Abstract
Lysophosphatidic acid (LPA) is a potent agonist that exerts various cellular functions on many cell types through binding to its cognate G protein-coupled receptors (GPCRs). Although LPA induces NF-κB activation by acting on its GPCR receptor, the molecular mechanism of LPA receptor-mediated NF-κB activation remains to be well defined. In the present study, by using MEKK3-, TAK1-, and IKKβ-deficient murine embryonic fibroblasts (MEFs), we found that MEKK3 but not TAK1 deficiency impairs LPA and protein kinase C (PKC)-induced IκB kinase (IKK)-NF-κB activation, and IKKβ is required for PKC-induced NF-κB activation. In addition, we demonstrate that LPA and PKC-induced IL-6 and MIP-2 production are abolished in the absence of MEKK3 but not TAK1. Together, our results provide the genetic evidence that MEKK3 but not TAK1 is required for LPA receptor-mediated IKK-NF-κB activation.
Keywords: NF-κB, LPA, GPCR, MEKK3, TAK1
1. Introduction
The nuclear factor-κB (NF-κB) family of transcription factors plays an important role in regulating the expression of genes responsible for innate and adaptive immunity, stress responses, anti-apoptosis, cell proliferation, and differentiation (1-4). In resting cells, NF-κB is retained in the cytosol in an inactive form through interaction with IκB inhibitory proteins. Release of NF-κB for translocation to the nucleus and activation of NF-κB dependent genes is accomplished through a signal-induced phosphorylation of IκB by IκB kinase (IKK) and subsequent IκBα degradation (5-8). Upon binding to their agonist ligands, various cell surface receptors, including receptors for proinflammatory cytokines such as TNFα and IL-1, Toll-like receptors (TLRs), antigen receptors, and GPCRs, induce distinct signaling pathways that eventually converge on IKK complex to activate NF-κB (9). A major challenge in the NF-κB field is to understand how these distinct receptor-mediated signaling effectors activate IKK/NF-κB in a signal-specific manner (10).
Lysophosphatidic acid (LPA) is a naturally occurring, water-soluble glycerophospholipid that exerts hormone- and growth factor-like activities on many cell types including fibroblasts, endothelial cells, and smooth muscle cells (11-12). LPA is involved in the regulation of various cellular responses such as cell proliferation, chemotaxis and survival through binding to its cognate G protein-coupled receptors (GPCRs) and activating LPA receptor-mediated multiple effector molecules, including NF-κB (11). Recently, the adaptor and scaffold proteins β-arrestin2, Bcl10, MALT1 and CARMA3 were identified as essential signal transducers to mediate LPA-induced NF-κB activation (13-17). Two members of MAP3K serine/threonine kinase family, MEKK3 and TAK1 have been demonstrated to be involved in regulating NF-κB activation through IKK (18-22). Surprisingly, it has been suggested that TAK1 is not essential in the LPA-mediated NF-κB activation (15). We therefore tested whether MEKK3 is required for LPA-induced NF-κB activation. Using MEKK3- and TAK1-deficient MEF cell lines, we demonstrate that MEKK3 but not TAK1 is required for LPA receptor-mediated IKK-NF-κB activation. These results reveal that MEKK3, but not TAK1, preferentially mediates GPCR-induced NF-κB activation.
2. Materials and methods
2.1. Antibodies, plasmids and reagents
Antibodies against ERK1/2, phospho-ERK1/2, JNK, phospho-JNK, IκBα, phospho-IκBα, IKKβ, phospho-IKKα/β, TAK1, and secondary antibodies conjugated to horseradish peroxidase were purchased from Cell Signaling Technology (Beverly, MA). Antibodies against PCNA (PC-10), NF-κB-p65 were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibody against MEKK3 was from BD Biosciences Pharmingen (San Diego, CA). Antibody against β-actin were from Sigma (St. Louis, MO). pBabe-vector and pBabe-HA-MEKK3 expression vector has been described previously (20). The NF-κB-dependent firefly luciferase reporter plasmid and pCMV promoter-dependent Renilla-luciferase reporter were purchased from Clontech (Mountain View, California). LPA, phorbol-12-myristate-13-acetate (PMA) and ionomycin (Iono) were purchased from Sigma. Mouse IL-6 and MIP-2 ELISA kits were purchased from BD Biosciences and R & D Systems (Minneapolis, MN), respectively. The ECL-Plus Western blotting system was purchased from GE Healthcare Bio-sciences Corp.
2.2. Cell Culture and transfection
MEKK3-/-, TAK1-/- and IKKβ-/- as well as the reconstituted MEF cell lines have been described previously (20;22;23). These cells are maintained in DMEM containing 10% FCS at 37°C with 5% CO2, and transfected with Lipofectamine 2000 (Invitrogen) following the manufacturer's protocol.
2.3. Luciferase reporter gene assay
Luciferase reporter gene assay was performed using a dual luciferase reporter assay system (Promega, Madison, WI) and a Monolight 3010 luminometer (BD Pharmingen) as described previously (Yang et al., 2001). Briefly, targeted cells were transiently cotransfected with specific vectors and an NF-κB-dependent firefly luciferase reporter construct as well as a Renilla-luciferase control construct. Cellular extracts were prepared 36 h post-transfection and the luciferase activities were determined. Relative NF-κB luciferase activity was normalized to Renilla-luciferase activity. Changes in luciferase activity with respect to control were calculated. Each experiment was conducted in triplicate.
2.4. Preparation of nuclear and cytosolic fractions
Nuclear and cytosolic extracts were made as described (20). In brief, cells were harvested in ice-cold PBS (pH 7.4) and were pelleted by centrifugation at 500 ×g for 3 min and then lysed for 30 min on ice in buffer B (10 mM HEPES buffer, pH 7.9, containing 0.1 mM EDTA, 10 mM KCl, 0.4% (v/v) IGEPAL, 0.5 mM dithiothreitol (DTT), and 1 mM phenylmethylsulfonyl fluoride (PMSF)). Lysates were centrifuged at 15,000 ×g for 10 min. The resulting supernatants constituted cytosolic fractions. The pellets were washed three times with buffer B and resuspended in buffer C (20 mM HEPES buffer, pH 7.9, containing 400 mM NaCl, 1 mM EDTA, 1 mM DTT and 1 mM PMSF) and incubated for 30 min on ice and centrifuged at 15,000 ×g for 10 min. The supernatants were used as nuclear extracts.
2.5. Electrophoretic mobility shift assay (EMSA)
NF-κB oligonucleotide probes were labeled with [γ-32P]ATP. MEF cells (1 × 106) were starved for 12 h and stimulated for the indicated time points. Nuclear extracts isolated from these cells were then incubated with 32P-labeled probes in 10 mM HEPES (pH 7.9), 40 mM NaCl, 1 mM EDTA, 4% glycerol, 3 μg of poly(dI·dC), and 0.5 mM DTT for 15 min at room temperature. The samples were then resolved in a nondenaturing polyacrylamide gel and exposed to X-ray film at -80°C.
2.6. Enzyme-linked immunosorbent assay (ELISA)
MEKK3-deficient MEF cell lines stably transfected with vector control or MEKK3 were treated with or without LPA (30 μM) or PMA (40 ng/ml) plus Iono (100 ng/ml), the supernatants were collected at different time points. Mouse IL-6 or MIP-2 concentrations in the medium were determined by ELISA according to the manufacturer's instructions.
2.7 Immunoblotting
Cells were harvested in ice-cold PBS (pH 7.4) and spun down. The pellet was dissolved in lysis buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% IGEPAL, 0.25% Na-deoxycholate, 1 mM PMSF, 1 mM DTT, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mM Benzamidine, 20 mM disodium p-nitrophenylphosphate (pNPP), 0.1 mM sodium orthovanadate (OV), 10 mM sodium fluoride (NaF), phosphatase inhibitor cocktail A and B (Sigma Aldrich)). The cell lysates were subjected directly to 10% SDS-PAGE and probed for the specific antibody for immunoblotting analysis.
3. Results
3.1. MEKK3 is required for LPA-induced IKK/NF-κB activation
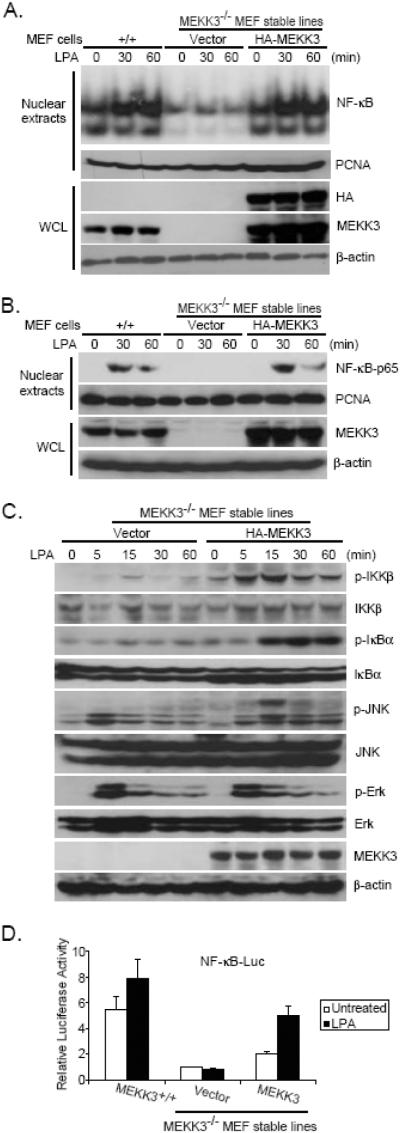
Previous study suggests that TAK1 is not required for LPA-induced NF-κB activation (15). Therefore, we hypothesized that MEKK3, another NF-κB activating MAP3K, is playing an essential role in LPA-mediated NF-κB activation. To test this hypothesis, we used MEF cells isolated from day 9.5 embryos of MEKK3 wild type (MEKK3+/+) and MEKK3-deficient (MEKK3-/-) mice. We first established MEKK3-/- MEF stable cell lines reconstituted with empty vector or HA-MEKK3 expression vector, respectively. The expression of HA-MEKK3 in the reconstituted MEF cell lines were verified by immunoblotting (Fig. 1A). Then MEKK3+/+ and MEKK3-/- MEF cell lines reconstituted with vector control or HA-MEKK3 were treated with LPA at different time points and nuclear translocation of NF-κB in these cells were examined by EMSA and immunoblotting of the nuclear extracts with anti-NF-κB-p65 antibody (Figs. 1A and B). In this assay, LPA stimulation effectively induced NF-κB nuclear translocation in the wild type and HA-MEKK3-reconstituted MEFs, whereas LPA stimulation failed to induce nuclear translocation of NF-κB in MEKK3-/- MEFs (Figs. 1A and B). These results suggest that MEKK3 is required for LPA-induced NF-κB nuclear translocation in the cells.
Fig. 1. MEKK3 is required for LPA-induced IKK-NF-κB activation.
(A) MEKK3+/+ and MEKK3-/- MEF cells reconstituted with empty vector or HA-MEKK3 were either untreated or treated with LPA (30 μM) for 0, 30, and 60 min, then harvested. Nuclear extracts were prepared and subjected to EMSA by using 32P-labeled NF-κB probes. Whole cell lysates (WCL) were subjected to SDS-PAGE and immunoblotting with antibodies indicated. PCNA was used as a loading control for nuclear extracts and β-actin was detected as a loading control for WCL. (B) MEKK3+/+ and MEKK3-/- MEF cells reconstituted with empty vector and MEKK3 were stimulated as in A. Then nuclear extracts were prepared and subjected to the immunoblotting analysis as indicated. (C) MEKK3-/- MEF cells reconstituted with empty vector and MEKK3 were either untreated or treated with LPA (30 μM) for 0, 5, 15, 30, and 60 min, then harvested. WCL were subjected to SDS-PAGE and immunoblotting analysis as indicated. (D) One μg of NF-κB luciferase reporter and 20 ng of Renilla-Luc plasmids were cotransfected into MEF cells indicated. Twenty-four hours after transfection, cells were starved for 12 h followed by the addition of LPA (30 μM) for 8 h. The relative luciferase activity was measured and normalized with the Renilla activity. Error bars indicate ± standard deviation in triplicate experiments.
MEKK3 has been shown to be involved in the regulation of IKK/NF-κB activation (18;20;23;24). To further characterize the physiological role of MEKK3 in LPA-mediated NF-κB activation, MEKK3-/- MEF cell lines reconstituted with vector control or HA-MEKK3 were treated with LPA at different time points and subsequently lysed. The cell lysates were immunoblotted with the indicated antibodies to examine the LPA-induced IKK and IκBα phosphorylation. In this assay, LPA-induced IKK and IκBα phosphorylation were significantly impaired in the vector control MEKK3-/- MEF cells compared to the MEKK3 reconstituted cells (Fig. 1C).
Previous studies have shown that MEKK3 is involved in JNK activation (25-27). We found that LPA failed to induce JNK2 phosphorylation in the MEKK3-/- MEF cells with vector control compared to the MEKK3 reconstituted cells whereas LPA-induced JNK1 phosphorylation was only slightly inhibited in the vector control MEKK3-/- MEF cells at later time points. Interestingly, LPA-induced-ERK phosphorylation was comparable in both cell lines (Fig. 1C).
Consistent with these results, luciferase analysis with NF-κB reporter showed that LPA failed to induce the luciferase reporter gene expression in MEKK3-/- MEF cells with the vector control whereas LPA induced higher level of reporter gene expression in the wild type and/or MEKK3 reconstituted MEF cells (Fig. 1D). Taken together, these results indicate that MEKK3 is required for LPA-induced optimal IKK/NF-κB activation.
3.2. MEKK3 is required for PMA/Iono-induced IKK/NF-κB activation
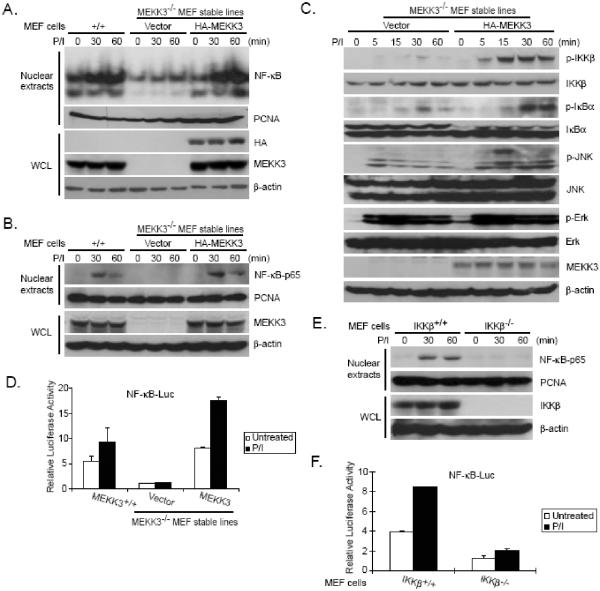
Protein kinase C (PKC) members have been reported to be involved in LPA-induced NF-κB activation (15). We therefore tested the role of MEKK3 in PKC-mediated NF-κB nuclear translocation in MEF cells. In our assays, the wild type, vector control and HA-MEKK3-reconstituted MEKK3-/- MEF cells were treated with or without PKC pharmacological agonists, PMA plus Iono at the indicated time points and nuclear translocation of NF-κB in these cells were examined by EMSA and immunoblotting of nuclear extracts with anti-NF-κB-p65 antibodies. We found that PMA plus Iono (PMA/Iono) co-stimulation effectively induced nuclear translocation of NF-κB in the wild type MEFs and MEKK3-/- MEFs reconstituted with HA-MEKK3 whereas PMA/Iono stimulation failed to induce NF-κB nuclear translocation in MEKK3-/- MEFs with vector control (Figs. 2A and B). These results suggest that MEKK3 is required for PMA/Iono-induced NF-κB nuclear translocation.
Fig. 2. MEKK3 is required for PKC-induced IKK/NF-κB activation.
(A) MEKK3+/+ and MEKK3-/- MEF cells reconstituted with empty vector and MEKK3 were either untreated or treated with PMA (40 ng/ml) plus Iono (100 ng/ml) for 0, 30, and 60 min. Nuclear extracts were prepared and subjected to EMSA by using 32P-labeled NF-κB probes. (B) MEKK3+/+ and MEKK3-/- MEF cells reconstituted with empty vector and MEKK3 were stimulated as in A. WCL and nuclear extracts isolated from the cells were subjected to SDS-PAGE and immunoblotting analysis as indicated. (C) MEKK3-/- MEF cells reconstituted with empty vector and MEKK3 were either untreated or treated with PMA (40 ng/ml) plus Iono (100 ng/ml) for 0, 5, 15, 30, and 60 min, then harvested. WCL were subjected to SDS-PAGE and immunoblotting analysis as indicated. (D) One µg of NF-κB luciferase reporter and 20 ng of Renilla-Luc plasmids were cotransfected into MEF cells indicated. Twenty-four hours after transfection, cells were starved for 12 h followed by the addition of PMA (40 ng/ml) plus Iono (100 ng/ml) for 8 h. The relative luciferase activity was measured and normalized with the Renilla activity. Error bars indicate ± standard deviation in triplicate experiments. (E) IKKβ+/+ and IKKβ-/- MEF cells were either untreated or treated with PMA (40 ng/ml) plus Iono (100 ng/ml) for 0, 30, and 60 min, then harvested. WCL and nuclear extracts were subjected to SDS-PAGE and immunoblotting analysis as indicated. (F) One μg of NF-κB luciferase reporter and 20 ng of Renilla-Luc plasmids were cotransfected into MEF cells indicated. Twenty-four hours after transfection, cells were starved for 12 h followed by the addition of PMA (40 ng/ml) plus Iono (100 ng/ml) for 8 h. The relative luciferase activity was measured and normalized with the Renilla activity. Error bars indicate ± standard deviation in triplicate experiments.
We further studied the role of MEKK3 in PMA/Iono-induced IKK-NF-κB activation and found that PMA/Iono-induced IKK and IκBα phosphorylation were significantly impaired in MEKK3-/- MEF cells with the vector control compared to the HA-MEKK3 reconstituted cells (Fig. 2C). We also found that PMA/Iono-induced JNK2 phosphorylation was completely blocked in MEKK3-/- MEF cells with the vector control and rescued in MEKK3 reconstituted MEF cells, whereas PMA/Iono-induced-JNK1 activation was only slightly inhibited in MEKK3-/- MEF cells with the vector control at later time points (Fig. 2C). However, PMA/Iono-induced-ERK phosphorylation was comparable in both cell lines (Fig. 2C). Consistent with these results, PMA/Iono failed to induce the NF-κB-responsive luciferase reporter gene expression in the vector control MEKK3-/- MEF cells whereas PMA/Iono induced high level of reporter gene expression in the wild type and MEKK3 reconstituted MEF cells (Fig. 2D). Taken together, these results indicate that MEKK3 is required for PKC-mediated IKK/NF-κB activation.
The IKKs and IKK-related kinases (IKKε/IKK-i and TBK1/NAK/T2K) play important roles in activation of the host defense system (28;29). IKKβ is essential for rapid NF-κB activation by proinflammatory signaling cascades, such as those triggered by TNFα or lipopolysaccharide (LPS), whereas IKKε/IKK-i and TBK1/NAK/T2K have been suggested to be involved in PKC-induced NF-κB activation (29-34). We therefore tested the role of IKKβ in PKC-mediated NF-κB activation in MEF cells. In our assays, IKKβ wild type (IKKβ+/+) and IKKβ-deficient (IKKβ-/-) MEF cells were treated with or without PMA plus Iono at the indicated time points and nuclear localization of NF-κB in these cells were examined. We found that PMA/Iono co-stimulation effectively induced nuclear localization of NF-κB in IKKβ+/+ MEFs whereas PMA/Iono stimulation failed to induce nuclear localization of NF-κB in IKKβ-/- MEFs (Fig. 2E). Consistent with this result, PMA/Iono induced a higher NF-κB dependent luciferase activity in the IKKβ+/+ MEF cells compared to IKKβ-/- MEF cells (Fig. 2F). These results suggest that IKKβ is required for PMA/Iono-induced NF-κB activation in the MEF cells.
3.3. TAK1 is not required for LPA and PMA/Iono-induced IKK/NF-κB activation
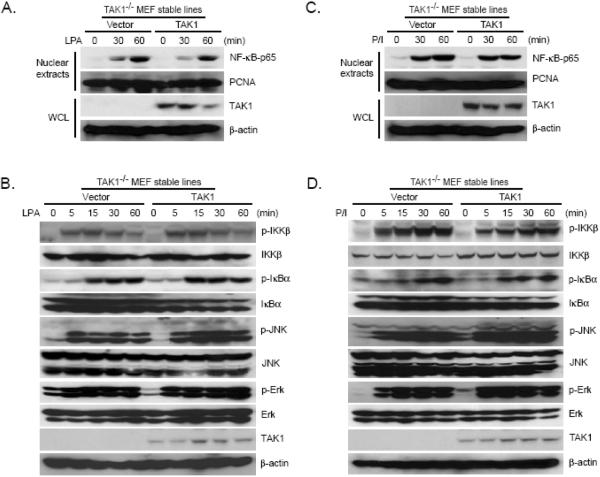
To further explore the role of TAK1 in the LPA and PMA/Iono-mediated NF-κB activation, we treated the TAK1-deficient (TAK1-/-) MEF cell lines reconstituted with vector control or TAK1 with LPA and PMA/Iono for the time points indicated. We found that LPA and PMA/Iono effectively induced IKK and IκB phosphorylation as well as nuclear translocation of NF-κB in both vector control and TAK1 reconstituted TAK1-/- MEF cells (Figs. 3A-3D). Thus, these results suggest that TAK1 is not required for the LPA and PMA/Iono-induced NF-κB activation.
Fig. 3. TAK1 is not required for LPA and PMA/Iono-induced IKK-NF-κB activation.
(A and C) TAK1-/- MEF cells reconstituted with empty vector and TAK1 were either untreated or treated with LPA (A) or PMA/Iono (C) for 0, 30, and 60 min, then harvested. WCL and nuclear extracts were subjected to SDS-PAGE and immunoblotting analysis as indicated. (B and D) TAK1-/- MEF cells reconstituted with empty vector and TAK1 expression plasmid were either untreated or treated with LPA (B) or PMA/Iono (D) for 0, 5, 15, 30, and 60 min, then harvested. WCL were subjected to SDS-PAGE and immunoblotting analysis as indicated.
3.4. MEKK3 is required for LPA and PMA/Iono-induced IL-6 and MIP-2 production
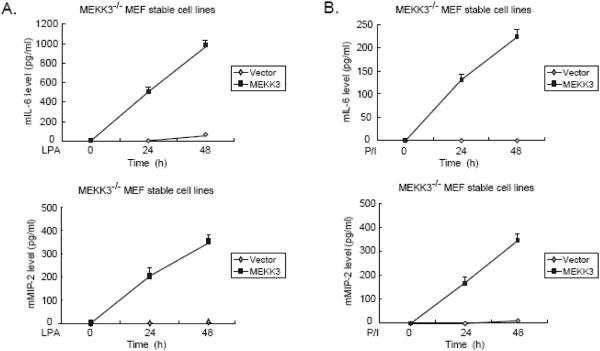
NF-κB activation has been shown to be required for LPA-induced IL-6 and macrophage inflammatory protein-2 (MIP-2) production in MEF cells (13;15;16). To determine the role of MEKK3 in LPA-induced IL-6 and MIP-2 production, the vector control and MEKK3-reconstituted MEKK3-/- MEF cell lines were treated with or without LPA and then analyzed for the IL-6 and MIP-2 production in the cells by ELISA (Fig. 4A). In this assay, LPA induced IL-6 and MIP-2 production in a time-dependent manner only in MEKK3-/- MEF cells reconstituted with MEKK3 whereas LPA completely failed to induce IL-6 and MIP-2 production in the MEKK3-/- MEFs with vector control (Fig. 4A). Consistent with this result, we also found that PMA/Iono-induced IL-6 and MIP-2 production in the cells were completely blocked in MEKK3-/- MEF cells with the vector control (Fig. 4B). In addition, we found that LPA and PMA/Iono effectively induced IL-6 production in both the vector control TAK1-/- and the TAK1 reconstituted cells (data not shown). These results demonstrate that MEKK3 but not TAK1 is essential for the LPA-induced physiological effects in the MEF cells.
Fig. 4. MEKK3 is required for the LPA and PKC-induced IL-6 and MIP-2 production.
(A and B) MEKK3-/- MEF cells reconstituted with empty vector and MEKK3 were untreated or treated with LPA (A) or PMA/Iono (B) for the time points indicated. The supernatants from these cultures were collected and subjected to the mouse IL-6 and MIP-2 ELISA analysis according to the manufacturer's instructions.
4. Discussion
LPA elicits its biological actions through its cognate G protein-coupled receptors. Binding of LPA to its GPCR receptor leads to NF-κB activation. However, the molecular regulation of GPCR-mediated NF-κB activation remains to be better defined. We report here that MEKK3, a member of MAP3K family, is a critical signaling component in LPA-induced NF-κB signal transduction pathway. Taking advantage of MEKK3 and TAK1-deficient MEF cell lines, we provide a genetic evidence that MEKK3 but not TAK1 is required for GPCR-mediated IKKβ and IκBα phosphorylation as well as NF-κB nuclear translocation and activation. In addition, we also show that LPA fails to induce production of the pro-inflammatory cytokine IL-6 and MIP-2 only in MEKK3-deficient but not TAK1 deficient MEF cell lines. These results suggest that MEKK3 but not TAK1 plays an essential role in LPA-induced cellular responses. In addition, we also found that IKKβ is required for PKC-mediated NF-κB activation in the MEF cells. These results suggest that MEKK3-mediated IKKβ activation is required for LPA and PKC-induced NF-κB activation.
Previous studies using knockout MEF cells demonstrated that β-arrestin 2, CARMA3, Bcl10 and TRAF6 are required for LPA-induced IKK-NF-κB activation whereas these proteins are not required for LPA-induced IKKβ phosphorylation (15;17). These results suggest that these proteins may be not required for LPA-mediated MEKK3 activation. However, further studies are needed to determine how MEKK3 is activated by LPA and PKC-mediated signaling events in non-hematopoietic cell types.
Interestingly, in our studies, we failed to observe an obvious LPA-induced IκBα degradation eventhough LPA induced IκBα phosphorylation. Consistent with our results, Qin et al reported that MEKK3 is required for TLR8-mediated NF-κB activation and TLR8-mediated signaling only causes IκBα phosphorylation but not degradation (26). Therefore, it is highly likely that the MEKK3-mediated IκBα phosphorylation might lead to dissociation of IκBα from NF-κB without IκBα degradation (26).
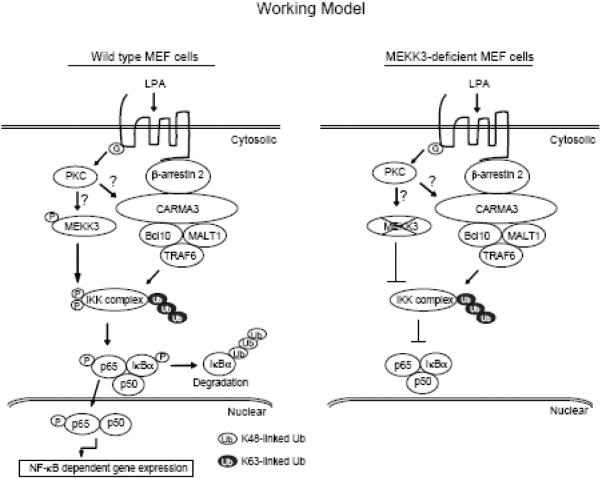
In conclusion, our data provide evidence that MEKK3 but not TAK1 is required for LPA and PKC-induced IKKβ/NF-κB activation in MEF cells. In view of the data presented here and in previous reports, we propose a working model (Fig. 5), in which LPA binding to its cognate GPCR induces PKC activation that leads to MEKK3-mediated phosphorylation of IKKβ and β-arrestin 2-CARMA3-Bcl10-MALT1-mediated ubiquitination of IKK complex that coordinately lead to IKKβ-mediated NF-κB activation in MEF cells.
Fig. 5. A working model for MEKK3 function in the LPA-induced IKK-NF-κB activation.
LPA induces PKC activation which in turn induces β-arrestin 2 and CARMA3-mediated ubiquitination of IKK complex via TRAF6/Bcl10/MALT1, as well as MEKK3-mediated IKKβ phosphorylation. IKK complex ubiquitination and phosphorylation coordinatively lead to optimal NF-κB activation.
Acknowledgements
We are grateful to Susan Burlingame for the excellent technical assistance. We thank Drs. Paul Chiao, Bing Su and Sankar Ghosh for providing IKKβ-, MEKK3- and TAK1-deficient MEFs. This research was supported in part by National Institutes of Health Grant 1R21CA106513-01A2 (to J.Y.), 5R01GM079451 (to X.L.), the American Cancer Society grant RSG-06-070-01-TBE (to J.Y.), and the Fleming and Davenport Award (to H.Z.).
Abbreviations used are
- NF-κB
nuclear factor-κB
- IKK
IκB kinase
- LPA
lysophosphatidic acid
- GPCR
G protein-coupled receptor
- PKC
protein kinase C
- TAK1
TGF-β activated kinase 1
- MEKK3
mitogen-activated protein kinase kinase kinase 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Baldwin AS., Jr. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- [2].May MJ, Ghosh S. Immunol. Today. 1998;19:80–88. doi: 10.1016/s0167-5699(97)01197-3. [DOI] [PubMed] [Google Scholar]
- [3].Li Q, Verma IM. Nat. Rev. Immunol. 2002;2:725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- [4].Karin M, Ben-Neriah Y. Annu. Rev. Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- [5].Beg AA, Baldwin AS., Jr. Genes Dev. 1993;7:2064–2070. doi: 10.1101/gad.7.11.2064. [DOI] [PubMed] [Google Scholar]
- [6].Beg AA, Finco TS, Nantermet PV, Baldwin AS., Jr. Mol. Cell Biol. 1993;13:3301–3310. doi: 10.1128/mcb.13.6.3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ghosh S, Karin M. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- [8].Verma IM, Stevenson JK, Schwarz EM, Van AD, Miyamoto S. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- [9].Hayden MS, Ghosh S. Genes Dev. 2004;18:2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- [10].Dixit V, Mak TW. Cell. 2002;111:615–619. doi: 10.1016/s0092-8674(02)01166-2. [DOI] [PubMed] [Google Scholar]
- [11].Ye RD. J. Leukoc. Biol. 2001;70:839–848. [PubMed] [Google Scholar]
- [12].Mills GB, Moolenaar WH. Nat. Rev. Cancer. 2003;3:582–591. doi: 10.1038/nrc1143. [DOI] [PubMed] [Google Scholar]
- [13].Klemm S, Zimmermann S, Peschel C, Mak TW, Ruland J. Proc. Natl. Acad. Sci. U. S. A. 2007;104:134–138. doi: 10.1073/pnas.0608388103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].lister-Lucas LM, Ruland J, Siu K, Jin X, Gu S, Kim DS, Kuffa P, Kohrt D, Mak TW, Nunez G, Lucas PC. Proc. Natl. Acad. Sci. U. S. A. 2007;104:139–144. doi: 10.1073/pnas.0601947103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grabiner BC, Blonska M, Lin PC, You Y, Wang D, Sun J, Darnay BG, Dong C, Lin X. Genes Dev. 2007;21:984–996. doi: 10.1101/gad.1502507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang D, You Y, Lin PC, Xue L, Morris SW, Zeng H, Wen R, Lin X. Proc. Natl. Acad. Sci. U. S. A. 2007;104:145–150. doi: 10.1073/pnas.0601894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sun J, Lin X. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17085–17090. doi: 10.1073/pnas.0802701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhao Q, Lee FS. J. Biol. Chem. 1999;274:8355–8358. doi: 10.1074/jbc.274.13.8355. [DOI] [PubMed] [Google Scholar]
- [19].Wang C, Deng L, Hong M, Akkaraju GR, Inoue J, Chen Z. J. Nature. 2001;412:346–351. doi: 10.1038/35085597. [DOI] [PubMed] [Google Scholar]
- [20].Yang J, Lin Y, Guo Z, Cheng J, Huang J, Deng L, Liao W, Chen Z, Liu Z, Su B. Nat. Immunol. 2001;2:620–624. doi: 10.1038/89769. [DOI] [PubMed] [Google Scholar]
- [21].Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Nat. Immunol. 2005;6:1087–1095. doi: 10.1038/ni1255. [DOI] [PubMed] [Google Scholar]
- [22].Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Schmidt C, Peng B, Li Z, Sclabas GM, Fujioka S, Niu J, Schmidt-Supprian M, Evans DB, Abbruzzese JL, Chiao PJ. Mol. Cell. 2003;12:1287–1300. doi: 10.1016/s1097-2765(03)00390-3. [DOI] [PubMed] [Google Scholar]
- [24].Nho CW, O'Dwyer PJ. J. Biol. Chem. 2004;279:26019–26027. doi: 10.1074/jbc.M309022200. [DOI] [PubMed] [Google Scholar]
- [25].Deacon K, Blank JL. J. Biol. Chem. 1999;274:16604–16610. doi: 10.1074/jbc.274.23.16604. [DOI] [PubMed] [Google Scholar]
- [26].Qin J, Yao J, Cui G, Xiao H, Kim TW, Fraczek J, Wightman P, Sato S, Akira S, Puel A, Casanova JL, Su B, Li X. J. Biol. Chem. 2006;281:21013–21021. doi: 10.1074/jbc.M512908200. [DOI] [PubMed] [Google Scholar]
- [27].Huang Q, Yang J, Lin Y, Walker C, Cheng J, Liu ZG, Su B. Nat. Immunol. 2004;5:98–103. doi: 10.1038/ni1014. [DOI] [PubMed] [Google Scholar]
- [28].Zandi E, Karin M. Mol. Cell Biol. 1999;19:4547–4551. doi: 10.1128/mcb.19.7.4547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Chau TL, Gioia R, Gatot JS, Patrascu F, Carpentier I, Chapelle JP, O'Neill L, Beyaert R, Piette J, Chariot A. Trends Biochem. Sci. 2008;33:171–180. doi: 10.1016/j.tibs.2008.01.002. [DOI] [PubMed] [Google Scholar]
- [30].Hawiger J, Veach RA, Liu XY, Timmons S, Ballard DW. Blood. 1999;94:1711–1716. [PubMed] [Google Scholar]
- [31].Shimada T, Kawai T, Takeda K, Matsumoto M, Inoue J, Tatsumi Y, Kanamaru A, Akira S. Int. Immunol. 1999;11:1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- [32].Li ZW, Chu W, Hu Y, Delhase M, Deerinck T, Ellisman M, Johnson R, Karin M. J. Exp. Med. 1999;189:1839–1845. doi: 10.1084/jem.189.11.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tanaka M, Fuentes ME, Yamaguchi K, Durnin MH, Dalrymple SA, Hardy KL, Goeddel DV. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- [34].Hacker H, Karin M. Sci. STKE. 2006:re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]