Abstract
Skeletal muscle is electrically anisotropic, with a tendency for applied electrical current to flow more readily along muscle fibers than across them. In this study, we assessed a method for non-invasive measurement of anisotropy to determine its potential to serve as a new technique for distinguishing neurogenic from myopathic disease. Measurements were made on the biceps brachii and tibialis anterior muscles in 15 normal subjects and 12 patients with neuromuscular disease (6 with amyotrophic lateral sclerosis and 6 with various myopathies) using 50 kHz applied current. Consistent multi-angle anisotropic patterns were found for reactance and phase in both muscles in normal subjects. Normalized anisotropy differences for each subject were defined, and group average values identified. The amyotrophic lateral sclerosis (ALS) patients demonstrated increased and distorted anisotropy patterns, whereas myopathic patients demonstrated normal or reduced anisotropy. These results suggest that non-invasive measurement of muscle anisotropy has potential for diagnosis of neuromuscular diseases.
Keywords: angle, anisotropy, current flow, electrical impedance, electrode, muscle
Electrical impedance myography (EIM) is a non-invasive method of muscle evaluation that relies on application of high-frequency, low-intensity alternating electrical current to a limb and measurement of the consequent surface voltages over a muscle or muscle group of interest.10,13 Abnormalities in muscle fiber structure and muscle membrane health are reflected in the impedance changes measured in this way.5,10,15 The major parameters measured include resistance (R) and reactance (X), and, from these, the phase (θ) of the tissue can be calculated. Although R and X are both potentially valuable variables in their own right, the phase tends to help account for size and shape effects and has thus been the focus of much of our EIM work to date. Indeed, phase declines in both localized and generalized neuromuscular disorders and has the potential to serve as a new approach for assessing disease severity. 5,10,11,15
EIM measurements are influenced by two distinct aspects of electrical conduction of muscle: (1) the properties of individual fibers, in particular via the capacitance of their membranes; and (2) the high degree of columnar order in the arrangement of the fibers. Indeed, the tightly bundled fiber structure of muscle confers an important additional feature accessible to EIM: the characteristic that the ease of electrical current flow varies according to its angle relative to the muscle fibers.1,2,4,8 This directional dependence of the impedance to current flow, termed anisotropy, impacts the three major EIM variables, X, R, and θ. Whereas the electrical anisotropy of muscle has been appreciated for decades, to our knowledge its potential measurement in the assessment of neuromuscular disorders has only recently been considered.3 Both muscle and nerve disorders might be expected to produce substantially different effects. The columnar structure is more disrupted in myopathic states, in contrast to a relative preservation of structure in neurogenic states. In principal then, direct measurement of anisotropy has the potential to become a new tool for assessment of neuromuscular disease.
We recently studied the anisotropy of the tibialis anterior muscle in a group of normal subjects and demonstrated that, for current flow parallel and perpendicular to the major muscle fiber direction, clear anisotropy could be identified at a variety of frequencies. Certain electrode sizes and ratios were most sensitive to this parameter.3 However, in order to make the technique more powerful and accurate for clinical use, it will likely be necessary to assess a variety of intermediate angles, because small inconsistencies in electrode position with muscle fiber alignment may lead to inaccuracies in the measured anisotropy. Thus, here we examine the anisotropic characteristics of the biceps brachii and tibialis anterior muscles in 15 normal subjects, at multiple angles relative to the major muscle fiber direction. In addition, 12 individuals with established neuro-muscular disease, 6 with amyotrophic lateral sclerosis (ALS) and 6 with myopathy, were also studied to assess the potential for anisotropy measurements to provide diagnostic information.
METHODS
Subjects
Fifteen normal healthy subjects (8 men and 7 women, mean age 53.7 years, range 30–79 years) participated in this study. None of the subjects had a history of neuromuscular disease affecting the limbs to be studied, including radicular symptoms. On examination, all of them had normal strength, sensation, and reflexes. In addition, 12 patients with neuromuscular disease, 6 with ALS and 6 with various myopathies (see Table 1 for patient demographics), were studied. All patients had extensive needle electromyography (EMG) during their initial evaluation to help confirm their respective diagnoses. All ALS patients had prominent evidence of neurogenic change, and the myopathy patients had myopathic change in several muscles from multiple body regions. The protocol was approved by the institutional review board of the Beth Israel Deaconess Medical Center, and all participants signed a hospital-approved informed consent form.
Table 1.
Neuromuscular disease patient demographics.
| Patient number |
Age (y) |
Gender | Diagnosis | MRC score of tibialis anterior studied with EIM |
Approximate mean MMT score for all muscles examined |
Disease duration (y) |
Weakest muscles |
|---|---|---|---|---|---|---|---|
| 1 | 40 | M | ALS | 4 | 3+ | 4 | Bilateral biceps |
| 2 | 54 | F | ALS | 0 | 4 | 2 | Bilateral tibialis anterior |
| 3 | 73 | M | ALS | 4 | 4 | 3 | Bilateral tibialis anterior |
| 4 | 75 | F | ALS | 0 | 4+ | 4 | Ipsilateral tibialis anterior |
| 5 | 76 | M | ALS | 3 | 4− | 3 | Ipsilateral tibialis anterior |
| 6 | 79 | F | ALS | 2 | 4 | 2 | Bilateral tibialis anterior |
| 7 | 32 | M | Dysferlin myopathy | 2 | 3 | 9 | Bilateral tibialis anterior |
| 8 | 44 | M | HIV-associated IBM | 5 | 4+ | 12 | Ipsilateral quadriceps |
| 9 | 44 | M | FSHD | 4+ | 4 | 28 | Bilateral biceps |
| 10 | 58 | M | s-IBM | 5− | 4+ | 4 | Bilateral triceps |
| 11 | 70 | F | s-IBM | 5− | 4+ | 1 | Bilateral finger flexors |
| 12 | 83 | F | s-IBM | 4− | 4− | 26 | Ipsilateral quadriceps |
ALS, amyotrophic lateral sclerosis; MRC, Medical Research Council; MMT,;HIV, human immunodeficiency virus; s-IBM, sporadic inclusion-body myositis; FSHD, fascioscapulohumeral muscular dystrophy.
EIM Measurement Equipment
Four-electrode (tetrapolar) impedance measurements were made using a wideband lock-in amplifier (Signal Recovery Model 7280; Advanced Measurement Technology, Inc., Oak Ridge, Tennessee), as described elsewhere.5 Subject current was drawn from the 1–V reference output channel, and the frequency was set at 50 kHz. Surface voltages were acquired using a very-low-capacitance probe (Model P6243; Tektronix, Beaverton, Oregon) at the input of the impedance instrument, which converted them into digital signals proportional to the resistance (R) and the reactance (X). The resulting data were stored in a standard computer (HP Pavilion a250n; Hewlett Packard, Palo Alto, California) through which additional data analysis was performed.
Electrode Placement and Measurement
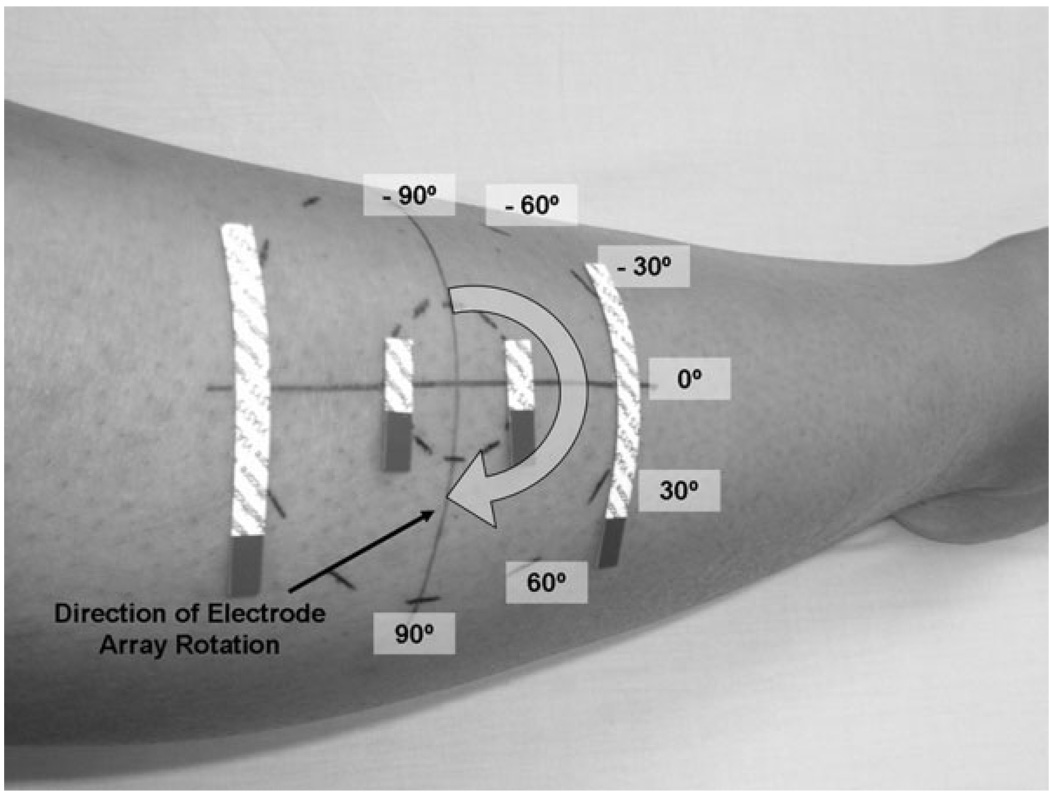
The electrodes used were 5.5-mm-wide × 9-cm-long adhesive Ag/AgCl strips (part no. 019-766400; Viasys Health-care/Nicolet Biomedical, Madison, Wisconsin). For current injection, the electrodes were cut to one-half length (4.5 cm), and for voltage measurement to one-eighth length (~1.13 cm). These were arranged in an array of four parallel segments, 2.5 cm apart, centered on the array axis, in standard tetrapolar order (Fig. 1).
FIGURE 1.
Technique for measuring skeletal muscle using the tibialis anterior. The electrode array is placed at multiple angles ranging from −90° to +90° (relative to the primary muscle fiber direction). The array here is shown at 0°. Current is applied via the outer electrodes, and the voltages are measured via the inner electrodes.
The tibialis anterior and biceps brachii were chosen for study because these two muscles are relatively accessible (i.e., superficial and sufficiently large) and are assessed commonly during neuromuscular disease evaluation. For measurements on tibialis anterior, the major muscle fiber direction was identified by drawing a reference line from a point just medial to the fibular head to a point just lateral to the medial malleolus. A point approximately one-third of the way down from the fibular head was chosen as the center of rotation for the electrode array and marked with an indelible marker. Using a stencil, additional marks were placed on the skin at 30° intervals starting from the reference line, ϕ = 0° (parallel to the major muscle fiber direction). The first measurements were made at ϕ = −90°, that is, the current direction perpendicular to the muscle fibers, after which all four electrodes were replaced and a fresh array positioned at ϕ = −60°. The procedure was then repeated at 30° intervals until 180° was subtended. The final set of measurements was made at ϕ = 90°. All measurements were made with the patient supine with a bolster placed under the knees for comfort. The entire procedure for a single muscle took about 20 minutes to perform.
The measurements for biceps were made in a similar fashion, with the center of the muscle being located by palpation at a point approximately one-third the distance up from the antecubital fossa toward the acromion. Measurements were made with the patient supine, arm slightly abducted, and the forearm supinated.
Data Analysis
Resistance (R) and reactance (X) were measured, and the phase was calculated using the relationship, θ = arctan (X/R). Each variable (± standard deviation) was plotted against the angle ϕ. Because R and X at a given angle may vary considerably among subjects, a normalization procedure was incorporated. For each subject, the average R and X were calculated from the 7 values obtained in the angular sweep. The individual R and X values for each ϕ were then divided by the relevant average, and the resulting “normalized” values, Rn(ϕ), Xn(ϕ), θn(ϕ) (± standard deviation), were plotted against ϕ. In an attempt to summarize the anisotropy in a single variable, the anisotropy difference (AD) was defined as the impedance data obtained at ϕ = 0° subtracted from the average of that obtained at = 90° and −90° and calculating intraclass correlation coefficients. Also, internal consistency of the measurements for each subject was assessed by comparing data for 90° and −90°. These values should be the same, because the two electrode placements are reversed but otherwise identical. For the 12 neuromuscular disease patients, the data were processed in a similar fashion, although, given the substantial distortion of the normal anisotropy pattern, the AD was calculated using the maximum and minimum values for each patient. Reproducibility was assessed by repeating the entire set of measurements in one patient. Comparisons between the myopathy and ALS patients were made using the Mann–Whitney U-test for continuous variables and Fisher’s exact test for categorical variables (two-tailed).
RESULTS
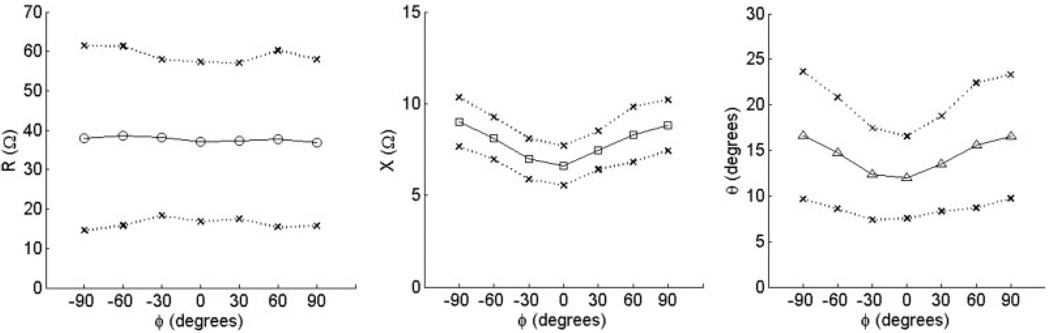
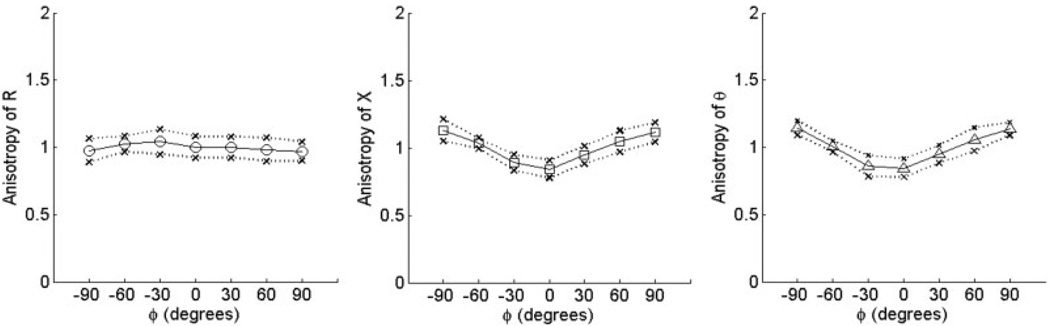
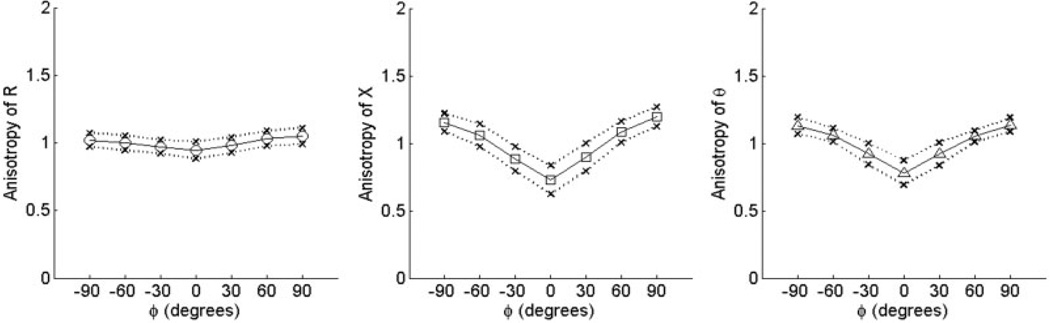
Values of R(phgr;), X(ϕ), and θ(ϕ) averaged over the 15 normal subjects are shown for biceps and tibialis anterior in Figure 2 and Figure 3, respectively. The outer points of the figures represent 1 standard deviation from the mean at each angle. As can be seen from both of these figures, the spread in raw resistance values among the subjects is considerably greater than that in reactance, and it is greater for biceps brachii than for tibialis anterior. Using the normalization procedure just described, however, the data become remarkably consistent across individuals and even between the two muscles (Fig. 4 and Fig. 5). For both reactance and phase at 90°, the values lie between 1.1 and 1.2, and at 0° are approximately 0.8, giving ADs ranging from 0.29 to 0.46 (see Table 2). Although there was evidence for weak anisotropy in Rn in some cases, very little survived averaging across the 15 subjects. The mean values of AD for RAD fell close to zero for both muscles.
FIGURE 2.
Raw anisotropy plots for the 15 subjects in a study of biceps brachii. Data show mean values ±1 standard deviation for R, X, and θ vs. angle ϕ.
FIGURE 3.
Raw anisotropy plots for tibialis anterior. Data show mean values ±1 standard deviation for R, X, and θ vs. angle ϕ.
FIGURE 4.
Normalized anisotropy plots for biceps. Data show mean values ±1 standard deviation for Rn, Xn, and θn vs. angle ϕ.
FIGURE 5.
Normalized anisotropy plots for tibialis anterior. Data show mean values ±1 standard deviation for Rn, Xn, and θn vs. angle ϕ. Note the similarity between these curves and those for biceps (Fig. 4).
Table 2.
Summary of data for normalized values (± standard deviation) for normal subjects.
| Biceps brachii | Tibialis anterior | |||||
|---|---|---|---|---|---|---|
| Resistance (Rn) | Reactance (Xn) | Phase (θn) | Resistance (Rn) | Reactance (Xn) | Phase (θn) | |
| 90° | 0.98 ± 0.076 | 1.13 ± 0.075 | 1.14 ± 0.051 | 1.05 ± 0.060 | 1.20 ± 0.073 | 1.14 ± 0.053 |
| 0° | 1.00 ± 0.075 | 0.84 ± 0.063 | 0.85 ± 0.067 | 0.94 ± 0.063 | 0.73 ± 0.11 | 0.78 ± 0.091 |
| AD | −0.015 ± 0.13 | 0.29 ± 0.12 | 0.29 ± 0.090 | 0.11 ± 0.11 | 0.46 ± 0.17 | 0.36 ± 0.13 |
As these measurements are calculated via subtracting ratios, the values are without unit designation. AD, anistrophy difference.
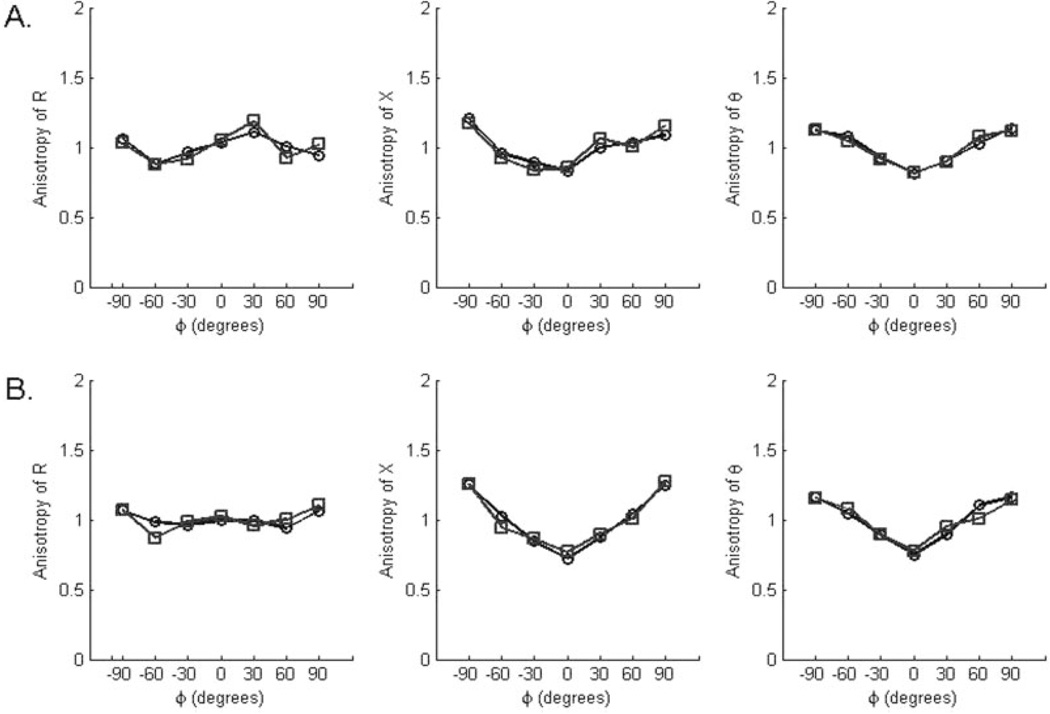
To assess reproducibility, intraclass correlation coefficients were calculated for the +90° and −90° measurements for each subject (because these represent identical measurements, with just the electrodes reversed). These were all quite high: biceps, 0.99 and 0.92 for R and X, respectively, and for tibialis anterior, 0.96 and 0.94, essentially demonstrating relatively strong test–retest reproducibility. In a single normal subject, the entire set of measurements was performed twice, and there was good consistency between sets of measurements (Fig. 6).
FIGURE 6.
Internal reproducibility of measurements taken from the last normal subject studied, a 21-year-old man: (A) biceps and (B) tibialis anterior. Repeat measurements were made immediately after completing the first set on each muscle.
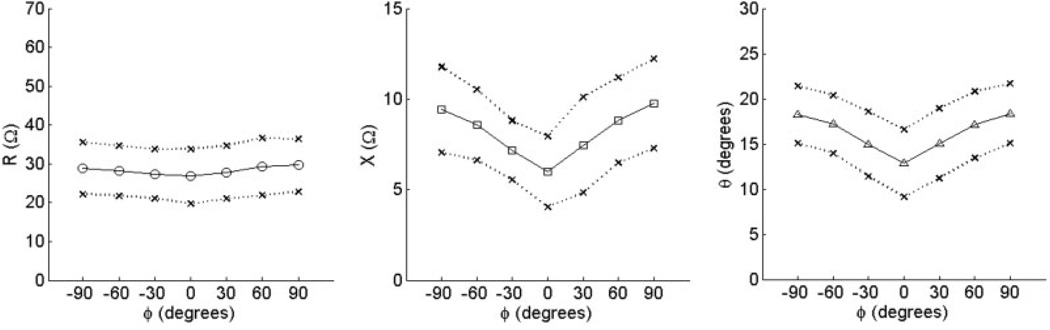
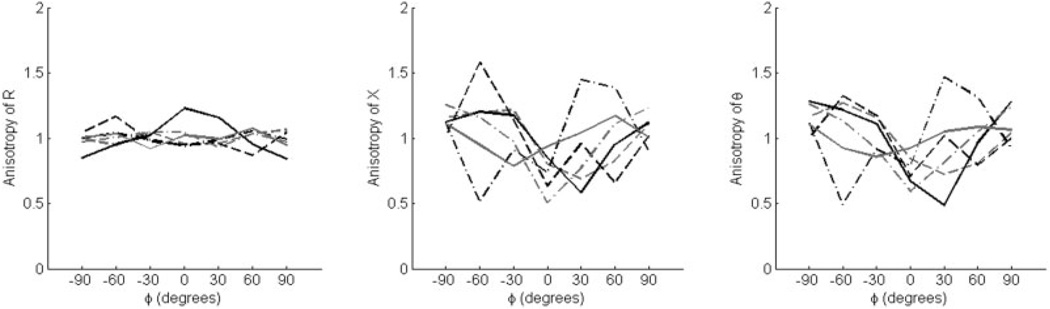
Only the tibialis anterior was studied in the ALS and myopathy patients. They were found, on average, to have relatively reduced raw phase and reactance compared with normal subjects, although this effect was more pronounced in the ALS patients. For example, the mean phase (± standard error) at 0° was 6.86 ±2.49° in the ALS patients. It was 10.51 ± 2.76° in the myopathy patients and 12.85 ± 0.96° in the normal subjects. These values also varied depending on the severity of weakness in tibialis anterior. For example, the dysferlin myopathy patient had 0/5 strength in the tibialis anterior and a very low phase of 2.18°, whereas the fascioscapulo-humeral dystrophy patient had 4+/5 tibialis anterior strength and a relatively normal value of 16.1°. We normalized the data, following the same procedure used for the normal subjects, and the results are shown in Figure 7 and Figure 8, plotted using the same scale. As can be seen, the myopathy cases show mildly reduced multi-angle anisotropy, whereas the ALS cases show markedly irregular behavior. There is evidence of increased ADs for several patients as well as a shift in the maxima and minima. Indeed, the difference between the myopathy and ALS patients in AD (Table 3) reaches significance for reactance (P = 0.015) and shows a trend for phase (P = 0.065). Similarly, the differences in the positions of the minima and maxima were also significant between the two groups. Only 1 of the 6 ALS patients had both a minimum and maximim phase at the expected ϕ = 0 and ±90°, respectively, yet all 6 myopathy patients had both (P = 0.015).
FIGURE 7.
Normalized anisotropy plots for tibialis anterior from 6 patients with ALS plotted against θ, showing the elevation and distortion of the normal anisotropic pattern in these individuals.
FIGURE 8.
Normalized anisotropy plots for tibialis anterior from 6 patients with myopathy, showing the diminution of the anisotropic pattern in these individuals.
Table 3.
Summary of anisotropy differences (± standard deviation) for ALS and myopathy patients in tibialis anterior.
| Resistance (Rn) | Reactance (Xn) | Phase (θn) | |
|---|---|---|---|
| ALS | 0.21 (0.11–0.39) | 0.69 (0.39–0.95) | 0.64 (0.25–0.98) |
| Myopathy | 0.16 (0.09–0.26) | 0.34 (0.11–0.53) | 0.32 (0.21–0.55) |
DISCUSSION
As these results reveal, the anisotropy of muscle is identifiable at multiple angles using a non-invasive technique that incorporates commercial Ag/AgCl adhesive electrodes, predetermined landmarks based on knowledge of the underlying anatomy, and standard equipment for measuring impedance. For both biceps brachii and tibialis anterior in normal subjects, reactance was at a minimum when current flow was parallel to the muscle fibers; however, for resistance, only minimal angular dependence was found in either muscle. The raw resistance, reactance, and phase values differed considerably among the normal individuals studied, and this is likely due to variability of a number of factors, including geometry of the limb, differences in skin–subcutaneous fat thickness, and variations in the muscle itself (including its vascular and connective tissue content). Nevertheless, the normalized anisotropy differences for the normal subjects fell within a relatively narrow range for all three variables, as shown in Figure 3 and Figure 4. Our assessment of the repeatability of the technique suggests relatively good intrasubject reproducibility.
Most importantly, however, our evaluation of patients suggests that this measurement approach has the potential of successfully discriminating myopathic from neurogenic disease. In this study, the ALS patients demonstrated prominent and distorted anisotropic patterns, with increased ADs for reactance and phase and shifts in the position of the maxima and minima for both variables. The myopathy patients, in contrast, demonstrated relatively “normal” anisotropic patterns, and all had low or low-normal ADs for these two parameters. Whether the phase or reactance will ultimately prove to be superior for discrimination of these diseases remains uncertain and requires further study.
The fact that muscle has marked electrical anisotropy was well established by the early 1960s. Most previous work focused on the resistivity of the tissue (or its reciprocal, the conductivity),2,4,6,7,9 and these studies have indicated that it is considerably lower for current flow in the longitudinal direction (parallel to the muscle fibers) than in the transverse direction. Measurements on exposed or excised dog muscle have given values ranging from 7 to 10 for the ratio of transverse:longitudinal resistivities.4,9 The values found in the present work are considerably smaller, but there is no contradiction. Indeed, calculations show that an actual ratio of 10:1 will yield only a 10% difference in surface-measured anisotropy. The actual values depend on the specifics of muscle size and shape and electrode shape, dimensions, and spacing.12 Simply put, the unavoidable price one must pay for the major increase in ease of measurement is a major decrease in the sensitivity of the technique to the intrinsic anisotropy. Moreover, in both the normal and diseased subjects, we found a nearly complete lack of evidence for anisotropy in the resistance, as compared to that for reactance and, consequently, the calculated phase. This is consistent with our previous bovine and human studies, both of which employed a similar adhesive electrode approach for measurement. 3,14
In our earlier human study,3 we found that a ratio of 1:1/4 for current:voltage electrode length is helpful in eliciting the anisotropy, and we incorporated that in the present study. In addition, we found the anisotropy to be maximal at frequencies of 50–150 kHz. Although 50 kHz is the lower limit of this range, we chose to use this frequency, because commercially available 50-kHz bioimpedance devices are readily available at this frequency. This will make it possible for other investigators to pursue similar investigations of electrical anisotropy in neuromuscular disease.
The influence of the subcutaneous fat layer on our results requires further discussion. Unlike endomysial fat, subcutaneous fat has the effect of causing the current to spread more widely prior to entering and upon leaving the muscle tissue. Thus, it is probable that with increasing thickness of subcutaneous fat, the measured surface anisotropy may begin to decrease. However, the fact that the normalized anisotropy among a group of healthy individuals remains so small in two different muscles suggests that this effect is likely to be minor, except in perhaps very obese subjects. Ultimately, to answer this question sufficiently we will need to perform a separate study to compare measurements of subcutaneous fat thickness on the measured anisotropy. Another potential limitation of this approach to disease assessment involves patients for whom there is some anatomical variation in the normal muscle fiber direction. In such cases, the anisotropy measurements may provide misleading data; however, given the narrow range of normal for the normalized anisotropy measurements in healthy individuals, we believe this is likely to be an infrequent problem.
The explanation as to why the anisotropy of reactance and phase in myopathy should differ from that in ALS is unclear and will require separate study. However, the pathology of the diseases is considerably different, with ALS showing grouped atrophy and myopathies showing fiber loss and more prominent fat and connective tissue infiltration. Although myopathic disorders often are thought to be patchy, the disease itself does not favor anisotropy in any specific fashion. Loss of muscle fibers and fat infiltration would not be expected to facilitate current flow in one direction or another—in fact, these occurrences could lead to a general reduction in anisotropy as the muscle fibers are replaced by isotropic tissue (fat, inflammatory cells, and connective tissue). Of course, not all myopathic diseases produce such typical changes in muscle. Some, especially metabolic myopathies, produce only very subtle disturbances in muscle fiber size and shape and are associated with minimal deposition of endomysial connective tissue or fat. In such cases, EIM may be unable to detect any abnormalities. Moreover, some chronic myopathies can develop a neurogenic appearance on electromyographic assessment, but it is unclear in this circumstance what EIM may reveal. Our anticipation is that it will still produce a reduction in anisotropy, because the typical pathological changes of myopathy will still be present. In stark contrast to most myopathies, however, in ALS and other neurogenic disorders, reinnervated groups of fibers could produce conduits of sorts that make the tissue more anisotropic when current is applied at particular angles. Nonetheless, long-standing neurogenic disorders (e.g., old polio) can be associated with myopathic change. Only through further dedicated study of such cases will we find what effect this will have on muscle anisotropy.
Further studies in anisotropy are now underway. First, we are continuing to obtain rotational data on individuals with a variety of neuromuscular disorders to assess the extent to which anisotropy can be used for the detection and classification of neuromuscular disease. In addition, we are now investigating animal models of neuromuscular disease to understand better the mechanisms of how anisotropy changes in disease states. Finally, we are in the process of developing a system capable of rapid data acquisition that is not dependent on adhesive electrodes and can provide a much finer angular resolution than that accomplished herein. Such a system may then be utilized in a similar fashion to the way needle electromyography is performed today, allowing the physician to survey a variety of muscles in an individual patient. Although this could be performed even with the approach presented in this study, at 15 minutes per muscle, it would take well over an hour to perform even a limited one-limb survey for radiculopathy. With further advances in the measurement technique and understanding of electrical anisotropy, it may be possible to put this distinctive property of muscle to effective practical use in the near future.
Acknowledgments
This study was funded by the National Institutes of Health (Grants RO1-NS42037-01A2 and RR01032) to the Beth Israel Deaconess Medical Center General Clinical Research Center.
Abbreviations
- AD
anisotropy difference
- ALS
amyotrophic lateral sclerosis
- EIM
electrical impedance myography
- EMG
electromyography
REFERENCES
- 1.Aaron R, Huang M, Shiffman CA. Anisotropy of human muscle via non-invasive impedance measurements. Phys Med Biol. 1997;42:1245–1262. doi: 10.1088/0031-9155/42/7/002. [DOI] [PubMed] [Google Scholar]
- 2.Burger HC, van Dongen R. Specific electrical resistance of body tissues. Phys Med Biol. 1961;5:431–447. doi: 10.1088/0031-9155/5/4/304. [DOI] [PubMed] [Google Scholar]
- 3.Chin AB, Garmirian LP, Nie R, Rutkove SB. Optimizing measurement of the electrical anisotropy of muscle. Muscle Nerve. 2008;37:560–565. doi: 10.1002/mus.20981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein BR, Foster KR. Anisotropy in the dielectric properties of skeletal muscle. Med Biol Eng Comput. 1983;21:51–55. doi: 10.1007/BF02446406. [DOI] [PubMed] [Google Scholar]
- 5.Esper GJ, Shiffman CA, Aaron R, Lee KS, Rutkove SB. Assessing neuromuscular disease with multifrequency electrical impedance myography. Muscle Nerve. 2006;34:595–602. doi: 10.1002/mus.20626. [DOI] [PubMed] [Google Scholar]
- 6.Gielen FLH, Wallinga-de Jongeand W, Boon KL. Electrical conductivity of skeletal muscle tissue: experimental results from different muscles in vivo. Med Biol Eng Comput. 1984;22:569–577. doi: 10.1007/BF02443872. [DOI] [PubMed] [Google Scholar]
- 7.Hart FX, Berner NJ, McMillen RL. Modelling the anisotropic electrical properties of skeletal muscle. Phys Med Biol. 1999;44:413–421. doi: 10.1088/0031-9155/44/2/009. [DOI] [PubMed] [Google Scholar]
- 8.Rush S. Methods of measuring the resistivities of anisotropic conducting media. J Res Nat Bur Stand. 1962;66C:217–222. [Google Scholar]
- 9.Rush S, Abildskov JA, McFee R. Resistivity of body tissues at low frequencies. Circ Res. 1963;12:40–50. doi: 10.1161/01.res.12.1.40. [DOI] [PubMed] [Google Scholar]
- 10.Rutkove SB, Aaron R, Shiffman CA. Localized bioimpedance analysis in the evaluation of neuromuscular disease. Muscle Nerve. 2002;25:390–397. doi: 10.1002/mus.10048. [DOI] [PubMed] [Google Scholar]
- 11.Rutkove SB, Esper GJ, Lee KS, Aaron R, Shiffman CA. Electrical impedance myography in the detection of radiculopathy. Muscle Nerve. 2005;32:335–341. doi: 10.1002/mus.20377. [DOI] [PubMed] [Google Scholar]
- 12.Shiffman CA, Aaron R. Angular dependence of resistance in non-invasive electrical measurements of human muscle: the tensor model. Phys Med Biol. 1998;43:1317–1323. doi: 10.1088/0031-9155/43/5/019. [DOI] [PubMed] [Google Scholar]
- 13.Shiffman CA, Aaron R, Amoss V, Therrien J, Coomler K. Resistivity and phase in localized BIA. Phys Med Biol. 1999;44:2409–2429. doi: 10.1088/0031-9155/44/10/304. [DOI] [PubMed] [Google Scholar]
- 14.Tarulli AW, Chin AB, Partida RA, Rutkove SB. Electrical impedance in bovine skeletal muscle as a model for the study of neuromuscular disease. Physiol Meas. 2006;27:1269–1279. doi: 10.1088/0967-3334/27/12/002. [DOI] [PubMed] [Google Scholar]
- 15.Tarulli AW, Esper GJ, Lee KS, Aaron R, Shiffman CA, Rutkove SB. Electrical impedance myography in the bedside assessment of inflammatory myopathy. Neurology. 2005;65:451–452. doi: 10.1212/01.wnl.0000172338.95064.cb. [DOI] [PubMed] [Google Scholar]