The thalassaemias are some of the most common genetic disorders worldwide and, wherever they occur, they constitute a major problem for patients, health providers and the society. The term β-thalassemia denotes a significant shortage or even complete absence of b globin chains resulting from the decreased or absent function of one (heterozygous carrier) or both β genes (homozygous form when the molecular defects are similar or compound heterozygotes when the molecular defects are different). The latter conditions result in an excess of a-chains which continue to be synthesised normally but cannot remain in solution; instead, they precipitate intracellularly causing premature erythroid cell death (ineffective erythropoiesis in the marrow and severe haemolysis in the peripheral blood). The end result is severe anaemia (thalassaemia major or Cooley's anaemia) and the patients need to be transfused for life.
The incidence of thalassaemia varies greatly across the world; it is definitely higher in Mediterranean countries and the near Middle East but the disease is also present in India, Thailand and Southern China, where thousands of patients are surviving.
The severity of the disease varies according to the underlying mutations. As a rule, it is milder in cases in which the defect allows some β-chain synthesis (β +) and more severe when β-chain synthesis is completely abolished (β0). The ability of the patients to compensate for the shortage of β-chains by reverting to γ-chain synthesis (formation of HbF), the simultaneous presence of α-thalassaemia, and other factors may also influence the severity of the phenotype1.
Untreated patients with bone marrow changes due to increased erythropoiesis have a characteristic "chipmunk" face and often growth retardation. The skin may have a peculiar copper colour from pallor, jaundice and melanin deposition. Hepatosplenomegaly is common. The disease, if left untreated, is uniformly fatal in childhood. Patients usually require regular blood transfusions to survive beyond the second decade of life. This intervention prolongs survival2, but the chronic administration of large amounts of blood combined with extravascular haemolysis and an increase in the intestinal absorption of iron inevitably leads - despite chelation therapy -to significant haemosiderosis of all organs, including the heart. Although iron deposition can affect seriously all body organs (endocrine glands, liver, etc.), cardiac complications, such as heart failure and arrhythmias, are the major causes of death in these patients3,4.
Although β-thalassaemia major is traditionally considered as an iron storage disease, it is not a simple haemochromatosis, but a combination of chronic haemolytic anaemia, iron storage disease and myopericarditis, probably related to the high incidence of infections due to abnormalities of the immune system5. Myocarditis has been reported to be one of the possible factors in the development of heart failure in thalassaemic patients6.
Heart failure and arrhythmias are the major causes of death in these patients. Iron cardiomyopathy is reversible, if chelation is started in time, but the diagnosis is often delayed due to the late appearance of symptoms and echocardiographic abnormalities. Once heart failure develops, the prognosis is poor with fast deterioration and death, despite intensive chelation. Conventional chelation treatment with subcutaneous desferrioxamine does not prevent cardiac iron deposition in two-thirds of patients, placing them at risk of heart failure. Additionally, desferrioxamine may cause skin reactions at the injection site or neurological side effects, particularly visual and auditory ones. Oral deferiprone is more effective than desferrioxamine in removing myocardial iron7. Many clinical trials have suggested that there is an increase in the T2* heart signal during deferiprone treatment although there were some limitations in these studies8–13.
Bone marrow transplantation is the only potentially complete cure for β-thalassaemia, but its use is limited to selected patients. Although it carries some risk of death due to the procedure itself, and in some patients the thalassaemic cells regrow, displacing the graft, it plays an important role in treatment. After a successful bone marrow transplant thalassaemic patients achieve normal haemoglobin concentrations using the donor bone marrow and no longer require transfusions. However, if transplantation is performed in patients with advanced disease, the correction of the thalassaemic defect is not sufficient, because they still have a degree of organ iron overload and dysfunction, acquired during the pre-transplantation years. According to recent data, serum ferritin and unbound iron binding capacity (UIBC) were moderately abnormal 7 years after transplantation, in a group of patients with moderate iron overload, and highly abnormal in a group of patients with marked iron overload14. These findings confirm the presence of iron overload at the time of transplantation and support the need for iron depletion treatment after the transplant. There is a great concern about persistent long-term iron overload in the liver, because it increases the risk of fibrosis, cirrhosis and hepatoma in these patients. However, hepatitis C virus infection is a more important determinant of fibrosis, cirrhosis and hepatoma than is persistent long-term hepatic iron overload15.
Long-term myocardial iron overload can contribute to cardiomyopathy and heart failure. It is, therefore, imperative to document myocardial iron deposition precisely in order to be able to apply targeted treatment. Using clinical criteria, it is impossible to predict, at an early stage, which patients are at high risk of dying from iron-related heart failure. Many indirect indices such as serum ferritin, liver biopsy, electrocardiographic and echocardiographic findings have been proposed in the past. The measurement of plasma ferritin provides an indirect estimate of total body iron stores, but the usefulness of this measurement is limited by many common clinical conditions such as inflammation, fever, and liver disease16; furthermore, it does not reflect myocardial iron overload17.
Tissue iron content measured in liver biopsies generally represents total body iron load but does not reflect myocardial iron deposition, which usually takes place later and to a lesser degree compared to that in the liver17. Additionally, liver biopsy is an invasive procedure, which cannot be repeated for routine follow-up. Some studies have suggested that maintaining serum ferritin levels below 2500 mg/L decreases the risk of cardiac death in thalassaemic patients18, but many patients with ferritin below this level have died from heart failure. Echocardiographic signs are late indicators of heart involvement in β-thalassaemia, revealing the cases in which impaired heart function is already present19. Furthermore, hyper-density on computed tomography scanss not specific for iron20.
The superconducting quantum interference device (SQUID) is an instrument that has been used in clinical studies for the non-invasive measurement of tissue iron stores, but the complexity, cost and technical demands of the technique have restricted clinical access to the method. Additionally, it can detect only liver and cardiac iron deposition21. There is clearly a need for a non-invasive, easily reproducible index, capable of accurate detection of iron in an individual organ and in an individual patient. This would help to relate iron deposition to other clinical findings and to evaluate the effectiveness of different chelation protocols.
Magnetic resonance imaging (MRI) uses the magnetic properties of the human body to provide pictures of any tissue. Hydrogen nuclei in water and lipid molecules are a principal constituent of body tissues. A hydrogen nucleus produces a dipole moment (magnetic field) that can interact with an external magnetic field. MRI machines generate a strong, homogeneous magnetic field using a large magnet made by passing an electric field through superconducting coils of wire. Patients placed in a horizontal cylinder are exposed to the magnetic field. Hydrogen nuclei in the body, which normally have randomly oriented spins, align in a direction parallel to the magnetic field. The MRI machine applies short electromagnetic pulses at a specific radiofrequency. The hydrogen nuclei absorb the radiofrequency energy and precess away from equilibrium. When the radiofrequency pulse is turned off, the precessing nuclei release the absorbed energy and return to normal. The strength of the signal varies, depending on the applied radiofrequency magnetic fields. A tissue examined returns to normal in the longitudinal plane over a characteristic interval called the T1 relaxation time. In the transverse plane, the return to normal occurs over a characteristic interval called the T2 relaxation time. These values may also be expressed as relaxation rates, R1 (1/T1) and R2 (1/T2).
Using MRI, tissue iron is detected indirectly by the effects on relaxation times of ferritin and haemosiderin iron interacting with hydrogen nuclei. The presence of iron in the human body results in marked alterations of tissue relaxation times22–25. While T1 decreases only moderately, T2 demonstrates a substantial decrease26– 29.
Myocardial T2 in experimental animals has been shown to be inversely correlated with myocardial iron content30. In a study by our group in which myocardial T2 was compared with iron content in heart biopsies, an agreement was found between the myocardial biopsy and MRI results31. Unfortunately, the magnetic resonance signal is affected by multiple acquisition variables. Although T2 is relatively independent of field strength, there is an exception in the case of iron overload. In these patients, there is a linear dependence of T2 relaxivity (1/T2) on field strength26. Most reports have measured T2 at relatively lower magnetic fields of 0.5T, where the field effect is less14. Using 1.5T, the T2 relaxation time was not measurable in heavily iron overloaded patients, because signal intensity approximated to background noise32.
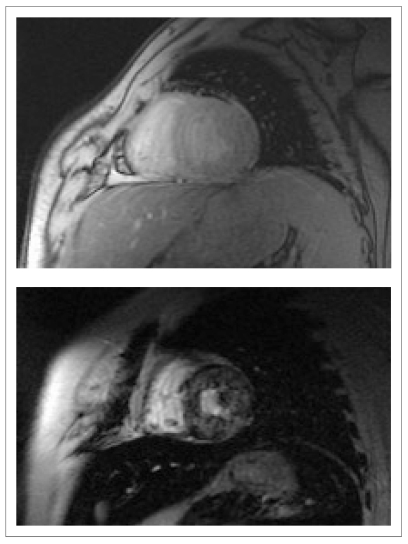
Anderson et al. reported on a new reproducible, non-invasive method for measuring liver and cardiac iron deposition, using a "T2-star" technique. A significant curvilinear, inverse correlation between iron concentration measured by biopsy and liver T2* was found33. In this study myocardial T2* was measured using a single short axis mid-ventricular slice in a 1.5T system (Figure 1).
Figure 1.
T2* image of a patient with b-thalassemia and low iron overload (above) and high iron overload (below).
Myocardial iron deposition can be reproducibly quantified using T2*. This is the most significant variable for predicting a requirement for targeted treatment of myocardial iron overload and it cannot be replaced by serum ferritin, liver iron or any other measurement33. Excellent T2* reproducibility between scanners produced by two different manufacturers supports the feasibility of widespread implementation of the technique 34. Comparing the single-breath-hold technique, used by Anderson, with the multi-echo technique for T2* measurement a close correlation was found35. This index has been successfully used for evaluation of patients taking oral deferiprone, which seems more effective than desferrioxamine in removing myocardial iron7. Myocardial T2* seems to be the most sensitive and easily reproducible index of myocardial iron deposition currently available. Ramazzotti et al. recently applied standardised T2* mapping of a normal human heart to correct T2* segmental artefacts. They also used the same technique in patients with thalassaemia intermedia and thalassaemia major with myocardial iron overload and fibrosis36. Additionally, using a multislice multiecho T2* approach it is possible to extend myocardial iron evaluation from the mid-ventricular septum to the whole left ventricle. This is important because histological and MRI studies have previously demonstrated that myocardial iron distribution is heterogeneous. The multislice multiecho T2* approach, accounting for the heterogeneous myocardial iron distribution, has enable the identification of three groups of patients (homogeneous, heterogeneous, and no myocardial iron overload) with statistically different serum ferritin levels and liver iron concentrations37,38. The T2* technique was also successfully used for the evaluation of different chelation protocols. In comparison to standard chelation monotherapy with deferoxamine, combination treatment with additional deferiprone reduced myocardial iron and improved the ejection fraction and endothelial function in patients with thalassaemia major with mild to moderate cardiac iron loading39.
At present MRI provides a simple way to measure excess iron in the body, but further efforts are needed to make this technique a clinically useful diagnostic tool. Priorities in MRI should include: (i) improved understanding of the contribution of ferritin and haemosiderin iron to magnetic resonance effects; (ii) development of optimal methods for measuring relaxation times (best techniques for data acquisition, choice of field strength, selection of timing parameters, reduction of noise, identification of region of interest and selection analysis); (iii) phantom studies for calibrating and validating iron concentration detected by MRI; (iv) standardisation between different laboratories; and (v) a widely acceptable imaging protocol for iron overload evaluation.
In conclusion, MRI is useful both in the diagnosis of iron deposition in asymptomatic iron overloaded patients and in the evaluation of chelation therapy. Since MRI can evaluate atrial and ventricular function at the same time and characterise tissue, this accurate, easily reproducible, non-invasive technique patients should be of benefit in patients with iron storage diseases. Overall, MRI plays a major role in the management, follow-up and understanding of the pathophysiology of iron cardiomyopathy.
References
- 1.Bunn HF. Disorders of hemoglobin. In: Wilson, et al., editors; Mc Graw-Hill, Intern, editors. Harrison's Principles of Internal Medicine. 12th Edition. 1991. pp. 1543–52. [Google Scholar]
- 2.Zurlo MG, Stefano P, Borgna-Pignatti C, et al. Survival and causes of death in thalassemia major. Lancet. 1989;2:27–30. doi: 10.1016/s0140-6736(89)90264-x. [DOI] [PubMed] [Google Scholar]
- 3.Sankul D, Thakerngpal K, Parcharee P. Cardiac pathology in 76 thalassemic patients. Birth Defects. 1988;23:177. [PubMed] [Google Scholar]
- 4.Ohene-Frempong K, Schwartz E. Clinical features of thalassemia. Pediatr Clin North Am. 1980;27:403. doi: 10.1016/s0031-3955(16)33858-5. [DOI] [PubMed] [Google Scholar]
- 5.Dwyer J, Wood C, McNamara J, et al. Abnormalities in the immune system of children with b-thalassemia major. Clin Exp Immunol. 1987;68:621–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Kremastinos DT, Tiniakos G, Theodorakis GN, et al. Myocarditis in b-thalassemia major. A cause for heart failure. Circulation. 1995;91:66–71. doi: 10.1161/01.cir.91.1.66. [DOI] [PubMed] [Google Scholar]
- 7.Anderson L, Wonke B, Prescott E, et al. Comparison of effects of oral deferiprone and subcutaneous desferrioxamine on myocardial iron concentrations and ventricular function in b-thalassemia. Lancet. 2002;360:516–20. doi: 10.1016/s0140-6736(02)09740-4. [DOI] [PubMed] [Google Scholar]
- 8.Pepe A, Lombardi M, Positano V, et al. Evaluation of the efficacy of oral deferiprone in beta-thalassemia major by multislice multiecho T2*. Eur J Haematol. 2006;76:183–92. doi: 10.1111/j.1600-0609.2005.00587.x. [DOI] [PubMed] [Google Scholar]
- 9.Neufeld EJ. Oral chelators deferasirox and deferiprone for transfusional iron overload in thalassemia major: new data, new questions. Blood. 2006;107:3436–41. doi: 10.1182/blood-2006-02-002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galia M, Midiri M, Bartolotta V, et al. Multicenter Trial Group of the Society for the Study of Thalassemia and Haemoglobinopathies. Potential myocardial iron content evaluation by magnetic resonance imaging in thalassemia major patients treated with deferoxamine or deferiprone during a randomized multicenter prospective clinical study. Hemoglobin. 2003;27:63–76. doi: 10.1081/hem-120021538. [DOI] [PubMed] [Google Scholar]
- 11.Di Tucci AA, Matta G, Deplano S, et al. Myocardial iron overload assessment by T2* magnetic resonance imaging in adult transfusion dependent patients with acquired anemias. Haematologica. 2008;93:1385–8. doi: 10.3324/haematol.12759. [DOI] [PubMed] [Google Scholar]
- 12.Kolnagou A, Kontoghiorghes GJ. Effective combination therapy of deferiprone and deferoxamine for the rapid clearance of excess cardiac iron and the prevention of heart disease in thalassemia. The Protocol of the International Committee on Oral Chelators. Hemoglobin. 2006;30:239–49. doi: 10.1080/03630260600642567. [DOI] [PubMed] [Google Scholar]
- 13.Tanner MA, Galanello R, Dessi C, et al. Combined chelation therapy in thalassemia major for the treatment of severe myocardial siderosis with left ventricular dysfunction. J Cardiovasc Magn Reson. 2008;10:12. doi: 10.1186/1532-429X-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mavrogeni S, Gotsis ED, Berdousi E, et al. Myocardial and hepatic T2* magnetic resonance evaluation in ex-thalassemic patients after bone-marrow transplantation. Int J Cardiovasc Imaging. 2007;23:739–45. doi: 10.1007/s10554-006-9203-7. [DOI] [PubMed] [Google Scholar]
- 15.Angelucci E, Muretto P, Nicolucci, et al. Effects of iron overload and hepatitis C virus positivity in determining progression of liver fibrosis in thalassemia following bone marrow transplantation. Blood. 2002;100:17–21. doi: 10.1182/blood.v100.1.17. [DOI] [PubMed] [Google Scholar]
- 16.Crosby WH. Serum ferritin fails to indicate hemochromatosis: nothing gold can stay. N Engl J Med. 1976;294:333–4. doi: 10.1056/NEJM197602052940611. [DOI] [PubMed] [Google Scholar]
- 17.Johnston DL, Rice L, Vick W, et al. Assessment of tissue iron overload by nuclear magnetic resonance imaging. Am J Med. 1989;87:40–7. doi: 10.1016/s0002-9343(89)80481-4. [DOI] [PubMed] [Google Scholar]
- 18.Olivieri NF, Nathan DG, McMillan JH, et al. Survival in medically treated patients with homozygous b-thalassemia. N Engl J Med. 1994;331:574–8. doi: 10.1056/NEJM199409013310903. [DOI] [PubMed] [Google Scholar]
- 19.Kremastinos D, Rentoukas E, Mavrogeni S, et al. Left ventricular inflow pattern in b-thalassemia major. A Doppler echocardiographic study. Eur Heart J. 1993;14:351–7. doi: 10.1093/eurheartj/14.3.351. [DOI] [PubMed] [Google Scholar]
- 20.Guyader D, Gandon Y, Robert JY, et al. Magnetic resonance imaging and assessment of liver content in genetic hemochromatosis. J Hepatol. 1992;15:304–8. doi: 10.1016/0168-8278(92)90060-3. [DOI] [PubMed] [Google Scholar]
- 21.Brittenham GM, Farrell DE, Harris JW, et al. Magnetic-susceptibility measurement of human iron stores. N Engl J Med. 1982;307:1671–5. doi: 10.1056/NEJM198212303072703. [DOI] [PubMed] [Google Scholar]
- 22.Gomori JM, Horev G, Tamary H, et al. Hepatic iron overload: quantitative MR imaging. Radiology. 1991;179:367–9. doi: 10.1148/radiology.179.2.2014276. [DOI] [PubMed] [Google Scholar]
- 23.Drayer B, Burger P, Darwin R, et al. Magnetic resonance imaging of brain iron. Am J Roentgenol. 1986;147:103–10. doi: 10.2214/ajr.147.1.103. [DOI] [PubMed] [Google Scholar]
- 24.Doyle FH, Pennock JM, Banks LM, et al. Nuclear MRI of the liver. Initial experience. Am J Roentgenol. 1982;138:193–200. doi: 10.2214/ajr.138.2.193. [DOI] [PubMed] [Google Scholar]
- 25.Berardino ME, Small W, Goldstein J, et al. Multiple NMR T2 relaxation values in human liver tissue. Am J Roentgenol. 1983;141:1203–8. doi: 10.2214/ajr.141.6.1203. [DOI] [PubMed] [Google Scholar]
- 26.Brasch RC, Wesbey G, Gooding CA, Koerper M. MRI of transfusional hemosiderosis in children with thalassemia major. Radiology. 1984;15:767–71. doi: 10.1148/radiology.150.3.6695078. [DOI] [PubMed] [Google Scholar]
- 27.Vymazal J, Brooks RA, Zak O, et al. T1 and T2 of ferritin at different field strengths: effect on MRI. Magn Res Med. 1992;27:368–74. doi: 10.1002/mrm.1910270218. [DOI] [PubMed] [Google Scholar]
- 28.Stark DD, Moseley ME, Bacon BR. Magnetic resonance imaging and spectroscopy of hepatic iron overload. Radiology. 1995;154:137–42. doi: 10.1148/radiology.154.1.3964933. [DOI] [PubMed] [Google Scholar]
- 29.Gomori J, Grossman R, Drott H. MR relaxation times and iron content of thalassemic spleens: an in vitro study. Am J Roentgenol. 1988;150:567–9. doi: 10.2214/ajr.150.3.567. [DOI] [PubMed] [Google Scholar]
- 30.Liu P, Henkelman M, Joshi J, et al. Quantification of myocardial tissue iron contents using NMR relaxation. Validation in a novel murine thalassemia model. JACC. 1992;19:187A. abstract 76. [Google Scholar]
- 31.Mavrogeni S, Markussis V, Kaklamanis L, et al. A comparison of magnetic resonance and cardiac biopsy in the evaluation of heart iron in patients with b-Thalassemia. Eur J Haematol. 2005;75:241–7. doi: 10.1111/j.1600-0609.2005.00474.x. [DOI] [PubMed] [Google Scholar]
- 32.Mavrogeni S, Gotsis E, Markussis V, et al. T2 relaxation time study of iron overload in b-thalassemia. MAGMA. 1998;6:7–12. doi: 10.1007/BF02662506. [DOI] [PubMed] [Google Scholar]
- 33.Anderson LJ, Holden S, Davis B, et al. Cardiovascular T2-star magnetic resonance for the early diagnosis of myocardial iron overload. Eur Heart J. 2001;22:2171–9. doi: 10.1053/euhj.2001.2822. [DOI] [PubMed] [Google Scholar]
- 34.Westwood MA, Anderson LJ, Firmin DN, et al. Interscanner reproducibility of cardiovascular magnetic resonance T2* measurements of tissue iron in thalassemia. J Magn Reson Imaging. 2003;18:616–20. doi: 10.1002/jmri.10396. [DOI] [PubMed] [Google Scholar]
- 35.Westwood M, Anderson LJ, Firmin DN, et al. A single breath-hold multiecho T2* cardiovascular magnetic resonance technique for diagnosis of myocardial iron overload. J Magn Reson Imaging. 2003;18:33–9. doi: 10.1002/jmri.10332. [DOI] [PubMed] [Google Scholar]
- 36.Ramazzotti A, Pepe A, Positano V, et al. Standardized T2* map of a normal human heart to correct T2* segmental artefacts; myocardial iron overload and fibrosis in thalassemia intermedia versus thalassemia major patients and electrocardiogram changes in thalassemia major patients. Hemoglobin. 2008;32:97–107. doi: 10.1080/03630260701879514. [DOI] [PubMed] [Google Scholar]
- 37.Pepe A, Positano V, Santarelli MF, et al. Multislice multiecho T2* cardiovascular magnetic resonance for detection of the heterogeneous distribution of myocardial iron overload. J Magn Reson Imaging. 2006;23:662–8. doi: 10.1002/jmri.20566. [DOI] [PubMed] [Google Scholar]
- 38.Positano V, Pepe A, Santarelli MF, et al. Standardized T2* map of normal human heart in vivo to correct T2* segmental artefacts. NMR Biomed. 2007;20:578–90. doi: 10.1002/nbm.1121. [DOI] [PubMed] [Google Scholar]
- 39.Tanner MA, Galanello R, Dessi C, et al. A randomized, placebo-controlled, double-blind trial of the effect of combined therapy with deferoxamine and deferiprone on myocardial iron in thalassemia major using cardiovascular magnetic resonance. Circulation. 2007;115:1876–84. doi: 10.1161/CIRCULATIONAHA.106.648790. [DOI] [PubMed] [Google Scholar]