Abstract
Background
The expression of drug-metabolizing enzymes cytochrome P450 (CYPs) is controlled by pregnane X receptor (PXR), and therefore understanding how PXR modulates CYP expression is important to minimize adverse drug interactions, one type of preventable adverse drug reaction.
Objective
We review the mechanisms of PXR-mediated repression of CYP expression
Methods
We discuss the clinical implications of CYP repression and the role of signal cross-talks, including protein-protein interactions and phosphorylation of PXR and coregulators, in inhibiting PXR and repressing CYP expression
Results/conclusion
Kinases such as cyclin-dependent kinase 2 (Cdk2), protein kinase A (PKA), protein kinase C (PKC), and 70kDa form of ribosomal protein S6 kinase (p70 S6K) repress CYP expression by phosphorylating and inhibiting PXR. Growth factor signaling represses CYP expression by phosphorylating and inhibiting forkhead in rhabdomyosarcoma (FKHR), a coactivator of PXR. During inflammation, nuclear factor κB (NF-κB) represses both PXR and CYP expression via protein-protein interactions with the PXR pathway.
Keywords: CYP3A expression, cytochrome P450 (CYP), drug metabolism, infection, inflammation, liver, kinase, phosphatase, phosphorylation, pregnane X receptor (PXR), proliferation, protein-protein interactions, regeneration, signal cross-talk
1. Introduction
1.1. Repression of cytochrome P450 (CYP) expression and adverse drug reactions (ADRs)
Adverse drug reactions (ADRs) are defined as the undesired side effects, including catastrophic outcomes, following administration of a certain medical substance with a desired therapeutic effect (1;2). Serious ADRs are a major health concern for hospitalized patients worldwide. In the US, more than 2 million ADRs are documented annually and are responsible for nearly 20% (approximately 100,000) of deaths that occur in hospitals (3). Deaths caused by ADRs have outnumbered those by other causes such as pulmonary diseases, diabetes, AIDS, and accidents (including automobile-related). ADRs are currently the fourth leading cause of deaths in hospitalized patients (4). The costs associated with managing ADRs have exceeded the combined expenses of treating cardiovascular diseases and diabetes, and currently exceed a staggering $100 billion annually in the US (5).
Adverse drug interactions mediated by the cytochrome P450 (CYP) pathway of drug metabolism are one type of preventable ADR. Drug metabolism is a major mechanism by which xenobiotics, including clinically used drugs, are cleared from the body. Drug-metabolizing enzymes play crucial roles in the highly regulated drug metabolism pathway. Variations in the expression levels of CYPs in the liver and other vital organs - caused by genetic, physiologic, pathologic, and environmental factors - can alter the therapeutic response and contribute significantly to adverse drug interactions. The pregnane X receptor (PXR) is critical in regulating the expression of genes of the CYP superfamily, thereby modulating the metabolism of xenobiotics and the occurrence of ADRs.
This review summarizes the mechanisms responsible for the repression of PXR-mediated CYP expression and subsequently of CYP-mediated drug metabolism primarily in the liver under different physiologic and pathologic conditions.
1.2. PXR and CYP expression
PXR plays a central role in activating the expression of CYPs such as CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP3A4, and CYP3A5 in the human liver and other organs (6). CYP3A4, one of the most important human CYPs, catalyzes the metabolism of more than 50% of clinically used drugs. PXR is the master receptor that controls CYP3A4 gene expression (Cyp3a11 and Cyp3A1 are the corresponding human CYP3A4 orthologs in mouse and rat, respectively; CYP3A is used when all 3 species are referred to) (6-8).
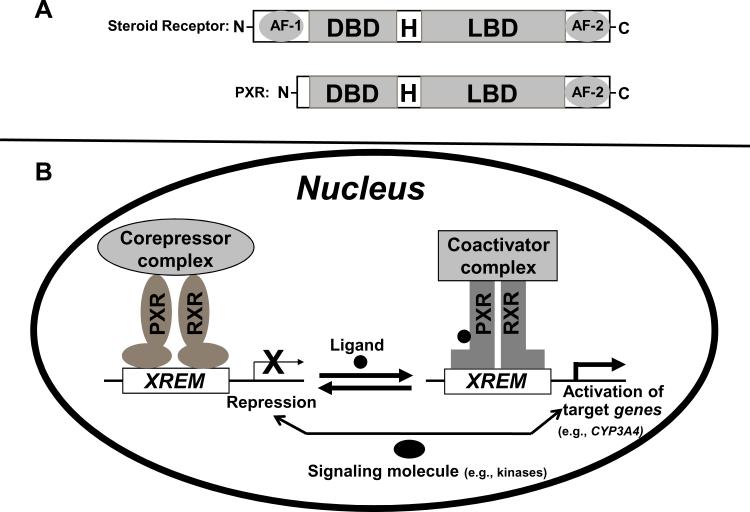
PXR is a member of the nuclear receptor (NR) superfamily of ligand-activated DNA-binding transcription factors that regulate the expression of their target genes, such as CYP3A4, by binding to the gene's promoter (Fig 1B). PXR is activated by binding to various chemically and structurally distinct endobiotics and xenobiotics, including clinically used drugs (6;9;10). As seen in steroid receptors and other DNA-binding NRs, the N-terminal part of the PXR protein contains a highly conserved DNA-binding domain (DBD) (Fig 1A) and the C-terminal part contains a ligand-binding domain (LBD) with an additional ligand-inducible transactivation function 2 (AF-2). The DBD and LBD are separated by a hinge region (Fig 1A). In contrast to most NRs, PXR does not have a ligand-independent activation function 1 (AF-1) (Fig 1A), which is the most variable region in NRs in terms of length and sequence similarities and is believed to be regulated by growth factor signaling.
Figure 1.
Mechanism of target gene induction by PXR. (A) A schematic comparison of the domain structures of a steroid receptor and PXR. AF-1, activation function 1; DBD, DNA binding domain; H, hinge region; LBD, ligand binding domain; AF-2, transactivation function 2. (B) A current model of PXR-mediated gene regulation. Ligand binding induces a dissociation of co-repressors, recruitment of co-activators and contributes to chromatin remodeling and transcriptional activation. Signaling molecules (e.g., protein kinases, phosphatases, or transcription factors) contribute to regulating the function of PXR. XREM, xenobiotic responsive enhancer module.
In the absence of an agonist, PXR is associated with transcriptional corepressors such as nuclear receptor corepressor 1 (NCoR1) and NCoR2 (also known as the silencing mediator of retinoid and thyroid hormone receptor [SMRT]) (Table 1) (Fig 1B) (10-13). NCoR1 and SMRT mediate repression of PXR basal transcription activity through the recruitment of histone deacetylases (HDACs) (13). SMRT exists in two major splicing isoforms, α and τ, with the α isoform containing an extra 46-amino acid sequence inserted immediately downstream to the distal or second corepressor motif (14). SMRTα interacts more strongly with PXR than SMRTτ does. In addition, the PXR-SMRTα interaction is resistant to PXR ligand-induced dissociation, where as PXR- SMRTτ interaction is sensitive to the PXR ligand-induced dissociation. Therefore, even though SMRTα and SMRTτ possess similar intrinsic repression activity and association with HDACs, SMRTα exerts a greater inhibition on PXR activity than SMRTτ does. Agonists such as rifampicin and pregnenolone 16α carbonitrile (PCN) bind to PXR and induce conformational changes that lead to dissociation of corepressors and recruitment of coactivators such as steroid receptor coactivator 1 (SRC-1) and SRC-3 (Table 1) (Fig 1B) (10-13), contributing to chromatin remodeling and subsequent transcriptional activation (10). Ligand-bound PXR binds to the promoter of its target gene as a heterodimer with the retinoid X receptor α (RXRα), and the heterodimer can form in the absence of the promoter (Table 1) (Fig 1B) (15).
Table 1.
Nuclear receptor coregulators, transcriptional factors (TF) and nuclear receptors (NR) regulating PXR transactivation of CYPs via protein-protein interactions
| Coregulator, TF or NR | Role or mechanism | Reference |
|---|---|---|
| NCoR1 | Inhibits basal transcription | (11;12;23) |
| NCoR2α (SMRTα) | Inhibits basal transcription | (13) |
| NCoR2τ (SMRTτ) | Inhibits basal transcription | (14) |
| SRC-1 | Enhances transcription | (10-13;23) |
| SRC-3 | Enhances transcription | (13) |
| PBP | Enhances transcription | (11) |
| PRMT1 | Enhances transcription | (77) |
| NF-κB | Repression of transcription | (53;70;71) |
| FKHR | Potentiation of transcription | (51;87) |
| SREBP-1 | Repression of transcription | (72) |
| RXRα | Obligate heterodimeric partner for PXR | (15) |
| SHP | Repression of transcription | (73;74) |
| *LXR | Repression of transcription | (74;76) |
It is not clear whether LXR repress PXR -mediated target gene expression via direct PXR-LXR protein-protein interactions.
NCoR, nuclear receptor corepressor; SMRT, silencing mediator for retinoid and thyroid hormone receptors; SRC, steroid receptor coactivator; PBP, peroxisome proliferator-activated receptor-binding protein; PRMT, protein arginine methyl transferase; NF-κB, nuclear factor-kappa B; FKHR, fork head in rhabdomyosarcoma; SREBP, sterol regulatory element binding protein; SHP, small heterodimer partner; LXR, liver X receptor; RXR, retinoid X receptor.
It is generally thought that in the absence of ligand, the subcellular localization of PXR is affected by the cellular context. When ectopically expressed in cultured cells such as HepG2, COS-1, COS-7 and HeLa, ligand-independent nuclear localization was observed for PXR (13;14;16-19). However, endogenous PXR in human PC-3 prostate cancer cells resides exclusively in the cytoplasm and treatment with PXR agonist SR12813 leads to the nuclear translocation (20). PXR has been shown to reside in the nuclei of some human endometrial and prostate cancer tissues (20;21). Similarly, PXR was reported to be localized in the nuclei of mouse liver cells irrespective of a ligand treatment (18). Conversely, Kawana et al. has shown that PXR was retained in the cytoplasm of hepatic cells of untreated mouse and was translocated to the nucleus after administration of a ligand (17). Along the same lines, mouse PXR expressed as yellow fluorescent protein (YFP) fusion was reported to be localized in the cytoplasm of mouse livers and ligand treatment translocated PXR into the nucleus (22). Because of the various observations of the subcellular localization of PXR, it is important that future studies are directed to determine the molecular mechanisms responsible for PXR localization in both physiological and pathological conditions.
Importantly, the activity of PXR can be modulated not only by ligand binding but also by signaling pathways (i.e., by signal cross-talking) (Fig 1B). Moreover, PXR activation is species specific in terms of ligand binding and signal cross-talking (23-25). Specific amino acids within the PXR sequence are responsible for species-specific ligand binding. For instance, rifampicin is an agonist of human PXR (hPXR) and induces CYP3A4 expression in humans but not in rodents, whereas PCN is a rodent-specific PXR agonist and induces the expression of Cyp3a11 (mouse) and Cyp3A1 (rat) but not CYP3A4 (human) (24;25). Because of this species specificity in terms of ligands, PXR “humanized” animal models have been developed to evaluate the enzyme induction, metabolism, and toxicity of drugs (26;27). In PXR humanized mice, mouse Cyp3a11 is no longer induced by PCN but is efficiently induced by the human-specific hPXR agonist rifampicin. For signal cross-talking, the signaling cascade itself, within a specific tissue/cellular context, is responsible for species specificity. Recently, Lichti-Kaiser et al. have found that activation of protein kinase A (PKA) signaling in human hepatocytes represses hPXR or mouse PXR (mPXR) activity and consequently represses corresponding CYP gene expression (23). Conversely, activation of PKA signaling in mouse hepatocytes enhances hPXR or mPXR activity and subsequently enhances corresponding CYP gene induction (23). Currently, no animal model exists that addresses the signaling-dependent species specificity for PXR-mediated CYP expression.
1.3. Physiologic and pathologic conditions that cause repression of CYP expression
Significant reductions in drug-metabolizing capacity caused by repression of CYP expression have been observed in numerous clinical settings, most notably under conditions that induce hepatocytes to proliferate or in hepatic or extra-hepatic pathologic states such as inflammation, infection, and cancer (28-37;37-45;45-49).
CYP expression has been seen to be significantly repressed in proliferating liver cells. These observations were made under nonphysiologic proliferating conditions such as liver carcinoma-derived cell lines (34;35) and isolated primary hepatocytes (36-38); physiologic proliferating condition such as livers of fetuses and young children (39-43); and clinical or pathologic proliferating conditions such as hepatomas (44;45) and regenerating livers (45-49). Unique characteristics associated with hepatocyte proliferation, including active cell cycle and elevated levels of growth factors such as hepatocyte growth factor (HGF) and augmenter of liver regeneration (ALR), are responsible for PXR-mediated repression of CYP expression. HGF decreases both CYP3A4 expression and activity (36); likewise, ALR, a tissue-specific hepatotrophic growth factor, downregulates CYP activity in the human liver (37). As discussed in the next section, cyclin-dependent kinase 2 (Cdk2) and the 70 kDa form of ribosomal protein S6 kinase (p70 S6K) are involved in PXR-mediated repression of CYP expression (Table 2) (16;50). In addition, signaling mediated by growth factor insulin represses PXR-mediated CYP promoter activity (Tables 1 & 3) (51) through the phosphatidylinositol 3'-kinase (PI3K)-protein kinase B (PKB, or Akt) pathway, and the forkhead in rhabdomyosarcoma (FKHR, or FOXO1) transcription factor.
Table 2.
Phosphorylation, phosphomimetic mutation or dephosphorylation-dependent PXR transactivation of CYPs
| Modification | Site | Enzyme | Mechanism | Reference |
|---|---|---|---|---|
| aPhosphomimetic mutation | Thr57 | - | Loss of transcription and promoter-binding activities | (16) |
| aPhosphomimetic mutation | Ser350 | - | Repression of transcription | (50) |
| Phosphorylation | Unknown | PKA | Repression of transcription and strengthen interaction of hPXR with NCoR | (23) |
| Phosphorylation | Unknown | PKA | Potentiation of transcription and strengthen interaction of mPXR with coactivators SRC-1 & PBP | (11) |
| Phosphorylation | Unknown | Cdk1 | Unknown | (23) |
| Phosphorylation | Unknown | CK2 | Unknown | (23) |
| Phosphorylation | Unknown | GSK3 | Unknown | (23) |
| Phosphorylation | Unknown | PKC | Repression of transcription. Strengthen interaction between PXR & NCoR and abolish interaction between PXR & SRC-1 | (12) |
| Phosphorylation | Unknown | Cdk2 | Repression of transcription | (50) |
| Dephosphorylation | Unknown | PP1/PP2A | Repression of transcription | (12) |
| Phosphorylation | Unknown | p70 S6K1 | Repression of transcription | (16;23) |
It is not known whether these functionally significant phosphomimetic mutation sites are actual sites of phosphorylation.
Thr, threonine; Ser, serine; PKA, protein kinase A; Cdk, cyclin-dependent kinase; CK, casein kinase; GSK, glycogen synthase kinase; PKC, protein kinase C; PP, protein phosphatase; p70S6K, 70kDa ribosomal S6 kinase; NCoR, nuclear receptor corepressor; SRC, steroid receptor coactivator; PBP, peroxisome proliferator-activated receptor-binding protein; hPXR, human PXR; mPXR, mouse PXR
Table 3.
Growth factors and cytokines regulating PXR transactivation of CYPs
| Molecules | Role or mechanism | Pathological or Physiological state | Reference |
|---|---|---|---|
| IL-6 | Repress mRNA levels of PXR and CYP | Inflammation | (29;31;91;92) |
| IL-1 | Repress mRNA levels of PXR and CYP | Inflammation | (29;31;97) |
| TNFα | Repress mRNA levels of PXR and CYP | Inflammation | (29;31;97) |
| Insulin | Repress mRNA levels of CYP | Proliferation | (51;105-109) |
| HGF | Repress mRNA levels of CYP | Proliferation | (36) |
| ALR | Repress mRNA levels of CYP | Proliferation | (37) |
IL, interleukin; TNF, tumor necrosis factor; HGF, hepatocyte growth factor; ALR, augmenter of liver regeneration.
In inflammation-associated pathologic states that originate from the liver or other organs, the reductions in hepatic drug-metabolizing capacity are mediated through cytokines (interleukin-1 [IL-1], interleukin-6 [IL-6] and tumor necrosis factor alpha [TNFα]), which are inflammatory mediators that modify the expression or function of specific transcription factors such as PXR and nuclear factor κB (NF- κB) in the liver (Table 1) (29;31;52;53). In addition, signaling mediated by PKA or protein kinase C (PKC) can alter PXR activity and subsequently CYP expression (Table 2) (11;12;23). These changes ultimately lead to downregulation of the overall activity levels of CYPs as a consequence of reduced gene expression, most notably through transcriptional suppression (29;52;54;55) and to some extent through reduced mRNA stability, increased protein degradation, and suppression of protein function (29;52;56). Extra-hepatic infections and tumors that are associated with inflammation reduce hepatic drug metabolism because CYPs are downregulated in the liver (29;57;58). This reduction probably involves the rapid transit of inflammatory components from the other organs to the peripheral circulation and then to the liver (59).
2. Mechanisms of repression of PXR-mediated CYP expression
2.1. Phosphorylation and protein-protein interaction events affect the function of PXR
As discussed below, inhibitory phosphorylation of PXR or its coactivator, or a protein-protein interaction between PXR and another signaling molecule, is responsible for the PXR-mediated repression of CYP expression. Phosphorylation and protein-protein interactions are dynamic regulatory mechanisms that not only affect the function of a protein in every conceivable way, resulting in increasing or suppressing activity, but also enable both specificity and cross-talk among diverse signaling pathways (60-62). It is well established for NRs that site-specific phosphorylation by kinases occurs on all domains and plays a vital role in regulating all aspects of NR function, including expression, stability, subcellular localization, dimerization, ligand binding, DNA binding, coregulator interaction, and transcriptional activity (63-69). PKA (11;23), PKC (12), Cdk2 (50), and p70 S6K (16) are involved in phosphorylating and regulating the activity of PXR. Furthermore, recently, Lichti-Kaiser et al. (23) have observed in metabolic labeling studies that ectopically expressed hPXR exists as a phosphoprotein in vivo in HepG2 cells and that immunopurified hPXR is phosphorylated by a wide variety of kinases such as Cdk1, casein kinase II (CK2), and glycogen synthase kinase 3 (GSK3), in addition to PKA, PKC, and p70 S6K (Table 2).
Protein-protein interactions define specificity in signal transduction pathways, in virtually every aspect of cellular function (62). Various signaling molecules interact and regulate the transcriptional activity of PXR as well as PXR-mediated CYP expression and activity (Table 1); including transcriptional factors such as NF-κB (53;70;71), FKHR (51), and sterol regulatory element binding protein 1 (SREBP-1) (72); NRs such as short heterodimer partner (SHP) (73-75) and liver X receptor (LXR) (74-76); and NR coregulators such as NCoR1, NCoR2, SRC-1, SRC-3, peroxisome proliferator-activated receptor-binding protein (PBP), and protein arginine methyltransferase 1 (PRMT1) (10-14;23;77).
2.2. Cdk2- and PI3K-Akt-mediated signaling in the repression of PXR-mediated CYP expression in proliferative hepatocytes
As discussed in the previous section, it has been well documented that the expression of CYPs is significantly reduced in proliferating hepatocytes. Studies by Greuet et al. (78), Donato et al. (36), and Thasler et al. (37) showed that the extent to which the expression of CYPs is repressed in proliferating hepatocytes changes over time, strongly suggesting the involvement of a cell cycle regulation mechanism. Interestingly, toward understanding a possible mechanism for CYP repression in proliferating hepatocytes, Thasler et al. (37) attempted to link the repression of CYPs to the downregulation of NRs. However, the molecular mechanism responsible for the repression of CYP expression was unknown until a recent study reported by Lin et al. (50). In their study, Lin et al. took a chemical biology approach and identified small-molecule inhibitors of Cdks as activators of hPXR-mediated CYP3A4 expression in proliferating HepG2 liver carcinoma cells (50). Their studies indicated that the inhibition of Cdk activity - but not the binding of Cdk inhibitors to hPXR - activates hPXR. This finding is significant, because it suggests that Cdk-mediated signaling might negatively regulate hPXR function. Lin et al. also showed that Cdk2 directly phosphorylates hPXR, most likely at a consensus phosphorylation site, Ser350 (Table 2), and that activation of Cdk2 leads to inhibition of hPXR-mediated CYP3A4 expression. Cdk2 belongs to the family of Cdks (catalytic subunit) that function with cyclins (regulatory subunit) to drive the cell cycle through each phase (G1, S, G2, and M). Since the activity of Cdk2 is maximal in the S phase, the activity of PXR is expected to be low in this cell cycle phase. Indeed, Lin et al. confirmed that PXR-mediated CYP3A4 expression is lower in the S phase than in the G1 phase. These results convincingly suggest that in proliferating hepatocytes, Cdk2 phosphorylates and inhibits hPXR, providing a possible explanation for the repression of CYP expression in proliferating hepatocytes. The studies by Lin et al. also suggest that multiple Cdk2 phosphorylation sites exist in hPXR. Whether other Cdks such as Cdk1 are involved and whether the activity of hPXR is modulated in other phases of the cell cycle remain unknown.
The most significant hepatic proliferation is associated with liver regeneration. Although the adult liver is quiescent (Go phase), the liver is constantly insulted by various physiologic, pathologic, and environmental factors that can cause liver injuries. As one of the largest internal organ of the body, the liver has developed a mechanism to protect itself from the loss of functional tissues through regeneration. A regenerative response in the liver can be triggered by the loss of liver mass because of chemical, traumatic, or infectious injuries. Liver regeneration is achieved mainly by driving quiescent mature hepatocytes to re-enter the cell cycle (79;80). The hepatocyte proliferative response is marked by the rapid release of growth stimulatory cytokines and growth factors, which are mediators of the liver regenerative response. The activation of cell cycle in hepatocytes after partial hepatectomy (a surgical procedure to trigger liver regeneration by removing up to two-third of the liver mass) (49) occurs in 2 phases: the priming phase, which represents the Go to G1 transition, and the progression or proliferative phase, which represents the G1 to S transition. Priming is induced by cytokines such as TNFα, IL-1, and IL-6, whereas progression or proliferation is induced by growth factors such as HGF and ALR (Table 3) (81;82). Cytokines mediate priming through NF-κB and STAT3 signaling (81;82), whereas growth factors mediate proliferation through various signaling pathways, including kinases such as Cdk2, PI3k-Akt, and p70 S6K (16;50;83-85).
Previous studies have reported that PXR and PI3K-Akt signaling are critical for hepatocyte proliferation in a mouse liver regeneration model after partial hepatectomy (83;86), and that p70 S6K expression and activity are upregulated in human hepatic tumors (84;85). These reports suggest that the PXR and kinase pathways might be linked to downregulation of CYP during hepatocyte proliferation. Indeed, Kodma et al. (51) and Pondugula et al. (16), have reported that both Akt and p70 S6K negatively regulate the transcriptional activity of PXR and PXR-mediated CYP expression in actively dividing HepG2 cells, providing additional possible mechanisms for CYP repression during growth factor-mediated hepatocyte proliferation in addition to Cdk2, which phosphorylates and attenuates the activity of PXR during the S phase of the cell cycle, as discussed above (50). However, as Cdk2 is active in actively cycling cells only, whether the effect of PI3K-Akt and p70 S6K on PXR is limited to cells in the active cell cycle is unknown.
The study by Dai et al. (86) showed that PXR-null mice have 17% less liver mass at the end of liver regeneration, suggesting that PXR is required for normal progression of liver regeneration. However, how PXR affects the normal progression of liver regeneration, and the activity level of PXR that is required for such a normal progression, is undefined. Lin et al. (50) showed that Cdk2 phosphorylates and attenuates the function of hPXR, providing a possible explanation for the repression of CYP expression during liver regeneration. It is possible that the reduced activity of the Cdk2-phosphorylated PXR is sufficient for the normal progression of liver regeneration. Alternatively, phosphorylation of PXR by Cdk2 might affect PXR's activity in inducing CYP expression without affecting its activity required for the normal progression of liver regeneration.
Forkhead box O factors (FOXO) are a family of insulin-sensitive transcription factors that influence NR transactivation by either repressing or activating transcription in an NR-specific manner (87). On the other hand, NRs function as inhibitors of FOXO-mediated transcription by interacting with FOXO factors (87). Central to insulin-mediated inhibition of FOXO is a shuttling mechanism that regulates FOXO localization from the nucleus to the cytosol, thereby terminating its transcriptional function. Since phosphorylation of FOXO factors influences their intracellular localization, it is assumed that phosphorylation of FOXO affects its interactions with NRs (87).
FKHR, also referred to as FOXO1, belongs to the FOXO family of transcription factors. FKHR interacts with and augments the transcriptional activity of PXR, whereas PXR represses FKHR-mediated transcription (51;87). These results suggested that the PI3K-Akt pathway, a major signaling pathway activated by insulin and other growth factors, is involved in negatively regulating the transcriptional activity of the PXR in HepG2 liver carcinoma cells by affecting the interaction between PXR and its coactivator FKHR. Akt possibly accomplishes this negative regulation by phosphorylating FKHR, resulting in the translocation of the nuclear FKHR into the cytoplasm for proteasomal degradation (88), consequently minimizing the levels of nuclear FKHR available for interacting with and activating PXR. However, it needs to be determined whether Akt phosphorylates PXR and other NR coregulators besides FKHR and whether Akt regulates PXR activity independently of FKHR.
Recently, Pondugula et al. (16) have shown that p70 S6K, a downstream kinase in the PI3K-Akt pathway, phosphorylates and negatively regulates the transcriptional activity of hPXR in HepG2 liver carcinoma cells (16) (Table 2). They found that a phosphorylation-deficient alanine mutation at Thr57 (T57A) confers partial but significant resistance to p70 S6K inhibition, suggesting that p70 S6K regulates hPXR activity possibly by phosphorylating Thr57 and that p70 S6K may have more than 1 functionally significant target residues in hPXR. In addition, introduction of a phosphomimetic mutation at Thr57 (T57D) impairs the transactivating activity of hPXR (16) (Table 2). The resistance to p70 S6K attenuation, conferred by the alanine mutation at Thr57, is consistent with the impaired hPXR activity when the phosphomimetic mutation was introduced at the same site (Table 2), and suggests that p70 S6K might regulate hPXR activity via Thr57 phosphorylation. Whether p70 S6K phosphorylates the coregulators of PXR to exert its inhibitory effect on PXR function is unknown.
Sterol regulatory element binding proteins (SREBPs) are lipogenic transcription factors of the basic helix-loop-helix family and play major role in lipid homeostasis (89). SREBPs activate the expression of genes that induce synthesis and uptake of cholesterol, fatty acids, phsopholipids, and triglycerides (89). Decreased drug clearance has been observed in obese, dyslipidemic, diabetic, and overfed rodents (72;89). A hallmark of these conditions is increased expression of SREBP-1 in the liver, hinting a possible link between regulation of CYPs and SREBP-1 and interconnection between drug metabolism and lipid metabolism.
Roth et al. (72) found that activation of SREBP-1 by insulin or cholesterol in mouse and human hepatocytes inhibits the transcriptional activity of PXR and consequently represses PXR-mediated CYP induction. SREBP-1 accomplishes this repression by directly interacting with PXR and by blocking the interaction of PXR with its coactivator SRC-1. These observations lead to the conclusion by Roth et al that PXR responds to lipid accumulation by directly interacting with SREBP-1 and that drug metabolism and lipid metabolism are interconnected.
2.3. Inflammatory cytokines, NF-κB, PKA, and PKC in the repression of CYP expression associated with inflammation
In disease states associated with inflammation, both CYP expression and drug-metabolizing activities are downregulated in the liver and other organs such as the intestine, where PXR is abundantly expressed (28;29;33). Interestingly, it has been reported that the expression levels and activities of inflammatory cytokines such as IL-1, IL-6, and TNFα and the activities of protein kinases such as PKA and PKC are upregulated during inflammation in the liver (11;12;23;29-31;52). These cytokines also mediate CYP repression in the liver and intestine during inflammation (28;29;33;90-92). Moreover, extra-hepatic infections and tumors associated with inflammation reduce the capacity of hepatic drug metabolism because CYPs are downregulated in the liver (57;58). The reduction in CYP expression and drug-metabolizing capacity is probably mediated through inflammatory cytokines circulated to the liver from remotely inflamed organs or tissues (59).
Inflammatory cytokines affect the expression and function of PXR either directly by unknown mechanisms or indirectly by modifying the expression and function of specific transcription factors such as NF-κB and STAT 3 in the liver (29;31;53). In addition, signaling mediated by PKA or PKC in the liver can alter PXR activity and thereby hepatic CYP expression (Table 2) (11;12;23). These changes ultimately downregulate the activity of CYPs because of reduced gene expression through transcriptional suppression.
Lipopolysaccharide (LPS) is a bacterial endotoxin commonly administered to rodents to induce local or systemic inflammation and to treat cultured cells to induce inflammatory changes. Following an LPS challenge, there is significant downregulation of mRNA or protein levels, or both, of PXR and CYP in mouse liver (91;93;94), rat liver and intestine (95;96), and human hepatocytes (90;92). LPS mediates such repression by upregulating the inflammatory cytokines IL-1β, IL-6, and TNFα (28-30;33;52) The downregulation of PXR and PXR-mediated CYP induction during inflammation can be mimicked by in vivo and in vitro treatment with these inflammatory cytokines in rodent livers and human hepatocytes (90-92). These results suggest that PXR and PXR-mediated CYP expressions are negatively regulated by LPS or LPS-induced cytokines, and provide a molecular mechanism for impaired drug metabolism during inflammation.
Inflammatory bowel disease (IBD) is associated with chronic inflammation of the intestinal tract. Recently, PXR was shown to be involved in IBD in humans (97). Langmann et al. observed a significant downregulation of PXR and CYP3A4 gene expression in the intestine of IBD patients and also found that inflammatory cytokines such as TNFα and IL-1β repress rifampicin-mediated induction of CYP3A4 in intestinal cell lines (97). Iizasa et al. (98) showed in dextran sodium sulfate (DSS)-induced colitis mice that mRNA for mPXR was significantly reduced in the intestine. These results suggest that inflammation in the intestine generates similar responses as in the liver in terms of the expression and activity of both PXR and CYP.
Signaling mediated by LPS and cytokines such as IL-1 and TNFα leads to the activation of NF-κB (99;100). Activation of NF-κB was recently shown to repress PXR activation and PXR-mediated CYP promoter activity (53;70;71). Gu et al. reported that activation of NF-κB by either LPS or TNF-α led to PXR suppression through interaction of NF-κB and the PXR-RXR heterodimer, and that inhibition of NF-κB by the NF-κB-specific suppressor SRIκBα reverses the suppressive effects of LPS and TNFα (70). Zhou et al. also reported that NF-κB activation inhibits hPXR activation, and that inhibition of NF-κB potentiates hPXR activation (53). This PXR-NF-κB axis provides a molecular explanation for the suppression of hepatic and intestinal CYP expression by inflammatory stimuli.
Some inflammatory cytokines such as IL-6 exert their biologic responses by activating STAT3 (29-31;82;101). When activated during inflammation, STAT 3 inhibits the transcriptional activity of NRs such as HNF4α and HNF4α-mediated CYP induction (29;31). Although IL-6 downregulates both PXR and CYP during inflammation (Table 3) (28;29;91;92), the molecular mechanisms responsible for IL-6- mediated repression of PXR and CYP and the effect of STAT3 on PXR activity during inflammation remain unknown.
Another protein kinase, PKC, plays key roles in the production of cytokines and in responding to cytokine signaling in the liver. Release of inflammatory cytokines such as IL-1, IL-6, and TNFα from liver Kupffer cells is dependent on PKC activity (102). During sepsis and inflammation, cytokine-mediated stimulation of hepatocytes initiates PKC-dependent intracellular signaling pathways (103). As discussed earlier, one of the most prominent responses following release of inflammatory cytokines is the drastic repression of hepatic CYP gene expression (90;104). It is also known that various pathologic stimuli, including inflammation, increase the intracellular concentration of cAMP in hepatocytes, resulting in activated PKA signaling followed by rapid decreases in CYP gene expression during inflammation (23). These findings lead to a logical speculation that both PKC and PKA signaling might be involved in repressing CYP3A gene expression by negatively regulating PXR activity in sepsis and inflammation.
Ding et al. (Table 2) (12) observed that activation of PKC signaling represses mPXR transcriptional activity, followed by reduced Cyp3a11 gene expression, possibly by strengthening the interaction between mPXR and NCoR while inhibiting the interaction between mPXR and SRC-1. In addition, Lichti-Kaiser et al. (Table 2) (23) showed that immunopurified hPXR can be phosphorylated in vitro by PKC. These observations suggest that phosphorylation of PXR or proteins involved in PXR signaling pathway following activation of the PKC signaling contribute to downregulation of hepatic CYPs. These data also provide the molecular basis for sepsis- and inflammatory-based repression of CYP3A gene expression through activation of PKC signaling, which then represses PXR activity. The effect of PKC might be mediated through alterations in the phosphorylation status of NCoR or SRC-1, or both, along with PXR. Further studies are required to determine the sites of phosphorylation in these proteins and whether phosphorylations are modified following stimulation with inflammatory cytokines and activation of the PKC signal transduction pathway.
PKA is also involved in regulating PXR activity. Ding et al. (Table 2) (11) and Lichti-Kaiser et al. (Table 2) (23) have shown that PKA phosphorylates hPXR in vitro. They showed that activation of PKA signaling by 8-Br-cAMP potentiates the induction of Cyp3a11 gene expression by PXR agonists such as PCN, taxol, and forskolin in mouse primary hepatocytes but represses the PCN-mediated induction of Cyp3A1 mRNA in rat primary hepatocytes and the rifampicin-mediated induction of CYP3A4 mRNA in human primary hepatocytes. Inhibition of PKA results in enhanced rifampicin-induced hPXR activity in HepG2 cells and attenuated PCN-mediated induction of Cyp3a11 gene expression in mouse primary hepatocytes (23). These data suggest that phosphorylation of PXR or proteins involved in the PXR signaling pathway by PKA plays a key role in regulating the induction of CYP3A gene expression in hepatocytes.
Interestingly, expression of constitutively active PKA in HepG2 cells inhibits both rifampicin and PCN-mediated hPXR and mPXR transactivation, respectively, whereas PKA activation with 8-Br-cAMP in humanized mouse hepatocytes results in potentiation of rifampicin-mediated induction of Cyp3a11 mRNA expression (23). These results led to the hypothesis by Lichti-Kaiser et al. that the observed species-specific interaction between PKA and PXR in hepatocytes is a function of how PKA signaling interfaces with CYP3A gene expression across species and not of the differences in primary amino acid sequences in the human and mouse PXR proteins. Moreover, these results provide compelling evidence for pronounced species-specific differences in the coupling of pivotal kinase cascades and PXR activity. The results of PKA suppression of hPXR activity and subsequent CYP3A4 gene expression in human primary hepatocytes and HepG2 cells provide a potential molecular mechanism for the repression of CYP3A gene expression during inflammatory conditions with enhanced PKA activity.
Expression of constitutively active PKA enhances the strength of the rifampicin-sensitive interaction between hPXR and NCoR in CV-1 cells (23). Activation of PKA signaling with 8-Br-cAMP strengthens the agonist-induced interaction between mPXR and coactivator proteins SRC-1 and PBP in CV-1 cells (11). These data suggest that PKA regulates PXR activity, in part, through its ability to modulate PXR-coregulator interaction. However, the site(s) on PXR phosphorylated by PKA and whether PKA phosphorylates coregulators to exert its effects on PXR remain to be studied.
3. Conclusion
ADRs are a serious public health problem. Adverse drug interactions mediated by the CYP-mediated drug-metabolizing pathway are one type of preventable ADRs. Repression of CYP expression contributes to adverse drug interactions. Since the expression of CYPs, especially CYP3A, is controlled by PXR, understanding the molecular mechanisms responsible for the PXR-mediated repression of CYP expression is critical to predict and prevent therapy-induced adverse drug interactions. Repression of CYP expression occurs under various clinical conditions, for example, when hepatocytes proliferate or during inflammation (hepatic or extra-hepatic). Several studies have begun to reveal the molecular mechanisms responsible for PXR-mediated repression of CYP expression. Direct phosphorylation and inhibition of PXR is one such mechanism, and kinases such as Cdk2, PKA, PKC, and p70 S6k have been shown to directly phosphorylate and inhibit PXR. Another mechanism is the phosphorylation and inhibition of PXR coregulators to indirectly attenuate the function of PXR: the PI3k-Akt pathway inhibits PXR indirectly by phosphorylating and inhibiting FKHR, a coactivator of PXR. Interaction of PXR with other signaling molecules is another mechanism for downregulation of PXR; NF-κB interacts with the PXR-RXR heterodimer and attenuates the function of PXR. Other possible mechanisms include downregulation of the expression or stability of PXR.
4. Expert opinion
Multiple mechanisms seem to be involved in downregulating the function of PXR and leading to the repression of CYP expression. Although multiple kinases (Table 2), such as Cdk2 (50), PKA (11;23), PKC (12;23), and p70 S6K (16), have been shown to directly phosphorylate PXR and possibly lead to the repression of CYP expression, and functionally significant amino acid residues (i.e., Ser350 and Thr57 of the hPXR) (Table 2) (16;50) have been linked to a phosphorylation-related function, no specific amino acid has been shown to be phosphorylated by these kinases. Future studies need to focus on comprehensively identifying all the functional kinase-specific phosphorylation sites on hPXR in order to define the mechanism leading to kinase-mediated inhibition of PXR and repression of CYP expression.
Species specificity occurs at both the ligand-binding and signaling-cascade levels (23). PXR humanized mice is a very useful animal model to address species specificity at the ligand-binding level (26;27). However, no animal model is available to address species specificity at the signaling-pathway level. Because of the effect of complex systematic function on signaling pathways, such an animal model will be extremely valuable but difficult to generate. We expect that such animal models will be specific for each relevant signaling pathway, and each specific pathway can be studied in each specific animal model. However, this might prove challenging because signaling pathways tend to interact (cross-talk) with each other. Transgenic mice with PXR engineered to mimic the function of a specific pathway might represent a valuable approach. For example, transgenic mouse harboring a phosphomimetic mutation at Ser350 (S350D) of PXR might be used to mimic the inhibition of PXR by Cdk2 (50). Similarly, if the species specificity of the effect of PKA on PXR can be mapped to specific PKA-mediated phosphorylation site on either PXR or PXR cofactors, phosphomimetic mutation can be created and used to generate transgenic mice.
Consistent with the role of PXR as a master xenobiotic receptor, numerous signaling pathways might be involved in regulating the function of PXR. Identifying all the signaling pathways that interact with PXR signaling will be critical to systematically dissect the regulation of PXR function. However, this will be an especially challenging task, and a high-throughput chemical biology or genomic approach will prove valuable. The discovery of Cdk2-mediated PXR inhibition (discussed above) (50) was initiated with a chemical biology approach. In such an approach, a collection of compounds with known bioactivity is used. The rationale is that the activity of PXR can be modulated either by ligands of PXR or by modulators of signaling pathways that cross-talk with the PXR signaling pathway. Therefore, compounds that modulate the activity of PXR in a ligand-independent manner will lead to the identification of signaling pathway(s) that cross-talk with PXR. We expect to see a greater use of the high-throughput approach to address the regulation of PXR and other hepatic function, such as regulation of liver regeneration.
Since phosphorylation has been shown to regulate the function of PXR, it is logical to speculate that not only protein kinases but also phosphatases are involved in this regulation. Phosphatase might be involved in regulating the function of PXR directly or indirectly by desensitizing the kinase pathway. It is vital to understand the contribution of both kinases and phosphatases in regulating PXR function to comprehensively address the role of phosphorylation in PXR function.
Expression of CYP is regulated by multiple NRs. It is therefore necessary to investigate the role of other relevant NRs such as the constitutive androstane receptor (CAR) in CYP inhibition.
Acknowledgements
This work was supported in part by the National Institutes of Health National Cancer Institute [Grant P30-CA21765], the American Lebanese Syrian Associated Charities (ALSAC) and St. Jude Children's Research Hospital. We thank members of the Chen group for their valuable discussions, Dr. Kip Guy for critical review of the manuscript, and Dr. Vani Shanker for editing the manuscript.
Footnotes
Declaration of interest This work was funded by the National Cancer Institute (Grant P30 CA021765-30), American Lebanese Syrian Associated Charities (ALSAC) and St. Jude Children's Hospital.
References
- (1).Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Ann Intern Med. 2004 May 18;140(10):795–801. doi: 10.7326/0003-4819-140-10-200405180-00009. [DOI] [PubMed] [Google Scholar]
- (2).Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000 Oct 7;356(9237):1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- (3).Leape LL, Brennan TA, Laird N, Lawthers AG, Localio AR, Barnes BA, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991 Feb 7;324(6):377–84. doi: 10.1056/NEJM199102073240605. [DOI] [PubMed] [Google Scholar]
- (4).Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998 Apr 15;279(15):1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- (5).Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med. 1995 Oct 9;155(18):1949–56. [PubMed] [Google Scholar]
- (6).Harmsen S, Meijerman I, Beijnen JH, Schellens JH. The role of nuclear receptors in pharmacokinetic drug-drug interactions in oncology. Cancer Treat Rev. 2007 Jun;33(4):369–80. doi: 10.1016/j.ctrv.2007.02.003. [DOI] [PubMed] [Google Scholar]
- (7).Zhou SF. Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr Drug Metab. 2008 May;9(4):310–22. doi: 10.2174/138920008784220664. [DOI] [PubMed] [Google Scholar]
- (8).Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- (9).Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest. 1998 Sep 1;102(5):1016–23. doi: 10.1172/JCI3703. ..The authors for the first time associate drug-drug interactions with PXR activation and CYP3A expression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Kliewer SA, Moore JT, Wade L, Staudinger JL, Watson MA, Jones SA, et al. An orphan nuclear receptor activated by pregnanes defines a novel steroid signaling pathway. Cell. 1998 Jan 9;92(1):73–82. doi: 10.1016/s0092-8674(00)80900-9. ..This is the first report on the identification of PXR. [DOI] [PubMed] [Google Scholar]
- (11).Ding X, Staudinger JL. Induction of drug metabolism by forskolin: the role of the pregnane X receptor and the protein kinase a signal transduction pathway. J Pharmacol Exp Ther. 2005 Feb;312(2):849–56. doi: 10.1124/jpet.104.076331. ..The authors show that PKA phosphorylates and regulates PXR function. [DOI] [PubMed] [Google Scholar]
- (12).Ding X, Staudinger JL. Repression of PXR-mediated induction of hepatic CYP3A gene expression by protein kinase C. Biochem Pharmacol. 2005 Mar 1;69(5):867–73. doi: 10.1016/j.bcp.2004.11.025. ..The authors show that activation of PKC signaling inhibits PXR function. [DOI] [PubMed] [Google Scholar]
- (13).Johnson DR, Li CW, Chen LY, Ghosh JC, Chen JD. Regulation and binding of pregnane X receptor by nuclear receptor corepressor silencing mediator of retinoid and thyroid hormone receptors (SMRT) Mol Pharmacol. 2006 Jan;69(1):99–108. doi: 10.1124/mol.105.013375. [DOI] [PubMed] [Google Scholar]
- (14).Li CW, Dinh GK, Chen JD. Preferential physical and functional interaction of pregnane X receptor with the SMRTalpha isoform. Mol Pharmacol. 2009 Feb;75(2):363–73. doi: 10.1124/mol.108.047845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Noble SM, Carnahan VE, Moore LB, Luntz T, Wang H, Ittoop OR, et al. Human PXR forms a tryptophan zipper-mediated homodimer. Biochemistry. 2006 Jul 18;45(28):8579–89. doi: 10.1021/bi0602821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Pondugula SR, Brimer-Cline C, Wu J, Schuetz EG, Tyagi RK, Chen T. A Phosphomimetic Mutation at Threonine-57 Abolishes Transactivation Activity and Alters Nuclear Localization Pattern of Human Pregnane X Receptor. Drug Metab Dispos. 2009 Jan 26; doi: 10.1124/dmd.108.024695. ..The authors identify a loss-of-function phosphomimetic mutant and present data for p70 S6K regulation of PXR function by direct phosphorylation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Kawana K, Ikuta T, Kobayashi Y, Gotoh O, Takeda K, Kawajiri K. Molecular mechanism of nuclear translocation of an orphan nuclear receptor, SXR. Mol Pharmacol. 2003 Mar;63(3):524–31. doi: 10.1124/mol.63.3.524. [DOI] [PubMed] [Google Scholar]
- (18).Saradhi M, Krishna B, Mukhopadhyay G, Tyagi RK. Purification of full-length human pregnane and xenobiotic receptor: polyclonal antibody preparation for immunological characterization. Cell Res. 2005 Oct;15(10):785–95. doi: 10.1038/sj.cr.7290348. [DOI] [PubMed] [Google Scholar]
- (19).Echchgadda I, Song CS, Oh TS, Cho SH, Rivera OJ, Chatterjee B. Gene regulation for the senescence marker protein DHEA-sulfotransferase by the xenobiotic-activated nuclear pregnane X receptor (PXR) Mech Ageing Dev. 2004 Oct;125(1011):733–45. doi: 10.1016/j.mad.2004.08.008. [DOI] [PubMed] [Google Scholar]
- (20).Chen Y, Tang Y, Wang MT, Zeng S, Nie D. Human pregnane X receptor and resistance to chemotherapy in prostate cancer. Cancer Res. 2007 Nov 1;67(21):10361–7. doi: 10.1158/0008-5472.CAN-06-4758. [DOI] [PubMed] [Google Scholar]
- (21).Masuyama H, Hiramatsu Y, Kodama J, Kudo T. Expression and potential roles of pregnane X receptor in endometrial cancer. J Clin Endocrinol Metab. 2003 Sep;88(9):4446–54. doi: 10.1210/jc.2003-030203. [DOI] [PubMed] [Google Scholar]
- (22).Squires EJ, Sueyoshi T, Negishi M. Cytoplasmic localization of pregnane X receptor and ligand-dependent nuclear translocation in mouse liver. J Biol Chem. 2004 Nov 19;279(47):49307–14. doi: 10.1074/jbc.M407281200. [DOI] [PubMed] [Google Scholar]
- (23).Lichti-Kaiser K, Xu C, Staudinger JL. Cyclic AMP-dependent protein kinase signaling modulates pregnane X receptor activity in a species-specific manner. J Biol Chem. 2009 Jan 13; doi: 10.1074/jbc.M807426200. ..The authors for the first time report species-specific PKA signaling on the function of PXR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Moore LB, Maglich JM, McKee DD, Wisely B, Willson TM, Kliewer SA, et al. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol Endocrinol. 2002 May;16(5):977–86. doi: 10.1210/mend.16.5.0828. [DOI] [PubMed] [Google Scholar]
- (25).Kliewer SA, Goodwin B, Willson TM. The nuclear pregnane X receptor: a key regulator of xenobiotic metabolism. Endocr Rev. 2002 Oct;23(5):687–702. doi: 10.1210/er.2001-0038. [DOI] [PubMed] [Google Scholar]
- (26).Xie W, Barwick JL, Downes M, Blumberg B, Simon CM, Nelson MC, et al. Humanized xenobiotic response in mice expressing nuclear receptor SXR. Nature. 2000 Jul 27;406(6794):435–9. doi: 10.1038/35019116. ..The first report on PXR humanized mouse models. [DOI] [PubMed] [Google Scholar]
- (27).Ma X, Shah Y, Cheung C, Guo GL, Feigenbaum L, Krausz KW, et al. The PREgnane X receptor gene-humanized mouse: a model for investigating drug-drug interactions mediated by cytochromes P450 3A. Drug Metab Dispos. 2007 Feb;35(2):194–200. doi: 10.1124/dmd.106.012831. [DOI] [PubMed] [Google Scholar]
- (28).Morgan ET. Regulation of cytochrome p450 by inflammatory mediators: why and how? Drug Metab Dispos. 2001 Mar;29(3):207–12. [PubMed] [Google Scholar]
- (29).Morgan ET, Goralski KB, Piquette-Miller M, Renton KW, Robertson GR, Chaluvadi MR, et al. Regulation of drug-metabolizing enzymes and transporters in infection, inflammation, and cancer. Drug Metab Dispos. 2008 Feb;36(2):205–16. doi: 10.1124/dmd.107.018747. ·The authors summarize the mechanisms of CYP repression in infection, inflammation and cancer. [DOI] [PubMed] [Google Scholar]
- (30).Kacevska M, Robertson GR, Clarke SJ, Liddle C. Inflammation and CYP3A4-mediated drug metabolism in advanced cancer: impact and implications for chemotherapeutic drug dosing. Expert Opin Drug Metab Toxicol. 2008 Feb;4(2):137–49. doi: 10.1517/17425255.4.2.137. [DOI] [PubMed] [Google Scholar]
- (31).Robertson GR, Liddle C, Clarke SJ. Inflammation and altered drug clearance in cancer: transcriptional repression of a human CYP3A4 transgene in tumor-bearing mice. Clin Pharmacol Ther. 2008 Jun;83(6):894–7. doi: 10.1038/clpt.2008.55. [DOI] [PubMed] [Google Scholar]
- (32).Renton KW. Alteration of drug biotransformation and elimination during infection and inflammation. Pharmacol Ther. 2001 Nov;92(23):147–63. doi: 10.1016/s0163-7258(01)00165-6. [DOI] [PubMed] [Google Scholar]
- (33).Renton KW. Cytochrome P450 regulation and drug biotransformation during inflammation and infection. Curr Drug Metab. 2004 Jun;5(3):235–43. doi: 10.2174/1389200043335559. [DOI] [PubMed] [Google Scholar]
- (34).Grant MH, Duthie SJ, Gray AG, Burke MD. Mixed function oxidase and UDP-glucuronyltransferase activities in the human Hep G2 hepatoma cell line. Biochem Pharmacol. 1988 Nov 1;37(21):4111–6. doi: 10.1016/0006-2952(88)90103-7. [DOI] [PubMed] [Google Scholar]
- (35).Donato MT, Bassi AM, Gomez-Lechon MJ, Penco S, Herrero E, Adamo D, et al. Evaluation of the xenobiotic biotransformation capability of six rodent hepatoma cell lines in comparison with rat hepatocytes. In Vitro Cell Dev Biol Anim. 1994 Sep;30A(9):574–80. doi: 10.1007/BF02631255. [DOI] [PubMed] [Google Scholar]
- (36).Donato MT, Gomez-Lechon MJ, Jover R, Nakamura T, Castell JV. Human hepatocyte growth factor down-regulates the expression of cytochrome P450 isozymes in human hepatocytes in primary culture. J Pharmacol Exp Ther. 1998 Feb;284(2):760–7. [PubMed] [Google Scholar]
- (37).Thasler WE, Dayoub R, Muhlbauer M, Hellerbrand C, Singer T, Grabe A, et al. Repression of cytochrome P450 activity in human hepatocytes in vitro by a novel hepatotrophic factor, augmenter of liver regeneration. J Pharmacol Exp Ther. 2006 Feb;316(2):822–9. doi: 10.1124/jpet.105.094201. [DOI] [PubMed] [Google Scholar]
- (38).Papeleu P, Vanhaecke T, Henkens T, Elaut G, Vinken M, Snykers S, et al. Isolation of rat hepatocytes. Methods Mol Biol. 2006;320:229–37. doi: 10.1385/1-59259-998-2:229. [DOI] [PubMed] [Google Scholar]
- (39).Giachelli CM, Omiecinski CJ. Developmental regulation of cytochrome P-450 genes in the rat. Mol Pharmacol. 1987 May;31(5):477–84. [PubMed] [Google Scholar]
- (40).Blake MJ, Gaedigk A, Pearce RE, Bomgaars LR, Christensen ML, Stowe C, et al. Ontogeny of dextromethorphan O- and N-demethylation in the first year of life. Clin Pharmacol Ther. 2007 Apr;81(4):510–6. doi: 10.1038/sj.clpt.6100101. [DOI] [PubMed] [Google Scholar]
- (41).Hakkola J, Pasanen M, Purkunen R, Saarikoski S, Pelkonen O, Maenpaa J, et al. Expression of xenobiotic-metabolizing cytochrome P450 forms in human adult and fetal liver. Biochem Pharmacol. 1994 Jul 5;48(1):59–64. doi: 10.1016/0006-2952(94)90223-2. [DOI] [PubMed] [Google Scholar]
- (42).Hines RN. Ontogeny of human hepatic cytochromes P450. J Biochem Mol Toxicol. 2007;21(4):169–75. doi: 10.1002/jbt.20179. [DOI] [PubMed] [Google Scholar]
- (43).Alcorn J, McNamara PJ. Using ontogeny information to build predictive models for drug elimination. Drug Discov Today. 2008 Jun;13(1112):507–12. doi: 10.1016/j.drudis.2008.03.016. [DOI] [PubMed] [Google Scholar]
- (44).Degawa M, Miura S, Yoshinari K, Hashimoto Y. Altered expression of hepatic CYP1A enzymes in rat hepatocarcinogenesis. Jpn J Cancer Res. 1995 Jun;86(6):535–9. doi: 10.1111/j.1349-7006.1995.tb02431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).George J, Liddle C, Murray M, Byth K, Farrell GC. Pre-translational regulation of cytochrome P450 genes is responsible for disease-specific changes of individual P450 enzymes among patients with cirrhosis. Biochem Pharmacol. 1995 Mar 30;49(7):873–81. doi: 10.1016/0006-2952(94)00515-n. [DOI] [PubMed] [Google Scholar]
- (46).von der DECK, HULTIN T. The enzymatic composition of rat liver microsomes during liver regeneration. Exp Cell Res. 1960 Apr;19:591–604. doi: 10.1016/0014-4827(60)90066-5. [DOI] [PubMed] [Google Scholar]
- (47).Marie IJ, Dalet C, Blanchard JM, Astre C, Szawlowski A, Saint AB, et al. Inhibition of cytochrome P-450p (P450IIIA1) gene expression during liver regeneration from two-thirds hepatectomy in the rat. Biochem Pharmacol. 1988 Sep 15;37(18):3515–21. doi: 10.1016/0006-2952(88)90705-8. [DOI] [PubMed] [Google Scholar]
- (48).Habib SL, Srikanth NS, Scappaticci FA, Faletto MB, Maccubbin A, Farber E, et al. Altered expression of cytochrome P450 mRNA during chemical-induced hepatocarcinogenesis and following partial hepatectomy. Toxicol Appl Pharmacol. 1994 Jan;124(1):139–48. doi: 10.1006/taap.1994.1017. [DOI] [PubMed] [Google Scholar]
- (49).Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007 Nov;213(2):286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Lin W, Wu J, Dong H, Bouck D, Zeng FY, Chen T. Cyclin-dependent kinase 2 negatively regulates human pregnane X receptor-mediated CYP3A4 gene expression in HepG2 liver carcinoma cells. J Biol Chem. 2008 Sep 9; doi: 10.1074/jbc.M806132200. ..The authors use high throughput screening chemical biology approach to discover the negative regulation of PXR function by Cdk2 in proliferating hepatocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Kodama S, Koike C, Negishi M, Yamamoto Y. Nuclear receptors CAR and PXR cross talk with FOXO1 to regulate genes that encode drug-metabolizing and gluconeogenic enzymes. Mol Cell Biol. 2004 Sep;24(18):7931–40. doi: 10.1128/MCB.24.18.7931-7940.2004. ..The authors discover that FOXO1 positively regulates the PXR function via direct protein-protein interaction. In addition, they describe that Akt regulation of FOXO1 affects PXR function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Aitken AE, Richardson TA, Morgan ET. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol. 2006;46:123–49. doi: 10.1146/annurev.pharmtox.46.120604.141059. [DOI] [PubMed] [Google Scholar]
- (53).Zhou C, Tabb MM, Nelson EL, Grun F, Verma S, Sadatrafiei A, et al. Mutual repression between steroid and xenobiotic receptor and NF-kappaB signaling pathways links xenobiotic metabolism and inflammation. J Clin Invest. 2006 Aug;116(8):2280–9. doi: 10.1172/JCI26283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Chen JQ, Strom A, Gustafsson JA, Morgan ET. Suppression of the constitutive expression of cytochrome P-450 2C11 by cytokines and interferons in primary cultures of rat hepatocytes: comparison with induction of acute-phase genes and demonstration that CYP2C11 promoter sequences are involved in the suppressive response to interleukins 1 and 6. Mol Pharmacol. 1995 May;47(5):940–7. [PubMed] [Google Scholar]
- (55).Jover R, Bort R, Gomez-Lechon MJ, Castell JV. Down-regulation of human CYP3A4 by the inflammatory signal interleukin-6: molecular mechanism and transcription factors involved. FASEB J. 2002 Nov;16(13):1799–801. doi: 10.1096/fj.02-0195fje. [DOI] [PubMed] [Google Scholar]
- (56).Ferrari L, Peng N, Halpert JR, Morgan ET. Role of nitric oxide in down-regulation of CYP2B1 protein, but not RNA, in primary cultures of rat hepatocytes. Mol Pharmacol. 2001 Jul;60(1):209–16. doi: 10.1124/mol.60.1.209. [DOI] [PubMed] [Google Scholar]
- (57).Charles KA, Rivory LP, Brown SL, Liddle C, Clarke SJ, Robertson GR. Transcriptional repression of hepatic cytochrome P450 3A4 gene in the presence of cancer. Clin Cancer Res. 2006 Dec 15;12(24):7492–7. doi: 10.1158/1078-0432.CCR-06-0023. [DOI] [PubMed] [Google Scholar]
- (58).Renton KW, Nicholson TE. Hepatic and central nervous system cytochrome P450 are down-regulated during lipopolysaccharide-evoked localized inflammation in brain. J Pharmacol Exp Ther. 2000 Aug;294(2):524–30. [PubMed] [Google Scholar]
- (59).Abdulla D, Goralski KB, Renton KW. The regulation of cytochrome P450 2E1 during LPS-induced inflammation in the rat. Toxicol Appl Pharmacol. 2006 Oct 1;216(1):1–10. doi: 10.1016/j.taap.2006.03.012. [DOI] [PubMed] [Google Scholar]
- (60).Cohen P. The regulation of protein function by multisite phosphorylation--a 25 year update. Trends Biochem Sci. 2000 Dec;25(12):596–601. doi: 10.1016/s0968-0004(00)01712-6. [DOI] [PubMed] [Google Scholar]
- (61).Cohen P. The role of protein phosphorylation in human health and disease. The Sir Hans Krebs Medal Lecture. Eur J Biochem. 2001 Oct;268(19):5001–10. doi: 10.1046/j.0014-2956.2001.02473.x. [DOI] [PubMed] [Google Scholar]
- (62).Pawson T, Nash P. Protein-protein interactions define specificity in signal transduction. Genes Dev. 2000 May 1;14(9):1027–47. [PubMed] [Google Scholar]
- (63).Orti E, Bodwell JE, Munck A. Phosphorylation of steroid hormone receptors. Endocr Rev. 1992 Feb;13(1):105–28. doi: 10.1210/edrv-13-1-105. [DOI] [PubMed] [Google Scholar]
- (64).Shao D, Lazar MA. Modulating nuclear receptor function: may the phos be with you. J Clin Invest. 1999 Jun;103(12):1617–8. doi: 10.1172/JCI7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Ismaili N, Garabedian MJ. Modulation of glucocorticoid receptor function via phosphorylation. Ann N Y Acad Sci. 2004 Jun;1024:86–101. doi: 10.1196/annals.1321.007. [DOI] [PubMed] [Google Scholar]
- (66).Weigel NL, Moore NL. Steroid receptor phosphorylation: a key modulator of multiple receptor functions. Mol Endocrinol. 2007 Oct;21(10):2311–9. doi: 10.1210/me.2007-0101. [DOI] [PubMed] [Google Scholar]
- (67).Blumberg B, Sabbagh W, Jr., Juguilon H, Bolado J, Jr., van Meter CM, Ong ES, et al. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998 Oct 15;12(20):3195–205. doi: 10.1101/gad.12.20.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Rochette-Egly C. Nuclear receptors: integration of multiple signalling pathways through phosphorylation. Cell Signal. 2003 Apr;15(4):355–66. doi: 10.1016/s0898-6568(02)00115-8. [DOI] [PubMed] [Google Scholar]
- (69).Sun K, Montana V, Chellappa K, Brelivet Y, Moras D, Maeda Y, et al. Phosphorylation of a conserved serine in the deoxyribonucleic acid binding domain of nuclear receptors alters intracellular localization. Mol Endocrinol. 2007 Jun;21(6):1297–311. doi: 10.1210/me.2006-0300. [DOI] [PubMed] [Google Scholar]
- (70).Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, et al. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006 Jun 30;281(26):17882–9. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- (71).Xie W, Tian Y. Xenobiotic receptor meets NF-kappaB, a collision in the small bowel. Cell Metab. 2006 Sep;4(3):177–8. doi: 10.1016/j.cmet.2006.08.004. [DOI] [PubMed] [Google Scholar]
- (72).Roth A, Looser R, Kaufmann M, Meyer UA. Sterol regulatory element binding protein 1 interacts with pregnane X receptor and constitutive androstane receptor and represses their target genes. Pharmacogenet Genomics. 2008 Apr;18(4):325–37. doi: 10.1097/FPC.0b013e3282f706e0. [DOI] [PubMed] [Google Scholar]
- (73).Ourlin JC, Lasserre F, Pineau T, Fabre JM, Sa-Cunha A, Maurel P, et al. The small heterodimer partner interacts with the pregnane X receptor and represses its transcriptional activity. Mol Endocrinol. 2003 Sep;17(9):1693–703. doi: 10.1210/me.2002-0383. [DOI] [PubMed] [Google Scholar]
- (74).Pascussi JM, Gerbal-Chaloin S, Duret C, ujat-Chavanieu M, Vilarem MJ, Maurel P. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: crosstalk and consequences. Annu Rev Pharmacol Toxicol. 2008;48:1–32. doi: 10.1146/annurev.pharmtox.47.120505.105349. [DOI] [PubMed] [Google Scholar]
- (75).Lim YP, Huang JD. Interplay of pregnane X receptor with other nuclear receptors on gene regulation. Drug Metab Pharmacokinet. 2008;23(1):14–21. doi: 10.2133/dmpk.23.14. [DOI] [PubMed] [Google Scholar]
- (76).Handschin C, Podvinec M, Amherd R, Looser R, Ourlin JC, Meyer UA. Cholesterol and bile acids regulate xenosensor signaling in drug-mediated induction of cytochromes P450. J Biol Chem. 2002 Aug 16;277(33):29561–7. doi: 10.1074/jbc.M202739200. [DOI] [PubMed] [Google Scholar]
- (77).Xie Y, Ke S, Ouyang N, He J, Xie W, Bedford MT, et al. Epigenetic regulation of transcriptional activity of pregnane X receptor by protein arginine methyltransferase 1. J Biol Chem. 2009 Jan 13; doi: 10.1074/jbc.M806193200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Greuet J, Pichard L, Ourlin JC, Bonfils C, Domergue J, Le TP, et al. Effect of cell density and epidermal growth factor on the inducible expression of CYP3A and CYP1A genes in human hepatocytes in primary culture. Hepatology. 1997 May;25(5):1166–75. doi: 10.1002/hep.510250520. [DOI] [PubMed] [Google Scholar]
- (79).Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997 Apr 4;276(5309):60–6. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- (80).Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006 Feb;43(2 Suppl 1):S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- (81).Fausto N, Laird AD, Webber EM. Liver regeneration. 2. Role of growth factors and cytokines in hepatic regeneration. FASEB J. 1995 Dec;9(15):1527–36. doi: 10.1096/fasebj.9.15.8529831. [DOI] [PubMed] [Google Scholar]
- (82).Galun E, Axelrod JH. The role of cytokines in liver failure and regeneration: potential new molecular therapies. Biochim Biophys Acta. 2002 Nov 11;1592(3):345–58. doi: 10.1016/s0167-4889(02)00326-9. [DOI] [PubMed] [Google Scholar]
- (83).Jackson LN, Larson SD, Silva SR, Rychahou PG, Chen LA, Qiu S, et al. PI3K/Akt activation is critical for early hepatic regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2008 Jun;294(6):G1401–G1410. doi: 10.1152/ajpgi.00062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Sahin F, Kannangai R, Adegbola O, Wang J, Su G, Torbenson M. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res. 2004 Dec 15;10(24):8421–5. doi: 10.1158/1078-0432.CCR-04-0941. [DOI] [PubMed] [Google Scholar]
- (85).Ostrowski J, Woszczynski M, Kowalczyk P, Wocial T, Hennig E, Trzeciak L, et al. Increased activity of MAP, p70S6 and p90rs kinases is associated with AP-1 activation in spontaneous liver tumours, but not in adjacent tissue in mice. Br J Cancer. 2000 Mar;82(5):1041–50. doi: 10.1054/bjoc.1999.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Dai G, He L, Bu P, Wan YJ. Pregnane X receptor is essential for normal progression of liver regeneration. Hepatology. 2008 Apr;47(4):1277–87. doi: 10.1002/hep.22129. [DOI] [PubMed] [Google Scholar]
- (87).Van Der Heide LP, Hoekman MF, Smidt MP. The ins and outs of FoxO shuttling: mechanisms of FoxO translocation and transcriptional regulation. Biochem J. 2004 Jun 1;380(Pt 2):297–309. doi: 10.1042/BJ20040167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Tang ED, Nunez G, Barr FG, Guan KL. Negative regulation of the forkhead transcription factor FKHR by Akt. J Biol Chem. 1999 Jun 11;274(24):16741–6. doi: 10.1074/jbc.274.24.16741. [DOI] [PubMed] [Google Scholar]
- (89).Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002 May;109(9):1125–31. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Muntane-Relat J, Ourlin JC, Domergue J, Maurel P. Differential effects of cytokines on the inducible expression of CYP1A1, CYP1A2, and CYP3A4 in human hepatocytes in primary culture. Hepatology. 1995 Oct;22(4 Pt 1):1143–53. [PubMed] [Google Scholar]
- (91).Teng S, Piquette-Miller M. The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. J Pharmacol Exp Ther. 2005 Feb;312(2):841–8. doi: 10.1124/jpet.104.076141. [DOI] [PubMed] [Google Scholar]
- (92).Pascussi JM, Gerbal-Chaloin S, Pichard-Garcia L, Daujat M, Fabre JM, Maurel P, et al. Interleukin-6 negatively regulates the expression of pregnane X receptor and constitutively activated receptor in primary human hepatocytes. Biochem Biophys Res Commun. 2000 Aug 11;274(3):707–13. doi: 10.1006/bbrc.2000.3219. [DOI] [PubMed] [Google Scholar]
- (93).Beigneux AP, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Reduction in cytochrome P-450 enzyme expression is associated with repression of CAR (constitutive androstane receptor) and PXR (pregnane X receptor) in mouse liver during the acute phase response. Biochem Biophys Res Commun. 2002 Apr 26;293(1):145–9. doi: 10.1016/S0006-291X(02)00196-1. [DOI] [PubMed] [Google Scholar]
- (94).Xu DX, Wei W, Sun MF, Wu CY, Wang JP, Wei LZ, et al. Kupffer cells and reactive oxygen species partially mediate lipopolysaccharide-induced downregulation of nuclear receptor pregnane x receptor and its target gene CYP3a in mouse liver. Free Radic Biol Med. 2004 Jul 1;37(1):10–22. doi: 10.1016/j.freeradbiomed.2004.03.021. [DOI] [PubMed] [Google Scholar]
- (95).Fang C, Yoon S, Tindberg N, Jarvelainen HA, Lindros KO, Ingelman-Sundberg M. Hepatic expression of multiple acute phase proteins and down-regulation of nuclear receptors after acute endotoxin exposure. Biochem Pharmacol. 2004 Apr 1;67(7):1389–97. doi: 10.1016/j.bcp.2003.12.012. [DOI] [PubMed] [Google Scholar]
- (96).Kalitsky-Szirtes J, Shayeganpour A, Brocks DR, Piquette-Miller M. Suppression of drug-metabolizing enzymes and efflux transporters in the intestine of endotoxin-treated rats. Drug Metab Dispos. 2004 Jan;32(1):20–7. doi: 10.1124/dmd.32.1.20. [DOI] [PubMed] [Google Scholar]
- (97).Langmann T, Moehle C, Mauerer R, Scharl M, Liebisch G, Zahn A, et al. Loss of detoxification in inflammatory bowel disease: dysregulation of pregnane X receptor target genes. Gastroenterology. 2004 Jul;127(1):26–40. doi: 10.1053/j.gastro.2004.04.019. ·The authors discover the association between PXR and inflammatory bowel disease. [DOI] [PubMed] [Google Scholar]
- (98).Iizasa H, Genda N, Kitano T, Tomita M, Nishihara K, Hayashi M, et al. Altered expression and function of P-glycoprotein in dextran sodium sulfate-induced colitis in mice. J Pharm Sci. 2003 Mar;92(3):569–76. doi: 10.1002/jps.10326. [DOI] [PubMed] [Google Scholar]
- (99).Abate A, Schroder H. Protease inhibitors protect macrophages from lipopolysaccharide-induced cytotoxicity: possible role for NF-kappaB. Life Sci. 1998;62(12):1081–8. doi: 10.1016/s0024-3205(98)00031-9. [DOI] [PubMed] [Google Scholar]
- (100).Lotem J, Sachs L. Differential suppression by protease inhibitors and cytokines of apoptosis induced by wild-type p53 and cytotoxic agents. Proc Natl Acad Sci U S A. 1996 Oct 29;93(22):12507–12. doi: 10.1073/pnas.93.22.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (101).Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004 Oct;5(10):836–47. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- (102).Bankey P, Carlson A, Ortiz M, Singh R, Cerra F. Tumor necrosis factor production by Kupffer cells requires protein kinase C activation. J Surg Res. 1990 Sep;49(3):256–61. doi: 10.1016/0022-4804(90)90130-t. [DOI] [PubMed] [Google Scholar]
- (103).Sayeed MM. Alterations in calcium signaling and cellular responses in septic injury. New Horiz. 1996 Feb;4(1):72–86. [PubMed] [Google Scholar]
- (104).bdel-Razzak Z, Loyer P, Fautrel A, Gautier JC, Corcos L, Turlin B, et al. Cytokines down-regulate expression of major cytochrome P-450 enzymes in adult human hepatocytes in primary culture. Mol Pharmacol. 1993 Oct;44(4):707–15. [PubMed] [Google Scholar]
- (105).Thummel KE, Schenkman JB. Effects of testosterone and growth hormone treatment on hepatic microsomal P450 expression in the diabetic rat. Mol Pharmacol. 1990 Jan;37(1):119–29. [PubMed] [Google Scholar]
- (106).Yamazoe Y, Murayama N, Shimada M, Yamauchi K, Kato R. Cytochrome P450 in livers of diabetic rats: regulation by growth hormone and insulin. Arch Biochem Biophys. 1989 Feb 1;268(2):567–75. doi: 10.1016/0003-9861(89)90324-x. [DOI] [PubMed] [Google Scholar]
- (107).Sotaniemi EA, Pelkonen O, Arranto AJ, Tapanainen P, Rautio A, Pasanen M. Diabetes and elimination of antipyrine in man: an analysis of 298 patients classified by type of diabetes, age, sex, duration of disease and liver involvement. Pharmacol Toxicol. 2002 Mar;90(3):155–60. doi: 10.1034/j.1600-0773.2002.900308.x. [DOI] [PubMed] [Google Scholar]
- (108).Sidhu JS, Omiecinski CJ. Insulin-mediated modulation of cytochrome P450 gene induction profiles in primary rat hepatocyte cultures. J Biochem Mol Toxicol. 1999;13(1):1–9. doi: 10.1002/(sici)1099-0461(1999)13:1<1::aid-jbt1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- (109).Yoshida Y, Kimura N, Oda H, Kakinuma A. Insulin suppresses the induction of CYP2B1 and CYP2B2 gene expression by phenobarbital in adult rat cultured hepatocytes. Biochem Biophys Res Commun. 1996 Dec 4;229(1):182–8. doi: 10.1006/bbrc.1996.1777. [DOI] [PubMed] [Google Scholar]