Abstract
The importance of the pathological changes in proteoglycans has driven the need to study and design novel chemical tools to control proteoglycan synthesis. Accordingly, we tested the fluorinated analogue of glucosamine (4-fluoro-N-acetyl-glucosamine (4-F-GlcNAc)) on the synthesis of heparan sulfate (HS) and chondroitin sulfate (CS) by murine airway smooth muscle (ASM) cells in the presence of radiolabeled metabolic precursors. Secreted and cell-associated CS and HS were assessed for changes in size by Superose 6 chromatography. Treatment of ASM cells with 4-F-GlcNAc (100 μm) reduced the quantity (by 64.1–76.6%) and decreased the size of HS/CS glycosaminoglycans associated with the cell layer (Kav shifted from 0.30 to 0.45). The quantity of CS secreted into the medium decreased by 65.7–73.0%, and the size showed a Kav shift from 0.30 to 0.50. Treatment of ASM cells with 45 μm and 179 μm 4-F-GlcNAc in the presence of a stimulator of CS synthesis, 4-methylumbelliferyl-β-d-xyloside, reduced the amount of the xyloside-CS chains by 65.4 and 87.0%, respectively. The size of xyloside-CS chains synthesized in the presence of 4-F-GlcNAc were only slightly larger than those with xyloside treatment alone (Kav of 0.55 compared with that of 0.6). The effects of 4-F-GlcNAc to inhibit CS synthesis were not observed with equimolar concentrations of glucosamine. We propose that 4-F-GlcNAc inhibits CS synthesis by inhibiting 4-epimerization of UDP-GlcNAc to UDP-GalNAc, thereby depleting one of the substrates required, whereas HS elongation is inhibited by truncation when the nonreducing terminus of the growing chain is capped with 4-F-GlcNAc.
The synthesis and physical properties (size and charge) of proteoglycans are altered under some pathological conditions such as cancer (1), spinal cord injury (2), atherosclerosis (3), and asthma (4). The importance of these pathological changes in proteoglycans has driven the need to study and design novel chemical tools which can control proteoglycan biosynthesis. Thus, we have studied the effect of a fluorinated analogue of glucosamine on proteoglycan synthesis in murine airway smooth muscle cells.
Mono-, di-, and oligosaccharides that contain fluorine have been developed to study the enzymes involved in carbohydrate metabolism, and some of these have been shown to be inhibitors. The atomic size of fluorine is only slightly smaller (van der Waals' radius (r′) of 135 pm) than that of oxygen (140 pm), and the C-F bond has a higher energy (485 kJ/mol) compared with that of C-O (370 kJ/mol) (5). The substitution of fluorine for oxygen at the 4-position of N-acetylglucosamine (4-F-GlcNAc)2 confers a greater electronegativity on the bond and makes it less likely to be removed from the GlcN carbon ring. It is the properties of fluorine that contribute to the unique characteristics of 4-F-GlcNAc.
4-F-GlcNAc used for cell culture experiments has O-acetyl groups at several of its ring positions, which in effect increases its cell permeability compared with that of unmodified forms (6). After hydrolysis to remove the O-acetyl residues, 4-F-GlcNAc, like GlcNAc, must be converted to UDP-4-F-GlcNAc, which in turn can be a substrate (or inhibitor) of enzyme reactions that use UDP-GlcNAc. GlcN is typically used as a control compound for 4-F-GlcNAc in vitro because of its superior cell permeability characteristics when compared with acetylated GlcN derivatives. Although acetylated GlcN derivatives enter the cell via passive diffusion, GlcN can enter by both passive diffusion and through the glucose transporter 4 (7).
4-F-GlcNAc and 4-F-N-acetylgalactosamine (4-F-GalNAc) have been specifically studied as potential inhibitors of cell growth for the treatment of leukemia. The IC50 values for 4-F-GlcNAc and 4F-GalNAc inhibition of leukemic cell proliferation are 34 and 35 μm, respectively (8). Moreover, by blocking polylactosamine synthesis necessary for elaboration of selectin ligands, 4-F-GlcNAc exhibits anti-inflammatory effects by reducing leukocyte homing to areas of contact allergic hypersensitivity in mice in vivo (9). Beyond effects on cell membrane glycoproteins, it has been proposed that the 4-fluorinated analogue of glucosamine truncates the GlcNAc-hexuronic acid chains on heparan sulfate (HS) by preventing the formation of the normal 1,4-glycosidic linkage between glucuronate (GlcUA) and on the nonreducing end of the growing chain (10). Thus, 4-F-GlcNAc has been suggested as a therapy for reducing amyloid deposition, which can feature HS accumulation (10, 11). Treatment of cultured hepatocytes in vitro with 4-F-GlcNAc and 4F-GalNAc (10–1000 μm) for 24 h reduced [3H]glucosamine and [35S]sulfate incorporation into cellular glycosaminoglycans (11). However, total protein synthesis was also reduced at 1000 μm (11).
Although the effects of 4-F-GlcNAc on HS production have been described (10), its effects on other extracellular matrix glycosaminoglycans, chondroitin/dermatan sulfate (CS/DS) and hyaluronan (HA), have not been reported.
Airway smooth muscle (ASM) cells produce HS- and CS/DS-containing proteoglycans, including perlecan, versican, and decorin (12). Using these cells, we observed that 4F-GlcNAc inhibits CS/DS synthesis nearly as effectively as it inhibits HS synthesis. Although the 4-F on a nonreducing terminal F-GlcNAc-HS chain would block further HS synthesis by preventing the formation of the GlcUAβ1,4 bond required for elongation, the glycosidic bond in CS/DS is β1,3 between hexuronic acid and GalNAc. Thus, UDP-4-F-GlcNAc could not interfere with CS/DS synthesis via the same mechanism because it cannot be 4-epimerized to UDP-4F-GalNAc. Thus, we hypothesized that UDP-4-F-GlcNAc is a potent inhibitor of the 4-epimerase required to convert UDP-GlcNAc to UDP-GalNAc, thereby depleting the cell of UDP-GalNAc, a necessary substrate for CS/DS synthesis. To explore this putative mechanism, we analyzed the inhibitory effects of 4-F-GlcNAc on intrinsic and xyloside-stimulated CS synthesis in ASM cells (13).
EXPERIMENTAL PROCEDURES
Materials
2-Acetamido-1,3,6-tri-O-acetyl-4-deoxy-4-fluoro-d-glucopyranose (4-F-GlcNAc) was synthesized and provided by the Chemical Resource Laboratory at the Roswell Park Cancer Institute (Buffalo, NY). Carrier-free sodium [35S]sulfate and [3H]glucosamine HCl (specific activity, 60 Ci/mmol) were from American Radiolabeled Chemicals Inc. (St. Louis, MO). [3H]Glucosamine HCl (specific activity, 25–40 Ci/mmol) was also from MP Biomedicals (Irvine, CA). Chondroitinase ABC (from Proteus vulgaris, EC number 4.2.2.4), heparitinase I (EC number. 4.2.2.8), heparitinase II (EC number pending), hyaluronidase SD (from Streptococcus dysgalactiae, EC 4.2.2), unsaturated chondro-disaccharide kit (c-Kit), and heparan sulfate/heparin-disaccharide kit (h-Kit) were from Seikagaku Corporation (Tokyo, Japan). Glucosamine and 4-methylumbelliferyl-β-d-xyloside (β-d-xyloside), chondroitin sulfate (from shark cartilage and bovine trachea), and heparan sulfate (from porcine intestinal mucosa) carriers were from Sigma. Cycloheximide was from Calbiochem Corp. (La Jolla, CA). Proteinase K was from Invitrogen. Pharmacia brand Sephadex G50 fine and the prepacked Superose 6 (HR10/30) column were from GE Healthcare. The Dionex DX 500 chromatography system was from Dionex Corp. (Sunnyvale, CA). Liquid scintillation mixture (Scintisafe 30%) was from Fisher. All of the other chemicals were from Sigma or of equivalent commercial grade.
Explant of ASM Cells
The tracheas of 1–2-month-old BALB/c mice were removed by dissection, cleaned of adipose and connective tissue, cut longitudinally, and kept in a solution of Pronase (0.15%) in Ham's F-12 medium (10 mm glucose) for 18 h at 4 °C. The airway epithelium was gently scraped with a cotton swab, and each intact trachea was allowed to attach to a scored plastic culture dish in 1:1 DMEM/Ham's F-12 medium containing 10% fetal bovine serum and 17.5 mm glucose at 37 °C, 5% CO2. After 3–4 days, the tracheal tissue was detached and removed, and the explanted ASM cells were allowed to grow for 3–4 days. The ASM cells were harvested by treatment with trypsin, and the cultures were maintained in DMEM-F12 (10% fetal bovine serum, 50 units/ml penicillin G, and 50 μg/ml streptomycin sulfate) and passaged weekly. The medium was changed every 3–4 days. The cultures were used for experiments at passage 3 or 4.
Metabolic Labeling of Glycosaminoglycans
Murine ASM cells grown to confluence on 35-mm plates were pretreated with 4-F-GlcNAc (45–716 μm) or glucosamine (45–716 μm) for 2 h in 1:1 DMEM/Ham's F-12 medium (17.5 mm glucose) without serum. The media were removed and replaced with DMEM/Ham's F-12 medium without serum (1 ml) containing the same concentrations of 4-F-GlcNAc or glucosamine in the presence or absence of 0.25 mm β-d-xyloside and with radiolabeled precursors [3H]glucosamine (75–150 μCi/ml) and [35S]sulfate (50 μCi/ml). Incorporation of precursors into secreted macromolecular material was monitored at 30 min, 1 h, 2 h, and 5 h by sampling the medium (25 μl). After 8 h the remaining medium (900 μl) was harvested and treated with proteinase K (0.1 mg/ml) at 37 °C for 16 h. The cell layer was treated with 1 ml proteinase K (0.1 mg/ml in 0.1 m ammonium acetate, pH 7.0) for 16 h at 37 °C. Unincorporated radiolabeled metabolic precursors were removed by loading samples onto a desalting column (Sephadex G-50 fine, 4-ml bed) in 0.1 m ammonium acetate, pH 7.0. The samples were incubated at 100 °C for 5 min to inactivate the proteinase K prior to enzyme digestions and analysis of molecular sizes on the Superose 6 column.
In an identical protocol, ASM cells were pretreated with cycloheximide (0.1, 10 μm) for 2 h prior to the addition of xyloside and radiolabeled precursors [3H]glucosamine (75–150 μCi/ml) and [35S]sulfate (50 μCi/ml) for a further 8 h. Analysis of incorporation into macromolecules was assessed following removal of unincorporated label by Sephadex G50.
In all experiments, the 3H and 35S counts were expressed per nanogram of DNA following quantitation using the Quant-iTTM PicoGreen® double-stranded DNA assay kit according to the manufacturer's protocol (Invitrogen).
Molecular Sieve Chromatography: Superose 6 for Intact Glycosaminoglycans
A proportion of each sample was mixed with CS carrier (5 μg) and HS carrier (5 μg) and applied to a Superose 6 molecular sieve column (Pharmacia) followed by elution at a constant flow rate of 0.38 ml/min in 0.5 m ammonium acetate, pH 7.0. Fractions were collected each minute using an automated fraction collector (RediFrac; Pharmacia). The radioactivity in each fraction was assessed by mixing with liquid scintillation mixture (1:5) and counting for 3H and 35S by liquid scintillation spectrometry on a Beckman LS-5801 β-counter. Fraction numbers were standardized to Kav values based on the Vt for glucuronic acid lactone (300 μg), which was added to the sample prior to column loading and assessed independently by the uronic acid assay (14). Samples that were treated with chondroitinase ABC (292 milliunits/ml) or a mixture of heparitinase I and II (100 milliunits/ml) were also applied to the Superose 6 column following the protocol described for the undigested samples.
RESULTS
Characterization of Glycosaminoglycans Produced by Murine ASM Cells
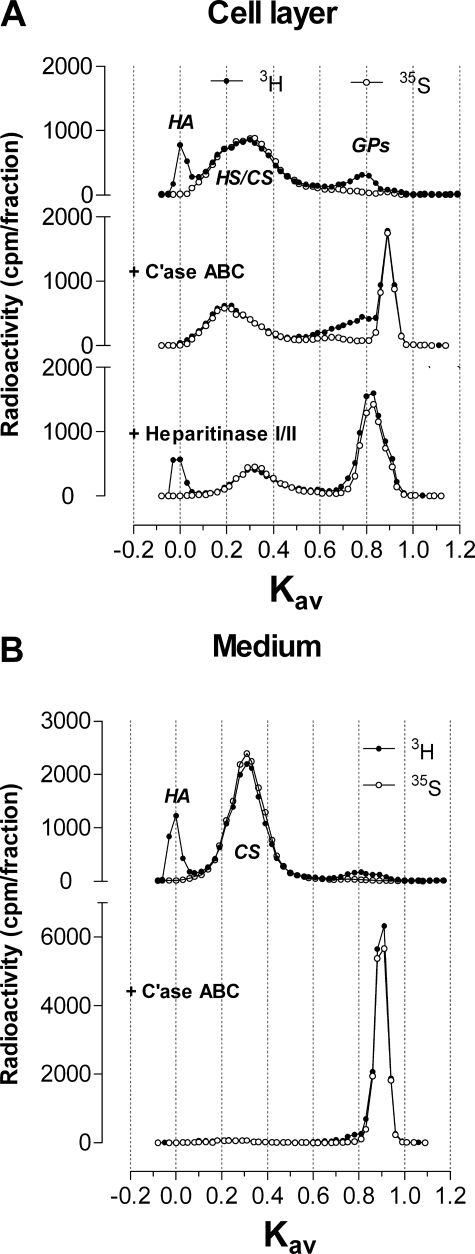
To understand the effects of 4-F-GlcNAc on gycosaminoglycan biosynthesis, we characterized the types of glycosaminoglycans synthesized by cultures of murine ASM cells that were radiolabeled with [3H]glucosamine and [35S]sulfate. After proteinase K digestion and removal of unlabeled precursors, the samples were eluted on a Superose 6 column. Macromolecules associated with the cell layer resulted in three major peaks labeled with 3H with Kav values of 0.0, 0.30, and 0.76 (Fig. 1A, top profile, filled circles). The peak with Kav value 0.30 was also labeled with 35S (Fig. 1A, top profile, open circles). Treatment of cell-associated macromolecules with chondroitinase ABC (C'ase ABC) completely removed the 3H-labeled peak with a Kav value of 0.0 (Fig. 1A, middle profile, filled circles). The 3H- and 35S-labeled digestion products following C'ase ABC treatment were recovered as 34.6 and 37.2% of the total, respectively, in the Vt region (peak Kav value of 0.9, Fig. 1A, middle profile). This suggests that after C'ase ABC treatment, the remaining peak with Kav value 0.20 contains HS chains (Fig. 1A, middle profile). Digestion of the cell-associated macromolecules with heparitinase I/II had no effect on the 3H-labeled peak with a Kav value of 0.0, and the proportions of 3H- and 35S-labeled products recovered in the Vt region were 59.4 and 62.3%, respectively (Fig. 1A, bottom profile). This suggests that after heparitinase I/II treatment, the remaining peak with Kav value 0.31 contains CS chains (Fig. 1A, bottom profile). Although the 3H-labeled peak with Kav value 0.76 is not visible after heparitinase digestion, it is unlikely that this peak was digested by heparitinase I/II, because this peak is not sulfated and is masked by the products of the heparitinase I/II digestion.
FIGURE 1.
Characterization of glycosaminoglycans from murine ASM cells by enzyme digestion and separation on a Superose 6 column. A, digestion of cell-associated macromolecules with C'ase ABC and heparitinase I/II. To facilitate visualization, multiple y axes are used to position the profiles for the control and C'ase ABC above the heparitinase I/II treatment profile. B, digestion of secreted macromolecules with chondroitinase ABC. Filled circles, 3H counts; open circles, 35S counts. Chromatographic profiles are representative of two independent experiments with similar results. GPs, glycopeptides.
The murine ASM cells secreted two major peaks into the medium that were labeled with 3H with Kav values 0.0 and 0.30 (Fig. 1B, filled circles, top profile). The peak with Kav value 0.30 was also labeled with 35S (Fig. 1B, open circles, top profile). The medium sample treated with C'ase ABC resulted in nearly complete digestion of both peaks with 91.7 and 96.2% of the total 3H- and 35S-labeled products, respectively, recovered in the Vt region (Fig. 1B, bottom profile).
In both the cell and medium, the murine ASM cells produce a large nonsulfated glycosaminoglycan (Kav value of 0.0) that is susceptible to digestion with C'ase ABC but not heparitinase I/II, therefore suggesting that this peak is HA. In the cell layer, the peak with Kav value 0.30, which was labeled with both 3H and 35S, is partially susceptible to both C'ase ABC and heparitinase I/II digestion and is a 2:1 mixture of HS with a Kav value of 0.20 to CS with a Kav value of 0.31. In the medium, the secreted glycosaminoglycan with a Kav value of 0.30, which labeled with both 3H and 35S, is predominantly chondroitin sulfate. In the cell layer, the smaller peak with Kav value 0.76, which labeled with 3H only and was not susceptible to C'ase ABC or heparitinase I/II, is likely to be glycopeptides.
4-F-GlcNAc Treatment of ASM Cells Reduces the Size of HS and CS
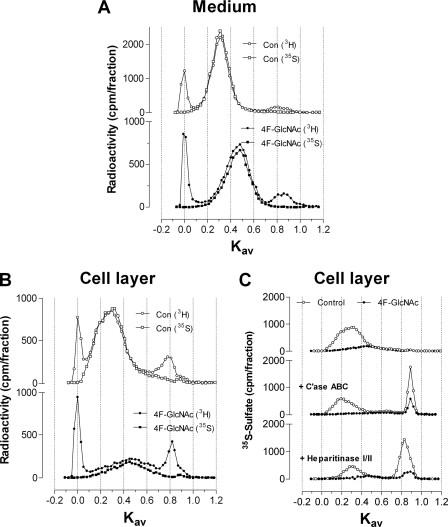
Cultures of murine ASM cells were treated with different concentrations of 4-F-GlcNAc for 8 h in the presence of [35S]sulfate. Analyses of 35S incorporation showed that a concentration of 100 μm 4-F-GlcNAc was sufficient to reach a plateau level of inhibition (data not shown). Therefore, cultures of murine ASM cells were treated with 4-F-GlcNAc or glucosamine for 2 h (both at 100 μm), and the size profiles of glycosaminoglycans, radiolabeled during the following 8 h, were assessed by Superose 6 chromatography.
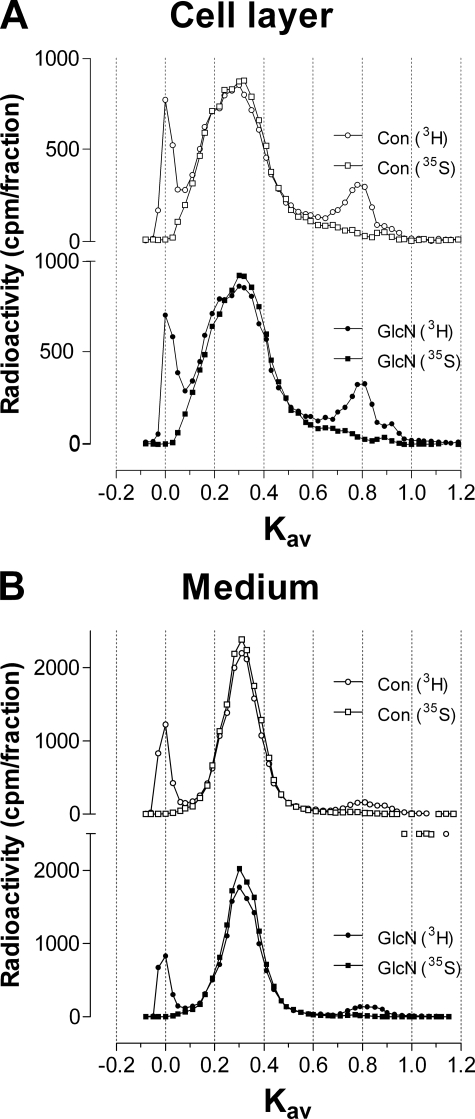
In the medium, the peak with a Kav value of 0.30 in the control was reduced in amount by 65.7% (3H) and 73.0% (35S), and the average size was smaller (peak with a Kav of 0.5) with 4-F-GlcNAc treatment (Fig. 2A). A comparison of the area under the curve of the cell-associated glycosaminoglycans with a Kav value of 0.30 in the control with that of glycosaminoglycans from 4-F-GlcNAc treatment showed reductions of 64.1% (3H) and 76.6% (35S) indicative of less production, and a shift in Kav value to 0.45 indicative of smaller glycosaminoglycan chain size (Fig. 2B). These effects of 4-F-GlcNAc treatment to reduce the amount and size of the major HS/CS peak in the cell layer and of the CS peak in the medium were not observed with an equimolar concentration of GlcN, which were nearly identical to untreated control values (Fig. 3, A and B, respectively).
FIGURE 2.
Size analysis of glycosaminoglycans from murine ASM cells treated with 4-F-GlcNAc. Murine ASM cells were pretreated with 4-F-GlcNAc (100 μm) in the absence of metabolic precursors for 2 h prior to the addition of [3H]glucosamine and [35S]sulfate for 8 h. Samples from the medium (A) and cell layer (B and C) were sized by Superose 6 in 0.5 m ammonium acetate, pH 7.0. Chromatographic profiles are representative of two independent experiments with similar results. To facilitate visualization, multiple y axes are used to position the chromatograms for the control above the treatment (4-F-GlcNAc).
FIGURE 3.
Size analysis of glycosaminoglycans from murine ASM cells treated with glucosamine. Murine ASM cells were pretreated with GlcN in the absence of metabolic precursors for 2 h prior to the addition of [3H]glucosamine and [35S]sulfate for 8 h. Samples from the cell layer (A) and medium (B) were sized by Superose 6 in 0.5 m ammonium acetate, pH 7.0. Chromatographic profiles are representative of two independent experiments with similar results. To facilitate visualization, multiple y axes are used to position the chromatograms for the control above the treatment (GlcN).
To determine whether 4-F-GlcNAc treatment changed either the size of cell-associated glycosaminoglycans and/or their HS:CS ratio, equal amounts of labeled material were treated with C'ase ABC or heparitinase I/II and compared with the profile of glycosaminoglycans from control cells treated with these enzymes (Fig. 2C). After digestion with C'ase ABC, glycosaminoglycans from cells treated with 4-F-GlcNAc showed 54.0% of 35S-labeled products recovered in the Vt region compared with 34.2% for the control (Fig. 2C, middle profile). The residual material resistant to C'ase ABC digestion, containing HS, is very broad, making it difficult to accurately assign a peak Kav value. Following degradation with heparitinase I/II, glycosaminoglycans from cells treated with 4-F-GlcNAc showed 51.5% of 35S-labeled products recovered in the Vt region compared with 62.3% for the control (Fig. 2C, bottom profile). The heparitinase I/II-resistant peak, determined to be CS described above, showed a shift from 0.31 in the control to 0.47 with 4-F-GlcNAc treatment (Fig. 2C, bottom profile). Thus, 4-F-GlcNAc treatment decreases the size of cell-associated CS and changes the ratio of HS:CS from 2:1 to 1:1.
Inhibition of Xyloside-initiated CS
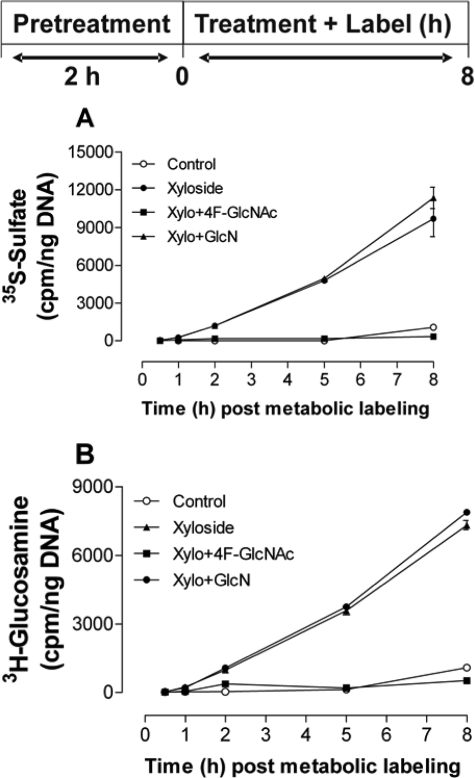
To test whether 4-F-GlcNAc treatment reduces the CS substrate UDP-GalNAc, we used methylumbelliferone-β-d-xyloside, which inhibits regular CS proteoglycan assembly and acts as an alternative acceptor for nonprotein-associated CS synthesis (9). We used 0.25 mm of this xyloside because other studies have shown that this concentration is sufficient to increase the net synthesis of CS by cultured cells to a plateau value (15). At this concentration ASM cells continuously increase incorporation of [35S]sulfate and 3H derived from [3H]glucosamine into secreted macromolecular material over 8 h (Fig. 4, filled circles), reaching levels more than 10-fold higher at 8 h than in the control cultures (open circles). After a 2-h pretreatment with 4-F-GlcNAc, the incorporation of 35S and 3H into macromolecular material was at basal levels, indicating that xyloside-initiated CS synthesis was not observed (Fig. 4, filled squares). Xyloside-initiated CS synthesis was not inhibited by the treatment with 100 μm GlcN (Fig. 4, filled triangles). Treatment of murine ASM cells with 4-F-GlcNAc inhibited xyloside-stimulated CS synthesis in a concentration-dependent manner both in the medium and cell layer (Fig. 5, A and B, respectively). Conversely, nonfluorinated GlcN at equimolar concentrations to 4-F-GlcNAc did not inhibit xyloside stimulated CS synthesis in either the medium or the cell layer (Fig. 5, A and B, respectively).
FIGURE 4.
Kinetics of precursor incorporation into macromolecules following treatment of murine ASM cells with 4-F-GlcNAc in the presence of xyloside. A, analysis of secreted macromolecules over 8 h labeled with [35S]sulfate following treatment of murine ASM cells with 4-F-GlcNAc or GlcN (both at 100 μm) in the presence of xyloside (0.25 mm). B, analysis of secreted macromolecules over 8 h labeled with [3H]glucosamine following treatment of murine ASM cells with 4-F-GlcNAc or glucosamine (both at 100 μm) in the presence of xyloside (0.25 mm). Open circles, DMEM/Ham's F-12 medium without xyloside; filled circles, xyloside treatment; filled squares, 4-F-GlcNAc treatment in the presence of xyloside; filled triangles, glucosamine treatment in the presence of xyloside. The data are expressed as the means ± S.D. (n = 2) from one experiment representing similar data from two independent experiments.
FIGURE 5.
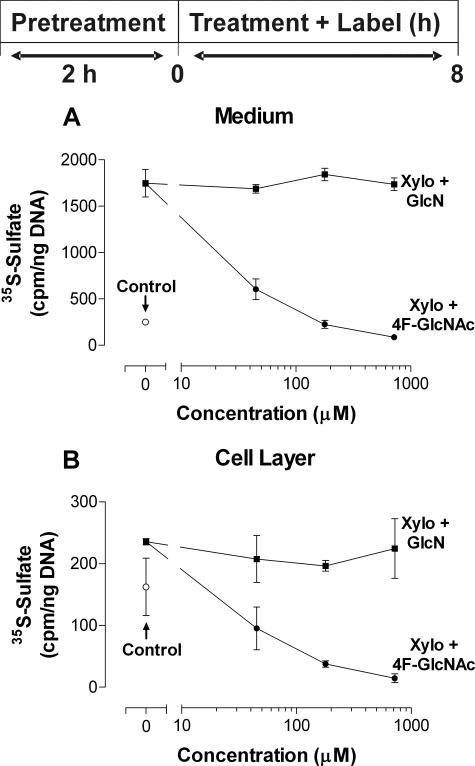
Concentration-response curve of 4-F-GlcNAc treatment in the presence of xyloside. A, analysis of secreted macromolecules following treatment of murine ASM cells with 4-F-GlcNAc or GlcN (both with 45–716 μm) in the presence of xyloside (0.25 mm). B, analysis of cell-associated macromolecules following treatment of murine ASM cells with 4-F-GlcNAc or glucosamine (both with 45–716 μm) in the presence of xyloside (0.25 mm). Open circles, DMEM/F12 without xyloside; filled circles, 4-F-GlcNAc treatment in the presence of xyloside; filled squares, glucosamine treatment in the presence of xyloside. The data are expressed as the means ± S.D. (n = 2) from one experiment representing similar data from two independent experiments.
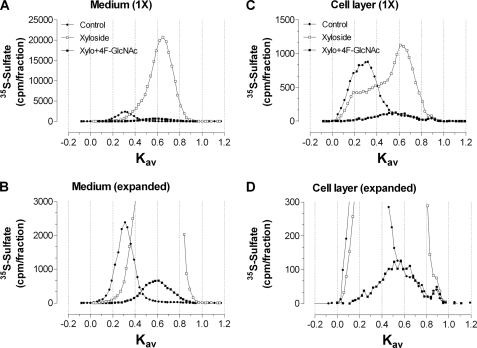
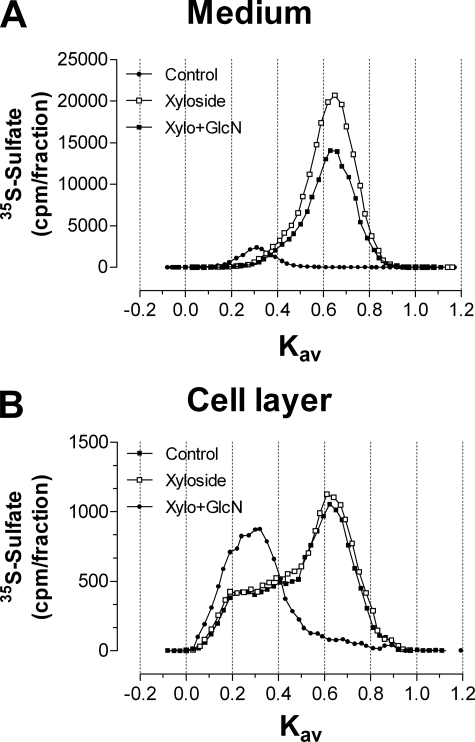
The size distributions of the xyloside-initiated glycosaminoglycans were analyzed by Superose 6 chromatography. Treatment of murine ASM cells with xyloside produced a single peak with a Kav of 0.65 in the medium, while producing a shoulder (Kav from 0.2 to 0.4) and a major peak (Kav of 0.60) in the cell layer (Fig. 6, A and C, open squares). Treatment of murine ASM cells with 4-F-GlcNAc and xyloside significantly reduced 35S incorporation into glycosaminoglycan (>90%) in both the medium and cell layer. The Kav values for the xyloside-CS peak after 4-F-GlcNAc treatment were 0.60 (medium) and 0.55 (cell layer; Fig. 6, B and D, filled squares). Nonfluorinated GlcN and xyloside treatment of ASM cells did not change the Kav value of the xyloside-CS peak in either the medium (Fig. 7A) or the cell layer (Fig. 7B). A small decrease (30.6%) in the area under the curve for 35S-labeled xyloside-CS was observed with GlcN treatment (Fig. 7A).
FIGURE 6.
Size analysis of macromolecules from murine ASM cells treated with 4-F-GlcNAc in the presence of xyloside. Murine ASM cells were pretreated with 4-F-GlcNAc (100 μm) in the absence of metabolic precursors and xyloside for 2 h prior to the addition of 4-F-GlcNAc with xyloside and [3H]glucosamine and [35S]sulfate for 8 h. Macromolecules from the medium (A) and the cell layer (C) were sized by Superose 6 in 0.5 m ammonium acetate, pH 7.0. The profiles from A and C are presented in B and D with a reduced scale on the y axis so that the peak for macromolecules from cells treated with 4-F-GlcNAc is visible. The chromatographic profiles are representative of two independent experiments with similar results.
FIGURE 7.
Size analysis of macromolecules from murine ASM cells treated with glucosamine in the presence of xyloside. Murine ASM cells were pretreated with GlcN (100 μm) in the absence of metabolic precursors and xyloside for 2 h prior to the addition of GlcN with xyloside and [3H]glucosamine and [35S]sulfate for 8 h. Macromolecules from the medium (A) and the cell layer (B) were sized by Superose 6 in 0.5 m ammonium acetate, pH 7.0. The chromatographic profiles are representative of two independent experiments with similar results.
Inhibition of New Protein Synthesis Does Not Alter Precursor Incorporation into Secreted Xyloside-initiated CS
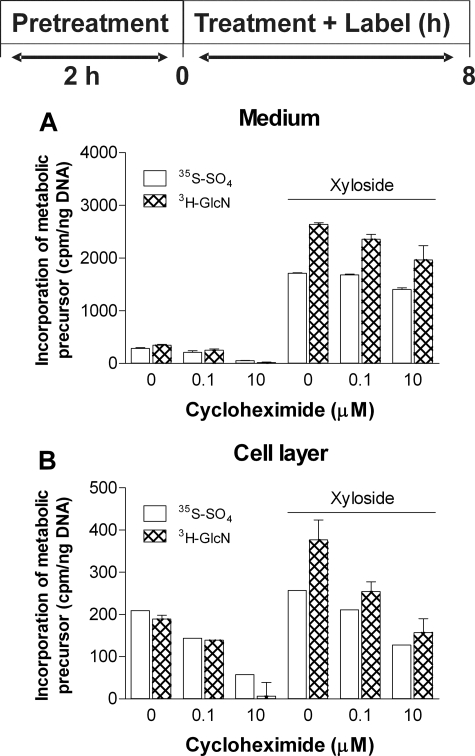
It has been observed in other studies that 4-F-GlcNAc treatment inhibits protein synthesis at high concentrations (8). To rule out the possibility that 4-F-GlcNAc treatment inhibits CS synthesis by nonspecifically inhibiting the production of transporters and enzymes associated with the assembly of xyloside-initiated glycosaminoglycans, murine ASM cells were treated with the protein synthesis inhibitor cycloheximide in the presence of xyloside in an identical protocol used for the analysis of 4-F-GlcNAc effects. The concentration of cycloheximide used in this study (100 nm) was close to its EC50 of 90 nm (16). Under both basal conditions and in the presence of xyloside, 0.1 μm cycloheximide treatment of murine ASM cells did not significantly reduce 35S and 3H incorporation into secreted macromolecular material compared with xyloside treatment alone (Fig. 8A). Under conditions without xyloside, 0.1 μm cycloheximide treatment reduced both 35S and 3H incorporation by ∼25% (Fig. 8B). Treatment of murine ASM cells with 0.1 μm cycloheximide and xyloside reduced 35S and 3H incorporation into cell-associated glycosaminoglycan by 20 and 33%, respectively, compared with xyloside treatment alone (Fig. 8B). Treatment of ASM cells with 10 μm cycloheximide, a concentration that is 100-fold greater than its EC50, inhibited 35S and 3H incorporation into xyloside-stimulated glycosaminoglycans in the medium by 15 and 25%, respectively (Fig. 8A), and in the cell layer by 50 and 58%, respectively (Fig. 8B), compared with xyloside treatment alone. These results indicate that the >90% inhibition of xyloside-stimulated CS synthesis by 4-F-GlcNAc treatment of ASM cells is unlikely to involve nonspecific inhibition of protein synthesis. Rather, the data suggest that 4-F-GlcNAc is more likely acting as a potent inhibitor of the 4-epimerase required to convert UDP-GlcNAc to UDP-GalNAc, thereby depleting the cell of this critical substrate for CS/DS synthesis.
FIGURE 8.
Effect of cycloheximide treatment in the presence xyloside on macromolecules produced by murine ASM cells. Murine ASM cells were pretreated with cycloheximide (0.1, 10 μm) in the absence of metabolic precursors and xyloside for 2 h prior to the addition of cycloheximide (0.1, 10 μm) with xyloside (0.25 mm) and [3H]glucosamine and [35S]sulfate for 8 h. The results represent macromolecular material eluted from a Sephadex G50 (fine) column in 0.1 m ammonium acetate, pH 7.0. The data are expressed as the means ± S.D. (n = 2) from one experiment.
DISCUSSION
The results from this current study using murine ASM cultures show both an inhibition of synthesis and a truncation of HS chain size by 4-F-GlcNAc treatment. HS synthesis is blocked by 4-F-GlcNAc addition to the nonreducing terminal GlcUA residue, thereby preventing further addition of GlcUA via a fluorine residue at the requisite 4-position for HS chain elongation. Our current observations are entirely consistent with this mode of action.
The equivalent inhibition of CS synthesis cannot occur by the same mechanism. The hexosamine substrate for CS synthesis is UDP-GalNAc, which has to be derived from UDP-GlcNAc by 4-epimerization. The 4-F substitution in UDP-4-F-GlcNAc would effectively prevent its epimerization. However, the 4-epimerase should normally continue to provide UDP-GalNAc from existing UDP-GlcNAc unless UDP-4-F-GlcNAc acts as a potent inhibitor of the epimerase. We tested this hypothesis by maximizing the synthesis of CS by ASM cells using treatment with 4-methylumbilliferal-xyloside, an artificial substrate for initiating CS synthesis. Untreated ASM cells increase CS synthesis to a maximum value of more than 10-fold over basal levels with saturating amounts of the xyloside. Pretreatment of the ASM cells for 2 h before adding the radiolabeled precursors completely inhibited any xyloside increase in CS synthesis, providing compelling evidence for a depletion of UDP-GalNAc through rapid and complete inhibition of 4-epimerase activity by 4-F-GlcNAc.
Interestingly, the incorporation of [3H]GlcN into hyaluronan was not significantly altered by the presence of 4-F-GlcNAc. In this case, the substrate UDP-GlcNAc would be added to the reducing end of the growing chain, i.e. to UDP-GlcUA-HA with displacement of UDP forming a β1,4 bond. The next step would be to add UDP-GlcUA to the reducing terminal UDP-GlcNAc-HA to form a β1,3 bond. Because this bond does not directly involve the 4-position, it is possible that UDP-4-F-GlcNAc could be incorporated into the growing hyaluronan chain. In any event, 4-F-GlcNAc does not appear to inhibit hyaluronan synthesis as it might if it were a competitive inhibitor through binding to the enzyme but not forming a glycosidic bond. Additional experiments will be needed to determine whether UDP-4-F-GlcNAc is indeed a substrate for HA synthesis.
There are no published studies on the pharmacokinetics of 4-F-GlcNAc including metabolism, excretion, and toxicity levels in animal models. However, 4-F-GlcNAc has been used in mice in vivo at 50–250 mg/kg/day where anti-inflammatory effects were observed at 100 mg/kg/day for 6 days with no reported toxicity (9). In this case, the effect appeared to be inhibition of the polylactosamine backbone for PSGL-1, a ligand for selectins, with consequent reduction in extravasation of inflammatory cells into sites of inflammation. Because the polylactosaminoglycan chain is synthesized at the nonreducing end and contains galactose-β1,4-GlcNAc repeats, 4-F-GlcNAc would truncate the growing chains in the same way that HS synthesis is truncated. Another report indicates that >200 mg/kg/day of 4-F-GalNAc for 5 days was toxic in DBA/2J mice inoculated with L1210 leukemia cells (8).
The ratio of 3H to 35S in the HS recovered digestion products is higher when cells are treated with 4-F-GlcNAc compared with untreated ASM cells. This would indicate that the equilibrium of UDP-GlcNAc/UDP-GalNAc has been altered by the presence of 4-F-GlcNAc such that there is more UDP-GlcNAc (and thus more radiolabeled UDP-GlcNAc) than UDP-GalNAc. A perturbation of the UDP-GlcNAc/UDP-GalNAc pool suggests that the activity of the UDP-GlcNAc/UDP-GalNAc 4-epimerase has changed. Chemically modified sugars appear to be substrates for the enzyme, UDP-GlcNAc pyrophosphorylase, which catalyzes the conversion of GlcNAc-1-phosphate and UTP to UDP-GlcNAc (17). Synthetically, UDP-GlcNAc pyrophosphorylase can convert both 4-F-GlcNAc-1-P and 4F-GalNAc-1-P to their UDP forms (17). However, this phenomenon has not been shown in cell culture systems. Importantly, whether or not the 4-epimerase can convert 4-F-GlcNAc-1-P to 4F-GalNAc-1-P is questionable, and we have provided evidence to show that the activity of the 4-epimerase is decreased in murine ASM cells treated with 4-F-GlcNAc. The net result of inhibition of 4-epimerase activity is a depletion of the UDP-GalNAc substrate for CS/DS glycosaminoglycan chain synthesis, leading to a reduction in the amount and length of the CS/DS chains and an abrogation of the response to xyloside. We conclude that 4-F-GlcNAc is a useful tool for studying glycosaminoglycan synthesis in cell culture systems that may assist the understanding of pathological changes in proteoglycans.
This work was supported, in whole or in part, by National Institutes of Health Grants P11 HL081064, HL073714 (to R. S.), and CA121335 (to R. S.).
- F
- fluorine
- HS
- heparan sulfate
- CS
- chondroitin sulfate
- HA
- hyaluronan
- ASM
- airway smooth muscle
- GlcUA
- glucuronate
- DS
- dermatan sulfate
- DMEM
- Dulbecco's modified Eagle's medium
- C'ase ABC
- chondroitinase ABC.
REFERENCES
- 1.Whitelock J. M., Melrose J., Iozzo R. V. ( 2008) Biochemistry 47, 11174– 11183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgenstern D. A., Asher R. A., Fawcett J. W. ( 2002) Prog. Brain Res. 137, 313– 332 [DOI] [PubMed] [Google Scholar]
- 3.Nakashima Y., Wight T. N., Sueishi K. ( 2008) Cardiovasc. Res. 79, 14– 23 [DOI] [PubMed] [Google Scholar]
- 4.Roberts C. R., Burke A. K. ( 1998) Can. Respir. J. 5, 48– 50 [PubMed] [Google Scholar]
- 5.Tsuchiya T. ( 1990) Chemistry and Developments of Fluorinated Carbohydrates, pp. 91– 277, Academic Press, San Diego, CA: [DOI] [PubMed] [Google Scholar]
- 6.Bernacki R. J., Sharma M., Porter N. K., Rustum Y., Paul B., Korythyk W. ( 1977) Journal of Supramolecular Structure 7, 235– 250 [DOI] [PubMed] [Google Scholar]
- 7.Uldry M., Ibberson M., Hosokawa M., Thorens B. ( 2002) FEBS Lett. 524, 199– 203 [DOI] [PubMed] [Google Scholar]
- 8.Sharma M., Bernacki R. J., Paul B., Korytnyk W. ( 1990) Carbohydr. Res. 198, 205– 221 [DOI] [PubMed] [Google Scholar]
- 9.Dimitroff C. J., Kupper T. S., Sackstein R. ( 2003) J. Clin. Investig. 112, 1008– 1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kisilevsky R., Szarek W. A. ( 2002) J. Mol. Neurosci. 19, 45– 50 [DOI] [PubMed] [Google Scholar]
- 11.Berkin A., Szarek W. A., Kisilevsky R. ( 2000) Carbohydr. Res. 326, 250– 263 [DOI] [PubMed] [Google Scholar]
- 12.Johnson P. R., Black J. L., Carlin S., Ge Q., Underwood P. A. ( 2000) Am. J. Respir. Crit. Care Med. 162, 2145– 2151 [DOI] [PubMed] [Google Scholar]
- 13.Potter-Perigo S., Braun K. R., Schönherr E., Wight T. N. ( 1992) Arch. Biochem. Biophys. 297, 101– 109 [DOI] [PubMed] [Google Scholar]
- 14.Blumenkrantz N., Asboe-Hansen G. ( 1973) Anal. Biochem. 54, 484– 489 [DOI] [PubMed] [Google Scholar]
- 15.Lohmander S., Madsen K., Hinek A. ( 1979) Arch. Biochem. Biophys. 192, 148– 157 [DOI] [PubMed] [Google Scholar]
- 16.Contreras A., Vazquez D., Carrasco L. ( 1978) J. Antibiot. 31, 598– 602 [DOI] [PubMed] [Google Scholar]
- 17.Feng F., Okuyama K., Niikura K., Ohta T., Sadamoto R., Monde K., Noguchi T., Nishimura S. ( 2004) Org. Biomol. Chem. 2, 1617– 1623 [DOI] [PubMed] [Google Scholar]