Abstract
The budding yeast formins, Bnr1 and Bni1, behave very differently with respect to their interactions with muscle actin. However, the mechanisms underlying these differences are unclear, and these formins do not interact with muscle actin in vivo. We use yeast wild type and mutant actins to further assess these differences between Bnr1 and Bni1. Low ionic strength G-buffer does not promote actin polymerization. However, Bnr1, but not Bni1, causes the polymerization of pyrene-labeled Mg-G-actin in G-buffer into single filaments based on fluorometric and EM observations. Polymerization by Bnr1 does not occur with Ca-G-actin. By cosedimentation, maximum filament formation occurs at a Bnr1:actin ratio of 1:2. The interaction of Bnr1 with pyrene-labeled S265C Mg-actin yields a pyrene excimer peak, from the cross-strand interaction of pyrene probes, which only occurs in the context of F-actin. In F-buffer, Bnr1 promotes much faster yeast actin polymerization than Bni1. It also bundles the F-actin in contrast to the low ionic strength situation where only single filaments form. Thus, the differences previously observed with muscle actin are not actin isoform-specific. The binding of both formins to F-actin saturate at an equimolar ratio, but only about 30% of each formin cosediments with F-actin. Finally, addition of Bnr1 but not Bni1 to pyrene-labeled wild type and S265C Mg-F actins enhanced the pyrene- and pyrene-excimer fluorescence, respectively, suggesting Bnr1 also alters F-actin structure. These differences may facilitate the ability of Bnr1 to form the actin cables needed for polarized delivery of nutrients and organelles to the growing yeast bud.
Bni1 and Bnr1 are the two formin isoforms expressed in Saccharomyces cerevisiae (1, 2). These proteins, as other isoforms in the formin family, are large multidomain proteins (3, 4). Several regulatory domains, including one for binding the G-protein rho, are located at the N-terminal half of the protein (4–7). FH1, FH2, and Bud6 binding domains are located in the C-terminal half of the protein (8). The formin homology 1 (FH1)2 domain contains several sequential poly-l-proline motifs, and it interacts with the profilin/actin complex to recruit actin monomers and regulate the insertion of actin monomers at the barbed end of actin (9–11). The fomin homology domain 2 (FH2) forms a donut-shaped homodimer, which wraps around actin dimers at the barbed end of actin filaments (12, 13). One important function of formin is to facilitate actin polymerization by stabilizing actin dimers or trimers under polymerization conditions and then to processively associate with the barbed end of the elongating filament to control actin filament elongation kinetics (13–18).
A major unsolved protein in the study of formins is the elucidation of the individual functions of different isoforms and their regulation. In vivo, these two budding yeast formins have distinct cellular locations and dynamics (1, 2, 19, 20). Bni1 concentrates at the budding site before the daughter cell buds from the mother cell, moves along with the tip of the daughter cell, and then travels back to the neck between daughter and mother cells at the end of segregation. Bnr1 localizes only at the neck of the budding cell in a very short period of time after bud emergence. Although a key cellular function of these two formins in yeast is to promote actin cable formation (8, 18), the roles of the individual formins in different cellular process is unclear because deleting either individual formin gene has limited impact on cell growth and deleting both genes together is lethal (21).
Although each of the two formins can nucleate actin filament formation in vitro, the manner in which they affect polymerization is distinctly isoform-specific. Most of this mechanistic work in vitro has used formin fragments containing the FH1 and FH2 domains. Bni1 alone processively caps the barbed end of actin filaments partially inhibiting polymerization at this end (14, 16, 18). The profilin-actin complex, recruited to the actin barbed end through its binding to Bni1 FH1 domain, possibly raises the local actin concentration and appears to allow this inhibition to be overcome, thereby, accelerating barbed end polymerization. It has also been shown that this complex modifies the kinetics of actin dynamics at the barbed end (9, 11, 18, 22). Moreover, Bni1 participation leads only to the formation of single filaments (8). In comparison, the Bnr1 FH1-FH2 domain facilitates actin polymerization much more efficiently than does Bni1. Moseley and Goode (8) showed Bnr1 accelerates actin polymerization up to 10 times better than does Bni and produces actin filament bundles when the Bnr1/actin molar ratio is above 1:2. Finally, the regulation of Bni1 and Bnr1 by formin binding is different. For example, Bud 6/Aip3, a yeast cell polarity factor, binds to Bni1, but not Bnr1, and also stimulates its activity in vitro.
For their studies, Moseley and Goode (8) utilized mammalian skeletal muscle actin instead of the S. cerevisiae actin with which the yeast formins are designed to function. It is entirely possible that the differences observed with the two formins are influenced quantitatively or qualitatively by the nature of the actin used in the study. This possibility must be seriously considered because although yeast and muscle actins are 87% identical in sequence, they display marked differences in their polymerization behavior (23). Yeast actin nucleates filaments better than muscle actin (24, 25). It appears to form shorter and more flexible filaments than does muscle actin (26, 27). Finally, the disposition of the Pi released during the hydrolysis of ATP that occurs during polymerization is different. Yeast actin releases its Pi concomitant with hydrolysis of the bound ATP whereas muscle actin retains the Pi for a significant amount of time following nucleotide hydrolysis (28, 29). This difference is significant because ADP-Pi F-actin has been shown to be more stable than ADP F-actin (30). Another example of this isoform dependence is the interaction of yeast Arp2/3 with yeast versus muscle actins (31). Yeast Arp2/3 complex accelerates polymerization of muscle actin only in the presence of a nucleation protein factor such as WASP. However, with yeast actin, no such auxiliary protein is required. In light of these actin behavioral differences, to better understand the functional differences of these two formins in vivo, we have studied the behavior of Bni 1 and Bnr 1 with WT and mutant yeast actins, and we have also explored the molecular basis underlying the Bnr 1-induced formation of actin nuclei from G-actin.
EXPERIMENTAL PROCEDURES
Protein Preparation
WT yeast actin and S265C yeast actin were prepared from frozen cells as described previously (32). Modification of the actins with N-(1-pyrenyl) maleimide (Sigma) on C374 of WT actin and C265C and C374 of S265C actin was carried out according to Feng et al. (33). The purified yeast actins were stored in the Ca2+-form in Ca-G-buffer (10 mm Tris-HCl, pH 7.5, 0.2 mm CaCl2, 0.2 mm ATP, and 1 mm DTT) for no more than a week. His-tagged FH1-FH2 fragments from the yeast formins Bnr1 and Bni1 were prepared in yeast strain BGY 502 from Gal-induced overexpression plasmids generously provided by Dr. Bruce Goode, Brandeis University, as previously described (8, 34). Purified formin fragments were stored in stock buffer (10 mm Tris, pH 7.5, 150 mm KCl, 0.2 mm MgCl2, and 1 mm DTT) at a concentration of 5–10 μm at −20 °C. Samples were retained no more than a week at 4 °C after thawing.
Formin/G-actin Binding, Actin Polymerization, and Fluorescence Measurements
Mg-G-actin was prepared by diluting Ca-G-actin at least 10-fold with Mg-G-buffer containing 10 mm Tris-HCl, pH 7.5, 0.2 mm MgCl2, 0.2 mm ATP, 100 μm EGTA, and 1 mm DTT at room temperature 2 min prior to use. To assay the binding of G-actin, different concentrations of formin were incubated with 1 μm G-actin in either Ca- or Mg-G-buffer. To induce actin polymerization, G-actin and formin were preincubated at room temperature for at least 15 min, and polymerization was induced by the addition of MgCl2 and KCl to final concentrations of 2 mm and 50 mm, respectively. The change in actin-pyrene fluorescence due to the binding of formin or to actin polymerization was recorded with either a Florolog-3 or a FlouroMax-3 instrument (Jobin Yvon-Spex). The excitation wavelength was 365 nm. The change in fluorescence intensity at emission wavelength 386 nm was recorded over time for kinetics analyses, and the emission spectrum from 375 nm to 600 nm was also recorded before and after each reaction. To calculate the net change in fluorescence intensity, the final intensity values at emission wavelength either 386 nm or 485 nm (pyrene excimer) of each reaction was subtracted from the control value obtained with actin alone. To normalize the change in fluorescence intensity, the net change in fluorescence with formin was divided by the net change of the control reaction.
Cosedimentation Assays
To assay the binding of formin to G-actin in G-buffer, the formin at the desired concentration was mixed with 1 μm Mg-G-actin in Mg-G-buffer in a final volume of 120 μl for 20 min at room temperature. For interaction with F-actin, the mixture of G-actin and formin was allowed to polymerize by the addition of salts at room temperature from 30 min as described above. The reaction solutions were centrifuged in a Beckman TL-100 ultracentrifuge in a TLA.1 rotor at 80 K rpm for 15 min at 25 °C. The supernatant was carefully removed, and the pellet was resuspended in 30 μl of Mg-G-buffer. The whole pellet sample and a quarter of the supernatant were analyzed by SDS-PAGE on 10% polyacrylamide gels, and the bands were stained with Coomassie Blue. Gels were quantitated by densitometry using an Epson Perfection 2450 Photo scanner (Epson America Inc.) with 600 dpi resolution. The intensity (histogram count) of the actin and formin bands in the image was further quantified by ImageJ (NIH) software making sure the density was in the linear detection range of the instrument. The ratio of formin to actin in each pellet was determined using the density values normalized to the MW of the two proteins.
Electron Microscopy
A 3-μl aliquot of the appropriate actin solution was deposited on a carbon-coated Formvar grid, which was then negatively stained with 1% uranyl acetate. The sample was observed on a JEOL 1230 transmission electron microscope in the University of Iowa Central Electron Microscopy Facility. The lengths of the filaments in the images were measured by ImageJ (NIH). At least 100 filaments from each sample were measured, and the statistic analysis was done by using Excel (Microsoft).
RESULTS
Interaction of Bnr1 and Bni1 with Pyrene-labeled Yeast G-actin
To begin to assess a possible difference in the ability of the two yeast formins to interact with G-actin, we examined their interaction with yeast G-actin in the Mg-form labeled at Cys-374 with pyrene maleimide. Because of the abundance of Mg2+ in the cell, this is the most physiologically relevant form of the actin. However, because actin conformation is known to be affected by the nature of the cation at the high affinity cation binding site (35, 36), we also examined the interaction of the formins with Ca-G-actin. This is the form in which actins are generally purified because it has been well established that the nature of the bound divalent cation can markedly affect actin polymerization properties.
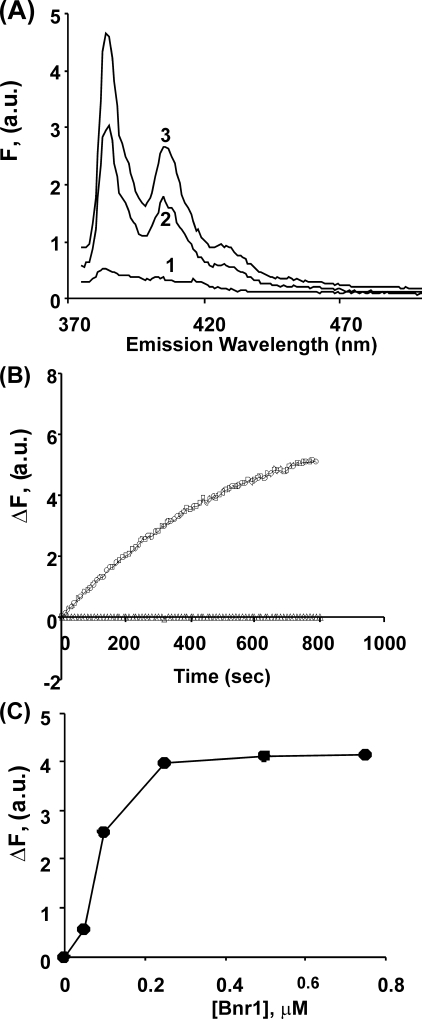
Addition of Bnr1 to Mg-G-actin in low ionic strength buffer caused a dose-dependent increase in actin pyrene fluorescence (Fig. 1A). If this fluorescence increase was due solely to the interaction of the formin with actin monomers, the increase in fluorescence would be expected to occur almost instantaneously, such as occurs in the binding of pyrene-labeled G-actin to profilin (26). However, this was not the case. Fig. 1B shows a slow increase in fluorescence in the presence of Bnr1 that plateaus over about 800 s, a kinetic profile possibly suggestive of actin polymerization but without the nucleation phase often observed. A titration curve with increasing amounts of Bnr1 against a fixed amount of actin shows saturation at a level of one Bnr1 monomer per two actins (Fig. 1C). Contrary to the case with Mg-actin, no increase in fluorescence was observed following the addition of Bnr1 to Ca-G-actin (data not shown). To determine whether this reaction was formin-specific, we repeated the experiments with Mg- and Ca-G-actin using Bni1. In this case, no increase in fluorescence was observed with either form of actin.
FIGURE 1.
Interaction of Bnr1 with pyrene-labeled Mg-G-actin. Panel A, aliquots of 0.5 μm pyrene-labeled Mg-G-actin were combined with increasing amounts of Bnr1 (line 1: 0 μm, line 2: 0.1 μm, and line 3: 0.5 μm) in Mg-G-buffer conditions (10 mm Tris-HCl pH 7.5, 0.2 mm MgCl2, 0.2 mm ATP, 100 μm EGTA, and 1 mm DTT), and the solutions were incubated at room temperature for 15 min at room temperature. The fluorescence emission spectra were recorded from 375 to 500 nm following excitation at 365 nm. Panel B, pyrene-labeled Mg G-actin was combined with Bnr1 (Δ: no Bnr1, and ○: 0.5 μm) in Mg-G-buffer, and the increase in pyrene fluorescence intensity at emission wavelength 385 nm was recorded over time. Excitation wavelength was 365 nm. Panel C, net change pyrene fluorescence intensity at emission wavelength 385 nm from panel A was plotted against the concentration of Bnr1 as described under “Experimental Procedures.” The experiments shown in each panel have been repeated twice with essentially the same results.
Electron Microscopy of G-actin/Bnr1 Mixtures
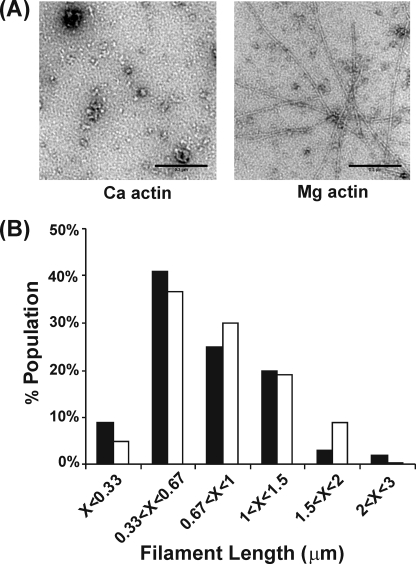
To further assess the possibility that Bnr1 induces actin polymerization in Mg-G-buffer, we examined negatively stained samples of G-actin/Bnr1 mixtures by electron microscopy. With Ca-G-actin (Fig. 2A), we observed only small non-descript aggregates that might be due to the actin alone. On the other hand, with Mg-G-actin under low ionic strength conditions, abundant single filamentous structures, not bundles, were observed. Length measurements show ranges of 0.2–3 μm with an average of about 0.8 μm (Fig. 2B), independent of the Bnr1/actin ratio. These filaments are about one-fourth the length of F-actin filaments formed in F-buffer in the absence of formin (26). With Bni1, no such filaments were observed, only the small non-descript aggregates (data not shown).
FIGURE 2.
Electron microscopic analysis of the structure and length of the Bnr1/G-actin complex. Panel A, either 1 μm Ca- or Mg-G-actin was mixed with 1 μm Bnr1 in either Ca- or Mg-G-buffer, respectively, at room temperature for 15 min, and the samples from each reaction were observed by EM as described under “Experimental Procedures.” Bar = 0.2 μm. Panel B, distribution of filament length in the reaction of 1 μm Mg-G-actin with Bnr1 (solid column: 0.1 μm, and open column: 1 μm) were determined by measuring at least 100 filaments per sample by ImageJ (NIH), and the distribution of filament length was analyzed with Excel (Microsoft).
Nature of the Bnr1/G-actin Filaments
Although Bnr1 can apparently facilitate the formation of filaments with the appearance of F-actin, we wished to gain further insight into the nature of these filaments. For these experiments, we utilized a mutant form of yeast actin we had previously characterized, S265C (33). Position 265 is near the tip of a hydrophobic loop between actin subdomains 3 and 4. Labeling of this actin with pyrene maleimide introduces two mol of probe per mol of actin, one at Cys-265 and one at Cys-374. In the filament, the pyrene at 265 interacts with the pyrene on Cys-374 of a monomer on the apposing strand of the two-stranded actin helix across the interstrand space. This interaction produces a new pyrene excimer fluorescence signal at 485 nm, which forms only in the context of F-actin.
Fig. 3 demonstrates that the combination of Bnr1 with Mg pyrene-labeled S265 yeast actin produces such an excimer band at 485 nm, identical to that observed with F-actin alone. The intensity of this band increases in a Bnr1 concentration-dependent manner saturating at or near a Bnr1/actin ratio of 1:2, based on the significant overlap of error bars for the final points on the saturation curve. This result is identical to that seen with the 385-nm peak using pyrene-labeled WT and S265C actins (Figs. 3B and 1C, respectively).
FIGURE 3.
Interaction of pyrene S265C G-actin with Bnr1. Panel A, pyreneS265C Mg-G-actin, 1 μm, was mixed with Bnr1 (line 1: no formin and line 2: 1 μm) in Mg- G-buffer at room temperature for 15 min, and the fluorescence emission spectra were recorded from 370–600 nm following excitation at 365 nm. Panel B, normalized net change in fluorescence at 485 nm from the titration was plotted against the concentration of Bnr1 in each reaction. The data are averaged from three individual experiments.
Sedimentation Analysis of the Bnr1/G-actin Interaction
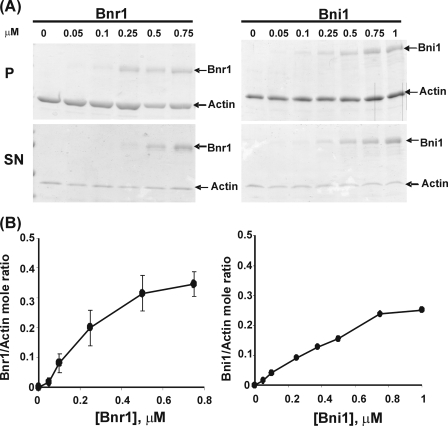
We next wished to gain information concerning the efficiency with which Bnr1 could induce G-actin to polymerize. Toward this aim, we assessed the extent of filament formation as a function of increasing Bnr1 concentration by centrifugation analysis. Samples of the two proteins were mixed, filaments were collected by centrifugation under conditions normally sufficient to pellet F-actin, and the distribution of the two proteins was assessed by SDS-PAGE followed by densitometric analysis of the Coomassie Blue-stained protein bands. Fig. 4A shows that increasing Bnr1 led to increasing amounts of actin in the pellet fraction until about 50–60% of the total actin had been pelleted at saturating formin levels. Assessment of the relative amount of Bnr1 and actin in the pellet fraction (Fig. 4B), again based on substantial overlap of the error bars for the final points on the saturation curve, showed the ratio increased until reaching a plateau value of about 1:2 as we observed in the fluorescence experiments in Figs. 1C and 3B.
FIGURE 4.
Cosedimentation analysis of the Bnr1/G-actin interaction. Panel A, Mg-G-actin, 1 μm, was mixed with different concentrations of Bnr1 (as indicated on the top of the gels) G-buffer conditions at room temperature for 20 min, and the mixture was then centrifuged. The entire pellet fraction (P) and one-fourth of the supernatant fraction (SN) were analyzed by 10% SDS-PAGE and Coomassie Blue staining. Details of the protocols are described under “Experimental Procedures.” Panel B, intensity of the actin and Bnr1 bands in the pellet fractions of each individual experiment were quantified by densitometry and corrected for the loading differences. The molar ratio of formin to actin in the pellet was calculated based on the intensity of each bands, corrected for differences in MW of the proteins, and plotted on the y axis against the Bnr1 concentration as described under “Experimental Procedures.” The data are averaged from four individual experiments.
Interaction of Bnr1 with TMR-modified Actin
Our work to this point demonstrates that formin can overcome the inhibition of actin polymerization caused by low ionic strength solutions. We next wanted to assess the extent to which other types of disruption of normal monomer-monomer interactions could be overcome by Bnr1 binding. We thus assessed the effects of Bnr1 on actin modified by tetramethylrhodamine (TMR) at Cys-374. Previous studies have shown that this actin is incapable of polymerization under normal polymerizing (F-salt) conditions (37, 38). We added Bnr1 to a mixture of Mg-G-actin containing 90% TMR-actin and 10% pyrene-labeled actin and recorded the change in pyrene fluorescence as a function of time. No increase in fluorescence was observed in the pyrene peak (data not shown), either in a G-actin sample or following addition of F-salts. EM examination of the samples before and after the addition of salt shows no identifiable filaments, only small aggregates (data not shown).
Salt-induced Polymerization of Yeast Actin in the Presence of Bnr1 or Bni1
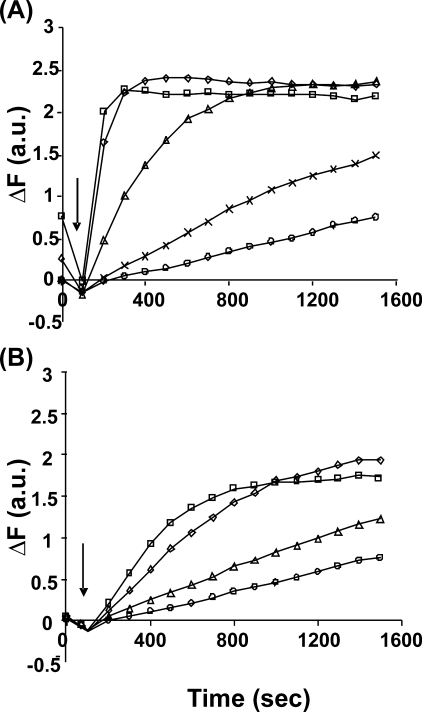
Previous work comparing the effects of the two yeast formins on actin polymerization utilized pyrene-labeled muscle actin (8). We thus wished to assess the differential effects of Bni1 and Bnr1 on the salt-induced polymerization of Cys-374 pyrene-labeled yeast actin. Fig. 5 demonstrates that, as seen previously with muscle actin, Bnr1 is much more effective than Bni1 in promoting actin polymerization. However, we saw a striking difference between the two formins in the extent of the change in pyrene fluorescence that occurred. With three different actin preparations, the total pyrene fluorescence change with Bnr1 was always about 25% greater than that caused by Bni1 and WT actin alone. To determine if this difference reflected a difference in the extent of polymerization with the two formins, we carried out a cosedimentation analysis on the material at the end of the reaction to verify possible changes in the amount of actin polymerization. Analysis of the actin amount in the pellet and supernatant fractions in the presence and absence of formin showed no significant difference in the extent of polymerization (Fig. 6). Thus, the fluorescence difference has to reflect a difference in the environment sampled by the actin probe in the presence of the two formins. Examination of the final states of the polymerization reactions by electron microscopy (Fig. 7) showed that, as reported before (34), in F-buffer Bnr1 bundled actin but Bni1 did not. However, the enlarged image in Fig. 7B demonstrated that the Bnr1-mediated actin bundle is not well organized.
FIGURE 5.
Copolymerization of G-actin with either Bnr1orBni1. Mg-G-actin (5% pyrene-labeled), 1 μm, was mixed with either Bnr1 (panel A) or Bni1 (panel B) for 15 min and was then induced to polymerize by the addition of MgCl2 and KCl to final concentrations of 2 mm and 50 mm, respectively. The increase in pyrene fluorescence over time was recorded as described under “Experimental Procedures.” These experiments have been repeated three times with essentially identical results, and only one data set is presented. Panel A, Bnr1 concentrations are no formin (○), 3.3 μm (Õ), 10 μm (▵), 50 μm (♢), and 150 μm (□). Panel B, Bnr1 concentrations are no formin (○), 10 μm (▵), 50 μm (♢), and 150 μm (□). Arrow, time of addition of salt.
FIGURE 6.
Cosedimentation assay for the binding of yeast formins to Mg-F-actin. 1 μm Mg-actin was copolymerized in Mg-G-buffer at room temperature for 30 min with yeast formin at different concentrations as indicated on the top of the gel, and then the mixture was centrifuged. The pellet (P) and supernatant (SN) were analyzed by 10% SDS-PAGE and Coomassie Blue staining as described as under “Experimental Procedures.” Panel B, the intensity of each actin and formin band in the pellet was quantified by densitometry. The molar ratio of formin to actin in the pellet was calculated based on the intensity of each protein band, corrected for their differences in MW, and plotted on the y axis against the Bnr1 concentration as described under “Experimental Procedures.” The Bnr1 binding data are averaged from three individual experiments, and Bni1 binding data are from two individual experiments.
FIGURE 7.
EM analysis of Mg-F-actin mixed with either Bnr1 or Bni1. 1 μm Mg-actin was polymerized in the presence of 0.25 μm of either Bnr1 (panels A and B) or Bni1 (panel C) at room temperature for 30 min. The product from each reaction was examined by EM as described under “Experimental Procedures.” Panel B, enlarged Bnr1/F actin bundle compared with the bundle in panel A. Bar = 0.2 μm.
Cosedimentation data (Fig. 6) show that the interaction of both formins with F-actin is saturable. Interestingly, at saturation, the ratio of formin: actin in the pellet was about 1:3 for Bnr1 and 1:4 for Bni1 suggesting that only limited binding sites are available on the actin filament.
Effect of Bnr1 on F-actin Structure
The difference in fluorescence change caused by the two formins during the copolymerization experiments suggested that formin binding to F-actin could alter its conformation. To test this possibility, we assessed the effect of Bnr1 on pyrene-labeled S265C F-actin by copolymerizing Bnr1 with S265C actin in F-salts and examining the emission fluorescence spectrum at the steady state. Fig. 8A shows that the addition of Bnr1 results in about a 40% maximum increase in the pyrene excimer fluorescence, indicative of a significant change in filament conformation as a result of the binding. Under the same condition, Bni1 produced no change (data not shown). We then assessed the effects of increasing Bnr1 concentration on excimer fluorescence. Fig. 8B demonstrates a saturable effect at a Bnr1:actin ratio of 1:5 that levels off substantially below the ratio (about 1:5 of Bnr1 to actin) at which saturable G-actin binding of the formin occurs.
FIGURE 8.
Pyrene-excimer resulting from the copolymerization of pyrene-S265C Mg-actin and Bnr1 in F-salts. Panel A, 1 μm pyreneS265C Mg-actin was copolymerized with Bnr1 (line 1: no formin and line 2: 0. 5 μm) following induction of polymerization with MgCl2 and KCl, and the fluorescence emission spectrum of this reaction was recorded from 375 to 600 nm using an excitation wavelength of 365 nm. Panel B, normalized net change in fluorescence intensity at 485 nm was plotted against the concentration of Bnr1 as described under “Experimental Procedures.” These experiments have been repeated twice with essentially same results, and the data presented here are the average of these two experiments.
DISCUSSION
We initially wished to gain insight into the mechanism underlying the difference in activity of the yeast formins Bnr1 and Bni1 toward muscle actin and to determine if these differences were dependent on the actin isoform used. Our initial experiments with yeast actin produced a very unexpected result. Addition of Bnr1, but not Bni 1, to yeast actin in low ionic strength conditions (G-buffer) led to actin filament formation. Furthermore, the pyrene-excimer results with labeled S265C yeast actin showed these filaments had a relatively normal F-actin structure. We observed the same result with muscle actin indicating that differences in formin behavior were inherent to the formins themselves. In their studies (8), Moseley and Goode had also demonstrated an increase pyrene fluorescence when they added Bnr 1 to their muscle actin sample just prior to adding salts to induce polymerization. They hypothesized that this may have resulted from some type of nucleus formation but did not further investigate the cause of this fluorescence increase.
Muscle actin, as opposed to yeast actin, will polymerize in the presence of F-buffer containing Ca2+ instead of Mg2+ (25), suggesting that the calcium form of muscle G-actin might be closer in conformation than yeast actin to that required for inducement of polymerization. However, the Bnr1-dependent polymerization of either actin in G-buffer required the actin in its Mg2+ form. Evidently, even with muscle actin, the potential stabilization of actin monomer-monomer conformation afforded by Bnr1 was insufficient to push calcium actin to a polymerization-competent state in the absence of added salt. The inability of Bnr1 to polymerize TMR-modified actin in G-buffer, even in the Mg2+ form, is further evidence of the actin structural limitations needed for low ionic strength polymerization to occur.
Two routes to this Bnr1-facilitated G-actin polymerization could theoretically be envisioned. Bnr1 could nucleate the filament causing a change in filament end conformation, which would allow elongation without a need for additional Bnr1, even in the absence of higher ionic strength conditions, in essence a propagated effect along the elongating filament. Alternatively, some type of Bnr1 binding along the entire length of the filament under G-buffer conditions stabilizes the filament as it is forming. Results of our titration experiments show that both of these alternatives might be involved. Increasing formin results in increasing filament formation until saturation is reached at a molar ratio of one FH2 domain per two actin monomers. However, by cosedimentation, the ratio of Bnr1:actin in the pelleted fraction increased as a function of the amount of added formin. This result suggests that initial stabilization of a filament nucleus by the formin under G-buffer conditions leads to an actin conformational change that propagates well beyond where the formin binds. Bugyi et al. (39) reached a similar conclusion based the application of an anisotropy decay assay using pyrene-labeled muscle actin in the presence of the mouse formin mDia 1 under F-buffer conditions. Finally, under G-buffer conditions, pelletable actin with the Bnr1 was only about 50–60% of the actin that would normally sediment under the usual F-buffer conditions. These results, coupled with the decreased length of the Bnr-1 G-actin filaments compared with normal yeast F-actin (26), suggest that filaments formed in G-buffer in the presence of Bnr1 have a higher critical concentration than does normal F-actin. Together, these findings suggest that a mechanism for Bnr1-initiated filament formation under G-buffer conditions is nucleation followed by elongation and subsequent polymer stabilization by the available formin in solution due to filament side binding. An alternative mechanism would involve the annealing of small formin-initiated actin oligomers. However, we cannot judge the relative contribution of these two routes to overall polymerization at the present time.
Mosley and Goode (8) showed that the addition of Bnr1 but not Bni1 to preformed muscle F-actin resulted in filament bundle formation. We found the same to be true when we copolymerized each of these formins with yeast actin in F-buffer. However, we observed no bundles with Bnr1 under G-buffer conditions, suggesting that bundle formation might require higher ionic strengths to allow access of the formin bundling motif to the surface of the actin filament.
Based on experiments with the mammalian bundling forming FLR1, Harris et al. (40) suggested that FLR1-dependent bundling resulted from the interaction of a basic patch of residues on the formin ring with an acidic patch on a neighboring filament. If the two apposing protein faces were constitutively exposed, and this simple hypothesis was correct, an increase in ionic strength should work to inhibit bundling, the opposite of what we observed. The formation of filament bundles in F- but not G-buffer gives credence to the idea that an ionic strength-dependent conformation change in either the formin or the actin is required for formin interaction with the side of the actin filament. A large body of data does suggest that ionic strength is a major determinant of actin conformation in agreement with this hypothesis (41, 42).
Our experiments demonstrated that the two yeast formins could differentially alter the conformation of yeast F-actin. Our pyrene fluorescence data, showing a greater change induced by Bnr1 compared with Bni1, suggest one of two possibilities. Either the Bnr1 preferentially interacts somehow with the probe, directly altering its fluorescence, or it causes a conformational change in the filament that secondarily alters the environment of the pyrene leading to an increase in its fluorescence yield.
Further insight into this differential alteration of filament conformation was obtained when we polymerized pyrene-S265C actin in the presence of Bnr1 in F-buffer resulting in a 40% greater pyrene excimer signal than that obtained with actin alone. One possible reason for this observation is that the interaction of the formin with the actin on the outside of the filament caused a propagated conformation change in the strand-strand interface within the filament leading to an increase in the total number of overlapping pyrene probes and hence a greater excimer signal. Alternatively, the formin may have produced a change in the environment of existing interacting pyrenes leading to improved inter-probe energy transfer. Significantly, a similar change was not caused by Bni1 binding, even though polymerization extent was the same as with Bnr1 and the formin:actin ratio in the pelleted fractions were nearly the same in both cases. Further evidence supporting a Bnr1-induced filament conformation change was the fact that the maximum increase in excimer fluorescence was reached at a Bnr1:actin ratio far below the 1:5 observed for saturable binding. This observation suggested that the interaction of Bnr1 with a pre-existing filament can exert a substantial propagated effect on conformation along the filament length.
In summary, our results show an enhanced ability of Bnr1 over Bni 1 to affect actin structure and behavior. Not only does it nucleate and bundle filaments under physiological conditions, but we show that its interaction with actin is strong enough to polymerize actin under G-conditions. We also show that it alters the conformation of preformed actin filaments, suggesting it can induce propagated changes along these filaments. The role of Bnr1 in vivo is to aid in the formation of polarized actin cables as tracks for optimal delivery of vesicles to the new cell bud. Our data suggest that the ability of Bnr1 to bundle filaments and to alter their conformation may play a role in the ability of actin binding regulatory proteins to properly facilitate cable deposition.
This work was supported, in whole or in part, by National Institutes of Health Grant GM211105 (to P. A. R.).
- FH1
- formin homology 1
- WT
- wild type
- DTT
- dithiothreitol
- TMR
- tetramethylrhodamine
- EM
- electron microscopy.
REFERENCES
- 1.Evangelista M., Pruyne D., Amberg D. C., Boone C., Bretscher A. ( 2002) Nat. Cell Biol. 4, 260– 269 [DOI] [PubMed] [Google Scholar]
- 2.Sagot I., Rodal A. A., Moseley J., Goode B. L., Pellman D. ( 2002) Nat. Cell Biol. 4, 626– 631 [DOI] [PubMed] [Google Scholar]
- 3.Kovar D. R. ( 2006) Curr. Opin. Cell Biol. 18, 11– 17 [DOI] [PubMed] [Google Scholar]
- 4.Goode B. L., Eck M. J. ( 2007) Annu. Rev. Biochem. 76, 593– 627 [DOI] [PubMed] [Google Scholar]
- 5.Wallar B. J., Alberts A. S. ( 2003) Trends Cell Biol. 13, 435– 446 [DOI] [PubMed] [Google Scholar]
- 6.Li F., Higgs H. N. ( 2003) Curr. Biol. 13, 1335– 1340 [DOI] [PubMed] [Google Scholar]
- 7.Evangelista M., Zigmond S., Boone C. ( 2003) J. Cell Sci. 116, 2603– 2611 [DOI] [PubMed] [Google Scholar]
- 8.Moseley J. B., Goode B. L. ( 2005) J. Biol. Chem. 280, 28023– 28033 [DOI] [PubMed] [Google Scholar]
- 9.Neidt E. M., Scott B. J., Kovar D. R. ( 2009) J. Biol. Chem. 284, 673– 684 [DOI] [PubMed] [Google Scholar]
- 10.Ezezika O., Younger N. S., Lu J., Kaiser D. A., Corbin Z. A., Nolen B. J., Kovar D. R., Pollard T. D. ( 2009) J. Biol. Chem. 284, 2088– 2097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kovar D. R., Wu J. Q., Pollard T. D. ( 2005) Mol. Biol. Cell 16, 2313– 2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu J., Meng W., Poy F., Maiti S., Goode B. L., Eck M. J. ( 2007) J. Mol. Biol. 369, 1258– 1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y., Moseley J. B., Sagot I., Poy F., Pellman D., Goode B. L., Eck M. J. ( 2004) Cell 116, 711– 723 [DOI] [PubMed] [Google Scholar]
- 14.Kovar D. R., Pollard T. D. ( 2004) Proc. Natl. Acad. Sci. U. S. A. 101, 14725– 14730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero S., Le Clainche C., Didry D., Egile C., Pantaloni D., Carlier M. F. ( 2004) Cell 119, 419– 429 [DOI] [PubMed] [Google Scholar]
- 16.Zigmond S. H., Evangelista M., Boone C., Yang C., Dar A. C., Sicheri F., Forkey J., Pring M. ( 2003) Curr. Biol. 13, 1820– 1823 [DOI] [PubMed] [Google Scholar]
- 17.Harris E. S., Li F., Higgs H. N. ( 2004) J. Biol. Chem. 279, 20076– 20087 [DOI] [PubMed] [Google Scholar]
- 18.Moseley J. B., Sagot I., Manning A. L., Xu Y., Eck M. J., Pellman D., Goode B. L. ( 2004) Mol. Biol. Cell 15, 896– 907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pruyne D., Gao L., Bi E., Bretscher A. ( 2004) Mol. Biol. Cell 15, 4971– 4989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallen E. A., Caviston J., Bi E. ( 2000) Mol. Biol. Cell 11, 593– 611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozaki-Kuroda K., Yamamoto Y., Nohara H., Kinoshita M., Fujiwara T., Irie K., Takai Y. ( 2001) Mol. Cell. Biol. 21, 827– 839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neidt E. M., Skau C. T., Kovar D. R. ( 2008) J. Biol. Chem. 283, 23872– 23883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubenstein P. A. ( 1990) Bioessays 12, 309– 315 [DOI] [PubMed] [Google Scholar]
- 24.Buzan J. M., Frieden C. ( 1996) Proc. Natl. Acad. Sci. U. S. A. 93, 91– 95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim E., Miller C. J., Reisler E. ( 1996) Biochemistry 35, 16566– 16572 [DOI] [PubMed] [Google Scholar]
- 26.McKane M., Wen K. K., Meyer A., Rubenstein P. A. ( 2006) J. Biol. Chem. 281, 29916– 29928 [DOI] [PubMed] [Google Scholar]
- 27.Belmont L. D., Orlova A., Drubin D. G., Egelman E. H. ( 1999) Proc. Natl. Acad. Sci. U. S. A. 96, 29– 34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melki R., Fievez S., Carlier M. F. ( 1996) Biochemistry 35, 12038– 12045 [DOI] [PubMed] [Google Scholar]
- 29.Yao X., Rubenstein P. A. ( 2001) J. Biol. Chem. 276, 25598– 25604 [DOI] [PubMed] [Google Scholar]
- 30.Orlova A., Egelman E. H. ( 1992) J. Mol. Biol. 227, 1043– 1053 [DOI] [PubMed] [Google Scholar]
- 31.Wen K. K., Rubenstein P. A. ( 2005) J. Biol. Chem. 280, 24168– 24174 [DOI] [PubMed] [Google Scholar]
- 32.Wen K. K., Giardina P. C., Blake M. S., Edwards J., Apicella M. A., Rubenstein P. A. ( 2000) Biochemistry 39, 8638– 8647 [DOI] [PubMed] [Google Scholar]
- 33.Feng L., Kim E., Lee W. L., Miller C. J., Kuang B., Reisler E., Rubenstein P. A. ( 1997) J. Biol. Chem. 272, 16829– 16837 [DOI] [PubMed] [Google Scholar]
- 34.Moseley J. B., Maiti S., Goode B. L. ( 2006) Methods Enzymol. 406, 215– 234 [DOI] [PubMed] [Google Scholar]
- 35.Kuang B., Rubenstein P. A. ( 1997) J. Biol. Chem. 272, 1237– 1247 [DOI] [PubMed] [Google Scholar]
- 36.Gershman L. C., Selden L. A., Kinosian H. J., Estes J. E. ( 1994) Adv. Exp. Med. Biol. 358, 35– 49 [DOI] [PubMed] [Google Scholar]
- 37.Kudryashov D. S., Phillips M., Reisler E. ( 2004) Biophys. J. 87, 1136– 1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pelikan Conchaudron A., Didry D., Le K. H., Larquet E., Boisset N., Pantaloni D., Carlier M. F. ( 2006) J. Biol. Chem. 281, 24036– 24047 [DOI] [PubMed] [Google Scholar]
- 39.Bugyi B., Papp G., Hild G., Lõrinczy D., Nevalainen E. M., Lappalainen P., Somogyi B., Nyitrai M. ( 2006) J. Biol. Chem. 281, 10727– 10736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris E. S., Rouiller I., Hanein D., Higgs H. N. ( 2006) J. Biol. Chem. 281, 14383– 14392 [DOI] [PubMed] [Google Scholar]
- 41.Tang J. X., Janmey P. A. ( 1996) J. Biol. Chem. 271, 8556– 8563 [DOI] [PubMed] [Google Scholar]
- 42.Hosek M., Tang J. ( 2004) X. Physical Review E 69, 051907 [DOI] [PubMed] [Google Scholar]