Phillips JM, Brown VJ. Attentional orienting is impaired by unilateral lesions of the thalamic reticular nucleus in the rat. J Neurosci. 1999;19:10135–10139. doi: 10.1523/JNEUROSCI.19-22-10135.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner IB. Differential diagnosis in amphetamine psychosis. Psychiatr Quart. 1964;38:707–716. doi: 10.1007/BF01573413. [DOI] [PubMed] [Google Scholar]
- Weiner DM, Meltzer HY, Veinbergs I, Donohue EM, Spalding TA, Smith TT, Mohell N, Harvey SC, Lameh J, Nash N, Vanover KE, Olsson R, Jayathilake K, Lee M, Levey AI, Hacksell U, Burstein ES, Davis RE, Brann MR. The role of M1 muscarinic receptor agonism of N-desmethylclozapine in the unique clinical effects of clozapine. Psychopharmacology. 2004;177:207–216. doi: 10.1007/s00213-004-1940-5. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Mangun GR, Woldorff MG. A role for top-down attentional orienting during interference between global and local aspects of hierarchical stimuli. Neuroimage. 2002;17:1266–1276. doi: 10.1006/nimg.2002.1284. [DOI] [PubMed] [Google Scholar]
- Wolgin DL. Effects of chronic amphetamine on the appetitive and consummatory phases of feeding. Appetite. 2002;38:221–223. doi: 10.1006/appe.2001.0483. [DOI] [PubMed] [Google Scholar]
- Wolgin DL, Jakubow JJ. Tolerance to amphetamine hypophagia: a real-time depiction of learning to suppress stereotyped movements in the rat. Behav Neurosci. 2004;118:470–478. doi: 10.1037/0735-7044.118.3.470. [DOI] [PubMed] [Google Scholar]
- Young D, Martinez V, Bruno JP, Sarter M. Neuronal mechanisms underlying the cognitive symptoms in a model of schizophrenia: prefrontal cholinergic inputs are necessary for attentional performance following repeated exposure to amphetamine. Soc Neurosci. 2007 Abs #606.9. [Google Scholar]
- Yui K, Ishiguro T, Goto K, Ikemoto S. Precipitating factors in spontaneous recurrence of methamphetamine psychosis. Psychopharmacology. 1997;134:303–308. doi: 10.1007/s002130050453. [DOI] [PubMed] [Google Scholar]
- Yui K, Goto K, Ikemoto S, Ishiguro T, Angrist B, Duncan GE, Sheitman BB, Lieberman JA, Bracha SH, Ali SF. Neurobiological basis of relapse prediction in stimulant-induced psychosis and schizophrenia: the role of sensitization. Mol Psychiatry. 1999;4:512–523. doi: 10.1038/sj.mp.4000575. [DOI] [PubMed] [Google Scholar]
- Yui K, Goto K, Ikemoto S, Ishiguro T. Stress induced spontaneous recurrence of methamphetamine psychosis: the relation between stressful experiences and sensitivity to stress. Drug Alcohol Depend. 2000;58:67–75. doi: 10.1016/s0376-8716(99)00060-5. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE. Cortical input to the basal forebrain. Neuroscience. 1997;79:1051–1078. doi: 10.1016/s0306-4522(97)00049-3. [DOI] [PubMed] [Google Scholar]
- Zmarowski A, Sarter M, Bruno JP. NMDA and dopamine interactions in the nucleus accumbens modulate cortical acetylcholine release. Eur J Neurosci. 2005;22:1731–1740. doi: 10.1111/j.1460-9568.2005.04333.x. [DOI] [PubMed] [Google Scholar]
- Zmarowski A, Sarter M, Bruno JP. Glutamate receptors in nucleus accumbens mediate regionally selective increases in cortical acetylcholine release. Synapse. 2007;61:115–123. doi: 10.1002/syn.20354. [DOI] [PubMed] [Google Scholar]