Abstract
Adult Upis ceramboides do not survive freezing in the summer but tolerate freezing to −60 °C in midwinter. The accumulation of two cryoprotective polyols, sorbitol and threitol, is integral to the extraordinary cold-hardiness of this beetle. U. ceramboides are the only animals known to accumulate high concentrations of threitol; however, the biosynthetic pathway has not been studied. A series of 13C-labeled compounds was employed to investigate this biosynthetic pathway using 13C{1H} NMR spectroscopy. In vivo metabolism of 13C-labeled glucose isotopomers demonstrates that C-3—C-6 of glucose become C-1—C-4 of threitol. This labeling pattern is expected for 4-carbon saccharides arising from the pentose phosphate pathway. In vitro experiments show that threitol is synthesized from erythrose 4-phosphate, a C4 intermediate in the PPP. Erythrose 4-phosphate is epimerized and/or isomerized to threose 4-phosphate, which is subsequently reduced by a NADPH-dependent polyol dehydrogenase and dephosphorylated by a sugar phosphatase to form threitol. Threitol 4-phosphate appears to be the preferred substrate of the sugar phosphatase(s), promoting threitol synthesis over that of erythritol. In contrast, the NADPH-dependent polyol dehydrogenase exhibits broad substrate specificity. Efficient erythritol catabolism under conditions that promote threitol synthesis, coupled with preferential threitol biosynthesis, appear to be responsible for the accumulation of high concentrations of threitol (250 mm) without concomitant accumulation of erythritol.
Cold-hardy terrestrial insects from temperate to arctic regions exhibit two physiological strategies to survive subzero temperatures: freeze tolerance and freeze avoidance (1–3). Freeze-tolerant organisms survive ice in their extracellular fluids but are generally susceptible to intracellular freezing (3). Freeze-avoiding insects do not survive freezing of their body fluids and typically supercool beyond the lowest environmental temperature they are likely to experience in nature (4).
Both freeze-tolerant and freeze-avoiding insects commonly produce polyols, also known as alditols, to mitigate the effects of low temperature (5). Polyols are biocompatible solutes that lack toxicity, even at molar concentrations, and stabilize native protein structure at low temperature (6). However, the functions of polyols depend on the overwintering strategy. In freeze-tolerant organisms, polyols reduce the fraction of frozen water, thereby preventing excessive intracellular dehydration. In contrast, polyols promote supercooling in freeze-avoiding organisms (5). Glycerol is the most commonly accumulated polyol in both freeze-tolerant and freeze-avoiding insects. However, overwintering insects are taxonomically diverse and produce various polyols in response to low temperature, including ethylene glycol, erythritol, mannitol, ribitol, sorbitol, and threitol (6).
The accumulation of sorbitol and threitol in the hemolymph of the freeze-tolerant beetle, Upis ceramboides, is associated with increasing cold tolerance (7). In the summer, when neither sorbitol nor threitol are detectable in the hemolymph, U. ceramboides do not survive freezing. As sorbitol and threitol accumulate in the hemolymph, there is a concomitant decrease in lethal temperature to −60 °C (8).
Polyols are typically produced in the fat body by the actions of two enzymes, a NADPH-dependent polyol dehydrogenase and a sugar phosphatase, on a sugar phosphate (e.g. glucose 6-phosphate) in constitutive metabolic pathways such as glycolysis or the pentose phosphate pathway (PPP)2 (9). However, threitol biosynthesis does not fit this pattern because T4P, the putative metabolic precursor of threitol, is not observed in normal constitutive metabolism. Threitol is a biologically rare compound, and U. ceramboides are the only organisms known to accumulate it at high concentrations (∼250 mm) (7, 10), although low concentrations (≤2 μg/mg fresh mass or ∼3 mm) have been reported in the overwintering spruce bark beetle, Ips typographus (11).
The threitol biosynthetic pathway has not been fully characterized in any organism. In humans, threitol is the major end product of xylose catabolism and is thought to be produced via the glucuronate pathway (12). Interestingly, xylans, containing predominantly β-(1→4)-linked xylose, are the major saccharide component of hemicellulose in plants (13). Thus, the wood-decomposing U. ceramboides have a potential dietary source of xylose, which could serve as the metabolic precursor of threitol.
Despite the absence of experimental evidence, it has been assumed that threitol production in insects involves the PPP. In addition to being a source of 4-carbon monosaccharides, flux through the PPP generates the reducing equivalents (NADPH) needed for polyol biosynthesis (9). However, the broad specificities observed for polyol dehydrogenases (14, 15) and sugar phosphatases (16, 17) lead to the question of how high concentrations of threitol arise from the PPP without the accumulation of other C4 and C5 alditols. Indeed, the ability of various insect species to accumulate high concentrations of a single C4 or C5 alditol originating from the PPP has not been investigated (9).
In this study, the threitol biosynthetic pathway in U. ceramboides has been investigated by 13C{1H} NMR spectroscopy using 13C-labeled metabolites. The results elucidate the threitol biosynthetic pathway and lead to a new metabolic model that explains the observed selective accumulation of threitol.
EXPERIMENTAL PROCEDURES
Collection of Insects
Adult U. ceramboides were collected in early to midwinter from near Fairbanks, Alaska. Insects were shipped overnight on cold packs, frozen at −20 °C, to the University of Notre Dame. If necessary, the insects were stored at −20 °C in darkness for up to 3 weeks to mimic the winter conditions in Fairbanks. Insects typically regained coordinated movement within 1 h of thawing at 15 °C. To reduce endogenous polyols to undetectable levels, all winter-collected insects were deacclimated for 4 days at 15 °C on a short photoperiod (4 h light/20 h dark) and stored at 4 °C for at least 3 days prior to experimental use. In contrast to many other species of insects, U. ceramboides are able to synthesize polyols after deacclimation or during any time of year (7).
Collection of Hemolymph
U. ceramboides collected in mid-January (avg. mass ∼150 mg) were bled by removing the elytra and wings and applying gentle pressure to the abdomen. Hemolymph pooled at the lesions created by the removal of the elytra/wings and was drawn up into 10-μl capillary tubes, typically ∼10–40 μl/individual. The hemolymph was removed from the capillary tubes by centrifuging at 1000 × g and consolidated in a 1.5-ml microcentrifuge tube. 600 μl of hemolymph was combined with 60 μl of 99% 2H2O in a 5-mm NMR tube prior to NMR analysis.
In Vivo Saccharide Metabolism
To monitor the metabolism of 13C-labeled saccharides, 4.5 μl of 13C-labeled aldose or alditol solution (∼330 mm for aldotetroses, otherwise 1–2 m; Omicron Biochemicals, Inc.) were injected directly into the hemolymph of a deacclimated U. ceramboides with a 5-μl Hamilton syringe. Following the injection of aldose, each beetle was acclimated at −4 °C for 7 h to 7 days to follow alditol production. In contrast, beetles injected with alditol were acclimated at 23 °C for 2–48 h to monitor the disappearance of alditol. Alternatively, alditol-injected beetles were acclimated at 4 °C for 5 days or at 0 °C for 6 days. After acclimation, the elytra and wings of each beetle were removed so that intact gut could be dissected out before homogenization. Each beetle (1–2/sample) was homogenized in 520 μl of 40 mm sodium phosphate buffer (pH 7.5 at 0 °C) and sonicated with a W-385 sonicator (Heat Systems-Ultrasonics, Inc.) for two 10-s intervals at power level 3. The homogenate was subsequently heated in a boiling water bath for 5 min to inactivate enzymes. After cooling on ice, 100 μl of chloroform was added to the homogenate, and the mixture was vortexed and subsequently centrifuged at 10,000 rpm for 20 min. The supernatant was removed and placed into a 5-mm NMR tube (Wilmad) containing 65 μl of 2H2O (Cambridge Isotope Laboratories, Inc.). The final volume was adjusted to 650 μl with phosphate buffer, and the sample was analyzed by 13C{1H} NMR spectroscopy.
Intermediary Threitol Biosynthesis
Polyol synthesis occurs within the insect fat body (9), the organ analogous to the vertebrate liver. However, the putative intermediate metabolites of threitol biosynthesis (E4P and T4P) are phosphorylated; thus, it was unlikely that these compounds would be transported across cell membranes. Intermediary threitol metabolism was therefore investigated in an in vitro cell-free extract of U. ceramboides.
In vitro metabolism samples were prepared in individual 5-mm NMR tubes with the following stock solutions: 60 μl of the appropriate d-[1-13C]aldose 4-phosphate; 65 μl of 99% 2H2O; 10 μl each of aqueous NADH and NADPH (Sigma-Aldrich) at 7 mg/ml; 515 μl of insect homogenate (recipe follows); and 0.8 or 1.6 μl of ∼2 m l-[1-13C]glucose (Omicron Biochemicals, Inc.) for the [1-13C]E4P and [1-13C]T4P experiments, respectively. Relative signal intensities were normalized to an internal control, the C-1 signal of β-l-[1-13C]glucose. The metabolism samples were made with pooled insect homogenate to reduce variability, and each reaction was replicated twice. For background spectra, deionized water was substituted for the tetrose 4-phosphates, reducing equivalents, and l-[1-13C]glucose solutions. Immediately before addition to the NMR tubes, the pH of each [1-13C]aldose 4-phosphate solution was adjusted to 5–7 with the dropwise addition of ice-cold 1 m NaOH with efficient stirring.
Ideally, the fat body should be the only tissue included in the in vitro homogenate, as it is the site of alditol synthesis. However, the fat body in U. ceramboides is not a consolidated organ and is difficult to remove. Thus, the whole body of the insect was homogenized after the gut was removed (elytra and wings also removed in the process) to prevent contamination with bacteria and/or proteolytic enzymes. The dissected beetle was placed in a 1.5-ml microcentrifuge tube and homogenized using a pair of small dissecting scissors in 520 μl of ice-cold 40 mm phosphate buffer (pH 7.5 at 0 °C) containing the following antioxidants/protease inhibitors (Sigma-Aldrich): 2 mg/ml glutathione, 1 mg/ml soybean trypsin inhibitor, 0.25 mg/ml antipain, 0.25 mg/ml chymostatin, 0.25 mg/ml leupeptin, 0.2 mg/ml aprotinin, and 0.05 mg/ml pepstatin A. Following homogenization, the homogenates were maintained on ice and sonicated with a W-385 sonicator for two 10-s intervals at power level 3. After sonication, 100 μl of chloroform (Sigma-Aldrich) was added to each homogenate to remove triglyceride contamination. Each homogenate was then vortexed and subsequently centrifuged at 10,000 rpm for 20 min at 4 °C. The aqueous supernatants from all insects (n = 6) were pooled, diluted with additional ice-cold buffer as necessary, and vortexed before being transferring into 5-mm NMR tubes. For the heat-treated control, 515 μl of pooled homogenate was transferred into 1.5-ml centrifuge tubes, placed in a boiling water bath for 5 min, and subsequently centrifuged for 10 min at 10,000 rpm to remove the precipitated protein prior to addition to the NMR tube. All in vitro aldose phosphate reactions were maintained at −2.5 °C in a cooling bath over the course of 7 days. Samples were transported on ice and maintained on ice until placed into the NMR spectrometer.
Synthesis of 13C-labeled Metabolites
d-[1-13C]Aldotetrose phosphates were prepared by the addition of K13CN to d-glyceraldehyde 3-phosphate to form two C-2-epimeric aldotetrononitrile phosphates, which were separated chromatographically before reduction to the tetrose 4-phosphates (18). The following modifications were made to the published protocol. Over-reduction of [1-13C]erythrononitrile 4-phosphate to [1-13C]erythritol 4-phosphate was observed after 8 h of reduction (∼40% over-reduction). A corresponding over-reduction was not observed with [1-13C]threononitrile 4-phosphate until the reduction time was increased to 16 h. In both cases, the alditol phosphates were separated from the aldose phosphates during final chromatography on DEAE-Sephadex A-25 anion exchange resin in the acetate form. Elution of the alditol 4-phosphate immediately preceded that of the aldose 4-phosphate, although some overlap of the two compounds was observed. The fractions containing predominantly alditol phosphate or aldose phosphate, as determined by 13C{1H} NMR analysis, were pooled. 13C NMR analysis of the alditol phosphates yielded chemical shifts consistent with the presence of phosphate at C-4 (Table 1). The alditol phosphates were used as standards to identify the products of in vitro tetrose 4-phosphate metabolism. Organic phosphate concentrations were determined by colorimetric assay (19).
TABLE 1.
13C chemical shifts of standard compounds in aqueous solution at 22 °C
Chemical shifts are expressed in parts per million (ppm) and are accurate to ±0.01 ppm. Spectra were referenced externally to d-[1-13C]mannose in 2H2O. The more intense signal, C-1 of α-d-mannopyranose, was set to 95.54 ppm. To externally reference the chemical shifts reported here to 2,2-dimethyl-2-silapentane-5-sulfonic acid dissolved in 2H2O, add 1.06 ppm.
| pH | C-1 | C-2 | C-3 | C-4 | C-5 | C-6 | |
|---|---|---|---|---|---|---|---|
| β-d-Glucopyranosea | 93.6 | 73.1 | 74.4 | 71.2 | 73.0 | 62.2 | |
| α-d-Glucopyranosea | 97.4 | 75.8 | 77.4 | 71.2 | 77.5 | 62.4 | |
| α,α-d-Trehalosea | 94.8 | 72.7 | 74.2 | 71.3 | 73.7 | 62.2 | |
| d-Sorbitola | 64.1 | 74.5 | 71.3 | 72.7 | 72.6 | 64.5 | |
| d-Erythrose 4-phosphate (hydrate)b | 1.5 | 90.3 | 73.8 | 71.4c | 68.0c | ||
| d-Erythritol 4-phosphated | ∼2 | 64.2 | 72.9 | 72.3c | 67.7c | ||
| d-Erythritole | 64.13 | 73.44 | 73.44 | 64.13 | |||
| d-Threose 4-phosphate (hydrate)b | 4.5 | 90.8 | 74.1 | 70.6c | 67.0c | ||
| d-Threitol 4-phosphated | ∼2 | 64.1 | 72.9 | 71.7c | 68.0c | ||
| d-Threitole | 64.16 | 73.11 | 73.11 | 64.16 | |||
| d-Erythrulose 4-phosphated | ∼2 | 67.5 | |||||
| l-Prolinea | 7.3 | 176.2 | 63.0 | 30.3 | 25.4 | 47.9 | |
| Betainea | 7.3 | 170.7 | 68.0 | 55.1 | |||
| d-Ribitole | 63.86 | 73.58 | 73.68 | 73.58 | 63.86 | ||
| Ethylene glycole | 64.06 | 64.06 |
d-[1-13C]Erythritol and d-[1-13C]threitol were prepared by reduction of the corresponding [1-13C]aldotetroses (Omicron Biochemicals, Inc.) with sodium borohydride (Sigma-Aldrich) (20). After workup to remove borate, the alditol samples were >95% pure as determined by NMR.
NMR Spectroscopy
13C{1H} NMR spectra were obtained on a Varian UNITY Plus 600 MHz (150.86 MHz for 13C) NMR spectrometer equipped with a Varian probe. Data acquisition parameters were as follows: 100–1,000 transients; 3-s recycle time; 295.15 K; −15 to 230 ppm spectral window. Free induction decays were zero-filled once, and a line-broadening function (1 Hz) was applied prior to Fourier transformation to give a final digital resolution of 7.14 points/Hz. Spectra were referenced externally using a solution of d-[1-13C]mannose in 2H2O. The most intense signal in the reference spectrum, arising from C-1 of α-d-mannopyranose, was set to 95.54 ppm. Spectra were collected shortly after the addition of aldose phosphate, and after 24 h, 72 h, and 7 days.
Primary alcoholic carbons (-CH2OH groups) resonate over a narrow range, making chemical shift-based assignments unreliable. For instance, the chemical shifts of threitol (C-1/C-4) and threitol 4-phosphate (C-1) differed by ∼0.03 ppm, but differences in temperature and pH resulted in chemical shift values that varied by more than 0.1 ppm for threitol 4-phosphate alone. Reaction mixtures were therefore spiked with unlabeled or 13C-labeled standards to allow firm identification of the chemical species producing specific signals in the spectra (supplemental Fig. 1).
In this study, both relative intensities (peak height) and relative integrals (peak area) of 13C signals were used to estimate relative concentrations. Signal intensities were compared when signal integration was not possible because signals were overlapping and could not be integrated accurately. The use of signal intensity, instead of signal area, was validated by measuring the resonance line widths at half peak height for the signals being compared. For instance, in the E4P in vitro metabolism sample after 7 days, the line widths of the four alditol signals were very similar, averaging 1.37 ± 0.10 Hz, thus allowing signal intensities to be used to determine relative concentrations. The intensity of each signal was measured from the base line to the highest point on the signal using Varian processing software. Raw intensities or areas were compared with the internal standard within a spectrum to generate relative intensities/areas, which were then compared across spectra.
Differential nuclear Overhauser effects and incomplete relaxation could potentially affect the relative intensities (or areas) of the signals (21). However, under suitable conditions, comparison of signal intensities or areas for carbons having the same number of attached protons is a reliable method of quantitation (21). A comparison of the intensities of the natural abundance 13C signals of sorbitol indicated that all six signals were approximately equally intense (Fig. 1) with a standard deviation of ±7%. The two natural abundance threitol signals behaved similarly; the ratio of the intensity of the C-1/C-4 signal to that of C-2/C-3 was 1.05:1.00. In the in vitro experiments, the ratio of the signals from the α- and β-l-glucose anomers served as an internal control, giving similar relative intensities between experiments. The ratio of the intensity of the α to β anomers for different samples during the course of the experiment was 0.45 ± 0.03:1 ± S.D. (n = 13). These results indicate that the error associated with using signal intensities to estimate saccharide concentration is ±7%.
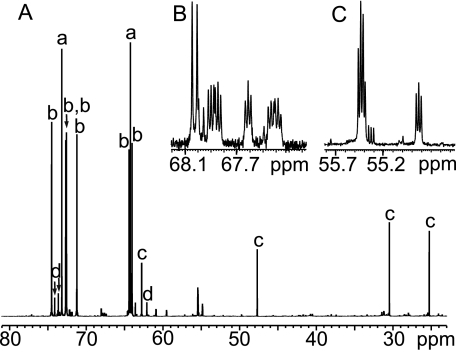
FIGURE 1.
13C{1H} NMR spectrum of native hemolymph from winter-acclimatized U. ceramboides. A, partial spectrum (150 MHz) showing signal assignments for threitol (a), sorbitol (b), proline (c), and trehalose (d). B and C are expansions of A showing weak signals tentatively assigned to betaine-like compounds based on their distinctive chemical shifts and multiplicities.
RESULTS
Characterization of Winter Hemolymph
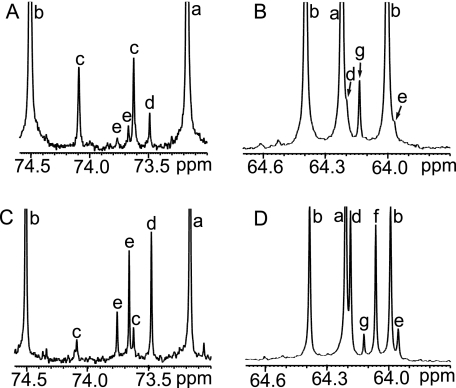
13C NMR analysis of the hemolymph from winter-acclimatized U. ceramboides confirmed that sorbitol and threitol are the predominant polyols (Fig. 1). In addition, weaker signals attributed to proline, trehalose, betaine-like compounds, erythritol, ribitol, and ethylene glycol were detected (Fig. 2). Signals assigned tentatively to erythritol and ribitol, expected by-products of threitol biosynthesis via the PPP, were confirmed by spiking the hemolymph with authentic alditols and observing the resulting enhanced signal intensities in the spectrum (Fig. 2). The relative signal intensities of both alditols provided further support for the assignments. The intensities of both erythritol 13C signals, each representing two carbon nuclei (Table 1), were approximately equal, whereas for ribitol the intensity of the C-3 signal was one-half that of the remaining two ribitol signals (Table 1), which arise from magnetically equivalent C-2/C-4 and C-1/C-5 pairs. Glycerol, the most commonly produced polyol in cold-hardy insects, was not observed in the winter hemolymph of U. ceramboides (Fig. 2). In other insect species, alditols are reported to freely penetrate cell membranes (9). In U. ceramboides, alditols observed in the hemolymph were qualitatively identical to those observed in the homogenate from the same beetle. (The hemocoel was rinsed with buffer to remove residual hemolymph polyols.) Furthermore, the relative proportions of polyols were very similar in both the hemolymph and the homogenate, suggesting that alditols penetrate cell membranes freely. Insects were homogenized in the in vivo experiments to increase the concentration of 13C nuclei in the NMR sample, which aided in detection of the labeled saccharides and increased the accuracy of quantification (21).
FIGURE 2.
Primary and secondary alcohol 13C signals in native hemolymph from winter-acclimatized U. ceramboides. A and B, expansions of the spectrum shown in Fig. 1. A, expansion showing secondary alcohol (-CHOH) carbons. B, expansion showing primary alcohol (-CH2OH) carbons. C and D correspond to A and B, respectively, after the sample was sequentially spiked with the following alditol standards: [1-13C]erythritol, [1-13C]glycerol, [1-13C]threitol, and natural abundance erythritol and ribitol. Spiking led to the following signal assignments: threitol (a), sorbitol (b), trehalose (c), erythritol (d), ribitol (e), glycerol (f), and ethylene glycol (g).
In Vivo Saccharide Metabolism
In Vivo Metabolism of d-Glucose 13C Isotopomers
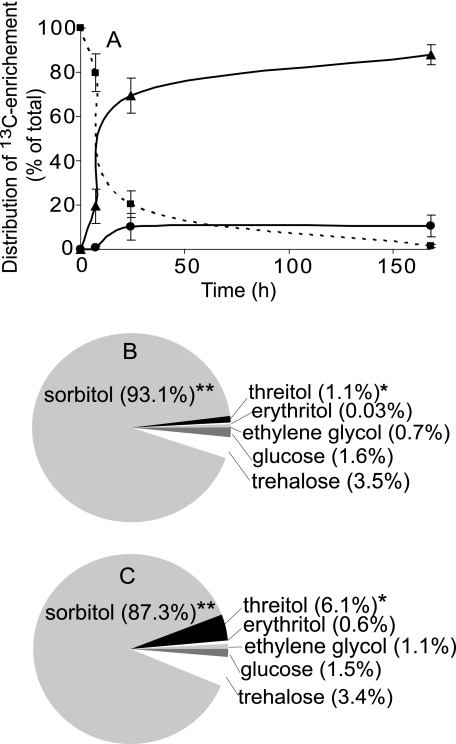
Changes in the relative distribution of isotopic enrichment over time (Fig. 3) provided insights into the metabolism of 13C-labeled glucose. Labeled glucose was rapidly metabolized at −4 °C; within 7 h, 20 ± 8% (n = 3) of the labeled glucose was reduced to correspondingly labeled sorbitol (Fig. 3A). Conversion to sorbitol accounted for >95% of the reduction in labeled glucose signal, although a low level of isotopic enrichment of trehalose was observed. Other alditols observed in the winter hemolymph of U. ceramboides, including threitol, were not detected. After 24 h, labeled glucose comprised ∼20% of the total 13C-enriched signals, suggesting that ∼80% of the glucose had been converted to other metabolites (Fig. 3A), of which sorbitol comprised ∼90%. The remaining 10% of 13C-enriched compounds consisted of trehalose, threitol, erythritol, and ethylene glycol. After 1 week (168 h) at −4 °C, very little residual labeled glucose was detected (∼1.5% of the total 13C signal), and sorbitol, trehalose, threitol, erythritol, and ethylene glycol were observed to be isotopically labeled (Fig. 3, B and C). The total integral of the 13C-enriched signals relative to natural abundance trehalose signals did not attenuate noticeably over time, suggesting that most of the 13C-labeled glucose was converted to the observed saccharides.
FIGURE 3.
Metabolic fate in vivo of 13C arising from singly labeled glucose isotopomers. A, time course showing metabolism of 13C-enriched glucose isotopomers over 168 h at −4 °C: glucose (squares), sorbitol (triangles), and all remaining saccharides (circles). The relative contribution of each saccharide to the 13C pool was calculated by integrating all signals within a spectrum. Signal integrals from a given molecule were compared with determine how much a specific site within the molecule (e.g. C-1, C-2, etc.) was enriched above natural abundance. This was not possible for ethylene glycol because it exhibits only one signal; thus, the enrichment was estimated based on the ratio of enriched to natural abundance threitol signals. The enrichment of individual saccharides, usually in terms of a standardized signal area, was then summed across saccharides to determine the proportional contribution of each. B and C, the proportion of the total 13C enrichment represented by a given saccharide at 168 h after the injection of singly labeled glucose 13C-labeled isotopomers labeled at C-1—C-2 (B) and C-3—C-6 (C). Asterisks indicate saccharides that comprised significantly different (p < 0.05, Student's t test, n = 10) proportions of the total 13C pool with the injection of C-1—C-2 versus C-3—C-6 glucose 13C-labeled isotopomers.
In Vivo Metabolism: PPP Versus Xylose-Glucuronate Pathway
To evaluate the potential contributions of both the PPP and xylose-glucuronate pathways (supplemental Fig. 2) to threitol biosynthesis, we monitored the metabolism of 13C-labeled xylose and glucose. d-[2-13C]Xylose was reduced predominantly to d-[2-13C]xylitol after 48 h at −4 °C, although low levels of 13C enrichment were also detected for threitol. The intensity of the C-1/C-4 threitol signal was enriched ∼2.2-fold over that of the C-2/C-3 signal, suggesting that a small amount of xylose was converted to threitol possibly though the glucuronate pathway. In contrast to d-xylose, d-glucose was converted primarily sorbitol (Fig. 3), not xylitol. As observed for xylose metabolism, small quantities of glucose were converted to saccharides other than the primary alditol product, including threitol.
It was observed that threitol comprised a significantly larger percentage of the 13C pool, 6.1 ± 2.5% (p < 0.0421, students t test, n = 10), when C-3—C-6 of glucose was 13C-labeled compared with when C-1—C-2 (1.1 ± 0.3%) was labeled (Fig. 3, B and C). These results show that threitol was generated from the bottom four carbons of d-glucose via the PPP (e.g. d-[3-13C]glucose yields d-[1-13C]threitol) (supplemental Fig. 2). The isotopic enrichment pattern of threitol resulting from the various singly labeled glucose isotopomers supports this argument because in every case the predicted site of 13C enrichment was at least 5-fold greater than the remaining threitol signal (Table 2). Injection of [1-13C] and [2-13C]glucose led to a 1.5–2-fold enrichment of one threitol signal relative to the remaining signal (Table 2). One possible explanation for this enrichment is that label scrambling might arise from combined actions of glycolytic and gluconeogenic enzymes. To investigate this possibility, we compared the intensity of the expected site of secondary enrichment for sorbitol to the intensities of the remaining four natural abundance sorbitol signals, revealing that this site was enhanced ∼1.8-fold.
TABLE 2.
Isotopic enrichment of threitol and sorbitol observed after injections of singly 13C-labeled glucose isotopomers into the hemolymph of U. ceramboides
| 13C-Labeled carbon in glucose | Predicted position of 13C enrichment in threitol | Ratio of threitol 13C signal intensities C-1/C-4:C-2/C-3 | Predicted position of secondary 13C enrichmenta | Ratio of sorbitol13C signal intensitiesb |
|---|---|---|---|---|
| 64.2:73.1 ppm | ||||
| C-1 | No enrichment | 1.5:1 | C-6 | 1.6 |
| C-2 | No enrichment | 1:2 | C-5 | 1.7 |
| C-3 | C-1 (64.2 ppm) | 5.2 ± 1.6:1 (n = 5) | C-4 | 1.8 ± 0.6 (n = 5) |
| C-4 | C-2 (73.1 ppm) | 1:5.9 | C-3 | 1.2 |
| C-5c | C-3 (73.1 ppm) | 1:5.5 | C-2 | 2.7 |
| C-6 | C-4 (64.2 ppm) | 27.5:1 | C-1 | 2.0 |
a Secondary enrichment of sorbitol due to label scrambling via the triosephosphate isomerase reaction and subsequent gluconeogenesis.
b Predicted site of secondary enrichment relative to the average of the remaining four natural abundance sorbitol signals.
c Stock solution contained ∼90% d-[5-13C]glucose and 10% d-[6-13C]glucose.
Polyol Dehydrogenase Specificity
Various d-[1-13C]aldoses were injected into the hemolymph of U. ceramboides to evaluate their propensities for reduction, potentially revealing differential affinities of polyol dehydrogenase enzymes for these substrates. For instance, when an approximately equimolar mixture (110 mm each; 330 mm overall) of d-[1-13C]erythrose, d-[1-13C]ribose, and d-[1-13C]threose was injected into the hemolymph of U. ceramboides held at −4.0 °C for 24 h, all three aldoses were >95% reduced to d-[1-13C]erythritol, d-[1-13C]ribitol, and d-[1-13C]threitol, respectively. d-Glucose and d-xylose were ∼75% reduced to their respective alditols after 24 h at −4 °C. The apparent disparity in the extent of reduction after 24 h arises from differences in concentration. The aldotetrose solutions were not concentrated beyond 330 mm to avoid oligomerization, whereas the other aldose solutions were 6-fold more concentrated (∼2 m). In contrast to the d-aldoses, l-[1-13C]glucose was not metabolized significantly under these conditions3 and was therefore used as an internal standard in the in vitro experiments.
In Vivo Metabolism of Threitol and Erythritol
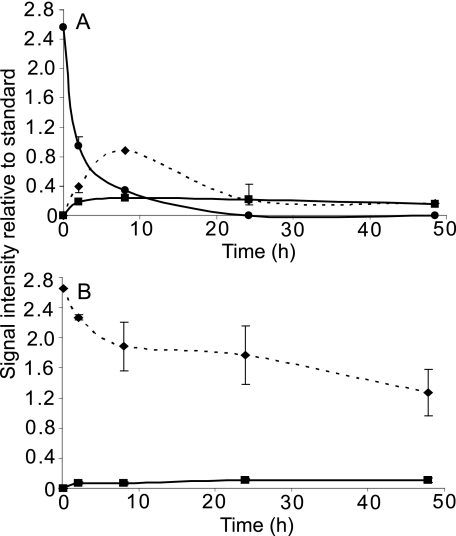
Injecting threitol and erythritol into the hemolymph of U. ceramboides allowed a determination of the relative stabilities of these alditols in vivo. These experiments showed that erythritol is catabolized more rapidly than threitol. When an approximately equimolar mixture of [1-13C]erythritol and [1-13C]threitol was injected into the hemolymph, the C-1 signal of erythritol was significantly attenuated after 6 days at 0 °C, whereas the threitol C-1 signal intensity was essentially unchanged (Fig. 4). After 5 days at 4 °C, the erythritol C-1 signal completely disappeared (Fig. 4).
FIGURE 4.
Relative tetritol catabolism in vivo at low temperature. A, partial 13C{1H} NMR spectrum (150 MHz) of an ∼equimolar mixture of [1-13C]erythritol and [1-13C]threitol showing signals arising only from the labeled carbons. B and C, the mixture in A was injected into the hemolymph of a deacclimated U. ceramboides and held either for 6 days at 0 °C (B) or 5 days at 4 °C (C). The homogenates of two insects are pooled for each sample. Signal assignments are as follows: C-1 threitol (a), C-1 erythritol (b), and ethylene glycol (c).
At higher temperatures, erythritol was considerably more labile. Within 8 h at 23 °C, >80% of the erythritol was metabolized, yielding two labeled metabolites, threitol and ethylene glycol (Fig. 5). These results were confirmed with the injection of [2-13C]erythritol (data not shown). In contrast, ∼40% of [1-13C]threitol remained after 48 h, indicating that threitol is metabolized much more slowly than erythritol. In all threitol and erythritol catabolism experiments, small quantities of ethylene glycol were observed. In addition, a small amount of the C-1 label from threitol was also incorporated into trehalose, enriching C-1/C-1′ 2.5 ± 0.8-fold (±S.D., n = 4).4
FIGURE 5.
In vivo tetritol catabolism at 23 °C. Equivalent amounts of [1-13C]erythritol (A) and [1-13C]threitol (B) were injected into the hemolymph of deacclimated U. ceramboides, which were held at 23 °C for 2, 8, 24, or 48 h before the homogenized insects were analyzed by 13C{1H} NMR. Signal intensities of labeled alditols observed in the homogenate were standardized against the C-1 signal of [1-13C]glycerol, which was added after heat inactivation of the homogenate. The standardized mean signal intensities ± S.D. of threitol (diamonds), erythritol (circles), and ethylene glycol (squares) are plotted against time. The sample size at each time point is from 1–4 individuals. (A) The 8 and 48 h time points represent only one individual. The initial time point (t = 0) for both alditols is estimated assuming 60% recovery of the labeled signal.
Intermediary Threitol Biosynthesis
[1-13C]Tetrose 4-Phosphate Concentrations
Organic phosphate concentrations in the d-[1-13C]E4P and d-[1-13C]T4P stock solutions were 114 ± 2 and 126 ± 8 mm, respectively, before being added to the insect homogenate. Thus, ∼68 and 76 μmol of organic phosphate were added to the in vitro [1-13C]E4P and [1-13C]T4P reaction mixtures, respectively. The actual tetrose 4-phosphate concentration was less than these values, however, because both solutions contained oligomers (18) and erythrulose-4-phosphate (Eu4P) (18, 22). For instance, [1-13C]E4P contributed ∼35% (∼24 μmol) of the total organic phosphate in solution at the initial time point (supplemental Fig. 3B). In contrast, [1-13C]T4P contributed ∼70% (∼53 μmol) (supplemental Fig. 4B).
In Vitro Metabolism of [1-13C]E4P
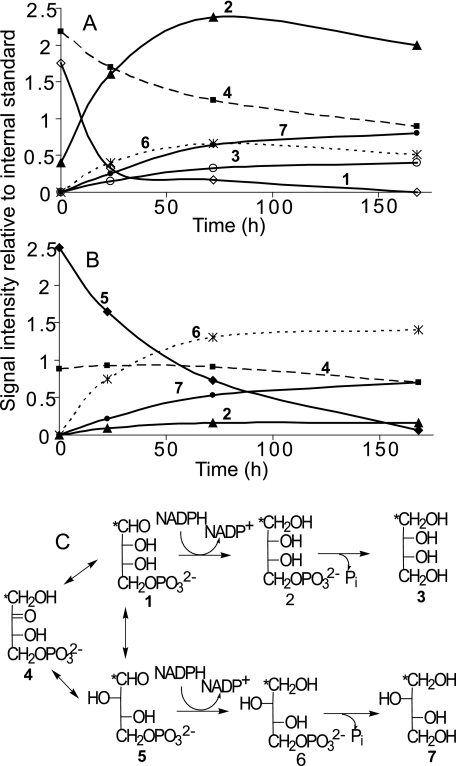
The intensity of the C-1 signal of [1-13C]E4P decreased by ∼70% within 24 h relative to the intensity of the internal standard, the C-1 signal of β-l-[1-13C]glucopyranose (97.4 ppm) (Fig. 6A). The rate of conversion to alditol over the first 24 h was ∼0.7 μmol/h, resulting in a 4-fold increase in alditol, which was composed predominantly of erythritol 4-phosphate, although threitol 4-phosphate, threitol, and/or erythritol were also observed (Fig. 6A). Erythritol and threitol could not be distinguished at 24 h because the spectrum was collected at −1 °C, where the alditol C-1 signals overlapped. However, subsequent time lapse 13C spectra were obtained at 22 °C where the signals were well resolved. These data indicated that both unphosphorylated alditols were present and that unphosphorylated threitol accumulated more rapidly than erythritol (Fig. 6A). After 7 days, the C-1 signal of [1-13C]E4P was undetectable, and the intensity of the C-1 signal of Eu4P had decreased by nearly 50% (Fig. 6A). The total alditol signal intensity increased nearly 10-fold during this same period of time. The alditol mixture after 7 days consisted of ∼64% erythritol/erythritol 4-phosphate with the majority (∼83%) being phosphorylated. In contrast, only ∼47% of the threitol remained phosphorylated (Fig. 6A).
FIGURE 6.
In vitro metabolism at −2.5 °C of putative intermediates in threitol biosynthesis. A and B, time course of signal intensities (relative to the internal standard) arising from in vitro metabolism of d-[1-13C]E4P (A) and d-[1-13C]T4P (B). The concentration of the internal standard was ∼2-fold higher in the T4P samples (relative to E4P samples), and both aldose phosphate stock solutions contained Eu4P arising from nonenzymatic isomerization prior to their addition to the homogenate. Only the total intensity of the erythritol and threitol signals was known at 24 h because the spectra were collected at −1 °C and their signals were coincident. Thus, the relative intensities at 24 h are estimates based on the total intensity. Subsequent spectra were collected at 22 °C, where the signals were well resolved. The number on each curve corresponds to a structure in the metabolic scheme (C). An asterisk indicates the site of 13C enrichment. Assignments are as follows: open diamonds, d-[1-13C]E4P (1); closed triangles, d-[1-13C]erythritol 4-phosphate (2); open circles, d-[1-13C]erythritol (3); closed squares, d-[1-13C]Eu4P (4); closed diamonds, d-[1-13C]T4P (5); asterisks, d-[1-13C]threitol 4-phosphate (6); closed circles, d-[1-13C]threitol (7).
Heat treatment of the U. ceramboides homogenate prevented the accumulation of alditol (supplemental Figs. 3 and 4). After 24 h, a small increase in the intensity of the C-1 signal of E4P was observed. However, after 7 days, a decrease in E4P intensity was observed with a corresponding increase in Eu4P, presumably because of the spontaneous (uncatalyzed) isomerization of the tetrose 4-phosphates under neutral pH conditions (18, 22).
In Vitro Metabolism of [1-13C]T4P
The intensity of the C-1 signal of [1-13C]T4P decreased substantially (∼36%) within 24 h (Fig. 6B). This apparently slow rate of reduction relative to the E4P experiment is an artifact of a 2-fold higher concentration of T4P relative to E4P. The difference in concentration is not apparent from the data because the concentration of the internal standard was adjusted accordingly (i.e. the concentration of l-[1-13C]glucose was 2-fold higher in the T4P sample). In fact, the rates of total alditol production over the first 24 h in both the E4P and T4P experiments were approximately equal, ∼0.7 versus ∼0.8 μmol/h, respectively. A 10-fold increase in alditol was observed in the first 24 h, consisting predominantly of threitol 4-phosphate and threitol (Fig. 6B), although erythritol 4-phosphate was also observed. After 7 days, the C-1 signal intensity of [1-13C]T4P decreased by more than 95% and there was also a small reduction in the Eu4P signal (Fig. 6B). Alditol increased more than 40-fold over the initial levels, whereas free threitol accounted for an increasingly larger proportion of total alditol as time progressed (Fig. 6B). After 7 days, the alditol mixture consisted of ∼93% threitol/threitol-4-phosphate and ∼7% erythritol 4-phosphate (no erythritol was observed). As observed in the E4P experiments, heat treatment prevented the accumulation of alditol and the ratio of aldose phosphate to Eu4P decreased with time (supplemental Fig. 4). Alditols accumulated more slowly when the homogenate was not supplemented with NAD(P)H, requiring several days for detectable alditol to accumulate (data not shown). In all in vitro experiments, the background spectra revealed that contributions of endogenous compounds to the overall spectra were minor and did not change with time.
DISCUSSION
Sorbitol and threitol were reported to be the predominant alditols present in the hemolymph of cold-acclimatized U. ceramboides, with in vivo concentrations of 400 and 250 mm, respectively (7). Our results confirm this finding and show that the threitol signals are well resolved from other major signals in the hemolymph (a necessary condition for a 13C NMR study of threitol biosynthesis) (Fig. 1). Furthermore, the relative intensities of the threitol and sorbitol signals in 13C{1H} NMR spectra agree well with the published concentrations, as threitol produces only two carbon signals, each with an intensity twice that expected from the concentration of the molecule.
The presence of betaine, proline, and ethylene glycol has not been reported previously in the hemolymph of winter-acclimated U. ceramboides (7, 8) and may contribute to freeze tolerance. The production of betaine compounds, such as glycine betaine, has been correlated with tolerance to osmotic stress in diverse organisms including plants and bacteria (23, 24). In addition, betaine has been found in both freeze-tolerant and freeze-avoiding insects (25, 26), where it presumably ameliorates the effects osmotic stress. Intracellular osmotic stress may arise from extracellular freeze/thaw events in freeze-tolerant organisms. Both proline and trehalose, which were observed in the winter hemolymph (Fig. 1), are commonly associated with insect cold tolerance (27–29) and have been shown to interact directly with membrane phospholipids and stabilize the lipid bilayer during freezing stress (30, 31). Nearly 1 m ethylene glycol has been reported to accumulate in the hemolymph of the freeze-avoiding beetle, Ips acuminatus, where it serves as the major colligative antifreeze (32). In U. ceramboides, ethylene glycol appears to be an intermediate metabolite of tetritol catabolism because the catabolism of both C-1 and C-2 13C-enriched erythritol and threitol yielded enriched ethylene glycol.
Although the metabolism of d-[2-13C]xylose resulted in 13C enrichment at C-1/C-4 of threitol, xylose metabolism does not appear to contribute to threitol biosynthesis, as xylitol, the predominant metabolite, is not observed in the winter hemolymph of U. ceramboides. In contrast glucose gives rise to sorbitol and threitol, in addition to other compounds, all of which are observed in the winter hemolymph.
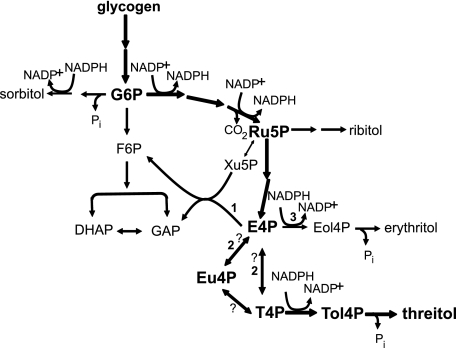
The following results from the in vivo studies support the biosynthesis of threitol via the PPP. 1) When singly labeled 13C isotopomers of d-glucose were labeled at C-3—C-6 instead of C-1—C-2, significantly more 13C was incorporated into threitol (Fig. 3). 2) The assumption that C-3—C-6 of glucose are used to generate 4-carbon saccharides in the PPP successfully predicted the sites of 13C enrichment in threitol (Table 2). 3) The presence of erythritol and ribitol in winter hemolymph from U. ceramboides (Fig. 2) provides additional support for a threitol biosynthesis pathway that proceeds through the PPP, as both alditols arise from saccharide intermediates in this pathway (Scheme 1). These alditols were not identified previously (7, 8, 10), presumably because of their low concentrations.
SCHEME 1.
Proposed alditol biosynthetic pathway in U. ceramboides. Metabolic scheme showing glycolysis and the pentose phosphate pathway. Biosynthetic pathway leading to threitol is in bold. Bold numbers indicate alternative metabolism pathways of E4P discussed under “Discussion.” Question marks indicate uncertainty associated with the mechanism of conversion of E4P to T4P. Eol4P, erythritol 4-phosphate; G6P, glucose 6-phosphate; F6P, fructose 6-phosphate; GAP, glyceraldehyde phosphate; Ru5P, ribulose 5-phosphate; Xu5P, xylulose 5-phosphate; Tol4P, threitol 4-phosphate. Ribulose 5-phosphate may first be isomerized to ribose 5-phosphate or reduced directly and dephosphorylated to form ribitol.
Moderate enrichment of threitol was also observed with d-[1-13C] and d-[2-13C]glucose (Table 2), possibly because of label scrambling via glycolysis/gluconeogenesis. To illustrate how this might occur, take the case of d-[1-13C]glucose, which gives rise to the glycolytic intermediates [1-13C]dihydroxyacetone phosphate (DHAP) and glyceraldehyde 3-phosphate. Subsequently, the glycolytic enzyme triosephosphate isomerase isomerizes [1-13C]DHAP to [3-13C]glyceraldehyde 3-phosphate, resulting in secondary enrichment of gluconeogenic glucose at C-6. Consequently, a portion of the saccharide metabolites arising from gluconeogenic glucose will be enriched at a secondary site. Indeed, the sorbitol signals reflect this pattern, because the predicted site of secondary enrichment was enhanced by ∼1.8-fold over the remaining natural abundance signals (Table 2). Furthermore, the magnitude of secondary sorbitol enrichment was comparable with that observed for threitol produced from [1-13C]- and [2-13C]glucose. The pattern of threitol enrichment resulting from the injection of [6-13C]glucose further strengthens the argument for active glycolysis/gluconeogenesis. In this case, enrichment of threitol is expected only at C-4 because the enriched C-1 of gluconeogenic glucose is not incorporated into threitol. Indeed, the fidelity of C-4 labeling of threitol was 5-fold higher than found when d-[3-13C]glucose or d-[4-13C]glucose was used (Table 2), because label scrambling with these isotopomers reduced the relative intensity of the major labeled threitol peak. The injection of d-[5-13C]glucose should also result in high fidelity enrichment of threitol at C-3. However, the original d-[5-13C]glucose solution contained ∼10% d-[6-13C]glucose, reducing the relative intensity of the labeled threitol peak.
In various species of insects, phosphofructokinase and fructose-1,6-bisphosphatase, the key enzymes regulating entrance into glycolysis and gluconeogenesis, respectively, are regulated seasonally by phosphorylation, temperature, and the abundance of each enzyme (9). Decreasing phosphofructokinase activity appears to potentiate the synthesis of cryoprotectants not arising from glycolytic intermediates, such as trehalose and sorbitol (33). As such, flux of carbohydrates into glycolysis during polyol synthesis in U. ceramboides should be minimal. However, even a small flux through glycolysis may be sufficient to produce the observed effect. In addition, U. ceramboides evaluated in the in vivo experiments were collected in midwinter, and the metabolic regulation observed during autumn polyol synthesis often becomes less rigid later in winter as the relative activities of enzymes change in preparation for polyol catabolism and assimilation of carbohydrates in the spring (9).
The in vitro metabolism studies demonstrate that E4P, a known metabolite in the PPP, is an intermediate in threitol biosynthesis. The reduction of aldose 4-phosphate to alditol was completely prevented by heat treatment of the insect homogenate and occurred much more rapidly when the sample was supplemented with NADPH, suggesting the involvement of NADPH-dependent polyol dehydrogenases. Furthermore, the production of threitol from E4P after 24 h at −2.5 °C strongly suggests that enzymes from U. ceramboides were responsible for the observed metabolism.
In vivo E4P has three likely metabolic fates (see Scheme 1): 1) metabolism in the nonlinear portion of the PPP, 2) epimerization and/or isomerization to T4P with subsequent reduction to threitol 4-phosphate, and 3) direct reduction to erythritol 4-phosphate. In vitro conditions favor the latter two pathways. The high concentration of E4P relative to the presumably unreplenished metabolic intermediates of the PPP marginalizes the contribution of pathway 1. In addition, the high availability of reducing equivalents should lead to alditol biosynthesis, favoring flux through the latter two pathways. Direct reduction to erythritol should be the most favored outcome because the conversion to threitol requires an additional step. Nevertheless, after 7 days threitol 4-phosphate and threitol collectively comprised ∼36% of the total alditol in the in vitro E4P sample.
There are two possible routes to convert E4P to T4P: direct epimerization of E4P to T4P or isomerization involving the 2-ketotetrose intermediate, Eu4P. A direct epimerization mechanism is not unprecedented; ribulose 5-phosphate 3-epimerase isolated from beef liver epimerizes E4P to T4P without a ketosugar intermediate (22). As expected, Eu4P was a very poor substrate for this enzyme. The latter route is analogous to the isomerization of glucose 6-phosphate to mannose 6-phosphate, which proceeds through the 2-ketohexose intermediate, fructose 6-phosphate. The results of the in vitro experiments do not distinguish conclusively between these pathways. Eu4P concentrations remained high over the first 24 h and decreased slowly after the aldose 4-phosphate was almost completely reduced. This is consistent with isomerization involving Eu4P, because at equilibrium Eu4P comprises ∼90% of the tetrose phosphate pool (22). However, the same results could also suggest that Eu4P is a very poor metabolic substrate in U. ceramboides and consequently could indicate a direct epimerization mechanism. The observed slow decrease of Eu4P may be explained in part by spontaneous isomerization to aldotetrose phosphate and subsequent reduction to alditol.
The in vitro results indicate that epimerization (isomerization) is followed by reduction and a subsequent dephosphorylation event (Fig. 6C). The order of the latter two reactions can vary with the specific alditol. For instance, in glycerol production, DHAP is first reduced to glycerol 3-phosphate and then dephosphorylated to form glycerol, whereas the order is reversed for sorbitol synthesis in insects (9). The accumulation of phosphorylated alditol in the in vitro experiments (Fig. 6) strongly suggests that dephosphorylation occurs after reduction. This conclusion is supported by the observation that alditol 4-phosphate concentrations initially increased more rapidly than those of unphosphorylated alditols (Fig. 6).
For the in vitro metabolism of E4P, threitol 4-phosphate and threitol comprised ∼36% of the total alditol after 7 days. In contrast, erythritol 4-phosphate comprised only ∼7% of the total alditol in the T4P experiment, and erythritol was not detected (Fig. 6). These results show that the net action of the last three steps in alditol biosynthesis, namely, epimerization (isomerization), reduction, and dephosphorylation, favor threitol synthesis over erythritol synthesis. Although all three steps may exert some control over the threitol biosynthetic pathway, neither the reduction by a NADPH-dependent polyol dehydrogenase nor epimerization appeared to significantly control flux through the pathway. When an equimolar mixture of ribose, erythrose, and threose was injected in the hemolymph, all three sugars were reduced to their respective alditols, indicating broad polyol dehydrogenase substrate specificity. The in vitro results confirm this observation. The similar rates of E4P and T4P reduction over the first 24 h, despite the 2-fold higher initial concentration of T4P, indicate that the polyol dehydrogenase exhibits saturation kinetics and has a similar affinity for both substrates. Polyol dehydrogenases studied in other insect species also exhibit broad substrate specificities (14, 15). It appears unlikely that epimerization itself has any effect on the observed kinetics, as equilibrium concentrations of E4P and T4P are approximately equal (22).
In contrast, in vitro E4P experiments indicate that threitol 4-phosphate is dephosphorylated preferentially over erythritol 4-phosphate even in the presence of a 4-fold higher concentration of the latter (Fig. 6A). Preferential dephosphorylation of threitol 4-phosphate may be critical to shifting the E4P ⇆ T4P equilibrium toward T4P, because dephosphorylation removes threitol 4-phosphate from the intracellular milieu, shifting the chemical equilibrium and allowing threitol to move out of the cytosol due to its greatly increased membrane permeability (34). Conversely, erythritol 4-phosphate remaining in the cytosol may be subject to further catabolism within fat body cells. This suggests that the affinity of the phosphatase for a specific sugar phosphate may affect its metabolic fate.
For many insect species, autumn polyol accumulation is initiated at temperatures near 5 °C, with the maximal accumulation rate occurring between 0 and −5 °C (9). The accumulation of threitol appears to fit this pattern, as threitol begins to accumulate in September when average night-time temperatures dip below 5 °C (8). Erythritol, in contrast, is catabolized at temperatures at least as low as 0 °C (Fig. 3). U. ceramboides actively accumulate threitol in September through October, when the average daytime highs in Fairbanks, Alaska, are at or above 4 °C (8), suggesting that U. ceramboides simultaneously accumulate threitol and catabolize erythritol during autumn polyol synthesis. We conclude that threitol is produced via the PPP and that the accumulation of this single alditol is achieved not only because its biosynthesis is favored over that of erythritol but also because erythritol is preferentially catabolized.
Supplementary Material
Acknowledgments
We thank Omicron Biochemicals, Inc. for generously providing the 13C-labeled compounds that made these experiments possible and Todd Sformo at the University of Alaska, Fairbanks, for help with collecting beetles during winter in Alaska.
This study was supported by National Science Foundation Grants OPP-0117104 and IOS-0618342.
 The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–4.
3 A small quantity of l-[1-13C]glucose (∼1.7%) was reduced to C-1-enriched sorbitol. This might be explained by the presence of a small amount of d-[1-13C]glucose in the sample.
4 The threitol catabolic pathway is not simply the reverse of the biosynthetic pathway, as this would yield glucose/trehalose enriched at C-3/C-6. Threitol catabolism may involve an ethylene glycol intermediate.
- PPP
- pentose phosphate pathway
- 13C{1H}NMR
- proton-decoupled carbon-13 nuclear magnetic resonance spectroscopy
- E4P
- erythrose 4-phosphate
- Eu4P
- erythrulose 4-phosphate
- DHAP
- [1-13C]dihydroxyacetone phosphate
- T4P
- threose 4-phosphate.
REFERENCES
- 1.Lee R. E. ( 1991) in Insects at Low Temperature, ( Lee R., Denlinger D. eds) pp. 17– 46, Chapman and Hall, New York [Google Scholar]
- 2.Block W. ( 1990) Philos. Trans. R. Soc. Lond. B Biol. Sci. 326, 613– 633 [Google Scholar]
- 3.Storey K. B., Storey J. M. ( 1988) Physiol. Rev. 68, 27– 84 [DOI] [PubMed] [Google Scholar]
- 4.Duman J. G. ( 2001) Annu. Rev. Physiol. 63, 327– 357 [DOI] [PubMed] [Google Scholar]
- 5.Zachariassen K. E. ( 1985) Physiol. Rev. 65, 799– 832 [DOI] [PubMed] [Google Scholar]
- 6.Storey K. B. ( 1990) Philos. Trans. R. Soc. Lond. B Biol. Sci. 326, 635– 654 [Google Scholar]
- 7.Miller L. K. ( 1978) J. Insect Physiol. 24, 791– 796 [Google Scholar]
- 8.Miller L. K. ( 1982) Comp. Biochem. Physiol. A 73, 595– 604 [Google Scholar]
- 9.Storey K. B., Storey J. M. ( 1991) in Insects at Low Temperature ( Lee R., Denlinger D. eds) pp. 64– 93, Chapman and Hall, New York [Google Scholar]
- 10.Miller L. K., Smith J. S. ( 1975) Nature 258, 519– 520 [DOI] [PubMed] [Google Scholar]
- 11.Kostál V., Zahradnícková H., Simek P., Zelený J. ( 2007) J. Insect Physiol. 53, 580– 586 [DOI] [PubMed] [Google Scholar]
- 12.Pitkänen E. ( 1977) Clin. Chim. Acta 80, 49– 54 [DOI] [PubMed] [Google Scholar]
- 13.Prade R. A. ( 1996) Biotechnol. Genet. Eng. Rev. 13, 101– 131 [DOI] [PubMed] [Google Scholar]
- 14.Chino H. ( 1960) J. Insect Physiol. 5, 1– 15 [Google Scholar]
- 15.Takahashi S. Y., Kajiura T., Kageyama T., Ohnishi E. ( 1974) Insect Biochem. 4, 33– 45 [Google Scholar]
- 16.Chino H. ( 1961) J. Insect Physiol. 6, 231– 240 [Google Scholar]
- 17.Kageyama T., Takahashi S. Y., Ohnishi E. ( 1973) Insect Biochem. 3, 373– 388 [Google Scholar]
- 18.Serianni A. S., Pierce J., Barker R. ( 1979) Biochemistry 18, 1192– 1199 [DOI] [PubMed] [Google Scholar]
- 19.Leloir L. F., Cardini C. E. ( 1957) Methods Enzymol. 3, 840 [Google Scholar]
- 20.Garrett E. C., Serianni A. S. ( 1990) Carbohydr. Res. 208, 23– 25 [DOI] [PubMed] [Google Scholar]
- 21.Bock K., Pedersen C. ( 1983) Adv. Carbohydr. Chem. Biochem. 41, 27– 66 [Google Scholar]
- 22.Terada T., Mukae H., Ohashi K., Hosomi S., Mizoguchi T., Uehara K. ( 1985) Eur. J. Biochem. 148, 345– 351 [DOI] [PubMed] [Google Scholar]
- 23.Wyn Jones R. G., Storey R. ( 1981) in The Physiology and Biochemistry of Drought Resistance in Plants ( Paleg L., Aspinall D. eds) pp. 172– 204, Academic Press, Sydney, Australia [Google Scholar]
- 24.Csonka L. N., Hanson A. D. ( 1991) Annu. Rev. Microbiol. 45, 569– 606 [DOI] [PubMed] [Google Scholar]
- 25.Kukal O., Serianni A. S., Duman J. G. ( 1988) J. Comp. Physiol. B 158, 175– 183 [DOI] [PubMed] [Google Scholar]
- 26.Bennett V. A., Sformo T., Walters K., Toien O., Jeannet K., Hochstrasser R., Pan Q., Serianni A. S., Barnes B. M., Duman J. G. ( 2005) J. Exp. Biol. 208, 4467– 4477 [DOI] [PubMed] [Google Scholar]
- 27.Fields P. G., Fleurat-Lessard F., Lavenseau L., Febvay G., Peypelut L., Bonnot G. ( 1998) J. Insect Physiol. 44, 955– 965 [DOI] [PubMed] [Google Scholar]
- 28.Ramlov H. ( 1999) J. Comp. Phys. B 169, 224– 235 [Google Scholar]
- 29.Shimada K., Riihimaa A. ( 1990) CryoLetters 11, 243– 250 [Google Scholar]
- 30.Crowe J. H., Crowe L. M., Carpenter J. F., Aurell Wistrom C. ( 1987) Biochem. J. 242, 1– 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudolph A. S., Crowe J. H. ( 1985) Cryobiology 22, 367– 377 [DOI] [PubMed] [Google Scholar]
- 32.Gehrken U. ( 1984) J. Insect Physiol. 30, 421– 429 [Google Scholar]
- 33.Hayakawa Y., Chino H. ( 1982) Insect Biochem. 12, 639– 642 [Google Scholar]
- 34.Walum E., Peterson A. ( 1982) Anal. Biochem. 120, 8– 11 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.