Abstract
Bundle-forming pili (BFP) promote the adherence of typical enteropathogenic Escherichia coli (EPEC) to human intestinal epithelial cells. BFP are polymers of bundlin and nine bundlin alleles have been identified in EPEC isolated from diverse sources. These alleles are divided into two main groups, α and β, based on their amino acid sequences. Alpha bundlins are also N-acetyllactosamine- (LacNAc) specific lectins and bind to HEp-2 cells, whereas β bundlins do not display these characteristics. The four surface-exposed regions of amino acid sequence heterogeneity between α and β bundlin were therefore investigated as potential LacNAc-specific carbohydrate binding domains in α bundlin. Mutation of one of these domains, 137-GENNI-141, in α1 bundlin to that of β bundlin (136-SPDST-140) resulted in BFP that no longer bound to LacNAc or HEp-2 cells. Conversely, mutating the β3 bundlin gene to encode the α bundlin sequence at this domain resulted in the gain of HEp-2 cell adherence. The importance of this domain in carbohydrate binding is supported by the finding that introducing the mutation GENNI➔GENNT altered the α1 bundlin carbohydrate-binding specificity from LacNAc to the Lewis X glycan sequence.
Introduction
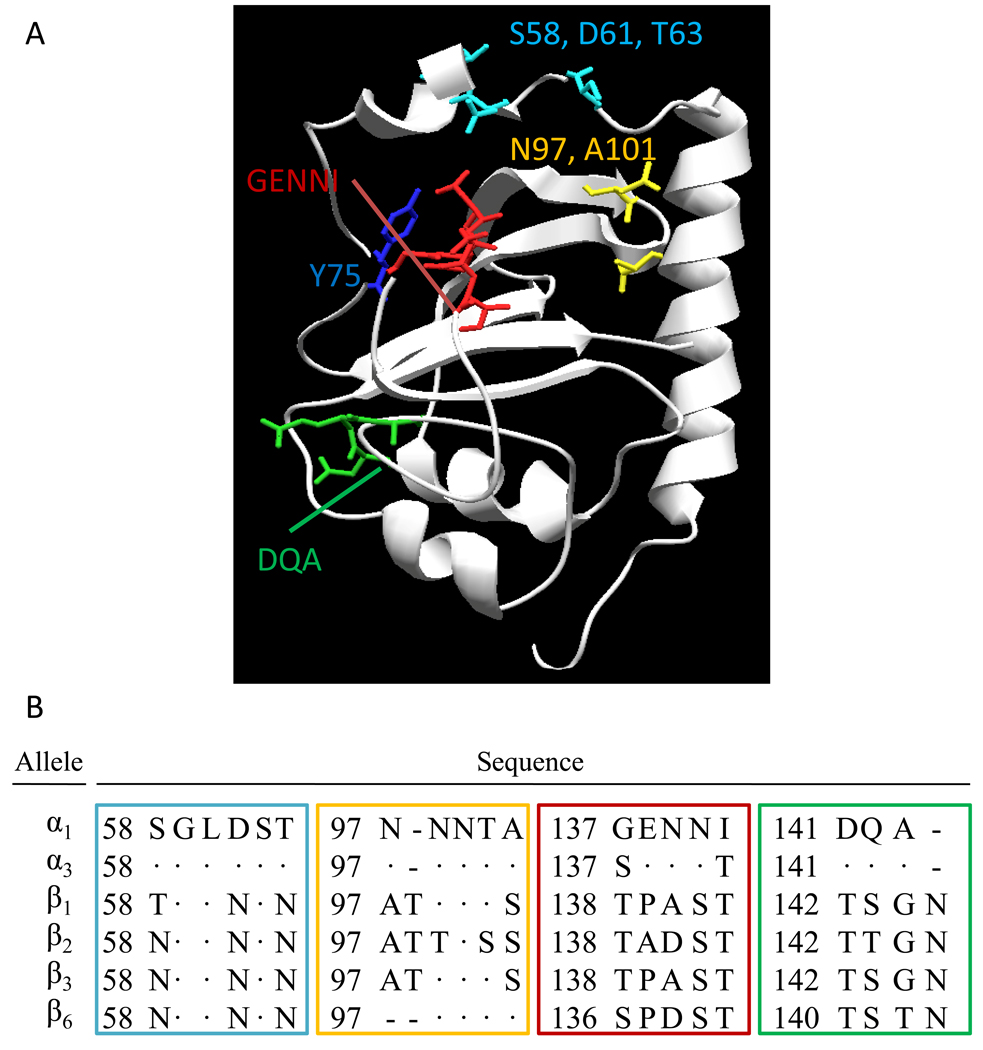
Typical enteropathogenic Escherichia coli (EPEC) strains bind as discrete microcolonies to the human intestinal epithelium, in a process known as localized adherence (LA) (Scaletsky et al., 1984). LA is mediated by the type IV bundle forming pili (BFP), which are required for both inter-bacterial adherence in the microcolony (Giron et al., 1991) as well as EPEC adherence to the host cell (Hyland et al., 2008). BFP are thought to be homopolymers of a protein called bundlin, which is expressed from the bfpA gene encoded on a 14-gene operon found on a large virulence-associated plasmid harbored by classical EPEC strains (Baldini et al., 1983). Nine bfpA alleles have been identified to date in EPEC isolates from diverse hosts (Blank et al., 2000; Blank et al., 2003). These alleles encode proteins that are 80% identical and are sub-grouped into two categories based on sequence similarity. The α group encompasses three highly homologous alleles and the β group is comprised of the remaining six alleles that are more divergent in their sequences (Blank et al., 2000). We previously demonstrated that synthetic N-acetyllactosamine (LacNAc) glycoside sequences coupled to BSA competitively inhibit early (i.e. 45-minute) HEp-2 cell LA of EPEC strains that express bundlin alleles from the α, but not β group (Hyland et al., 2008). Furthermore, purified α1 bundlin specifically binds synthetic LacNAc in nano electrospray mass spectrometry (nanoES-MS) binding assays (Hyland et al., 2008). Herein, we confirm that the β bundlin group, represented by β6 bundlin, does not demonstrate LacNAc specific lectin-like carbohydrate binding activity. Since the amino acid sequences of α and β bundlin proteins differ at four surface-exposed regions on the protein (Figure 1A) (Ramboarina et al., 2005), we sought to determine whether these regions represent the potential α bundlin LacNAc-specific carbohydrate binding domain (CBD). This was accomplished by mutating the α1 bundlin protein in order to convert it into a β6 bundlin at these regions. The α1 bundlin mutants were then assessed for bundlin expression, BFP assembly, and the ability to bind to HEp-2 cell monolayers in a LacNac-dependant fashion.
Figure 1.
(A) Cartoon of bundlin structure. Protein tertiary structure is shown in the ribbon and wire format, whereas amino acids targeted for mutation in this study are shown in the stick format. Amino acids are colored according to the block in which they were mutated. Cartoon was produced in SwissPbdViewer, version 3.7 from the NMR-solved α1 bundlin structure, reference number 1ZWT. (B) Predicted amino acid sequences for α1, α3, β1- β3, and β6 bundlins at the domains mutated in this study. Coloured boxes around each domain correspond to the color of the amino acids in panel A. Amino acid number is given for the mature bundlin protein in each case. Dots represent invariant amino acids and dashed lines represent absent amino acids relative to the α1 allele.
Results
β6 bundlin is not a LacNAc-specific lectin
Previously, we demonstrated that cloned, soluble α1 bundlin binds synthetic LacNAc-Benzene (LacNAc-Bn) (Hyland et al., 2008). Herein, we sought to confirm that β bundlin, represented by the β6 allele, does not bind LacNAc, a result predicted by the inability of the bundlin-null EPEC strain, UMD901, to bind HEp-2 cells in the early LA assay when complemented in trans with β1, β2, β3 or β6 bundlins (Hyland et al., 2008).
In nanoES-MS experiments, purified hexa-His-β6 bundlin appeared at a molecular weight of 34,660 Da (data not shown), twice the expected molecular weight of the β6 bundlin monomer (17,332 Da), suggesting that the protein dimerizes in solution. However, in contrast to our previous findings using purified α1 bundlin, no specific interaction between β6 bundlin and LacNAc-Bn was found in the nano-ES MS binding assay (data not shown), after correcting for nonspecific binding complexes, as described previously (Hyland et al., 2008).
Mutation of α1 bundlin amino acids GENNI (α1 allele) to SPDST (β6 allele) results in the loss of the early LA phenotype and LacNAc-specific binding activity
Four mutant α1 bfpA alleles were constructed in pRPA100, a plasmid expressing the α1 bundlin allele from its native promoter. The mutated domains (Figure 1) were selected by aligning the primary amino acid sequences of the α1 and β bundlins and selecting those surface-exposed regions where these two proteins were most different. The resulting plasmids were then introduced by electroporation into the bundlin-null EPEC strain, UMD901.
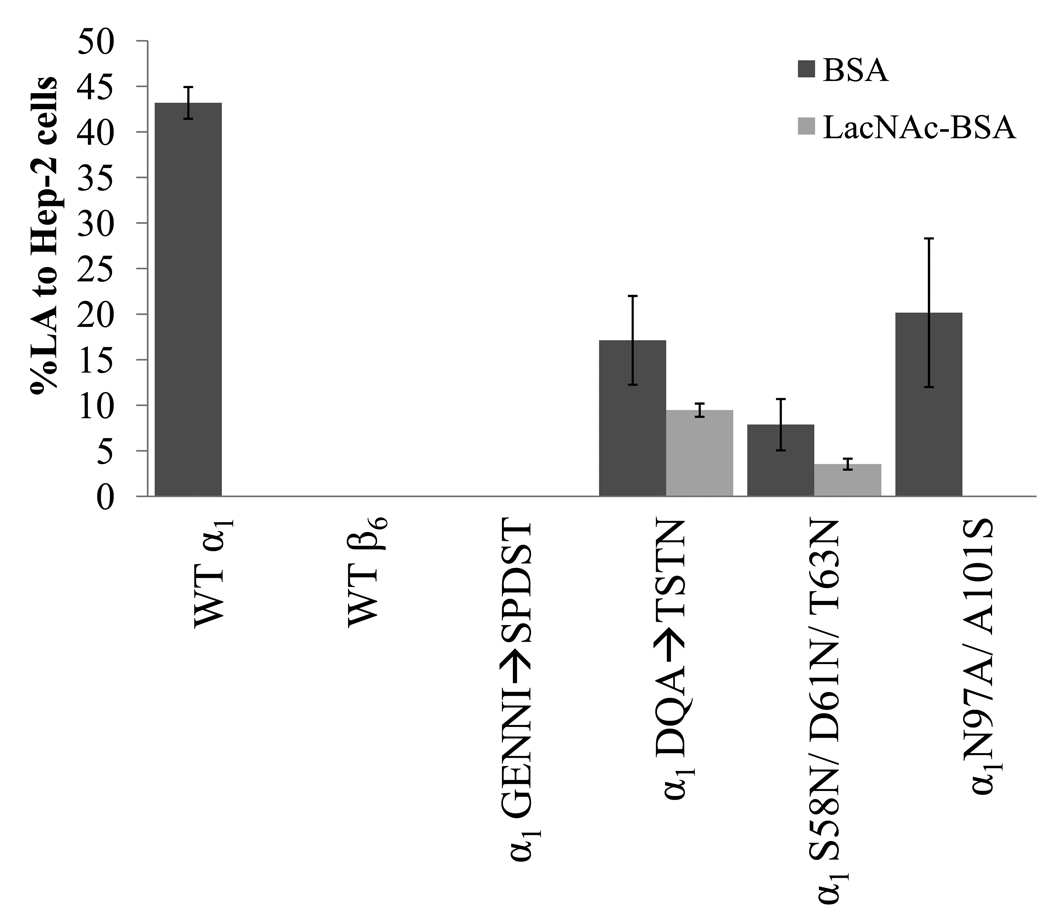
When grown in liquid culture, BFP-expressing EPEC aggregate into large clusters, a phenotype known as autoaggregation. Autoaggregation is quantified by the autoaggregation index described by Anantha and colleagues (Anantha et al.,1998,2000), a test we employed to functionally characterize BFP expression by our mutant strains. UMD901 complemented with the mutated bundlins α1GENNI➔SPDST, α1DQA➔TSTN, α1S58N/D61N/T63N and α1N97A/A101S all displayed similar autoaggregation indices to that of UMD901 complemented with the wild-type α1 bundlin (WT α1) (Table 1), indicating wild-type expression of BFP by all these strains. This was confirmed by ELISA quantification of BFP expressed by these strains (Table 1). The mutation α1GENNI➔SPDST resulted in the loss of EPEC early LA to HEp-2 cells (Figure 2), a phenotype that is similar to that of β bundlin-expressing UMD901 strains (Figure 2). The α1DQA➔TSTN and α1S58N/D61N/T63N mutations resulted in a reduction in early LA to HEp-2 cells, as compared to WT α1 (Figure 2). The early HEp-2 cell LA of strains bearing the α1N97A/A101S mutation was also reduced as compared to WT α1, but this was not statistically significantly (p>0.4, n=3, t-test) (Figure 2). The addition of 280 µM LacNAc-BSA to the early LA assay resulted in the complete (100%) inhibition of WT α1 and α1N97A/A101S early LA, whereas α1DQA➔TSTN and α1S58N/D61N/T63N were only inhibited by 55 (±7.6) % and 45 (± 4.3) %, respectively (Figure 2).
Table 1.
Autoaggregation Phenotype and Relative BFP Expression by UMD901 complemented with WT and mutant bundlins
| Bundlin Type (mutation) | Autoaggregation Index a (% of WT, (± standard deviation), n=3) |
BFP Expression b relative to WT α1 complemented strain (% of WT, (± standard deviation), n=3) |
|---|---|---|
| No bundlin | 0.0 (0.0) | 0.0 (0.0) |
| α1 (WT) | 9.9 (0.5) | 100.0 (6.80) |
| α1 (GENNI➔SPDST) | 7.25 (0.2) | 93.13 (37.4) |
| α1 (DQA➔TSTN) | 8.5 (0.3) | 90.19 (14.73) |
| α1 (S58N, D61N, T63N) | 11.5 (0.7) | 88.81 (11.05) |
| α1 (N97A, A101N) | 10.4 (0.2) | 72.12 (3.39) |
| α1 (Y75A) | 0.05 (0.0) | 0.0 (0.0) |
| α1 (Y75S) | 5.0 (0.5) | 89.40 (10.0) |
| α1 (Y75T) | 5.0 (0.4) | 86.43 (2.31) |
| α1 (Y75F) | 10.0 (1.2) | 167.8 (15.8) |
| α1 (GENNI➔SENNI) | 9.8 (0.2) | 96.01 (2.53) |
| α1 (GENNI➔GENNT) | 9.9 (0.5) | 95.25 (4.71) |
| β6 (WT) | 10.9 (0.5) | 88.53 (5.89) |
| β2 (WT) | 8.1 (0.1) | n.d.c |
| β3 (WT) | 9.2 (0.1) | n.d.c |
| β2 (TANST➔GENNI) | 2.0 (0.3) | 13.37 (0.33) |
| β3 (TPAST➔GENNI) | 9.0 (0.2) | 87.5 (7.895) |
| β6 (SPDST➔GENNI) | 0.8 (0.5) | 10.55 (0.589) |
Autoaggregation index was measured by the % increase in optical density at 600nm of the cultures after vortex mixing
BFP expression was determined by ELISA of BFP-expressing cultures using polyclonal anti-α1 bundlin rabbit sera and goat anti-rabbit IgG conjugated to horse radish peroxidase, as detailed in the materials and methods section.
n.d., not done.
Figure 2.
Early LA of UMD901 strains complemented with WT and mutated α1 bfpA or WT β6 bfpA to HEp-2 cells in the presence of 280 µM LacNAc-BSA (light gray bars) or BSA (dark gray bars). Prior to each experiment, EPEC were incubated at 37°C in DMEM to induce BFP expression, and subsequently with 280 µM LacNAc-BSA or BSA. Bacteria were then incubated with sub-confluent HEp-2 cells for 45 minutes, followed by vigorous washing in PBS and enumeration by oil immersion light microscopy, as described previously (Vanmaele et al., 1999). X-axis labels represent bundlin type cloned into UMD901. Bars represent the mean of three experiments, and error bars indicate the standard deviations from the mean.
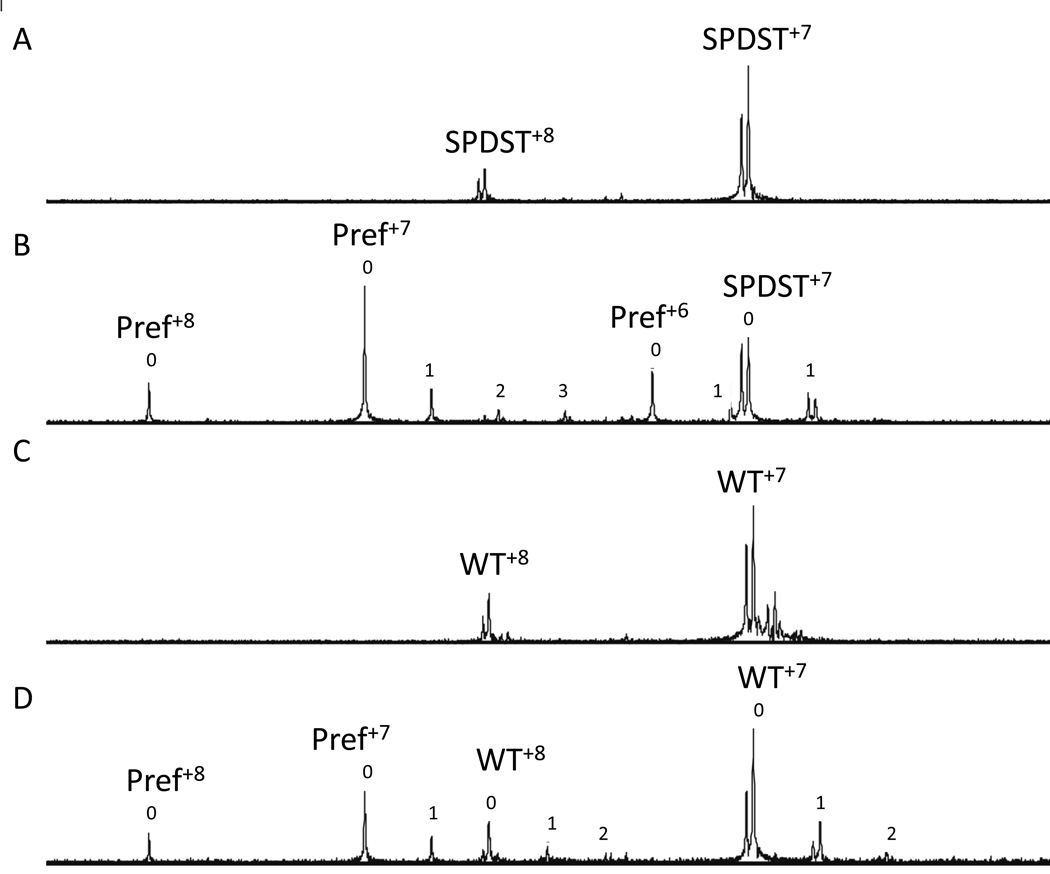
These results were confirmed by testing the LacNAc-specific lectin-like activity of a soluble recombinant α1 bundlin containing the mutation GENNI➔SPDST in the nano-ES-MS. In this experiment, the mutated protein appeared at two different molecular weights, of 17 434 Da and 17 493 Da (Figure 3A), as predicted based on the mutations introduced, whereas the WT recombinant α1 bundlin appeared at 17472 Da and 17528 Da (Figure 3C). The 59 Da shift in the protein weights is likely due to a nickel adduct leftover from our Ni-agarose purification procedure for these proteins. No interaction between synthetic LacNAc-Bn and the mutated α1 bundlin was observed (Ka=0, Figure 3B), once the data were corrected for background; whereas WT α1 bundlin bound LacNAc-Bn (Figure 3D) with an association constant, calculated directly from the nano-ES-MS data, of (6 +/− 4) × 102 M−1. This value is in agreement with our previously published results (Hyland et al., 2008), and significantly different (p<0.0001, t-test) from the Ka calculated for the mutated bundlin.
Figure 3.
NanoES mass spectra of aqueous solutions of α1GENNI➔SPDST and WT α1 bundlin. (A) 10µM solution of pure α1GENNI➔SPDST (labeled SPDST), which, typical of bundlin proteins, appears at two masses at each charge state: 17434 and 17493 Da. (B) Solution of pure α1GENNI➔SPDST (labeled SPDST) in the presence of 90 µM LacNAc-Bn and 5µM lysozyme (Pref). (C) 10µM solution of pure WT α1 bundlin, which appears at two masses: 17 472 and 17 528 Da. (D) 10µM solution of pure WT α1 bundlin in the presence of 90 µM LacNAc-Bn and 10µM Pref. Superscript numbers indicate the charge state of each protein. Peak numbers in panels C and D represent the number of LacNAc ligands associated with each protein. The relative proportion of Pref which was non-specifically associated with LacNAc was subtracted from the relative proportion of bundlin associated with LacNAc to calculate specific binding.
Mutation of β bundlin amino acids TPAST (β3 allele) to GENNI (α1 allele) imparts early adherence to HEp-2 cells
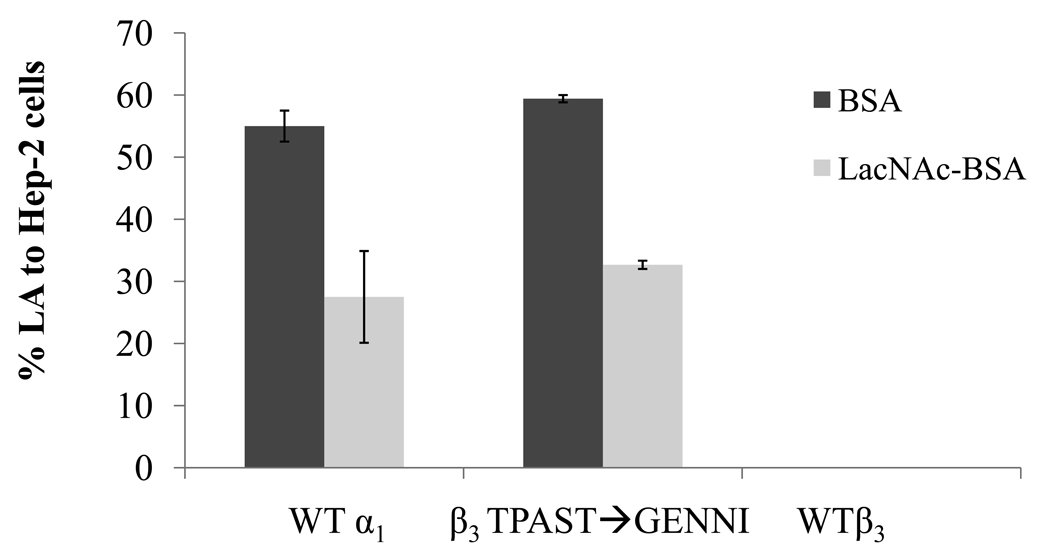
In order to confirm the role of the amino acids GENNI in the LacNAc-specific lectin like activity of bundlin, the mutations β2TADST➔GENNI, β3TPAST➔GENNI and β6SPDST➔GENNI (Figure 1B) were constructed in the plasmids pXLW16, 17 and 15, respectively (Table S1). The β2TADST➔GENNI and β6SPDST➔GENNI mutants displayed significantly (p<0.01, n=3, t-test) reduced autoaggregation indices as compared to WT β1 bundlin expressing strains, as well as compared to the mutant, β3TPAST➔GENNI (Table 1). Both of these strains produced very low amounts of BFP (Table 1), as assessed by the ELISA assay, and did not express the early LA phenotype on HEp-2 cells (data not shown). However, β3TPAST➔GENNI, which expressed wild-type levels of BFP (Table 1), bound to HEp-2 cells in the early LA assay, a phenotype that was inhibited by 55 (±0.5)% by LacNAc-BSA (Figure 4).
Figure 4.
Effect of the mutation β3TPAST➔GENNI on early LA to HEp-2 cells. LA was assessed in the presence of 280µM LacNAc-BSA (light gray bars) or BSA (dark gray bars). X-axis labels represent bundlin type cloned into UMD901. Bars represent the mean of three experiments, and error bars indicate the standard deviation from the mean.
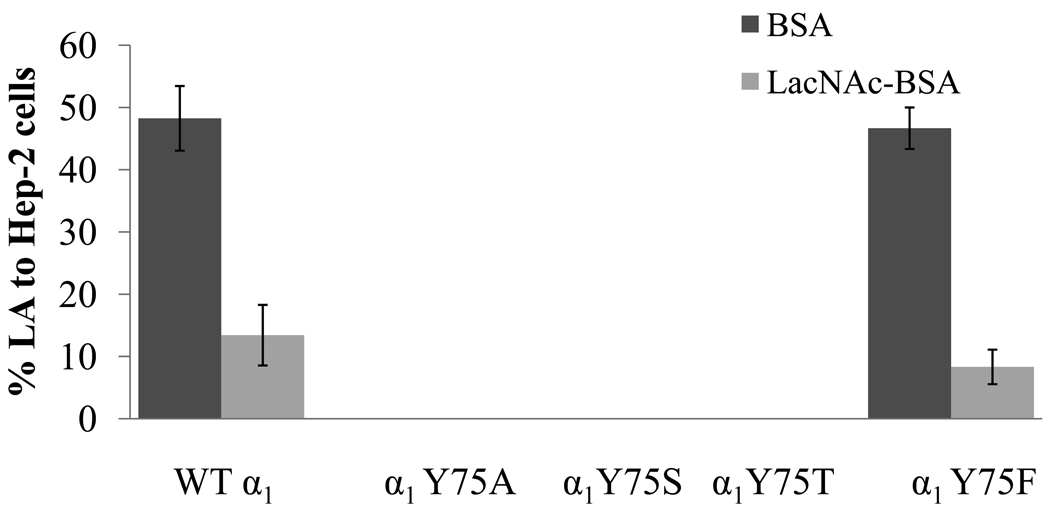
An aromatic amino acid at α1 bundlin amino acid position 75 is necessary for the EPEC LA phenotype
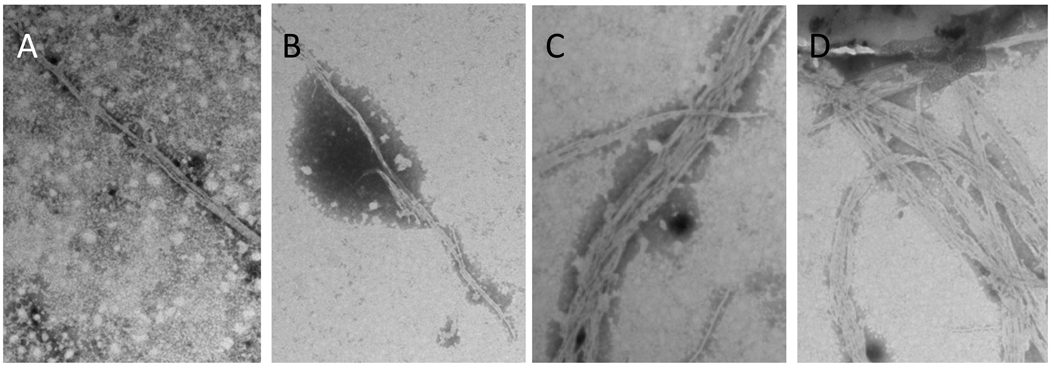
The amino acids GENNI lie in close proximity to the only solvent-exposed aromatic amino acid in α1 bundlin, tyrosine 75 (Y75, Figure 1A). Aromatic amino acids are often found in the CBD of glycan binding proteins since the planar surface of the aromatic side chain often participates in hydrophobic interactions with hexose rings in the glycan sequence (Toone, 1994). To elucidate the potential role of Y75 in coordinating the binding of LacNAc to α1 bundlin, point mutations were made in bfpA of pRPA100 (Table S1) which would introduce alanine, serine, threonine or phenylalanine at position 75 in α1 bundlin (Table S1). These plasmids were transferred into UMD901, and BFP expression, BFP assembly, autoaggregation, and LA to HEp-2 cells, were all subsequently assessed. The mutation α1Y75A did not display the autoaggregation phenotype (Table 1) and the loss of BFP expression was confirmed by ELISA analysis of this strain (Table 1). As anticipated from this result, α1Y75A also did not bind to HEp-2 cells in the early LA assay (Figure 5). By contrast, we found that α1Y75S and α1Y75T displayed a reduced autoaggregation index, as compared to the strain expressing WT α1 (Table 1), despite apparently expressing equivalent amounts of BFP (Table 1). TEM analysis of these strains revealed that the BFP did not resemble WT α1 BFP (Figure 6), in that they appeared to be much less “bundled” than wild-type BFP. This observation may explain the reduction in their autoaggregation phenotype. Additionally, both α1Y75S and α1Y75T were unable to adhere to HEp-2 cells in the early LA assay (Figure 5), despite producing wild-type amounts of BFP (Table 1).
Figure 5.
Effect of point mutations in α1 bundlin at Y75 on early LA to HEp-2 cells. LA was assessed in the presence of 280 µM LacNAc-BSA (light gray bars) or BSA (dark gray bars). X-axis labels represent bundlin type cloned into UMD901. Bars represent the mean of three experiments, and error bars indicate the standard deviations from the mean.
Figure 6.
TEM analysis of BFP expression by the mutants (A) α1Y75S (B) α1Y75T, (C) α1Y75, and (D) WT α1. All samples were stained with 1.0% (w/v) PTA, pH 7.2, and were visualized at 20,000x magnification using a Hitachi X-7000 TEM.
α1Y75F displayed the WT α1 autoaggregation phenotype (Table 1), and BFP expression (Table 1), and bound to HEp-2 cells in numbers equivalent to those observed for WT α1 bundlin (Figure 5). The LA of α1Y75F was also inhibited by the addition of LacNAc-BSA to the binding assay (Figure 5).
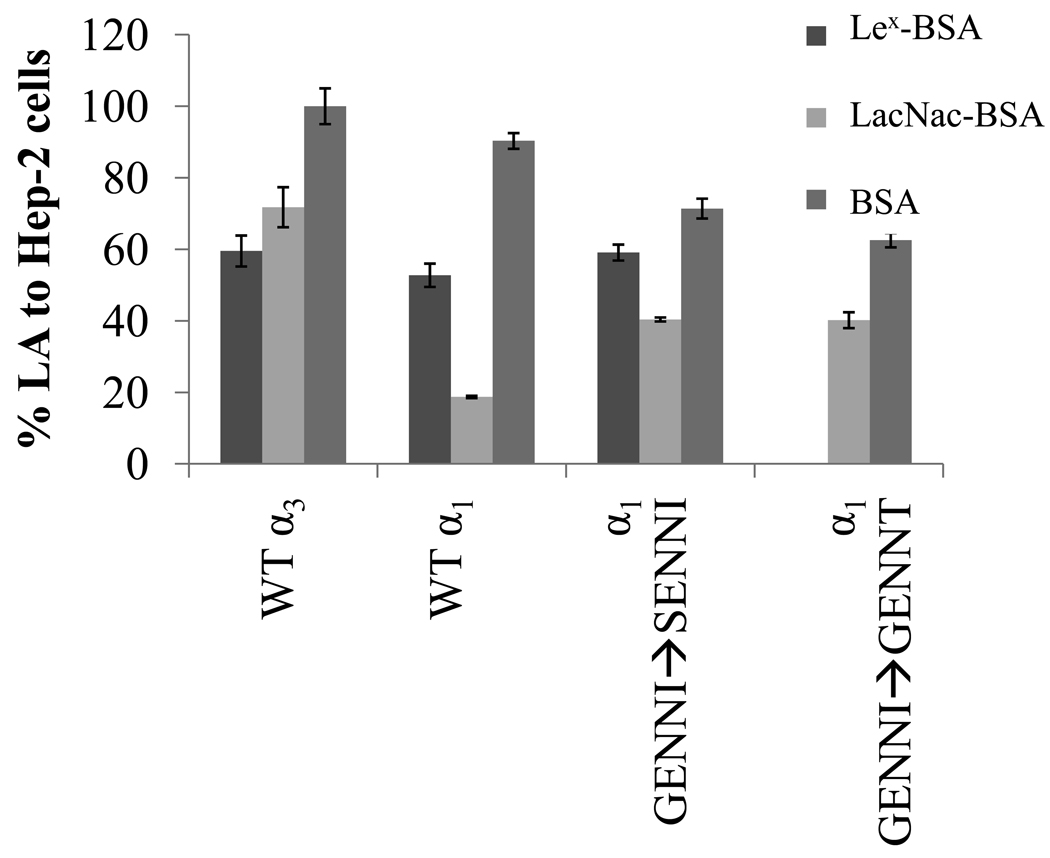
The α3 bundlin allele has a greater affinity for LewisX over LacNAc glycosides
We previously demonstrated that the LA of E2348/69, an α1 bundlin expressing strain, is inhibited by the LacNAc glycoside better than by its fucosylated derivatives, Lewisx (Lex)and Lewisy, or common sulfo and sialyl modified versions of these glycosides (Hyland et al., 2006b; Vanmaele et al., 1999). However, in the previous work, we found that the LA of one EPEC strain, serotype O119:H6, was best inhibited by a Lex-BSA glycoconjugate (Vanmaele, 1999). Sequencing this strain revealed that it contained an α3 bfpA allele (data not shown). The α1 and α3 bundlin alleles are 98% homologous, and differ at six amino acids. K81 of α1 bundlin is replaced by R in α3 bundlin, R110 by G, N168 by K and T169 by P, and, in the GENNI cluster, G137 is replaced by S, and I141 by T (Figure 1B). Since these latter two amino acid changes might alter the structure of bundlin and permit entry of the more bulky Lex glycan sequence into the LacNAc-specific CBD, we produced two mutations in α1 bundlin: GENNI➔SENNI and GENNI➔GENNT to test the importance of each amino acid in LacNAc versus Lex binding. Neither of the mutations had an apparent effect on the ability of the bacteria to express BFP (Table 1) and the HEp-2 cell LA phenotypes (Figure 7) of these mutants were the same as those of organisms expressing WT α1 and α3 bundlin (p > 0.05, n=3, t-test). Consistent with our previous results, Lex-BSA, at a concentration of 280 µM, inhibited EPEC serotype O119:H6 LA to HEp-2 cells significantly better than LacNAc-BSA (p = 0.003, n=3, t-test). Also, as expected, LA of the strain expressing the WT α1 allele was inhibited better (p < 0.001, n=3, t-test) by LacNAc-BSA than by Lex-BSA (Figure 8). LacNAc-BSA inhibited the LA of α1GENNI➔SENNI by 44.4 ± 0.56%, whereas Lex-BSA only inhibited the LA of this strain by 17.2 ± 2.2%, a result not significantly different than that observed for the WT α1 bundlin. In contrast, the early LA of α1GENNI➔GENNT was completely (100 ± 0.0%) abolished by Lex-BSA, but only inhibited by 35.7 ± 2.2% by LacNAc-BSA, suggesting that this mutation may be responsible for the altered glycan binding specificity observed for α3 bundlin.
Figure 7.
Effect of the mutations α1GENNI➔SENNT and α1GENNI➔GENNT on the inhibition of early LA to HEp-2 cells with LacNAc-BSA and Lex-BSA. LA was assessed in the presence of 280 µM LacNAc-BSA, Lex-BSA, or BSA. X-axis labels represent bundlin type cloned into UMD901. The error bars represent the range of two experiments.
Discussion
Bundlin is the major structural subunit of BFP (Giron et al., 1991), a type IV pilus that appears to be important for the early stages of EPEC adherence to cells and infection of the host (Cleary et al., 2004; Hyland et al., 2008). In a previous article, we demonstrated that α bundlins are LacNAc-specific lectins, whereas bundlins expressed from the β bfpA alleles are not (Hyland et al., 2008). Herein, we explored the basis for this phenotypic difference through mutational analysis of the bfpA gene.
The α and β bundlin types differ most at four regions in their amino acid sequences (Figure 1), sites which are predicted to be surface-exposed when bundlin is assembled into BFP (Ramboarina et al., 2005). We therefore performed site-specific mutagenesis to convert these four regions of α1 bundlin into the β6 allelic sequence (Figure 1). These experiments demonstrated that amino acids 137–141 in α1 bundlin (GENNI) and amino acids 136–140 in β6 bundlin (SPDST) are responsible for the differences observed in the early LA phenotype of α1 and β6 bundlin-expressing EPEC. This result is supported by the nano-ES-MS data, which demonstrates that α1GENNI➔SPDST no longer displayed LacNAc-specific lectin-like activity (Figure 3). Furthermore, conversion of a β3 bundlin allele to the α1 allele with the mutation β3TPAST➔GENNI resulted in a gain of the LA phenotype (Figure 4), albeit not to WT α1 levels, possibly due to other amino acid sequence differences between these two proteins.
The reduced LA phenotypes expressed by α1DQA➔TSTN and α1S58N/D61N/T63N, and the reduced ability of LacNAc-BSA to inhibit the early LA of these two strains (Figure 2), may have resulted from remote conformational effects of the mutations on the bundlin CBD. The amino acids that were altered in α1DQA➔TSTN and α1S58N/D61N/T63N are both within 10 angstroms of GENNI on the surface of wild-type α1 bundlin. This distance is roughly the diameter of a pyranose ring structure, and these mutations may therefore alter access of LacNAc glycan sequences to the CBD of α1 bundlin. Mutation of α1N97A/A101S did not alter the early LA phenotype, a result that was remarkable, given the non-conserved nature of the mutations and their location (5 angstroms away) relative to the putative CBD identified in this work.
The role of Y75, which lies adjacent to GENNI (Figure 1), in the putative LacNAc CBD of α1 bundlin was also investigated. The mutation α1Y75F, which had no effect on LA or the inhibitory effect of LacNAc-BSA, suggests that the hydroxyl group on tyrosine is not required for the LacNAc-specific lectin-like activity of α1 bundlin. The loss of the LA phenotype with the α1Y75S and α1Y75T mutations therefore may be due to the loss of the planar aromatic amino acid surface at this site. However, we cannot discount the possibility that the mutations introduced to α1 bundlin at Y75 simply altered the protein fold in such a way that carbohydrate binding by the mutants was lost.
The loss of an aromatic amino acid (α1Y75S and α1Y75T), also appears to have an effect on the ability of the individual BFP filaments to form bundles, since reduced autoaggregation was observed in these strains (Table 1), despite the apparent wild-type expression levels of the bundlin protein expression by these strains (Table 1). Y75 lies in a surface-exposed variable region known as the αβ-loop that, along with the D-region, makes up the functional surface of the type IV pili (Craig et al., 2006; Craig and Li, 2008). Mutation of the D-region of PilX, a pilin protein found in the type IV pili expressed by Neisseria meningitidis results in the loss of bacterial aggregation (Helaine et al., 2007), but the αβ-loop has not been implicated to date in inter-filament interactions. However, the αβ-loop of bundlin is predicted to be more solvent-exposed than those of other type IV pili (Ramboarina et al., 2005), and therefore, as suggested by our data, may play a role in pilus-pilus interactions. While the mutations α1GENNI➔SPDST and β3TPAST➔GENNI drastically altered the early LA phenotype (Figure 2 and 4), no effect was seen on the autoaggregation (Table 1) of strains expressing these mutated bundlins, a result that suggests the GENNI domain itself does not mediate inter-BFP interactions. BFP have also been shown to bind to phosphotidylethanolamine (PE) (Barnett Foster et al., 1999; Khursigara et al., 2001) in both EPEC and human epithelial lipid extracts, which may also mediate the dual roles of BFP in LA and autoaggregation (Khursigara et al., 2001). The identity of the PE-specific binding site remains to be elucidated, but it is possible that the residual binding in the presence of LacNAc-BSA is due to host cell PE-BFP interactions.
Previously, we demonstrated that the LA phenotype of the O119:H6 EPEC serotype, which expresses an α3 bundlin allele, was inhibited by Lex-BSA better than by LacNAc-BSA (Vanmaele, 1999). This different glycan specificity also appears to be mediated by the amino acids GENNI; and specifically by I141, as the mutation, I141T resulted in an EPEC strain that was better inhibited by Lex-BSA than by LacNAc-BSA (Figure 8). It is tempting to speculate, based on these results, that during the bundlin-LacNAc interaction, the galactose moiety of LacNAc interfaces with Y75, whereas the glucosamine moiety is coordinated by I141. If so, then substituting the latter residue with a less bulky amino acid (such as T) might accommodate for the presence of fucose on glucosamine, as is found in Lex.
Through evolution, EPEC has evolved 9 known bundlin types, presumably as a means to avoid the host immune response (Fernandes et al., 2007). Together, the experiments herein reveal that different bundlin types also exhibit varying receptor affinities, a characteristic which may allow EPEC strains to colonize a more diverse range of host species. In this regard, EPEC strain B171-8 demonstrates tropism for human over murine epithelial cell lines (Tobe and Sasakawa, 2002), a phenotype dependant on bundlin. Furthermore, β bundlin expressing-EPEC have been isolated from canine and avian hosts (Blank et al., 2000), and do not bind human epithelial cell lines. However, it would appear that EPEC expressing α1 bundlin bind via a LacNAc-related receptor to both the human adult (Hyland et al., 2006a), and pediatric (manuscript in preparation) intestine, as this adherence can be inhibited by LacNAc glycoconjugates.
EPEC colonization of the host is a complex process that involves as many as three stages: initial adherence, signal transduction, and intimate adherence. Initial adherence is characterized by LA, and would appear to occur via the interaction between α1 bundlin and LacNAc-related receptors on host cells. Following this, EPEC inject effector proteins into the host cell cytoplasm via a type three secretion system, the cumulative effect of which is the disappearance of microvilli from the apical surface of the host cell. Also injected into the host cell is the Translocated Intimin Receptor (Tir) which is inserted into the host cell plasma membrane and bound by the EPEC surface protein, Intimin, to consolidate intimate adherence. In the context of EPEC infections, it would appear therefore that LacNAc glycoconjugates can be used to inhibit the earliest stage of adherence, potentially terminating the infection before EPEC can achieve intimate adherence.
Several other oligosaccharides, including N-acetylgalactosamine (Cravioto et al., 1991; Jagannatha et al., 1991; Vanmaele et al., 1999), have been proposed as BFP receptors in EPEC strains different from those tested in this study, but the bundlin alleles of these strains were not determined. It is possible, therefore, that these results represent another example of different bundlin types exhibiting alternate receptor affinities; and potentially represent receptors for the β bundlins. This is of particular interest, as no receptor has been described to date for any of the β bundlins. Whether the reduced immunogenicity associated with the β bundlins (Fernandes et al., 2007) came at the evolutionary cost of losing adhesion activity, requiring those EPEC strains to adopt alternate adhesins for LA, or simply changed the receptor specificity in the β bundlins, remains to be elucidated. Further testing of the receptor specificity of the β bundlins will shed more light on the nature of EPEC’s interaction with host intestinal epithelial cells.
Materials and Methods
Bacterial strains, plasmids, and recombinant proteins used in this study
The bacterial strains and plasmids used in this study are listed in Table S1 of the supplementary material. Bacteria were routinely cultured overnight at 37°C in TSB from single colonies picked from an overnight TSA plate. Media were supplemented with 50 µg ml−1 ampicillin, 50 µg ml−1 kanamycin and/or 25 µg ml−1 chloramphenicol, as appropriate. UMD901, which is a bundlin-null strain (Zhang and Donnenberg, 1996), was complemented with the α1 bfpA allele (pRPA100), the β3 allele (pXLW17), the β6 allele (pXLW15), or with various mutated versions of the α1 bundlin allele (pRMH1-13), as described below, and in Table S1. These plasmids all contain a bfpA gene cloned behind the native BFP promoter from EPEC strain E2348/69 into the low-copy-number vector pWKS30 as described previously (Anantha et al., 2000; Fernandes et al., 2007).
Soluble, recombinant bundlin with a hexahistidine N-terminal tag was expressed from the plasmids pPF401 (WT α1 bundlin), pPF402 (WT β6 bundlin), and pRMH1exp (α1GENNI➔SPDST) as described previously (Fernandes et al., 2007; Hyland et al., 2008). The recombinant proteins were purified under native conditions using a nickel-NTA column (Qiagen, Missasauga ON), following the manufacturer’s recommendations. The proteins were further purified by FPLC size exclusion chromatography on a Superdex 75 column.
Mutation of the α bundlin genes
Predicted surface-exposed regions displaying amino acid sequence heterogeneity between the bfpA genes of the EPEC strains E2348/69 (which expresses α1 bundlin, NCBI accession number AF304474) and RN587/1 (which expresses β6 bundlin, NCBI accession number AF474407) were targeted for mutation (Figure 1). Mutations were introduced into α1 bfpA in pRPA100, β2 bfpA in pXLW16, β3 bfpA in pXLW17 and β6 bfpA in pXLW15 (Table S1) using the Stratagene Quikchange mutagenesis strategy (Deng et al., 2007). Oligonucleotide primers were designed such that a minimum of 10 homologous base pairs flanked the mutated sequence. The sense primers are listed in Table S2; antisense primers were the reverse compliment of the sense primers.
Thermalcycling and DpnI digestion were performed as recommended by Stratagene (LaJolla, CA). The resultant plasmids (pRMH1-13, Table S1) were introduced into DH5α and the bfpA sequence of 5 colonies was confirmed by amplification of the bfpA gene with the sense primer bfpAFnew (5’actatgatatcctgtctttgattgaatctgca) and the antisense primer bfpARnew (5’atattagatctttacttcataaaatatgtaac), and subsequent sequencing using the primer bfpAnestF (5’agcgcaacgtctgcaattaatggtctg) employing standard methods on a Applied Biosystems Genetic Analyzer by the University of Calgary Core DNA Services. Those plasmids that contained mutated bfpA were introduced into UMD901 by electroporation using a GenePulser (Bio-Rad, Hercules, CA), as previously described (Fernandes et al., 2007).
The α1 bundlin gene of pPF401, an expression vector for soluble α1 bundlin under the control of a T7 promoter (Fernandes et al., 2007), was mutated using the primer RMH1 and its complement, to introduce the mutation GENNI➔SPDST to produce the plasmid pRMH1exp (Table S1).
Phenotypic assessment of α1 bundlin mutations in UMD901
All mutant and WT bundlin expressing strains (Table S1) were assessed for BFP expression by measuring the autoaggregation index, which is a phenotype that requires the expression of BFP (Anantha et al., 2000). Briefly, overnight TSB cultures were diluted 1:100 in DMEM and incubated for 4 h at 37°C in a CO2 incubator. The A600 of each culture was recorded and, after each measurement, the culture was mixed vigorously using a Vortex mixer for 30 s and the A600 was subsequently recorded again. The percent increase in A600 after mixing was recorded as a quantitative autoaggregation index. Each experiment was repeated three times.
BFP expression was also assessed by ELISA. Briefly, bacterial cultures were grown statically at 37°C overnight in TSB with no glucose. The cultures were then inoculated, at a 1:100 dilution, into DMEM which had been pre-equilibrated in the CO2 incubator overnight. The cultures were grown in the CO2 incubator for an additional 3 hours, and harvested by centrifugation at 14,000 × g for 3 minutes. 500 uL of pre-warmed PBS was then added and BFP were sheared from the bacterial cells using a Vortex mixer at top speed for 1 minute, thereby preventing retraction and degradation of the pili by the bacteria during further processing. These mixtures were split into two portions, half of which were used to coat, in triplicate, 96 well microtiter ELISA plates overnight at 4°C, and half of which were used for total protein determination, in duplicate. For protein determinations, 1% (w/v) SDS was added and the preparations were incubating at 37°C for 30 minutes. The concentration of protein in each of these samples was then determined by the conventional BCA assay (Pierce, Rockford, IL). ELISA was performed by conventional methods, using α1-bundlin-specific polyclonal rabbit antisera and a commercial goat anti-rabbit IgG HRP conjugate (Sigma, Mississauga, ON) for detection. A standard curve for the ELISA was prepared by adding increasing amounts of purified recombinant α1-bundlin to UMD901 cells which produces no BFP. These preparations were then processed and assayed in the same manner as UMD901 strains expressing the various BFP mutants. ELISA data were normalized to total protein concentration for each preparation, and reported as % BFP expression relative to the signal produced by the UMD901 (pRPA100) strain which expresses wild type α1bundlin BFP (Table 1).
BFP morphology was assessed by observing negatively stained (phosphotungstic acid) bacteria using the transmission electron microscope as described (Hyland et al., 2006b).
Early LA binding and inhibition assays
The early LA to HEp-2 cell monolayers was assessed in the 45 minute modified LA assay described in our previous articles (Hyland et al., 2006b; Hyland et al., 2008). LA was quantified by light microscopy of the HEp-2 cell monolayers under oil immersion, by two observers blinded to the sample identities. Additionally, the ability of 280 µM (0.8 mg ml−1) synthetic LacNAc-BSA or, in some experiments, Lex-BSA to inhibit LA was determined, as previously described (Hyland et al., 2008; Vanmaele et al., 1999). The incorporation of ligands into BSA was determined by mass spectroscopy to be 19:1 (mol/mol) for LacNAc-BSA and 14:1 (mol/mol) for Lex-BSA. In these experiments, native BSA was used as a negative control, also at a concentration of 280 µM.
Nanoelectrospray mass spectrometry binding assay
The interaction between the recombinant bundlins and LacNAc was determined by the nanoES-MS technique, exactly as described in our previous manuscript (Hyland et al., 2008).
Supplementary Material
Acknowledgements
Financial support was from the Alberta Ingenuity Centre for Carbohydrate Science (E.K., J.S.K., P.I.K. and G.D.A.) and by Public Health Service award R01 AI-37606 (M.S.D.) from the National Institutes of Health. R.M.H. and T.P.G. were the recipients of a Canadian Graduate Scholarship from NSERC. We thank Dr. David Bundle for supplying the synthetic ligands used in the nanoES-MS experiments.
Literature Cited
- Anantha RP, Stone KD, Donnenberg MS. Role of BfpF, a member of the PilT family of putative nucleotide-binding proteins, in type IV pilus biogenesis and in interactions between enteropathogenic Escherichia coli and host cells. Infect Immun. 1998;66:122–131. doi: 10.1128/iai.66.1.122-131.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantha RP, Stone KD, Donnenberg MS. Effects of bfp mutations on biogenesis of functional enteropathogenic Escherichia coli type IV pili. J Bacteriol. 2000;182:2498–2506. doi: 10.1128/jb.182.9.2498-2506.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini MM, Kaper JB, Levine MM, Candy DC, Moon HW. Plasmid-mediated adhesion in enteropathogenic Escherichia coli. J Pediatr Gastroenterol Nutr. 1983;2:534–538. doi: 10.1097/00005176-198302030-00023. [DOI] [PubMed] [Google Scholar]
- Barnett Foster D, Philpott D, Abul-Milh M, Huesca M, Sherman PM, Lingwood CA. Phosphatidylethanolamine recognition promotes enteropathogenic E. coli and enterohemorrhagic E. coli host cell attachment. Microb Pathog. 1999;27:289–301. doi: 10.1006/mpat.1999.0305. [DOI] [PubMed] [Google Scholar]
- Blank TE, Zhong H, Bell AL, Whittam TS, Donnenberg MS. Molecular variation among type IV pilin (bfpA) genes from diverse enteropathogenic Escherichia coli strains. Infect Immun. 2000;68:7028–7038. doi: 10.1128/iai.68.12.7028-7038.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank TE, Lacher DW, Scaletsky IC, Zhong H, Whittam TS, Donnenberg MS. Enteropathogenic Escherichia coli O157 strains from Brazil. Emerg Infect Dis. 2003;9:113–115. doi: 10.3201/eid0901.020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary J, Lai LC, Shaw RK, Straatman-Iwanowska A, Donnenberg MS, Frankel G, Knutton S. Enteropathogenic Escherichia coli (EPEC) adhesion to intestinal epithelial cells: role of bundle-forming pili (BFP), EspA filaments and intimin. Microbiology. 2004;150:527–538. doi: 10.1099/mic.0.26740-0. [DOI] [PubMed] [Google Scholar]
- Craig L, Volkmann N, Arvai AS, Pique ME, Yeager M, Egelman EH, Tainer JA. Type IV pilus structure by cryo-electron microscopy and crystallography: implications for pilus assembly and functions. Mol Cell. 2006;23:651–662. doi: 10.1016/j.molcel.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Craig L, Li J. Type IV pili: paradoxes in form and function. Curr Opin Struct Biol. 2008;18:267–277. doi: 10.1016/j.sbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cravioto A, Tello A, Villafan H, Ruiz J, del Vedovo S, Neeser JR. Inhibition of localized adhesion of enteropathogenic Escherichia coli to HEp-2 cells by immunoglobulin and oligosaccharide fractions of human colostrum and breast milk. J Infect Dis. 1991;163:1247–1255. doi: 10.1093/infdis/163.6.1247. [DOI] [PubMed] [Google Scholar]
- Deng Q, Luo W, Donnenberg MS. Rapid site-directed domain scanning mutagenesis of enteropathogenic Escherichia coli espD. Biol Proced Online. 2007;9:18–26. doi: 10.1251/bpo130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes PJ, Guo Q, Donnenberg MS. Functional consequences of sequence variation in bundlin, the enteropathogenic Escherichia coli type IV pilin protein. Infect Immun. 2007;75:4687–4696. doi: 10.1128/IAI.00009-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giron JA, Ho AS, Schoolnik GK. An inducible bundle-forming pilus of enteropathogenic Escherichia coli. Science. 1991;254:710–713. doi: 10.1126/science.1683004. [DOI] [PubMed] [Google Scholar]
- Helaine S, Dyer DH, Nassif X, Pelicic V, Forest KT. 3D structure/function analysis of PilX reveals how minor pilins can modulate the virulence properties of type IV pili. Proc Natl Acad Sci U S A. 2007;104:15888–15893. doi: 10.1073/pnas.0707581104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland RM, Beck P, Mulvey GL, Kitov PI, Armstrong GD. N-acetyllactosamine conjugated to gold nanoparticles inhibits enteropathogenic Escherichia coli colonization of the epithelium in human intestinal biopsy specimens. Infect Immun. 2006a;74:5419–5421. doi: 10.1128/IAI.00739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyland RM, Griener TP, Mulvey GL, Kitov PI, Srivastava OP, Marcato P, Armstrong GD. Basis for N-acetyllactosamine-mediated inhibition of enteropathogenic Escherichia coli localized adherence. J Med Microbiol. 2006b;55:669–675. doi: 10.1099/jmm.0.46344-0. [DOI] [PubMed] [Google Scholar]
- Hyland RM, Sun J, Griener TP, Mulvey GL, Klassen JS, Donnenberg MS, Armstrong GD. The bundlin pilin protein of enteropathogenic Escherichia coli is an N-acetyllactosamine-specific lectin. Cell Microbiol. 2008;10:177–187. doi: 10.1111/j.1462-5822.2007.01028.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagannatha HM, Sharma UK, Ramaseshan T, Surolia A, Balganesh TS. Identification of carbohydrate structures as receptors for localised adherent enteropathogenic Escherichia coli. Microb Pathog. 1991;11:259–268. doi: 10.1016/0882-4010(91)90030-e. [DOI] [PubMed] [Google Scholar]
- Khursigara C, Abul-Milh M, Lau B, Giron JA, Lingwood CA, Foster DE. Enteropathogenic Escherichia coli virulence factor bundle-forming pilus has a binding specificity for phosphatidylethanolamine. Infect Immun. 2001;69:6573–6579. doi: 10.1128/IAI.69.11.6573-6579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramboarina S, Fernandes PJ, Daniell S, Islam S, Simpson P, Frankel G, Booy F, Donnenberg MS, Matthews S. Structure of the Bundle-forming Pilus from Enteropathogenic Escherichia coli. J Biol Chem. 2005;280:40252–40260. doi: 10.1074/jbc.M508099200. [DOI] [PubMed] [Google Scholar]
- Scaletsky IC, Silva ML, Trabulsi LR. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;45:534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobe T, Sasakawa C. Species-specific cell adhesion of enteropathogenic Escherichia coli is mediated by type IV bundle-forming pili. Cell Microbiol. 2002;4:29–42. doi: 10.1046/j.1462-5822.2002.00167.x. [DOI] [PubMed] [Google Scholar]
- Toone EJ. Structure and energetics of protein-carbohydrate complexes. Curr Opin Struct Biol. 1994;4:719–728. [Google Scholar]
- Vanmaele RL. Medical Microbiology and Immunology. Vol. University of Alberta: PhD Edmonton; 1999. Role of carbohydrates in EPEC adherence; p. 204. [Google Scholar]
- Vanmaele RP, Heerze LD, Armstrong GD. Role of lactosyl glycan sequences in inhibiting enteropathogenic Escherichia coli attachment. Infect Immun. 1999;67:3302–3307. doi: 10.1128/iai.67.7.3302-3307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HZ, Donnenberg MS. DsbA is required for stability of the type IV pilin of enteropathogenic escherichia coli. Mol Microbiol. 1996;21:787–797. doi: 10.1046/j.1365-2958.1996.431403.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.