Preface
Forces associated with blood flow are major determinants of vascular morphogenesis and physiology. Blood flow is crucial for blood vessel development during embryogenesis and for regulation of vessel diameter in adult life. It is also a key factor in atherosclerosis, which, despite the systemic nature of major risk factors, occurs mainly at regions of arteries that experience disturbances in fluid flow. Recent data have highlighted the potential endothelial mechanotransducers that mediate responses to flow, the effects of atheroprotective versus atherogenic flow, and the mechanisms that contribute to progression of the disease over time and how systemic factors interact with flow patterns to cause atherosclerosis.
Introduction
Vertebrates have evolved a pressurized vascular system that consists of the heart as a pump and a closed network of blood vessels that deliver oxygen and nutrients to every tissue. It has been proposed that a major fraction of the vertebrate-specific genome evolved to support vascular development and physiology, which is crucial in large animals with high metabolic rates 1. Indeed, the vascular system is exquisitely regulated throughout life to match blood flow to demand so that all tissues receive adequate perfusion with maximal efficiency under widely varying conditions. Mechanical forces that determine vessel distribution and diameter are among the stimuli that mediate these regulatory effects.
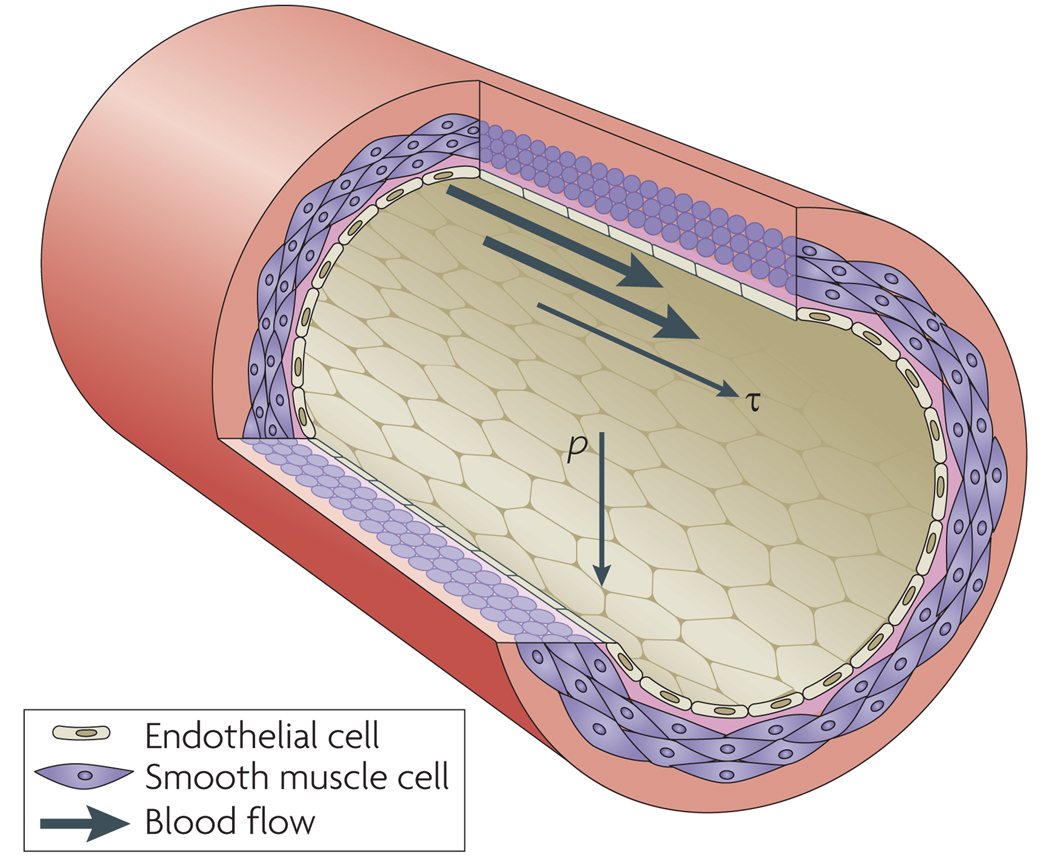
Fluid shear stress (τ), the frictional force per unit area from flowing blood, acts on the endothelial cells (ECs) that line the vessels (Fig 1). Blood pressure (p), which drives fluid flow, exerts a circumferential stretch normal to the vessel wall on both the ECs and the vascular smooth muscle cells (VSMCs) that surround the endothelium in arteries. Hydrostatic pressure per se may also alter cellular physiology but is much less important than shear stress or circumferential stretch and will not be discussed further.
Figure 1. Mechanical forces on the vessel wall.
A section of an artery wall shows endothelial cells (ECs) that form the inner lining and align longitudinally, and smooth muscle cells (SMCs) that form the outer layers and align circumferentially. Pressure (p) is normal to vessel wall, resulting in circumferential stretch of the vessel wall. Shear stress (τ) is parallel to vessel wall and is exerted longitudinally in the direction of blood flow.
Fluid shear stress is a key determinant of embryonic morphogenesis of the heart and blood vessels, since altering shear can inhibit both reorganization of the primitive vascular plexus into a properly organized vascular tree and development of the outflow tract of the heart 2,3 (Box 1). Shear and pressure also control vascular physiology in adults (discussed below). However, an inevitable consequence of the branched structure of the arterial tree is the appearance of irregularities in flow at places where arteries branch or turn sharply (Fig 2). These sites show a low level of chronic inflammation, not only in healthy individuals but even in newborn babies, mice and other mammals that are highly resistant to atherosclerosis. This mild inflammation is generally benign; however, in older humans where other risk factors (diabetes, obesity, hyperlipidemia, lack of exercise, smoking and circulating inflammatory mediators) are present, it can become more severe and progress to atherosclerotic plaques.
Box 1. Developmental roles of fluid shear stress.
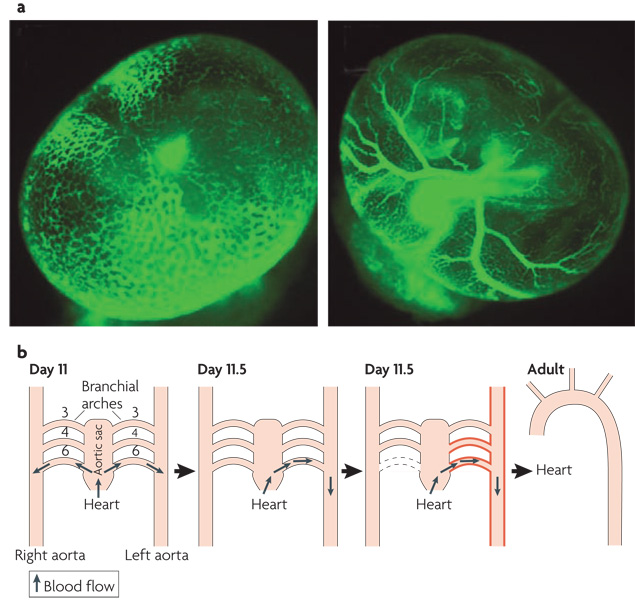
The general concept that high shear stress stabilizes vessels and promotes their enlargement, whereas low flow leads to vessel shrinkage or complete regression is important in development of the vascular system. Endothelial cells (ECs) in the yolk sac first assemble into a primitive vascular plexus, a net-like meshwork of tubes 105. After the heart begins to beat and blood cells enter the circulation, this meshwork reorganizes into a typical vascular tree in which large arteries branch into smaller arteries and then capillaries that rejoin to form veins (see figure panel a). Lucitti et al 3 used genetic, surgical and biomechanical manipulations to elegantly show that fluid shear stress is crucial for this reorganization of the plexus into a hierarchical vascular system.
A second study concerned the formation of an aorta that curves to the left as it exits the heart before descending to the abdomen. In early embryos (day 11 of gestation) , the outflow tract from the primitive heart leads into a structure called the aortic sac, which connects through short vessels called the aortic arches to two symmetric arteries, the left and right aortas (see figure panel b). A rotation of structures within the outflow tract directs higher flow toward the left side of the aortic sac. Thus, the left aortic arches receive higher fluid flow (shown in red) . As a result, the right 6th aortic arch regresses completely while the left arch enlarges and thickens. After acquiring this asymmetry, these vessels rearrange during later development into the mature aorta and its major branches.
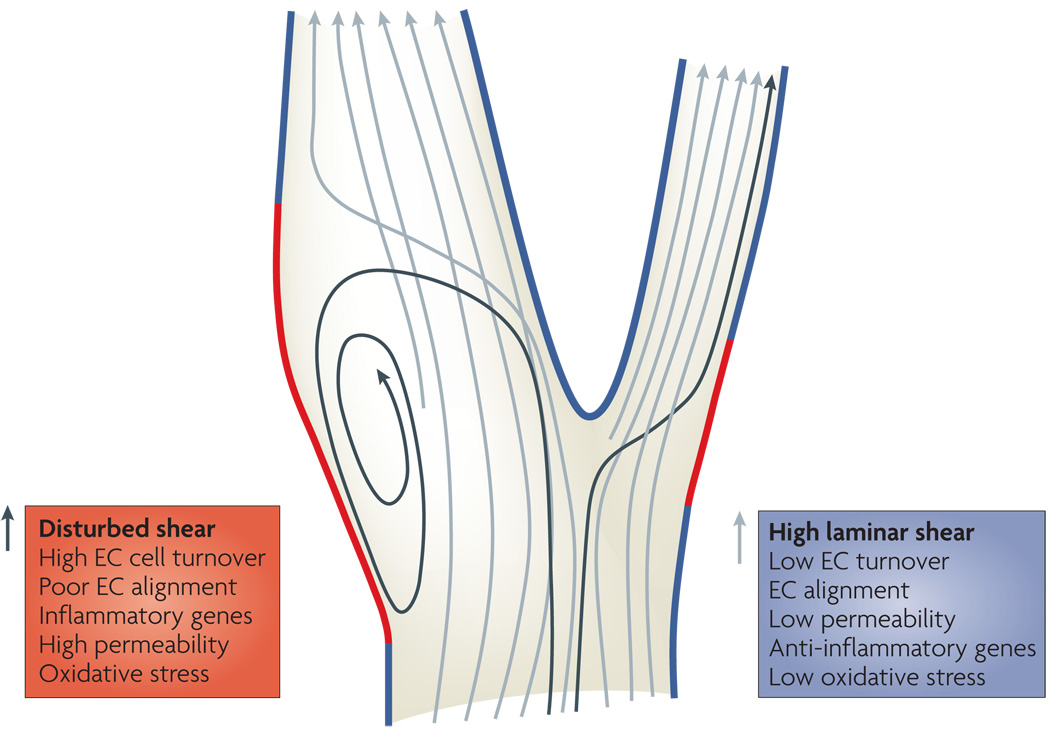
Figure 2. Vascular bifurcation and flow patterns.
In straight regions of arteries, the rate of blood flow changes during the cardiac cycle but flow is always in the same direction and patterns are laminar (blue segments). In regions where arteries divide or curve sharply, there are regions where complex flow patterns develop (red segments). Flow in these regions is lower and can reverse direction during the cardiac cycle, so-called oscillatory flow. Endothelial cells (ECs) in regions of high, laminar shear have a quiescent, anti-inflammatory phenotype characterized by alignment in the direction of flow, expression of anti-inflammatory genes, and low levels of oxidative stress, cell turnover and permeability and are protected from atherosclerosis. By contrast, endothelial cells in regions of disturbed shear have an activated, pro-inflammatory phenotype characterized by poor alignment, high turnover, oxidative stress, expression of inflammatory genes and high turnover, associated with high susceptibility to atherosclerosis.
Atherosclerosis occurs in large and medium-sized arteries where lesions form that contain lipids, leukocytes, smooth muscle cells and, at late stages, necrotic cores with cholesterol crystals and calcification 4 (Box 2). These plaques can gradually narrow the arteries, decreasing blood flow and resulting in pain or limited function, as in angina, congestive heart failure or peripheral vascular disease. Of greater clinical import, plaques can also suddenly rupture, leading to thrombus formation and vessel occlusion. These events often manifest in the form of myocardial infarction or stroke, which together are responsible for approximately half the deaths in developed nations. The risk factors mentioned above play major roles in the incidence and progression of atherosclerosis. However, these risk factors are relatively uniform throughout the vasculature, whereas atherosclerosis is initially highly focal, occurring mainly at artery bifurcations, branch points and regions of high curvature that result in complex blood flow patterns. In this review, we will discuss the basic mechanisms by which mechanical forces regulate normal physiology, how endothelial cells respond to fluid flow, and how chronic exposure to disturbed flow leads to vessel dysfunction and disease.
Box 2. Atherosclerosis.
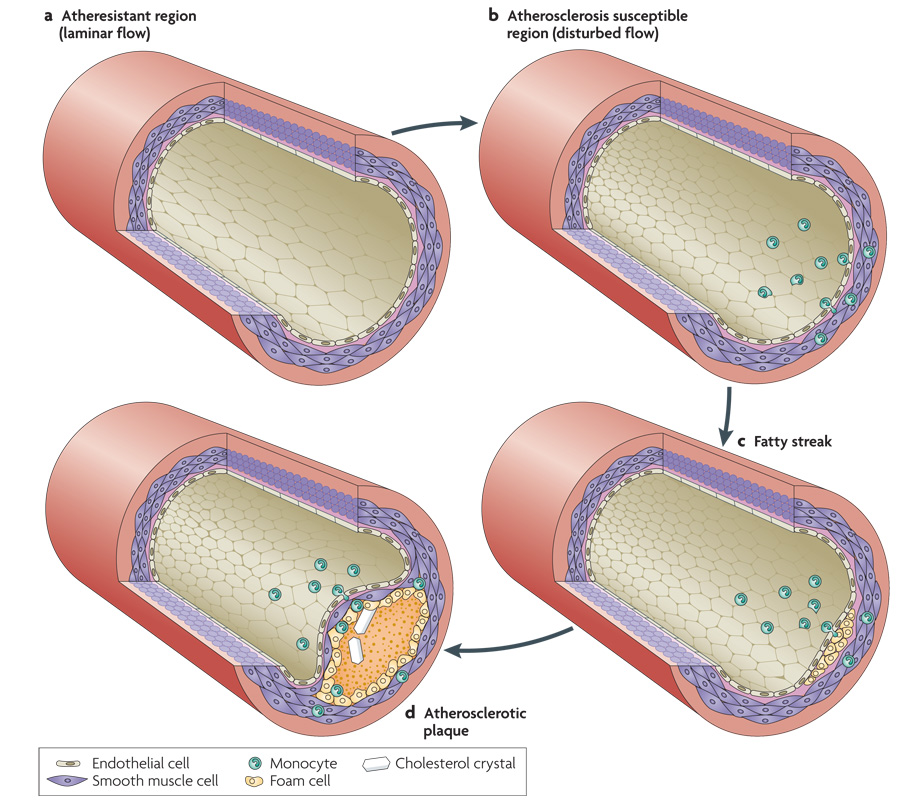
In a healthy, athero-resistant artery with high, laminar shear stress, endothelial cells (ECs) are aligned in the direction of flow and remain in a quiescent state with low rates of both proliferation and death (see figure panel a)106. They also express low levels of adhesion receptors and cytokines that attract leukocytes(not shown). In an atherosclerosis-susceptible region where flow is disturbed, ECs are poorly aligned. They are in an activated state with high rates of cell turnover and high expression of leukocyte adhesion receptors such as E-selectin, vascular cell adhesion-molecule-1 (VCAM-1) VCAM-1 and intracellular adhesion-molecule-1 (ICAM-1), and cytokines such as monocyte chemotactic protein 1 (MCP-1). As a result, increased numbers of leukocytes, mainly monocytes, bind to the endothelium and migrate into the vessel wall (see figure panel b).
Even in children and healthy young adults, fatty streaks form in regions of disturbed flow. Monocytes in these regions differentiate into macrophages, a highly phagocytic type of immune cell. They take up lipoproteins, mainly low density and very low density lipoproteins (LDL and VLDL) that carry cholesterol and triglycerides to the tissues. By contrast, high density lipoproteins carry lipids away from these cells and oppose the effects of LDL and VLDL. Macrophages that are engorged with lipid are called foam cells. These cells are more activated and secrete cytokines and enzymes that alter the surrounding extracellular matrix. These factors also act on the smooth muscle cells, leading to increased growth and migration, and thickening of the smooth muscle layer (see figure panel c).
Small fatty streaks can progress to larger, more inflamed lesions called atherosclerotic plaques. The rate of progression is determined mainly by risk factors such as levels of plasma lipoproteins, oxidants from smoking or other sources, elevated blood glucose, circulating inflammatory mediators and exercise. Plaques can vary substantially in their composition but typically contain a core of necrotic cells and fatty debris with cholesterol crystals, in addition to further accumulation of foam cells, other immune cells and smooth muscle (see figure panel d).
Mechanotransduction in vessel physiology
The periodic contractions of the heart cause large, pulsatile changes in blood pressure on the arterial side of the circulation. Large arteries respond to blood pressure passively due to their intrinsic elasticity. These arteries, especially the aorta, expand at each peak of pressure from the heart (systole), then, as pressure from the heart drops (diastole), they gradually deflate, releasing blood downstream. The elasticity of large vessels thus dampens the periodic variations in pressure, evening out the blood flow in smaller, less elastic vessels during the cardiac cycle. The resultant cyclic stretch of artery walls promotes a quiescent, contractile state where VSMCs express a full range of differentiation markers 5.
Blood pressure is determined mainly by the diameter of smaller resistance arteries that lead into capillary beds. VSMCs in these vessels actively respond to acute changes in blood pressure via a mechanism called the myogenic effect 6. Elevated pressure triggers VSMC contraction, which narrows small resistance arteries to keep blood flow constant in downstream capillaries. If pressure remains elevated over longer times, VSMCs remodel, thickening the vascular wall to resist these forces. Indeed, arterial diameters and wall thickness seem to be calibrated to maintain a constant value for tension/length and thickness of the vessel wall according to Laplace’s Law 7. However, under pathological conditions in which pressures stay high, this remodelling can eventually compromise vessel elasticity, decreasing the ability to accommodate sudden changes in pressure 8.
ECs also respond to stretch, however, fluid shear stress appears to be the main determinant of EC function. ECs in arteries respond to increased blood flow by causing relaxation of the surrounding smooth muscle. They do so by producing substances such as nitric oxide (NO), prostacyclin and a poorly characterized arachidonic acid metabolite that induces smooth muscle hyperpolarization, and by releasing potassium ions through membrane channels 9–11. Hyperpolarization is associated with relaxation since it makes the SMCs less likely to activate the voltage-dependent calcium channels that open when the cell depolarizes. These channels admit calcium ions to trigger activation of myosin and thus cell contraction. VSMC relaxation in response to flow occurs over seconds to minutes, widening arterial diameters to restore wall shear stresses to initial levels. If high flow persists, flow-dependent signals from the ECs remodelling of the artery wall to enlarge the lumens on time scales of weeks to months 12.
Conversely, decreased flow induces vessel narrowing that is also mediated by signals from the endothelium 13. In the extreme case, low flow leads to complete vessel regression, which involves apoptosis of the ECs 14,15. Interestingly, flow is a potent survival signal for ECs, via effects on multiple signalling pathways, which include phosphatidylinositol 3-kinase (PI3K), extracellular signal-regulated kinase 5 (ERK5) and NO 16. In summary, both ECs and VSMCs respond to mechanical forces to modulate artery diameters so that the blood flow meets the demands of the tissues.
Potential mechanotransducers
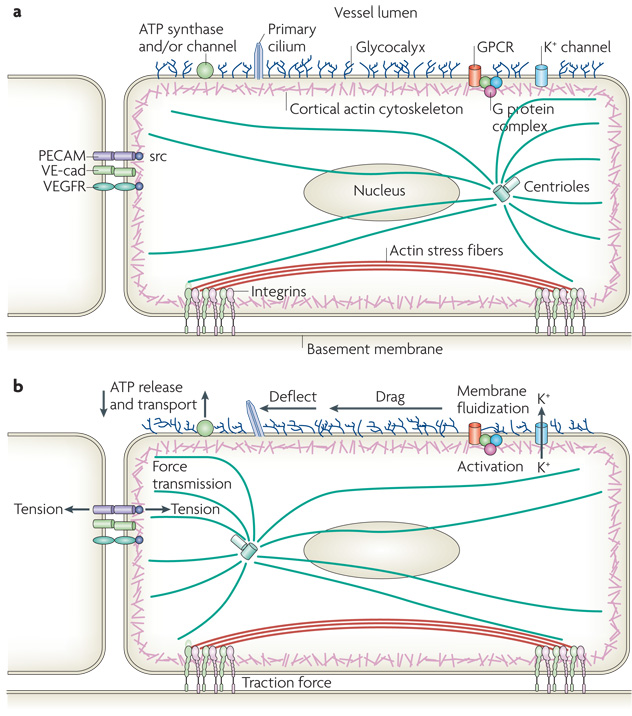
Most of the responses to flow mentioned above are restricted to ECs, which indicates that ECs must express specific mechanotransducers that convert physical stresses into biochemical signals. Many putative mechanotransducers have been proposed to function in sensing flow, including ion channels, integrins, receptor tyrosine kinases, the apical glycocalyx, primary cilia, heterotrimeric G proteins, platelet-endothelial-cell adhesion molecule-1 (PECAM-1) and VE-cadherin 16,17 (Fig 3). However, much work remains to be done before we understand in detail how these various components orchestrate responses to shear stress.
Figure 3. Endothelial mechanotransducers.
a | The upper surface of the endothelium has a carbohydrate-rich glycocalyx that extends several hundred microns into the vessel lumen. A fraction of cells in regions of low shear also have a luminal primary cilium several microns long. G protein coupled receptors (GPCRs), heterotrimeric G proteins and ion channels may also reside in the upper plasma membrane. Cells also have ATP channels and/or cell surface ATP synthase. The lateral cell membrane contains the homophilic adhesion receptors platelet-endothelial-cell adhesion molecule-1 (PECAM-1) and VE-cadherin, which bind their counterparts on adjacent cells. Vascular endothelial growth factor (VEGF) receptor (VEGFR) associates laterally with VE-cadherin in these domains. The cortical actin cytoskeleton, actin stress fibers, microtubules and intermediate filaments (not shown) mechanically connect different regions of the cell. Integrin-dependent complexes anchor the cell to the basement membrane.
b |. Under fluid shear stress, the glycocalyx experiences drag that is transmitted to the cortical cytoskeleton. The cilium is deflected by flow and bends relative to the apical membrane or cytoskeleton. Both ATP release and its transport [what is the significance of the arrow that points down on the left hand side of ATP synthase? Does it indicate ATP transport?] near the cell surface are modified by flow. Tension is transmitted to the lateral borders and basal membrane where adhesion receptors such a PECAM-1 or integrins experience changes in tension. Changes in fluidity of the apical membrane have been observed and may activate potassium channels, GPCRs or G proteins.
Cytoskeleton
The cytoskeleton has an important role in EC responses to shear. Microtubules, actin and intermediate filaments physically connect different regions of the endothelial cell to transmit forces from the apical domain, where shear is applied, to the basal or lateral domains, where mechanotransduction events have been observed 18. Imaging of a green fluorescent protein (GFP) fusion of the intermediate filament protein vimentin demonstrated that flow induces non-homogenous displacements of these filaments within the cells; regions of high strain were most often observed at lateral and basal structures, suggesting transmission of forces to these sites 19. These results therefore support models in which cell-matrix or cell-cell adhesions mediate mechanotransduction (see also article by Geiger in this issue). Consistent with the idea that force from flow is transmitted through the cytoskeleton, inhibition of actin, microtubules or intermediate filaments by drugs or genetic methods blocks many EC responses to flow 20–22. However, there is little evidence that the cytoskeleton functions as a direct mechanotransducer of shear stress per se.
Adhesion receptors
The idea that force is transmitted to lateral structures in the cell was further supported when a protein complex consisting of PECAM-1, VE-cadherin and the transmembrane tyrosine kinase vascular endothelial growth factor (VEGF) receptor, Flk-1, which is found at cell-cell junctions, was found to mediate multiple responses to flow 23. PECAM-1 is a transmembrane immunoglobulin family protein that participates in homophilic adhesion at cell-cell junctions. Evidence implicates PECAM-1 in the activation of a Src family kinase in direct response to force, which is the earliest known event in this pathway. VE-cadherin, which is a classical cadherin specific to ECs, is required for mechanotransduction in this system but, surprisingly, does not require binding to cadherins on other cells; instead, VE-cadherin appears to function as an adaptor that associates with Flk-1 and brings it into proximity to PECAM-1, which facilitates the transactivation of Flk-1 by active Src. This model is consistent with previous studies demonstrating ligand-independent activation of Flk-1 by flow 24,25.
Once activated, Flk-1 recruits and activates PI3K, which mediates crucial downstream signals 25–27. One such signal is the Akt-dependent phosphorylation of endothelial nitric oxide synthase (eNOS), which contributes to stimulation of NO release and vessel relaxation 28. Another important event that is activated downstream of the junctional complex through PI3K is the conformational activation of integrins at the basal surface of cells 27,29. These newly activated integrins then bind to the extracellular matrix (ECM) beneath the ECs. New ECM binding triggers many of the same pathways seen in cells freshly plated on ECM, including activation of the small GTPases Rac, Rho and Cdc42 29–31. Rac, Rho and Cdc42 together cooperate to mediate the alignment of cells in the direction of flow. Activation of Rac also stimulates NF-κB, an inflammatory transcription factor implicated in atherosclerosis 32.
Although a more direct role for integrins in mechanotransduction cannot be excluded, this model of integrin activation by PI3K probably explains published results where integrin antibodies or other perturbations of integrin function inhibit responses to flow 33–36. Although integrins can be conformationally activated by tension, forces from physiological flow are 100–1000 times less than the typical traction forces between a cell and its substratum 37. Thus, it is unlikely that the forces exerted by shear stress could activate integrins on their own, and there is little evidence to suggest that integrins function as direct mechanotransducers in the shear stress response.
Luminal membrane proteins
Elements on the luminal side of the cell that directly experience shear stress may also mediate flow responses. Flow induces an increase in the fluidity of the plasma membrane that is restricted to the upstream sides of cells relative to flow 38. Consistent with this result, both flow and increased membrane fluidity were reported to activate heterotrimeric G proteins 39. G protein activation was suggested to occur either directly 39 or indirectly, via ligand-independent conformational effects on the bradykinin receptor 40. However, the evidence for ligand-dependence versus independence is contradictory. Effects in vitro suggested ligand-independence 40, whereas studies in mice demonstrated that genetic deletion of kinin, the precursor of bradykinin, blocked responses to flow 41. Thus, the role of G proteins or G protein-coupled receptors as direct transducers of flow remains unclear.
Mechanosensitive ion channels have been studied extensively and can also be directly gated by tension within the lipid bilayer; thus, changes in membrane fluidity or tension could trigger signalling through ion channels in cells under flow 42. An inward rectifying potassium channel has been identified that is rapidly activated by shear 43, and the resultant potassium ion flux has been proposed to induce vessel dilation by hyperpolarizing and thereby decreasing contraction of VSMCs 44. Interestingly, activation of these channels is inhibited by high plasma membrane cholesterol 45. This result is consistent with a role for membrane fluidity in channel regulation and also suggests a mechanism by which high plasma cholesterol might interfere with vascular regulation.
Release of ATP is also stimulated by flow and appears to stimulate the ECs in an autocrine fashion by activation of purinergic receptors 46. There is evidence for both release of intracellular ATP via ion channels 47,48 and for synthesis at the extracellular cell surface by ATP synthase by ECs 49. Computational modelling studies suggest that different flow patterns should modulate concentrations of ATP at the cell surface via changes in rates of transport between bulk medium and the cell surface, which suggests that differential signalling in atheroprotected versus atheroprone regions of the vasculature could contribute to endothelial phenotypes 50. But how flow stimulates ATP release and/or synthesis are poorly understood.
Additionally, a dense glycocalyx several hundred microns thick has been identified on the luminal side of the endothelium 51,52. While its composition is not fully characterized, it appears to consist of several types of long proteoglycans anchored to the plasma membrane. The glycocalyx serves as a barrier to both diffusion of solutes in the blood and blood flow. Interestingly, its ability to block flow implies that shear stress is not exerted directly on the plasma membrane but must be transmitted through the glycocalyx 53. Evidence that the glycocalyx participates in mechanotransduction comes from studies in which enzymes that digest proteoglycans, such as heparinase and chondroitinase, inhibited responses to fluid shear stress 53. However, these approaches suffer from the drawback that they would also interfere with proteoglycans at the basal surface of cells that function as co-receptors with integrins for ECM proteins 54. Since integrins are strongly implicated in many responses to flow 37 these treatments may not be specific for apical mechanotransduction. In summary, whereas the role of the glycocalyx in flow-sensing is intriguing, its exact contribution remains uncertain.
Primary cilia
Finally, many cell types contain an apical primary cilium, a microtubule-containing rod several microns long. Primary cilia are implicated in sensing low levels of shear in the kidney 55 and mutations in genes for cilia proteins give rise to polycystic kidney disease, possibly due to failure of kidney tubule cells to sense the flow of urine down the nephron (see article by Lammerding on mechanotransduction in disease in this issue). Interestingly, the cilia-associated proteins polycystin-1 and polycystin-2, that mediate flow-sensing in the kidney, are also expressed in ECs. Both humans and mice with mutations or genetic deletions of polycystin genes have vascular defects 56. Whether primary cilia exist in ECs has been controversial. In arteries they are seen only rarely; on ECs in culture they have been observed, but rapidly disassemble in response to flow 57. However, a recent study showed primary cilia on a modest fraction of ECs at sites that are susceptible to atherosclerosis in vivo 58. Thus, cilia could contribute to sensing the low shear stress present at these regions of arteries. Alternatively, the polycystins also localize to cell-cell junctions 59, and could mediate mechanotransduction from these sites within the cell.
Blood flow and atherosclerosis
Atherogenic flow patterns include low flow, flow separation, gradients, flow reversal and, in limited locations, turbulence. These flow patterns are often grouped under the term ‘disturbed flow’ 60. Regions of disturbed flow in vivo are associated with high rates of both EC proliferation and apoptosis, higher permeability to solutes, failure to align in the direction of flow, increased production of reactive oxygen species (ROS) and increased expression of inflammatory mediators 16,61. In vitro, application of oscillatory or other types of disturbed shear recapitulates these events. Disturbed flow induces expression of leukocyte adhesion receptors such as intracellular adhesion-molecule-1 (ICAM1) and vascular cell adhesion-molecule-1 (VCAM1), and chemokines such as monocyte chemotactic protein 1 (MCP1), which together recruit leukocytes, thereby initiating and maintaining inflammation within the vessel wall 62. These events appear to be largely mechanical, since increased levels of both endothelial adhesion molecules and leukocytes are seen in areas of disturbed flow in wild type, atherosclerosis-resistant mice 63. When additional systemic risk factors are present, monocytes are retained in the vessel wall and differentiate into macrophages that take up lipoproteins to become foam cells. These cells have a more highly activated, inflammatory phenotype; they secrete additional inflammatory mediators and further promote progression toward atherosclerosis 4.
Unidirectional laminar or pulsatile blood flow, as occurs in most of the vascular system, actively suppresses atherogenesis. Compared to cells under static (no flow) conditions, high laminar shear decreases EC turnover, suppresses expression of inflammatory mediators and activates multiple antioxidant pathways that decrease ROS levels 64. Cells exposed to unidirectional flow in vitro or in vivo show expression of multiple atheroprotective genes, many of which are dependent on increased levels of the transcription factor Kruppel-like factor-2 (KLF-2) 65–68. Induction of KLF-2 is itself dependent on the mitogen-activated protein (MAP) kinase Erk5, whose activity is specifically stimulated by atheroprotective flow 69. High laminar shear also promotes EC alignment in the direction of flow, an important adaptive mechanism that alters the way shear acts upon the cytoskeleton. In summary, the localized appearance of plaques at regions of disturbed flow involves two spatially complementary sets of responses: activation of pro-atherosclerotic pathways in regions of disturbed shear and activation of anti-atherosclerotic pathways in regions of laminar, atheroprotective shear (Fig 2). These two mechanisms give rise to the highly local appearance of atherosclerotic plaques in regions of disturbed shear.
Time course of atherogenic events
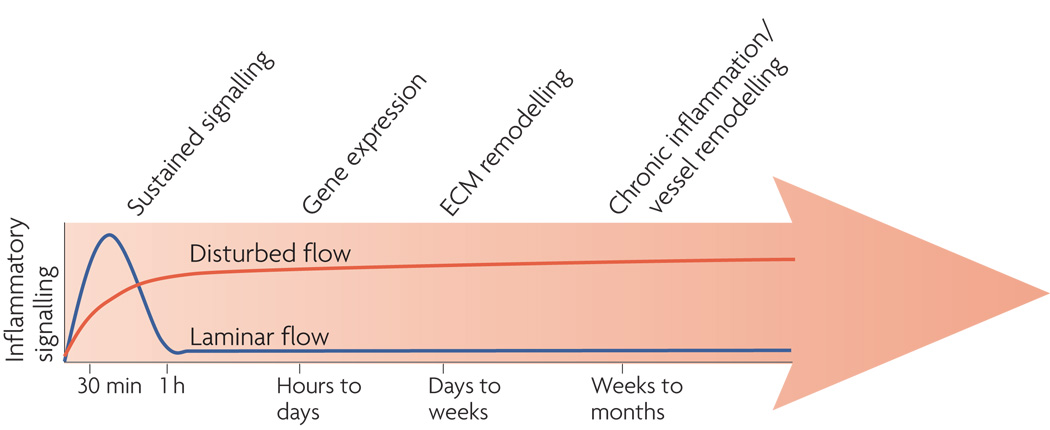
How do the immediate responses to shear over seconds to hours lead to atherosclerotic plaque formation and progression over multiple decades (Fig 4)? A detailed description that spans this time frame is well beyond current knowledge. However, in vitro studies that examine events up to a few days have been informative.
Figure 4. Time course of endothelial activation.
Cells under laminar flow (blue line) transiently activate a variety of inflammatory signaling pathways including production of reactive oxygen species (ROS), c-Jun-N-terminal-kinase (JNK), NF-kB, p21-activated kinase (PAK) and expression of a variety of cytokines. However, these are downregulated over times on the order of 1h. Disturbed shear activates the same pathways in a sustained manner. Changes in gene expression that occur over hours to days sustains oxidative stress and the activated phenotype. Alterations in the extracellular matrix (ECM) that occur over days to weeks lead to engagement of integrins that enhance inflammatory pathways. These changes give rise to a persistently activated phenotype that leads to chronic inflammation and vessel remodelling.
Shear-dependent signalling
To summarize results from many studies, applying laminar shear to a naïve endothelial monolayer initiates rapid signalling events that, as mentioned previously, include opening of ion channels, release of prostacyclin and NO, activation of integrins, production of ROS and activation of many kinases and GTPases. Within an hour, transcription factors are activated or induced, including NF-κB, activator protein-1 (AP1), early growth response-1 (EGR1) and KLF2.
Strikingly, in laminar flow, many of these events are transient. Over one to several hours, production of ROS and activity of many of the kinases, GTPases and transcription factors return to baseline 70. The activity of pro-inflammatory pathways such as NF-κB, ROS and c-Jun-N-terminal-kinase (JNK) eventually go significantly below the baseline defined by cells under static conditions, consistent with the atheroprotective effects of laminar flow. Cell cycle regulators such as cyclins also decrease below baseline and cell division is inhibited. In disturbed flow, many of the same events, including activation of NF-κB, ROS production and expression of pro-inflammatory genes are stimulated; however, in this case the induction is sustained. These pathways and genes are also generally activated in atheroprone regions of arteries in vivo, even in wild type mice that do not develop atherosclerosis 63. These results suggest that the crucial difference between atheroprotective laminar flow and athero-promoting, disturbed flow may be the ability of cells to adapt and downregulate relevant pathways. In support of this idea, the sustained activation of inflammatory events correlates very well with failure of ECs to align in the direction of flow 71.
In addition to disturbed flow, low levels of laminar shear are also atherogenic. In vitro, ECs in low flow (typically <4 dynes/cm2, as opposed to atheroprotective flow above 10 dynes/cm2) fail to align and show sustained activation of atherogenic pathways 72,73. Although activation of inflammatory pathways is less than in oscillatory flow, low flow may well be functionally relevant. A study in hypercholesterolemic apolipoprotein E (ApoE)-deficient mice that develop atherosclerosis in regions of disturbed flow similar to humans showed that imposing low flow on an arterial segment was sufficient to induce atherosclerotic plaque formation in these animals 74. Mechanistically, the observation that EC alignment in the direction of flow requires a higher shear stress than for the activation of atherogenic signalling pathways suggests that alignment requires additional mechanosensors that respond to higher shear forces. A mathematical modelling approach hypothesized that transfer of shear force to focal adhesions could cause directional remodelling of adhesions to promote alignment 75, suggesting that these sites could function as a second mechanosensor. However, the idea has not been explored further.
In vivo, the development and progression of atherosclerotic plaques is a highly complex process, involving multiple risk factors and occurring over decades. The mechanisms involved are beyond both our current understanding and the scope of this review. However, the studies of gene expression and matrix remodelling described below suggest two mechanisms by which early responses to disturbed flow can become entrained and promote disease progression over longer time scales.
Flow patterns and gene expression
Gene expression in ECs under disturbed versus atheroprotective flow has been evaluated in some detail, both in vivo and in vitro. In vitro studies comparing oscillatory to high laminar shear demonstrated that laminar shear induces protective genes that suppress inflammation and oxidative stress 70,76–78. These results correlate with the resistance to oxidative stress and reduced expression of inflammatory mediators seen in cells under atheroprotective flow. Among the flow-sensitive antioxidant genes are heme oxygenase-1, which degrades heme to produce the antioxidants biliverdin and bilirubin; thioredoxin reductase, which reduces hydroperoxides and regenerates the antioxidant form of thioredoxin; and peroxiredoxins, which catalyze the destruction of peroxides and other oxidant species 76,77. High laminar shear also reduces expression of thioredoxin interacting protein, an inhibitor of thioredoxin 78. Thus, shear boosts the EC’s ability to eliminate oxidants by multiple mechanisms. The transcription factor Nrf-1, which is activated and translocates to the nucleus in response to atheroprotective, but not oscillatory flow, mediates activation of several of these genes, which include heme oxygenase-1 and thioredoxin reductase 77.
In vivo analysis of gene expression has given conflicting results. Some studies verified the upregulation of antioxidant gene expression and reduced oxidant production in atheroprotected regions of mouse arteries compared with atheroprone regions 77. However, an unbiased analysis of EC gene expression in different regions of porcine arteries showed upregulation of many of the anti-oxidant genes in atheroprone regions 79. Given the long latency and dependence on additional risk factors for disease progression, it is perhaps not surprising that in vivo analyses have yielded complex results. Overall, the results suggest that disturbed flow biases EC phenotype toward a sustained activated, pro-inflammatory state, which, in the presence of other risk factors, eventually results in atherogenesis.
Extracellular matrix remodelling and inflammation
Matrix remodelling may provide a second mechanism by which early changes in phenotype become fixed to generate sustained alterations in cell behaviour. The normal subendothelial ECM is a basement membrane (BM) consisting mainly of collagen IV, laminin, nidogen and proteoglycans. When a tissue is wounded, injured or inflamed, ECM proteins including fibronectin, fibrinogen and thrombospondin are produced and incorporated into the matrix. These proteins are believed to aid growth and migration to promote wound healing 80,81. BM-binding integrins have distinct signalling properties from fibronectin- and fibrinogen-binding integrins 54. For example, integrins αvβ3 and α5β1, which bind fibronectin and fibrinogen, show strong synergies with growth factor receptors and promote cell cycle progression and cell motility, consistent with a role in wound repair.
Interestingly, fibronectin has been observed in the subendothelial ECM at atheroprone regions of arteries, even in wild-type mice 81. In atheroprone ApoE−/− mice, atherosclerotic progression is associated with increased staining for fibronectin and at later stages, for fibrinogen. Human atherosclerotic plaques are also enriched in fibronectin and fibrinogen 82,83. Since integrin activation and matrix binding are flow-sensitive and mediate a subset of shear signals, these observations suggest that the subendothelial ECM might modulate how EC respond to shear. Indeed, flow triggers activation of p38 MAP kinase in cells adherent to collagen but not fibronectin 81, consistent with data showing that the collagen binding integrin α2β1 specifically activates p38 during cell adhesion 84. Flow also triggers activation of NF-κB in cells on fibronectin or fibrinogen but not on collagen or laminin 81, again resembling the behaviour of this signalling pathway during cell adhesion 85,86. Furthermore, shear stress activates p21-activated kinase (PAK) in cells on fibronectin, but not on collagen or BM proteins, and this activation was associated with control of endothelial monolayer permeability 87.
The importance of matrix remodelling in atherogenesis is supported by an analysis of mice in which the alternatively spliced extra type III domain A (ED-A) of fibronectin was deleted 88. The ED-A domain is normally present in cell-derived fibronectin but is missing from plasma fibronectin, and enhances the ability of fibronectin to assemble into matrix fibrils 89. Atherosclerotic plaques stain with antibodies against this domain, indicating that at least a portion of plaque fibronectin is cell-derived 88. When ED-A−/− mice were crossed into the ApoE−/− background, atherosclerosis was significantly reduced compared to ED-A+/+/ApoE−/− mice, indicating that fibronectin is functionally important in atherosclerosis.
The reasons for ECM remodelling in athero-prone regions are unknown. ECs in culture constitutively assemble a fibronectin matrix instead of a basement membrane, making the problem difficult to study. However, in vitro studies have shown that flow modulates both the levels and organization of ECM proteins 90,91. Moreover, fibronectin expression in ECs can be increased by NF-κB activation 92. These data suggest that disturbed flow initiates some fibronectin production but that further events promote a positive feedback loop. It is tempting to hypothesize that the initial deposition of fibronectin may sensitize ECs to the pro-inflammatory effects of disturbed flow, activating NF-κB to produce more fibronectin and promoting further inflammation. However, further work will be needed to address these issues.
Systemic risk factors and atherosclerosis
A detailed discussion of how the well characterized risk factors for atherosclerosis synergize with flow and with each other to promote atherosclerosis is beyond the subject of this review. In fact, the interplay between biomechanical and clinical risk factors is not well understood. However, some ideas about possible mechanisms of synergy have been proposed.
Hypertension
Hypertension has been identified as a significant risk factor for atherosclerotic plaque formation or progression 93. It may also be an important reason why atherosclerosis is seen in arteries but not veins where blood pressure is much lower. Although blood pressure is not uniform throughout the entire arterial tree, progressively declining with vessel size, it is nearly equivalent in adjacent atheroprone and atheroresistant regions. Thus, as with other risk factors, there appears to be a synergy between flow patterns and hypertension.
Several explanations for the effects of hypertension have been advanced. The MAP kinase JNK and its downstream transcription factor AP-1 are activated by mechanical stretch and can mediate the expression of multiple inflammatory mediators that contribute to leukocyte recruitment in atherosclerosis 94. Activation of JNK by stretch is dependent on the direction of stretch relative to the actin stress fibres, such that stretch along the fibres activates JNK but stretch perpendicular to the fibres does not 95. Properly aligned ECs would therefore show little JNK activation in response to pressure-induced circumferential strain of artery walls. Since Ecs are poorly aligned in regions of disturbed flow, these cells should be more sensitive to stretch-induced activation of this inflammatory pathway.
It has also been proposed that since both stretch and flow are pulsatile, their relative phases are important. Computational analyses showed that shear and strain are in phase in atheroprotected regions, but out of phase in atheroprone regions 96. When pulsatile shear and stretch were applied simultaneously to an endothelial monolayer, altering their phasing had significant effects on NO production, consistent with this hypothesis.
Finally, hypertension also promotes oxidative stress 8. Since the effects of flow and stretch on oxidant production may be additive, combined stretch and disturbed flow could overwhelm the available anti-oxidant machinery. Once the balance shifts toward ROS production, inflammatory pathways leading to JNK and NF-κB would be activated. Additionally, ROS react with and eliminate NO, inhibiting vasorelaxation and the ability to maintain homeostasis 97.
Hyperlipidemia
Hyperlipidemia is a major risk factor for atherosclerosis. High levels of cholesterol carried by low density and very low density lipoproteins (LDL and VLDL) that bring cholesterol to the tissues contribute to atherosclerosis 98. Increased endothelial permeability in atheroprone regions favours deposition of lipoproteins in the vessel wall 87, and matrix remodelling in the plaque increases retention of these components 98. Cholesterol loading of monocytes and macrophages then activates these cells to promote further inflammation. Additionally, oxidant stress synergizes with local permeability/retention of cholesterol, leading to the generation of oxidized LDL 99. A number of the resultant oxidized lipids possess potent cytokine-like activity and further promote inflammation.
Exercise
Sedentary lifestyle is also a significant risk factor. Although exercise has beneficial effects on blood pressure, glucose and lipoprotein levels, the decrease in the incidence of vascular disease is well beyond what can be expected based purely on changes in these other risk factors 100. One possible explanation is that increased cardiac output during exercise increases fluid shear stress within the arteries 101. Higher, atheroprotective levels of shear for even a limited time may increase eNOS expression and thus reduce the inflammatory status of these regions.
Diabetes
Diabetes is also a potent risk factor for atherosclerosis though how it accelerates this disease is largely a mystery. Interestingly, in both human and animal models, diabetes leads to broader, more diffuse and complex lesions 102. Furthermore, elevated glucose is known to stimulate fibronectin gene expression in ECs, as well as widespread deposition of fibronectin beneath the endothelium in vivo 92,103. If, as discussed above, fibronectin sensitizes ECs to the inflammatory effects of flow, then increased fibronectin deposition would be predicted to accelerate inflammation, broadening the affected regions. However, this idea also remains to be tested. Elevated glucose in diabetics also increases production of ROS, and the elevated oxidative stress may accelerate atherosclerosis 104.
Conclusions
The mammalian vasculature is precisely regulated so that oxygen and nutrients are delivered to each tissue according to demand. Circumferential stretch and fluid shear stress from blood flow are among the stimuli that regulate vessel development and physiology. Homeostatic mechanisms operating on time scales from seconds to years regulate vessel diameter and wall thickness in response to changes in applied forces. A number of mechanoreceptors have been proposed to mediate these effects. Subsequent changes in nitric oxide, reactive oxygen, classical signalling pathways involving kinases and GTPases, and multiple transcription factors mediate the responses of endothelial and smooth muscle cells to changes in flow and stretch.
Difficulties arise at regions of arteries where flow patterns develop a variety of disturbances. Atheroprotective effects of high, laminar shear are lost, while low or disturbed flow activates pro-inflammatory pathways. Endothelial cells are unable to adapt to these pathways, resulting in sustained activation of pathways associated with inflammation and tissue remodelling. Local, chronic inflammation synergizes with additional risk factors such as hypertension, high plasma cholesterol, diabetes, etc, leading to more severe inflammation and atherosclerosis.
Despite substantial progress, we still lack answers to major questions. Current and future work is needed to identify all of the EC-specific shear sensors, elucidate how resultant signals are processed and interpreted by cells, and how these signals synergize with systemic risk factors to promote disease progression. Answers to these questions may identify therapeutic targets for specific blockade of pathological inflammation and remodelling without inhibiting physiological mechanisms that govern efficient transport of blood to the tissues.
Box 1.
Box 2.
Acknowledgments
Work from the laboratory of M. S. was supported by NIH grants RO1 HL75092 and 80956 to MAS.
Glossary
- Apolipoprotein E
A major constituent of the high density lipoprotein that carries ‘good cholesterol’ from the tissues to the liver.
- Blood pressure
The hydraulic pressure (force per area) in the blood vessels that is due to the pumping action of the heart. Pressure is highest in the aorta and successively decreases as it travels into smaller arteries, then capillaries then veins. Blood pressure exerts force that causes a circumferential stretch of the vessel wall.
- Foam cell
Macrophage in the artery wall that become engorged with cholesterol ester and triglycerides.
- Hyperlipidemia
Blood carrying high levels of lipoproteins with cholesterol and triglycerides.
- Laplace’s law
This law states that tension (T) in the vessel wall equals the difference in pressure (P) across the vessel times the radius (R) of the vessel divided by the thickness (W) of the wall. Thus, higher blood pressure or vessels of larger radius require thicker walls to be mechanically stable.
- Nephron
The kidney consists of millions of these functional units. Each nephron consists of a glomerulus, where blood is filtered through a specialized basement membrane. The resultant cell-free fluid enters a tube lined with epithelial cells that transport valuable components back into the blood. The remainder is secreted as urine.
- Shear stress
The frictional force per unit area that a fluid exerts when it flows over a surface. This force is parallel to the surface and is proportional to the viscosity and the velocity of the fluid, and inversely proportional to the radius of the vessel.
- Vasorelaxation
Release of factors such as nitric oxide (NO) by the endothelium causes relaxation of the smooth muscle layer and widening of the artery lumen. Extra type III domain A (ED-A)
References
- 1.Hynes RO, Zhao Q. The evolution of cell adhesion. J Cell Biol. 2000;150:F89–F96. doi: 10.1083/jcb.150.2.f89. [DOI] [PubMed] [Google Scholar]
- 2.Hove JR, et al. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- 3. Lucitti JL, et al. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. This work provides rather elegant evidence that fluid shear stress mediates the rearrangement of a primitive vascular plexus into a mature vascular tree in early mouse embryos.
- 4. Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. A good review that discusses the initiation and progression of atherosclerosis with emphasis on genetics, lipids and cellular interactions.
- 5.Birukov KG, et al. Intraluminal pressure is essential for the maintenance of smooth muscle caldesmon and filamin content in aortic organ culture. Arterioscler Thromb Vasc Biol. 1998;18:922–927. doi: 10.1161/01.atv.18.6.922. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Lemus LA, et al. Integrins as unique receptors for vascular control. J Vasc Res. 2003;40:211–233. doi: 10.1159/000071886. [DOI] [PubMed] [Google Scholar]
- 7.Folkow B. Early structural changes in hypertension: pathophysiology and clinical consequences. J Cardiovasc Pharmacol. 1993;22(Suppl 1):S1–S6. [PubMed] [Google Scholar]
- 8.Lehoux S, Castier Y, Tedgui A. Molecular mechanisms of the vascular responses to haemodynamic forces. J Intern Med. 2006;259:381–392. doi: 10.1111/j.1365-2796.2006.01624.x. [DOI] [PubMed] [Google Scholar]
- 9.Campbell WB, Falck JR. Arachidonic acid metabolites as endothelium-derived hyperpolarizing factors. Hypertension. 2007;49:590–596. doi: 10.1161/01.HYP.0000255173.50317.fc. [DOI] [PubMed] [Google Scholar]
- 10.Vanhoutte PM, Boulanger CM, Mombouli JV. Endothelium-derived relaxing factors and converting enzyme inhibition. Am J Cardiol. 1995;76 3E-12E. [PubMed] [Google Scholar]
- 11.Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R546–R552. doi: 10.1152/ajpregu.00491.2005. [DOI] [PubMed] [Google Scholar]
- 12.Di Stefano I, Koopmans DR, Langille BL. Modulation of arterial growth of the rabbit carotid artery associated with experimental elevation of blood flow. J Vasc Res. 1998;35:1–7. doi: 10.1159/000025559. [DOI] [PubMed] [Google Scholar]
- 13.Brownlee RD, Langille BL. Arterial adaptations to altered blood flow. Can J Physiol Pharmacol. 1991;69:978–983. doi: 10.1139/y91-147. [DOI] [PubMed] [Google Scholar]
- 14.Baffert F, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006;290:H547–H559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 15.Meeson A, Palmer M, Calfon M, Lang R. A relationship between apoptosis and flow during programmed capillary regression is revealed by vital analysis. Development. 1996;122:3929–3938. doi: 10.1242/dev.122.12.3929. [DOI] [PubMed] [Google Scholar]
- 16.Li YS, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38:1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 17.Orr AW, Helmke BP, Blackman BR, Schwartz MA. Mechanisms of mechanotransduction. Dev Cell. 2006;10:11–20. doi: 10.1016/j.devcel.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Davies FP. Flow-mediated endothelial mechanotransduction. Physiol Rev. 1995;75:519–560. doi: 10.1152/physrev.1995.75.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Helmke BP, Thakker DB, Goldman RD, Davies PF. Spatiotemporal analysis of flow-induced intermediate filament displacement in living endothelial cells. Biophys J. 2001;80:184–194. doi: 10.1016/S0006-3495(01)76006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffers PM, et al. Altered flow-induced arterial remodeling in vimentin-deficient mice. Arterioscler Thromb Vasc Biol. 2000;20:611–616. doi: 10.1161/01.atv.20.3.611. [DOI] [PubMed] [Google Scholar]
- 21.Hutcheson IR, Griffith TM. Mechanotransduction through the endothelial cytoskeleton: mediation of flow- but not agonist-induced EDRF release. Br J Pharmacol. 1996;118:720–726. doi: 10.1111/j.1476-5381.1996.tb15459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Malek AM, Zhang J, Jiang J, Alper SL, Izumo S. Endothelin-1 gene suppression by shear stress: pharmacological evaluation of the role of tyrosine kinase, intracellular calcium, cytoskeleton, and mechanosensitive channels. J Mol Cell Cardiol. 1999;31:387–399. doi: 10.1006/jmcc.1998.0873. [DOI] [PubMed] [Google Scholar]
- 23. Tzima E, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. This study identifies a complex consisting of PECAM-1, VE-cadherin and VEGFR2 in the pathway leading to integrin activation and induction of NF-kB by flow.
- 24.Jin ZG, et al. Ligand independent activation of VEGF receptor 2 by fluid shear stress regulates activation of endothelial nitric oxide synthesis. Circ Res. 2003;93:354–363. doi: 10.1161/01.RES.0000089257.94002.96. [DOI] [PubMed] [Google Scholar]
- 25.Shay-Salit A. VEGF receptor 2 and the adherens junction as a mechanical transducer in vascular endothelial cells. Proc. Natl. Acad. Sci. 2002;99:9462–9467. doi: 10.1073/pnas.142224299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin ZG, Wong C, Wu J, Berk BC. Flow Shear Stress Stimulates Gab1 Tyrosine Phosphorylation to Mediate Protein Kinase B and Endothelial Nitric-oxide Synthase Activation in Endothelial Cells. J Biol Chem. 2005;280:12305–12309. doi: 10.1074/jbc.M500294200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tzima E, et al. Identification of a mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 28.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 29.Tzima E, Pozo MAD, Shattil SS, Chien S, Schwartz AM. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J. 2001;20:4639–4647. doi: 10.1093/emboj/20.17.4639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzima E, et al. Activation of Rac in endothelial cells in response to fluid shear stress mediates gene expression and cell alignment. EMBO J. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tzima E, Kiosses WB, delPozo MA, Schwartz AM. Localized Cdc42 activation detected using a novel assay mediates MTOC positioning in endothelial cells in response to fluid shear stress. J Biol Chem. 2003;278:31020–31023. doi: 10.1074/jbc.M301179200. [DOI] [PubMed] [Google Scholar]
- 32.Tzima E, et al. Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. Embo J. 2002;21:6791–6800. doi: 10.1093/emboj/cdf688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhullar IS, et al. Fluid shear stress activation of IkB kinase is integrindependent. J. Biol. Chem. 1998;273:30544–30549. doi: 10.1074/jbc.273.46.30544. [DOI] [PubMed] [Google Scholar]
- 34.Chen KD, et al. Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. J. Biol. Chem. 1999;274:18393–18400. doi: 10.1074/jbc.274.26.18393. [DOI] [PubMed] [Google Scholar]
- 35.Muller JM, Chilian WM, Davis MJ. Integrin signaling transduces shear stress--dependent vasodilation of coronary arterioles. Circ. Res. 1997;80:320–326. doi: 10.1161/01.res.80.3.320. [DOI] [PubMed] [Google Scholar]
- 36.Ishida T, Peterson TE, Kovach NL, Berk BC. MAP kinase activation by flow in endothelial cells. Role of beta 1 integrins and tyrosine kinases. Circ. Res. 1996;79:310–316. doi: 10.1161/01.res.79.2.310. [DOI] [PubMed] [Google Scholar]
- 37.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279:12001–12004. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 38.Butler PJ, Norwich G, Weinbaum S, Chien S. Shear stress induces a time- and position-dependent increase in endothelial cell membrane fluidity. Am J Physiol Cell Physiol. 2001;280:C962–C969. doi: 10.1152/ajpcell.2001.280.4.C962. [DOI] [PubMed] [Google Scholar]
- 39.Gudi S, Nolan JP, Frangos JA. Modulation of GTPase activity of G proteins by fluid shear stress and phospholipid composition. Proc Natl Acad Sci U S A. 1998;95:2515–2519. doi: 10.1073/pnas.95.5.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White CR, Frangos JA. The shear stress of it all: the cell membrane and mechanochemical transduction. Philos Trans R Soc Lond B Biol Sci. 2007;362:1459–1467. doi: 10.1098/rstb.2007.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergaya S, et al. Decreased flow-dependent dilation in carotid arteries of tissue kallikrein-knockout mice. Circ Res. 2001;88:593–599. doi: 10.1161/01.res.88.6.593. [DOI] [PubMed] [Google Scholar]
- 42.Maroto R, et al. TRPC1 forms the stretch-activated cation channel in vertebrate cells. Nat Cell Biol. 2005;7:179–185. doi: 10.1038/ncb1218. [DOI] [PubMed] [Google Scholar]
- 43. Hoger JH, Ilyin VI, Forsyth S, Hoger A. Shear stress regulates the endothelial Kir2.1 ion channel. Proc Natl Acad Sci U S A. 2002;99:7780–7785. doi: 10.1073/pnas.102184999. This work shows that an inward rectifying potassium channel opens in response to flow and analyzes the mechanism of activation.
- 44.Zaritsky JJ, Eckman DM, Wellman GC, Nelson MT, Schwarz TL. Targeted disruption of Kir2.1 and Kir2.2 genes reveals the essential role of the inwardly rectifying K(+) current in K(+)-mediated vasodilation. Circ Res. 2000;87:160–166. doi: 10.1161/01.res.87.2.160. [DOI] [PubMed] [Google Scholar]
- 45.Fang Y, et al. Functional expression of Kir2.x in human aortic endothelial cells: the dominant role of Kir2.2. Am J Physiol Cell Physiol. 2005;289:C1134–C1144. doi: 10.1152/ajpcell.00077.2005. [DOI] [PubMed] [Google Scholar]
- 46.Bodin P, Bailey D, Burnstock G. Increased flow-induced ATP release from isolated vascular endothelial cells but not smooth muscle cells. Br J Pharmacol. 1991;103:1203–1205. doi: 10.1111/j.1476-5381.1991.tb12324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Faigle M, Seessle J, Zug S, El Kasmi KC, Eltzschig HK. ATP release from vascular endothelia occurs across Cx43 hemichannels and is attenuated during hypoxia. PLoS ONE 3. 2008:e2801. doi: 10.1371/journal.pone.0002801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabirov RZ, Okada Y. ATP release via anion channels. Purinergic Signal. 2005;1:311–328. doi: 10.1007/s11302-005-1557-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamamoto K, et al. Involvement of cell surface ATP synthase in flow-induced ATP release by vascular endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1646–H1653. doi: 10.1152/ajpheart.01385.2006. [DOI] [PubMed] [Google Scholar]
- 50.Choi HW, Ferrara KW, Barakat AI. Modulation of ATP/ADP concentration at the endothelial surface by shear stress: effect of flow recirculation. Ann Biomed Eng. 2007;35:505–516. doi: 10.1007/s10439-006-9247-9. [DOI] [PubMed] [Google Scholar]
- 51.Smith ML, Long DS, Damiano ER, Ley K. Near-wall micro-PIV reveals a hydrodynamically relevant endothelial surface layer in venules in vivo. Biophys. J. 2003;85:637–645. doi: 10.1016/s0006-3495(03)74507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vink H, Duling BR. Capillary endothelial surface layer selectively reduces plasma solute distribution volume. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H285–H289. doi: 10.1152/ajpheart.2000.278.1.H285. [DOI] [PubMed] [Google Scholar]
- 53.Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- 54.Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci. 2001;114:2553–2560. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- 55.Weimbs T. Polycystic kidney disease and renal injury repair: common pathways, fluid flow, and the function of polycystin-1. Am J Physiol Renal Physiol. 2007;293:F1423–F1432. doi: 10.1152/ajprenal.00275.2007. [DOI] [PubMed] [Google Scholar]
- 56.Kim K, Drummond I, Ibraghimov-Beskrovnaya O, Klinger K, Arnaout MA. Polycystin 1 is required for the structural integrity of blood vessels. Proc Natl Acad Sci U S A. 2000;97:1731–1736. doi: 10.1073/pnas.040550097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol. 2004;164:811–817. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van der Heiden K, et al. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 59.Wilson PD. Polycystin: new aspects of structure, function, and regulation. J Am Soc Nephrol. 2001;12:834–845. doi: 10.1681/ASN.V124834. [DOI] [PubMed] [Google Scholar]
- 60.Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. Jama. 1999;282:2035–2042. doi: 10.1001/jama.282.21.2035. [DOI] [PubMed] [Google Scholar]
- 61.Brooks AR, Lelkes PI, Rubanyi GM. Gene expression profiling of vascular endothelial cells exposed to fluid mechanical forces: relevance for focal susceptibility to atherosclerosis. Endothelium. 2004;11:45–57. doi: 10.1080/10623320490432470. [DOI] [PubMed] [Google Scholar]
- 62.Chappell DC, Varner SE, Nerem RM, Medford RM, Alexander RW. Oscillatory shear stress stimulates adhesion molecule expression in cultured human endothelium. Circ Res. 1998;82:532–539. doi: 10.1161/01.res.82.5.532. [DOI] [PubMed] [Google Scholar]
- 63.Jongstra-Bilen J, et al. Low-grade chronic inflammation in regions of the normal mouse arterial intima predisposed to atherosclerosis. J Exp Med. 2006;203:2073–2083. doi: 10.1084/jem.20060245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berk BC, Abe JI, Min W, Surapisitchat J, Yan C. Endothelial atheroprotective and anti-inflammatory mechanisms. Ann N Y Acad Sci. 2001;947:93–109. doi: 10.1111/j.1749-6632.2001.tb03932.x. discussion 109-11. [DOI] [PubMed] [Google Scholar]
- 65.Dekker RJ, et al. Prolonged fluid shear stress induces a distinct set of endothelial cell genes, most specifically lung Kruppel-like factor (KLF2) Blood. 2002;100:1689–1698. doi: 10.1182/blood-2002-01-0046. [DOI] [PubMed] [Google Scholar]
- 66.Wang N, et al. Shear stress regulation of Kruppel-like factor 2 expression is flow pattern-specific. Biochem Biophys Res Commun. 2006;341:1244–1251. doi: 10.1016/j.bbrc.2006.01.089. [DOI] [PubMed] [Google Scholar]
- 67.Dekker RJ, et al. KLF2 provokes a gene expression pattern that establishes functional quiescent differentiation of the endothelium. Blood. 2006;107:4354–4363. doi: 10.1182/blood-2005-08-3465. [DOI] [PubMed] [Google Scholar]
- 68.SenBanerjee S, et al. KLF2 Is a novel transcriptional regulator of endothelial proinflammatory activation. J Exp Med. 2004;199:1305–1315. doi: 10.1084/jem.20031132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parmar KM, et al. Integration of flow-dependent endothelial phenotypes by Kruppel-like factor 2. J Clin Invest. 2006;116:49–58. doi: 10.1172/JCI24787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hsieh HJ, et al. Increase of reactive oxygen species (ROS) in endothelial cells by shear flow and involvement of ROS in shear-induced c-fos expression. J. Cell. Physiol. 1998;175:156–162. doi: 10.1002/(SICI)1097-4652(199805)175:2<156::AID-JCP5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 71.Cicha I, Goppelt-Struebe M, Yilmaz A, Daniel WG, Garlichs CD. Endothelial dysfunction and monocyte recruitment in cells exposed to non-uniform shear stress. Clin Hemorheol Microcirc. 2008;39:113–119. [PubMed] [Google Scholar]
- 72.Zhao S, et al. Synergistic effects of fluid shear stress and cyclic circumferential stretch on vascular endothelial cell morphology and cytoskeleton. Arterioscler Thromb Vasc Biol. 1995;15:1781–1786. doi: 10.1161/01.atv.15.10.1781. [DOI] [PubMed] [Google Scholar]
- 73.Mohan S, Mohan N, Sprague EA. Differential activation of NF-kB in human aortic endothelial cells conditioned to specific flow environments. Am. J. Physiol. (Cell Physiol.) 1997;273:C572–C578. doi: 10.1152/ajpcell.1997.273.2.C572. [DOI] [PubMed] [Google Scholar]
- 74.Cheng C, et al. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 75.Civelekoglu-Scholey G, et al. Model of coupled transient changes of Rac, Rho, adhesions and stress fibers alignment in endothelial cells responding to shear stress. J. Theor. Biol. 2005;232:569–585. doi: 10.1016/j.jtbi.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 76.Mowbray AL, Kang DH, Rhee SG, Kang SW, Jo H. Laminar shear stress up-regulates peroxiredoxins (PRX) in endothelial cells: PRX 1 as a mechanosensitive antioxidant. J Biol Chem. 2008;283:1622–1627. doi: 10.1074/jbc.M707985200. [DOI] [PubMed] [Google Scholar]
- 77.Dai G, et al. Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ Res. 2007;101:723–733. doi: 10.1161/CIRCRESAHA.107.152942. [DOI] [PubMed] [Google Scholar]
- 78. Yamawaki H, Pan S, Lee RT, Berk BC. Fluid shear stress inhibits vascular inflammation by decreasing thioredoxin-interacting protein in endothelial cells. J Clin Invest. 2005;115:733–738. doi: 10.1172/JCI200523001. An important study that reveals a novel mechanism by which laminar shear stress inhibits oxidative stress and inflammatory activation of endothelial cells.
- 79.Passerini AG, et al. Coexisting proinflammatory and antioxidative endothelial transcription profiles in a disturbed flow region of the adult porcine aorta. Proc Natl Acad Sci U S A. 2004;101:2482–2487. doi: 10.1073/pnas.0305938101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Canfield AE, et al. The involvement of matrix glycoproteins in vascular calcification and fibrosis: an immunohistochemical study. J Pathol. 2002;196:228–234. doi: 10.1002/path.1020. [DOI] [PubMed] [Google Scholar]
- 81.Orr AW, et al. The subendothelial extracellular matrix modulates NF-kappaB activation by flow: a potential role in atherosclerosis. J Cell Biol. 2005;169:191–202. doi: 10.1083/jcb.200410073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shekhonin BV, Domogatsky SP, Idelson GL, Koteliansky VE, Rukosuev VS. Relative distribution of fibronectin and type I, III, IV, V collagens in normal and atherosclerotic intima of human arteries. Atherosclerosis. 1987;67:9–16. doi: 10.1016/0021-9150(87)90259-0. [DOI] [PubMed] [Google Scholar]
- 83.Smith EB. Fibrinogen, fibrin and fibrin degradation products in relation to atherosclerosis. Clin Haematol. 1986;15:355–370. [PubMed] [Google Scholar]
- 84.Klekotka PA, Santoro SA, Zutter MM. alpha 2 integrin subunit cytoplasmic domain-dependent cellular migration requires p38 MAPK. J Biol Chem. 2001;276:9503–9511. doi: 10.1074/jbc.M006286200. [DOI] [PubMed] [Google Scholar]
- 85.Klein S, et al. Alpha 5 beta 1 integrin activates an NF-kappa B-dependent program of gene expression important for angiogenesis and inflammation. Mol Cell Biol. 2002;22:5912–5922. doi: 10.1128/MCB.22.16.5912-5922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Scatena M, et al. NF-kappaB mediates alphavbeta3 integrin-induced endothelial cell survival. J Cell Biol. 1998;141:1083–1093. doi: 10.1083/jcb.141.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Orr AW, et al. Matrix-specific p21-activated kinase activation regulates vascular permeability in atherogenesis. J Cell Biol. 2007;176:719–727. doi: 10.1083/jcb.200609008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tan MH, et al. Deletion of the alternatively spliced fibronectin EIIIA domain in mice reduces atherosclerosis. Blood. 2004;104:11–18. doi: 10.1182/blood-2003-09-3363. [DOI] [PubMed] [Google Scholar]
- 89.Guan JL, Trevithick JE, Hynes RO. Retroviral expression of alternatively spliced forms of rat fibronectin. J Cell Biol. 1990;110:833–847. doi: 10.1083/jcb.110.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thoumine O, Nerem RM, Girard PR. Changes in organization and composition of the extracellular matrix underlying cultured endothelial cells exposed to laminar steady shear stress. Lab Invest. 1995;73:565–576. [PubMed] [Google Scholar]
- 91.Thoumine O, Nerem RM, Girard PR. Oscillatory shear stress and hydrostatic pressure modulate cell-matrix attachment proteins in cultured endothelial cells. In Vitro Cell Dev Biol Anim. 1995;31:45–54. doi: 10.1007/BF02631337. [DOI] [PubMed] [Google Scholar]
- 92.Chen S, Mukherjee S, Chakraborty C, Chakrabarti S. High glucose-induced, endothelin-dependent fibronectin synthesis is mediated via NF-kappa B and AP-1. Am J Physiol Cell Physiol. 2003;284:C263–C272. doi: 10.1152/ajpcell.00192.2002. [DOI] [PubMed] [Google Scholar]
- 93.Jankowski P, Bilo G, Kawecka-Jaszcz K. The pulsatile component of blood pressure: its role in the pathogenesis of atherosclerosis. Blood Press. 2007;16:238–245. doi: 10.1080/08037050701428166. [DOI] [PubMed] [Google Scholar]
- 94.Adhikari N, Charles N, Lehmann U, Hall JL. Transcription factor and kinase-mediated signaling in atherosclerosis and vascular injury. Curr Atheroscler Rep. 2006;8:252–260. doi: 10.1007/s11883-006-0081-1. [DOI] [PubMed] [Google Scholar]
- 95. Kaunas R, Usami S, Chien S. Regulation of stretch-induced JNK activation by stress fiber orientation. Cell Signal. 2006 doi: 10.1016/j.cellsig.2006.02.008. This study demonstrates a fascinating dependence of JNK activation on the orientation of mechanical stretch relative to the actin stress fibers.
- 96.Dancu MB, Tarbell JM. Large Negative Stress Phase Angle (SPA) attenuates nitric oxide production in bovine aortic endothelial cells. J Biomech Eng. 2006;128:329–334. doi: 10.1115/1.1824120. [DOI] [PubMed] [Google Scholar]
- 97.Harrison DG, et al. Endothelial mechanotransduction, nitric oxide and vascular inflammation. J Intern Med. 2006;259:351–363. doi: 10.1111/j.1365-2796.2006.01621.x. [DOI] [PubMed] [Google Scholar]
- 98.Maxfield FR, Tabas I. Role of cholesterol and lipid organization in disease. Nature. 2005;438:612–621. doi: 10.1038/nature04399. [DOI] [PubMed] [Google Scholar]
- 99.Stocker R, Keaney JF., Jr Role of oxidative modifications in atherosclerosis. Physiol Rev. 2004;84:1381–1478. doi: 10.1152/physrev.00047.2003. [DOI] [PubMed] [Google Scholar]
- 100.Blair SN, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. Jama. 1996;276:205–210. [PubMed] [Google Scholar]
- 101.Britten MB, Zeiher AM, Schachinger V. Clinical importance of coronary endothelial vasodilator dysfunction and therapeutic options. J Intern Med. 1999;245:315–327. doi: 10.1046/j.1365-2796.1999.00449.x. [DOI] [PubMed] [Google Scholar]
- 102.Gaba MK, Gaba S, Clark LT. Cardiovascular disease in patients with diabetes: clinical considerations. J Assoc Acad Minor Phys. 1999;10:15–22. [PubMed] [Google Scholar]
- 103.Kaur H, et al. Diabetes-induced extracellular matrix protein expression is mediated by transcription coactivator p300. Diabetes. 2006;55:3104–3111. doi: 10.2337/db06-0519. [DOI] [PubMed] [Google Scholar]
- 104.Stephens JW, Khanolkar MP, Bain SC. The biological relevance and measurement of plasma markers of oxidative stress in diabetes and cardiovascular disease. Atherosclerosis. 2008 doi: 10.1016/j.atherosclerosis.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 105.Rossant J, Howard L. Signaling pathways in vascular development. Ann. Rev. Cell Dev. Biol. 2002;18:541–573. doi: 10.1146/annurev.cellbio.18.012502.105825. [DOI] [PubMed] [Google Scholar]
- 106.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]