Abstract
Reduced lipolysis in hormone-sensitive lipase-deficient mice is associated with impaired glucose-stimulated insulin secretion (GSIS), suggesting that endogenous β-cell lipid stores provide signaling molecules for insulin release. Measurements of lipolysis and triglyceride (TG) lipase activity in islets from HSL−/− mice indicated the presence of other TG lipase(s) in the β-cell. Using real time-quantitative PCR, adipose triglyceride lipase (ATGL) was found to be the most abundant TG lipase in rat islets and INS832/13 cells. To assess its role in insulin secretion, ATGL expression was decreased in INS832/13 cells (ATGL-knockdown (KD)) by small hairpin RNA. ATGL-KD increased the esterification of free fatty acid (FFA) into TG. ATGL-KD cells showed decreased glucose- or Gln + Leu-induced insulin release, as well as reduced response to KCl or palmitate at high, but not low, glucose. The KATP-independent/amplification pathway of GSIS was considerably reduced in ATGL-KD cells. ATGL−/− mice were hypoinsulinemic and hypoglycemic and showed decreased plasma TG and FFAs. A hyperglycemic clamp revealed increased insulin sensitivity and decreased GSIS and arginine-induced insulin secretion in ATGL−/− mice. Accordingly, isolated islets from ATGL−/− mice showed reduced insulin secretion in response to glucose, glucose + palmitate, and KCl. Islet TG content and FFA esterification into TG were increased by 2-fold in ATGL−/− islets, but glucose usage and oxidation were unaltered. The results demonstrate the importance of ATGL and intracellular lipid signaling for fuel- and non-fuel-induced insulin secretion.
Free fatty acids (FFA)5 and other lipid molecules are important for proper glucose-stimulated insulin secretion (GSIS) by β-cells. Thus, deprivation of fatty acids (FA) in vivo (1) diminishes GSIS, whereas a short term exposure to FFA enhances it (1–3). In contrast, a sustained provision of FA, particularly in the presence of high glucose in vitro, is detrimental to β-cells in that it reduces insulin gene expression (4) and secretion (5) and induces β-cell apoptosis (6). The FA supply to the β-cells can be from exogenous sources, such as plasma FFAs and lipoproteins, or endogenous sources, such as intracellular triglyceride (TG) stores. Studies from our laboratory (7–10) and others (11, 12) support the concept that the hydrolysis of endogenous TG plays an important role in fuel-induced insulin secretion because TG depletion with leptin (13) or inhibition of TG lipolysis by lipase inhibitors such as 3,5-dimethylpyrazole (7) or orlistat (11, 12) markedly curtail GSIS in rat islets. Furthermore, mice with β-cell-specific knock-out of hormone-sensitive lipase (HSL), which hydrolyzes both TG and diacylglycerol (DAG), show defective first phase GSIS in vivo and in vitro (14).
Lipolysis is an integral part of an essential metabolic pathway, the TG/FFA cycle, in which FFA esterification onto a glycerol backbone leading to the synthesis of TG is followed by its hydrolysis with the release of the FFA that can then be re-esterified. Intracellular TG/FFA cycling is known to occur in adipose tissue of rats and humans (15, 16) and also in liver and skeletal muscle (17). It is generally described as a “futile cycle” as it leads to the net hydrolysis of ATP with the generation of heat (18). However, several studies have shown that this cycle has important functions in the cell. For instance, in brown adipose tissue, it contributes to overall thermogenesis (17, 19). In islets from the normoglycemic, hyperinsulinemic, obese Zucker fatty rat, increased GSIS is associated with increased glucose-stimulated lipolysis and FA esterification, indicating enhanced TG/FFA cycling (10). Stimulation of lipolysis by glucose has also been observed in isolated islets from normal rats (12) and HSL−/− mice (8) indicating the presence of glucose-responsive TG/FFA cycling in pancreatic β-cells.
The identity of the key lipases involved in the TG/FFA cycle in pancreatic islets is uncertain. HSL is expressed in islets (20), is up-regulated by long term treatment with elevated glucose (21), and is associated with insulin secretory granules (22). In addition, our earlier results suggested that elevated HSL expression correlates with augmented TG/FFA cycling in islets of Zucker fatty rats (10). However, it appears that other lipases may contribute to lipolysis and the regulation of GSIS in islet tissue. Thus, results from studies using HSL−/− mice showed unaltered GSIS (8, 23), except in fasted male mice (8, 9) in which lipolysis was decreased but not abolished. Furthermore, HSL−/− mice show residual TG lipase activity (8) indicating the presence of other TG lipases.
Recently, adipocyte triglyceride lipase (ATGL; also known as Desnutrin, TTS-2, iPLA2-ζ, and PNPLA2) (24–26) was found to account for most if not all of the residual lipolysis in HSL−/− mice (26, 27). Two homologues of ATGL, Adiponutrin and GS2, have been described in adipocytes (24). All three enzymes contain a patatin-like domain with broad lipid acyl-hydrolase activity. However, it is not known if adiponutrin and GS2 are actually TG hydrolases. An additional lipase, TG hydrolase or carboxylesterase-3, has been identified in rat adipose tissue (28, 29). Although the hydrolysis of TG is catalyzed by all these lipases, HSL can hydrolyze both TG and DAG, the latter being a better substrate (30).
In this study, we observed that besides HSL, ATGL (31), adiponutrin, and GS2 are expressed in rat islets and INS832/13 cells, with ATGL being the most abundant. We then focused on the role of ATGL in fuel-stimulated insulin secretion in two models, INS832/13 β-cells in which ATGL expression was reduced by RNA interference-knockdown (ATGL-KD) and ATGL−/− mice.
EXPERIMENTAL PROCEDURES
Cell Culture
Rat insulinoma INS832/13 cells (32) (passages 54–63) were cultured at 11.1 mm glucose in RPMI 1640 medium supplemented with 10% (w/v) fetal bovine serum, 10 mm HEPES, 2 mm glutamine, 1 mm sodium pyruvate, and 50 μm β-mercaptoethanol (complete RPMI) at 37 °C in a humidified atmosphere (5% CO2, 95% air). Cells were seeded at 4 × 106 cells 2 days before transfection to reach a 60–70% confluence at the day of transfection.
Animals
10-Week-old overnight fasted male ATGL−/− mice (33) backcrossed to the C57BL/6 strain for more than nine generations were used. Control mice used in this study were C57BL/6 wild type littermates. The mice are not from the C57BL/6J background and therefore do not harbor a mutation in the nicotinamide nucleotide transhydrogenease gene (34). Wistar rats (200–250 g) were obtained from Charles River Laboratories (St. Constant, Quebec, Canada). Mice and rats were housed under control temperature (23 °C) and light conditions (12-h light/dark cycle) with free access to water and standard diet (11% fat by energy). Serum insulin, glucose, nonesterified FFA, and TG were measured in overnight fasted pentobarbital-anesthetized mice (8). The measurements of whole body fat and lean mass were done in fed mice by quantitative magnetic resonance (EchoMRI, Echo Medical Systems). All procedures were approved by the Institutional Committee for the Protection of Animals at the Centre de Recherche du Centre Hospitalier de l'Université de Montréal.
Islet Isolation and Culture
Fed or overnight fasted male Wistar rats and male ATGL−/− mice and their wild type controls were anesthetized with sodium pentobarbital (Somnotol, MTC Pharmaceuticals, Hamilton, Ontario, Canada) and killed by exsanguination. Pancreatic rat or mice islets were isolated by collagenase digestion of the pancreas according to the method of Gotoh et al. (35). After digestion and washing, islets were separated from digested exocrine tissue by Histopaque gradient, after which rat islets were hand-picked for immediate extraction of RNA or protein preparation for Western blotting. Mouse islets were handpicked and kept before experiments for 2 h in culture in complete RPMI medium containing 2.8 mm glucose without β-mercaptoethanol at 37 °C in a humidified atmosphere containing 5% CO2 (8).
Short Hairpin RNA-mediated ATGL Gene Suppression
Short hairpin (sh) RNA-mediated gene suppression was performed as described previously (36). In brief, five 19-nucleotide shRNAs against rat ATGL (GenBankTM accession number NM_001108509) with a 9-nucleotide loop were synthesized, annealed, and ligated to the PmeI and XbaI sites of pDLDU6 (37). Efficiency of the various shRNA constructs on ATGL mRNA levels was verified in INS832/13 cells, and the most efficient shRNA was chosen for subsequent experiments. The sense target sequence of shRNA-ATGL chosen is 5′-AAA GAC CAT CCG TGG TTG TCT-3′ (beginning at nucleotide 371 of ATGL sequence). The sense sequence with no known rat gene homology for scrambled control shRNA is 5′-CTG AGC ATT CAT TGG TCG C-3′ (scrATGL). The pDLDU6 empty-vector (referred to as Mock) was also used as control.
Cell Transfection
shRNA-pDLDU6 constructs were introduced into INS832/13 cells by nucleofection (36) at a concentration of 5 μg of DNA for 6.9 × 106 cells. After transfection, cells were seeded in 12-well plates with 3 × 105 cells for mRNA preparation and insulin secretion assays; 6-well plates with 6 × 105 cells for immunoblot analysis, TG hydrolase activity, TG content, fatty acid (FA) esterification, and lipolysis measurements; 48-well plates with 6 × 104 cells for mitochondrial membrane potential determinations; 96-well plates with 2 × 104 cells for the assessment of ATP content; and in 25-cm2 flasks with 1.5 × 106 cells for glucose oxidation determination. Experiments with shRNA-ATGL were performed 96 h post-transfection.
Real Time Quantitative PCR Analysis
Total RNA was extracted from rat adipocytes using the RNeasy lipid tissue mini kit (Qiagen, Mississauga, Ontario, Canada) and from rat and mouse islets and cells using the RNeasy mini kit (Qiagen) with RNase-free DNase (Qiagen). RNA (3 μg) was reverse-transcribed to cDNA using Moloney murine leukemia virus reverse transcriptase (Invitrogen) and hexamers as described previously (38). The primers (IDT, Coralville, IA) used are listed in supplemental Table 1. Gene expression was determined by the standard curve method and normalized to the expression of cyclophilin or β-actin. Real time PCR analysis was performed using the Rotor-Gene R3000 (Corbett Research, Mortlake, New South Wales, Australia) and the LCR Faststart DNA MasterPLUS SYBR Green reagent (Roche Applied Science). The number of mRNA molecules of the different lipases per μg of total RNA was evaluated employing a corresponding standard curve. For the determination of the effect of glucose on ATGL mRNA expression, INS832/13 cells at 70–80% confluence were employed after washing twice with PBS and culturing in complete RPMI medium at 3, 11, or 16 mm glucose for 24 h.
Triglyceride Lipase Activity
Transfected cells were washed twice in PBS and centrifuged for 5 min at 200 × g. Cell homogenates were then prepared in 0.2 ml of homogenization buffer (0.25 mm sucrose, 1 mm EDTA, 10 mm Tris, pH 7.0, 20 μg/ml leupeptin, 2 μg/ml antipain, and 1 μg/ml pepstatin A). Triglyceride lipase activity assay was performed on the cell homogenates as described previously (8).
Western Blotting Analysis
Transfected cells collected by trypsinization and isolated rat islets were washed three times with PBS. Total protein extracts from islets and cells were obtained as described previously (36).
Inguinal fat pads were surgically removed from fed rats, pulverized under liquid nitrogen, and extracted with the use of ice-cold lysis buffer (20 mm Tris-HCl, pH 7.2, containing 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 1% (v/v) Triton X-100, 0.1% SDS, and protease inhibitors) for 30 min at 4 °C. Insoluble material was removed by centrifugation at 10,000 × g at 4 °C for 10 min. The supernatant was collected and the protein assayed with a BSA protein assay kit (Pierce). Protein extracts from cells, islets, and fat (15 μg/lane) were separated on 10% SDS-PAGE and electroblotted to nitrocellulose membrane (Whatman, Hanestrabe, Dassel, Germany). Membranes were blocked with 5% (w/v) nonfat dry skimmed milk in Tris-buffered saline, pH 7.5, with 0.1% (v/v) Tween (TBS-T) for 30 min at 37 °C and incubated with rabbit anti-ATGL antibody (1/200 dilution in TBS-T, Cayman Chemical Co., Ann Arbor, MI) overnight at 4 °C. Blots were washed with TBS-T, blocked 15 min at 37 °C with TBS-T, 5% milk, and exposed to horseradish peroxidase-conjugated goat anti-rabbit IgG (1/10,000 dilution in TBT-T, Bio-Rad) for 1 h at room temperature. Membranes were washed again and developed by enhanced chemiluminescence using a standard kit (SuperSignal West Pico chemiluminescent substrate, Pierce). Band intensity was measured by densitometry and analyzed using image analysis Genesnap software from the G-Box (PerkinElmer Life Sciences). For the normalization of ATGL protein levels, membranes were incubated with anti-actin antibody (1/500 dilution in TBS-T).
Triglyceride Content
TG content was measured in transfected cells and mice islets as described (9).
Insulin Secretion and Insulin Content Measurements
Transfected cells (96 h post-transfection) were washed with PBS and cultured for 2 h in complete RPMI medium at 1 mm glucose. They were then preincubated for 40 min at 37 °C in 1 ml of Krebs-Ringer bicarbonate (KRBH) buffer containing 10 mm HEPES, 1 mm glucose, and 0.5% defatted BSA (d-BSA), after which they were incubated for 45 min in KRBH, 0.5% d-BSA plus different test agents as indicated in figure legends. At the end of the incubation, media were kept for insulin measurement by radioimmunoassay using a human insulin standard (Linco Research, St. Charles, MO). Total insulin content was measured following acid/ethanol (0.2 mm HCl in 75% ethanol) extraction of cells, and protein cellular content was determined by using a BCA protein assay kit.
For insulin secretion in islets, freshly isolated islets that were cultured as described above in RPMI medium for only 2 h for recovery from isolation were distributed in 12-well plates (10 islets/well) and preincubated for 45 min at 37 °C in KRBH, 0.5% d-BSA, and 2.8 mm glucose. They were then incubated for 1 h in KRBH, 0.5% d-BSA, and 2.8, 8.3, or 16.7 mm glucose, in the presence or absence of 0.4 mm palmitate, or 35 mm KCl. Insulin in the media at the end of the incubation and insulin contents of islets were quantified as indicated above.
Intraperitoneal Glucose Tolerance Test
Intraperitoneal glucose tolerance tests (IPGTT) were performed in conscious mice in the morning after a 16-h fast. A 15% glucose solution (1.5 g of glucose/kg of body weight) was administrated intraperitoneally. Tail blood samples (∼70 μl) were collected into heparinized tubes at 0, 15, 30, 60, and 120 min for measurement of glycemia and insulinemia.
Hyperglycemic Clamp
One-step hyperglycemic clamps (HGC) were performed on conscious mice. A 20% dextrose solution (Baxter Healthcare Corp.) was infused through the jugular vein to clamp plasma glucose at ∼300 mg/dl for 60 min. Glucose infusion rate of the exogenous glucose infusion was adjusted based on instantaneous glycemia assessments using a handheld glucometer (Accu-Check, Roche Applied Science). At time 60 min of the clamp, an arginine bolus injection was performed (intravenously, 1 mmol/kg; Sandoz Canada Inc.) to assess the maximal insulin response. Blood samples were collected from the tail vessels, rapidly spun to separate erythrocytes, and kept frozen until insulin determination by enzyme-linked immunosorbent assay (insulin mouse ultrasensitive electroimmunoassay, ALPCO Diagnostics, Salem, NH) at times 0, 5, 15, 30, 60, 61, and 70 min.
Glucose Oxidation in INS Cells
Transfected INS832/13 cells, cultured and preincubated as described under insulin secretion experiments, were incubated for 2 h in 2 ml of KRBH, 0.5% d-BSA with 1 mm glucose plus 0.1 μCi/ml [U-14C]glucose (290 mCi/mmol; Amersham Biosciences) or 10 mm glucose plus 0.2 μCi/ml of [U-14C]glucose. At the beginning of the incubation, 25-cm2 flasks were sealed with a stopper containing a piece of Whatman GF/B paper soaked in 5% KOH. At the end of the incubation, perchloric acid was injected into each flasks, and the liberated CO2 was trapped into Whatman paper. The trapped 14CO2 was measured by liquid scintillation counting.
Islets Glucose Metabolism
Groups of 20 freshly isolated islets, cultured and preincubated as described for insulin secretion, were incubated at 37 °C for 2 h in KRBH, 0.25% d-BSA containing 0.5 μCi of d-[5-3H]glucose (16 Ci/mmol) and 1 μCi/ml d-[U-14C]glucose (250 mCi/mmol), 2.8 or 16.7 mm glucose. Incubation was stopped by the addition of citrate/NaOH buffer (400 mm, pH 4.9) containing antimycin-A (10 μm), rotenone (10 μm), and potassium cyanide (5 mm) as described previously (39). Glucose oxidation was measured by the generation of potassium hydroxide-trapped 14CO2 after 60 min of incubation at room temperature. Glucose utilization was determined by measuring the amount of 3H2O.
Fatty Acid Esterification and Oxidation
For fatty acid esterification determination in INS832/13 cells, transfected cells cultured and preincubated as for insulin secretion experiments, were incubated for 45 min in 2 ml of KRBH containing 0.1 μCi/ml [14C]palmitate (57.5 mCi/mmol; Amersham Biosciences), 0.2 mm palmitate, 0.5% d-BSA, 1 mm carnitine, and 1 or 10 mm glucose. For FA esterification and oxidation in mice islets, batches of 50 freshly isolated islets cultured as for insulin secretion experiments were preincubated for 45 min in 1 ml of KRBH containing 0.1 mm (FA oxidation) or 0.2 mm palmitate, 0.25% d-BSA (FA esterification) and 2.8 mm glucose. They were then incubated for 2 h in 0.5 ml of KRBH, 0.25% d-BSA, 0.1 mm (oxidation) or 0.2 mm palmitate (esterification), 1 mm carnitine, 2 μCi/ml [9,10-3H]palmitate (51 Ci/mmol, Amersham Biosciences), and 2.8 or 16.7 mm glucose. At the end of the incubation, cells or islets were collected for FA esterification determination, washed in cold PBS, and resuspended in 3 ml of Folch reagent (40). Total lipids were extracted and separated by thin layer chromatography using a solvent for neutral lipids (petroleum ether/ether/acetic acid; 70/30/1) to measure the incorporation of labeled palmitate into complex lipids as described previously (10). For FA oxidation, after 2 h, incubation media were transferred into Eppendorf tubes for separation of 3H2O from labeled fatty acids as described previously (41).
Lipolysis Measurement
Transfected INS832/13 cells cultured and preincubated as for insulin secretion experiments were incubated for 2 h in 1 ml of KRBH, 0.5% d-BSA and 1 or 10 mm glucose. Batches of 60 freshly isolated islets, cultured as for insulin secretion experiments, were incubated for 3 h in 0.2 ml of KRBH, 0.07% d-BSA at 2.8 or 16.7 mm glucose. At the end of the incubation, media were kept to measure glycerol release as an index of lipolysis. An enzymatic luminescence detection method, based on the reduction of NAD+ to NADH in a series of enzymatic reactions (8), was used to measure glycerol in the medium.
ATP Content and Mitochondrial Membrane Potential
Transfected INS832/13 cells cultured as described for insulin secretion experiments were preincubated for 1 h in KRBH, 0.07% d-BSA at 1 mm glucose, after which they were incubated for 10 min in KRBH, 0.07% d-BSA at 1 or 10 mm glucose. At the end of the incubation, ATP was extracted from the cells and assayed using an ATP bioluminescence assay (ATPlite kit, PerkinElmer Life Sciences) according to the manufacturer's instructions.
For mitochondrial membrane potential determination, transfected cells were loaded with rhodamine 123 (Rh123, Invitrogen) for 20 min. They were then washed and incubated in KRBH, 0.07% d-BSA at 1 mm glucose for 30 min, after which they were washed and incubated in the same solution for 10 min. At the end of the incubation, basal fluorescence (Rh123 excited at 485 nm and the emitted fluorescence monitored at 530 nm) was measured on a FLUOstar microplate reader (BMG Labtech, Offenburg, Germany). Then 1 or 10 mm glucose with or without 0.3 mm fluoro-carbonyl cyanide phenylhydrazone, a mitochondrial membrane uncoupler, was added, and 10 min later Rh123 fluorescence was recorded.
Statistical Analysis
All results are expressed as means ± S.E. Statistical significance was calculated with the Student's t test or, for multiple comparisons, one-way or two-ways analysis of variance (ANOVA) with Bonferroni post hoc testing as indicated. A p value of <0.05 was considered significant.
RESULTS
ATGL Is Expressed in Rat Islets and INS832/13 Cells and Regulated by Fasting
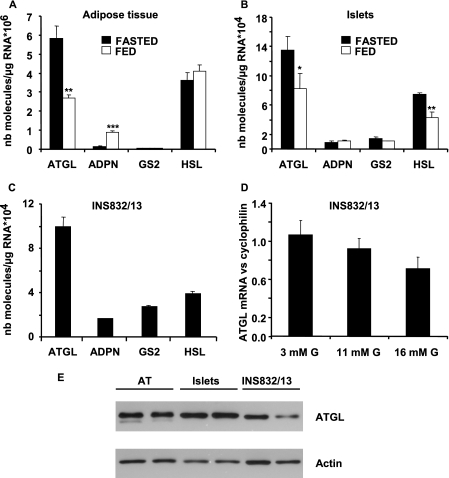
We first determined the level of expression of TG lipases that have recently been characterized, including ATGL, adiponutrin, and GS2 (24), in white adipose tissue (WAT) of inguinal fat pads, rat islets, and INS832/13 cells. As shown in Fig. 1A, ATGL and HSL are the most highly expressed TG lipases in WAT. Adiponutrin is detectable in WAT from fed rats, but it is almost absent in WAT from fasted rats. GS2 mRNA was not detected. ATGL mRNA was up-regulated in WAT of overnight fasted rats, as described previously (25). In rat islets, ATGL and HSL mRNA were the most abundantly expressed lipases, and their levels were significantly increased by fasting. Adiponutrin and GS2 mRNA were present at low levels (Fig. 1B). The mRNA for carboxylesterase 3 (also named TG hydrolase), another TG lipase identified in WAT and possibly involved in basal lipolysis in adipocytes (29), was not detected in either rat islets or INS832/13 cells (data not shown). ATGL was the main TG lipase in INS832/13 cells cultured in complete RPMI medium at 11.1 mm glucose (Fig. 1C). Because HSL expression is up-regulated by long term exposure to high glucose (21), the effect of glucose on ATGL expression was evaluated by RT-quantitative PCR in INS832/13 cells incubated for 24 h at different glucose concentrations. No significant effect of glucose on ATGL mRNA levels was observed, although it tended to decrease with increasing glucose concentrations (Fig. 1D). Possibly, reduced expression of ATGL may become significant with longer incubation times at high glucose. The presence of ATGL protein in rat WAT, rat islets, and INS832/13 cells was established by Western blot analysis (Fig. 1E).
FIGURE 1.
ATGL expression relative to other triglyceride lipases in adipose tissue, islets, and INS832/13 cells and its regulation by the dietary state. ATGL, adiponutrin (ADPN), GS2, and HSL mRNA levels were determined by real time RT-PCR in adipose tissue (A) and islets (B) from overnight fasted and fed rats, and in INS832/13 cells cultured in complete RPMI medium at 11.1 mm glucose (C). The effect of glucose on ATGL expression was studied in INS832/13 exposed for 24 h at 3, 11, or 16 mm glucose (G) in complete RPMI medium and normalized to cyclophilin (D). Immunoblot analysis of ATGL (E) in adipose tissue (AT) (adipose tissue from two different rats, 1st and 2nd lanes), rat islets (two different rats, 3rd and 4th lanes), and INS832/13 cells (two different passages, 5th and 6th lanes) is shown. The data are expressed as means ± S.E. of four rats (A and B) or four different passages for INS832/13 cells (C and D). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus nutritional state, by unpaired two-tailed Student's t test. NB, number.
ATGL Knockdown Decreases TG Lipase Activity and Induces Accumulation of Intracellular TG
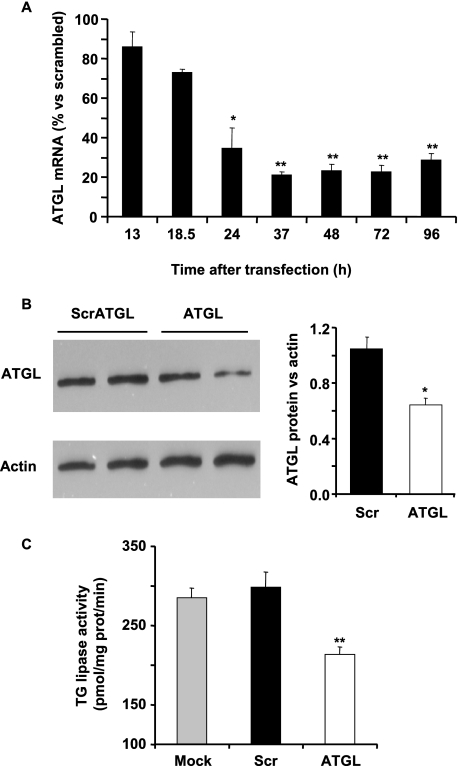
As ATGL appears to be the most expressed TG lipase in β-cells, we assessed the effect of silencing it in INS832/13 cells using shRNA (shATGL). Transfection efficiency was ∼85%, as evaluated with a green fluorescence protein reporter plasmid (data not shown). ATGL mRNA expression was reduced by 65% 24-h post-shATGL transfection and by 75% from 37 to 96 h (Fig. 2A). For subsequent experiments, the effects of shATGL were studied 96 h post-transfection. At this time, there was a 40% decrease in cellular ATGL protein (Fig. 2B) and a 30% reduction in total triglyceride lipase activity (Fig. 2C). It should be noted that ATGL is not the only TG lipase expressed in INS832/13 cells and that other lipases, including HSL, contribute to the measured TG lipase activity. We verified that the decrease in ATGL expression did not lead to a compensatory increase in HSL expression (data not shown).
FIGURE 2.
shATGL treatment of INS832/13 cells reduces the expression of ATGL mRNA and protein and decreases TG lipase activity. INS832/13 cells were electroporated in the presence of empty vector (Mock), scrATGL (Scr), or shATGL. Nontransfected cells served as additional control. A, time course of the effect of shATGL on ATGL mRNA expression in INS832/13 cells cultured in complete RPMI medium at 11.1 mm glucose. B, ATGL protein expression was determined by Western blot analysis using actin as a control. C, triglyceride lipase activity of cytosolic extracts of INS832/13 cells using radiolabeled triolein as a substrate. Means ± S.E. are of three experiments performed in duplicate. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus time 13 h after transfection (A) or versus scrambled ATGL (B and C), by unpaired two-tailed Student's t test.
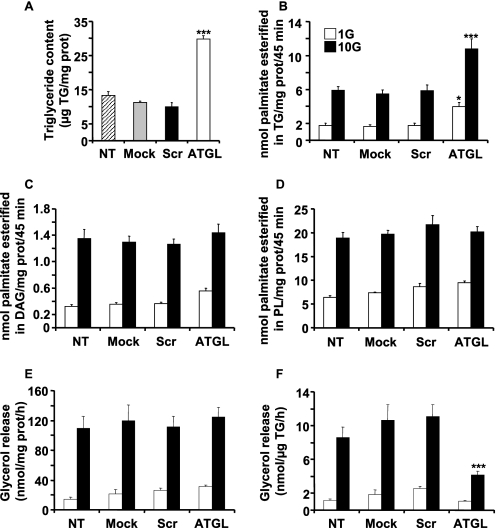
ATGL is responsible for the initial step of TG catabolism (26). It is anticipated that reduced ATGL expression should reduce TG/FFA cycling and cause accumulation of cellular TG. In keeping with this prediction, ATGL-KD in INS832/13 cells resulted in a 3-fold increase in TG content (Fig. 3A). To measure the effect of ATGL-KD on FA esterification, transfected cells were labeled for 45 min with [14C]palmitate. Palmitate esterification into TG was enhanced about 2-fold at both 1 and 10 mm glucose in ATGL-KD cells (Fig. 3B). In contrast, no changes in palmitate incorporation into DAG (Fig. 3C) or phospholipids (Fig. 3D) or in cellular nonesterified palmitate (NEFA) was observed (data not shown). A modest reduction in esterification of FA to cholesterol esters in ATGL-KD cells cultured at 10 mm glucose was noted (data not shown).
FIGURE 3.
Knockdown of ATGL expression in INS832/13 cells increases FA esterification into TG and causes TG deposition. Cellular TG content was measured 96 h post-transfection with scrATGL, shATGL, or under mock or nontransfected (NT) conditions (A). Means ± S.E. are of 15 different wells in four separate experiments. B–D, cells were incubated for 45 min in KRBH containing [1-14C]palmitate at 1 and 10 mm glucose (G). B–D show palmitate esterification into TG (B), DAG (C), and PL (D). Means ± S.E. of three independent experiments are done in triplicate. Glycerol release is shown in E and F and expressed per protein content (E) or per TG content (F). Data represent means ± S.E. of five independent experiments performed in duplicate or triplicate. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus scrambled ATGL for the same glucose concentration, one way-ANOVA, Bonferroni post hoc test.
The effect of modulating ATGL expression on lipolysis was evaluated by measuring glycerol release. There was no change in the release of glycerol expressed per mg of protein in shATGL cells (Fig. 3E). In ATGL-KD cells, the TG content was increased 3-fold, and if lipolysis is expressed per unit of TG, it was reduced in shATGL cells (Fig. 3F). Overall the data indicate that TG metabolism of INS832/13 cells is affected upon reduced expression of ATGL.
Knockdown of ATGL Expression Does Not Affect Glucose and Energy Metabolism
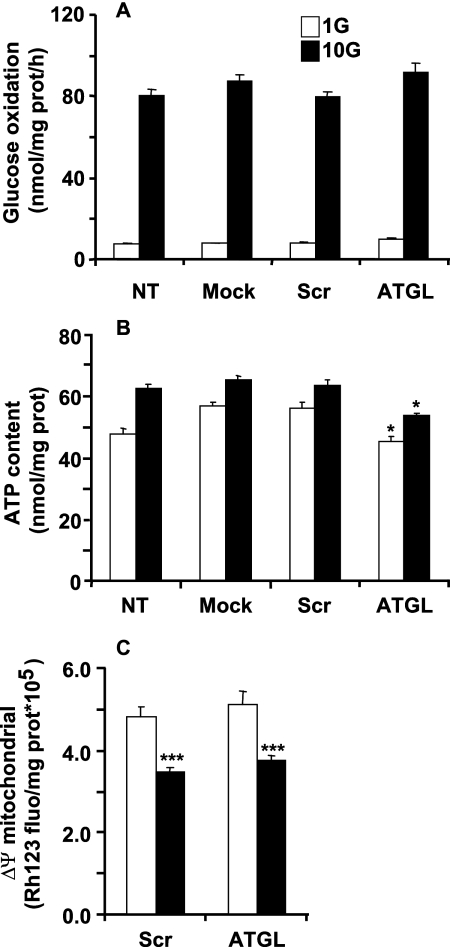
Various parameters of glucose and energy metabolism were determined to assess the potential toxicity of the constructs or of reducing ATGL expression. ATGL-KD had no effect on glucose oxidation at low or high glucose concentrations in INS832/13 cells (Fig. 4A). A very modest reduction in ATP content was observed at both low and high glucose in ATGL-KD cells; however, there was no difference in the fractional increase in ATP content from 1 to 10 mm glucose between scrATGL and shRNA-ATGL cells (Fig. 4B). Insulin granules contain a large amount of ATP (42). Because ATGL-KD causes a modest reduction in the insulin content of INS832/13 cells (see below), the slight reduction in ATP content is possibly due to a reduced number of secretory granules. Mitochondrial membrane potential was measured in INS832/13 cells using rhodamine 123, a fluorescent lipophilic cation. In scrATGL- and shATGL-transfected cells, 10 mm glucose caused mitochondrial membrane potential hyper-polarization to the same extent, indicated by a fall in the intensity of Rh123 fluorescence, and membrane potential at basal glucose was similar (Fig. 4C). These results indicate that ATGL-KD does not affect glucose and energy metabolism in INS832/13 cells.
FIGURE 4.
Decreased ATGL expression in INS832/13 cells does not affect glucose and mitochondrial metabolism. Nontransfected cells (NT) or INS832/13 cells were subjected to electroporation in the presence of either control (mock and scrATGL), or shATGL plasmids were cultured for 96 h prior to experiments. A, glucose (G) oxidation was measured in cells incubated for 2 h in KRBH at 1 or 10 mm glucose with [U-14C]glucose. Means ± S.E. are of nine separate determinations in three independent experiments. B, total ATP content was determined in cells incubated for 10 min in KRBH at 1 or 10 mm glucose. Means ± S.E. are of 18 separate determinations in three independent experiments. C, mitochondrial membrane potential was monitored as rhodamine 123 fluorescence. After dye loading, basal fluorescence was determined in cells cultured at 1 mm glucose, and then fluorescence was recorded for 10 min at 10 mm glucose. Means ± S.E. are of 12 separate determinations in two independent experiments. *, p < 0.05 versus scrambled ATGL for the same glucose concentration; ***, p < 0.001 versus 1 mm glucose for the same group; one way-ANOVA, Bonferroni post hoc test.
Reduction in ATGL Expression Lowers Fuel-induced Insulin Secretion
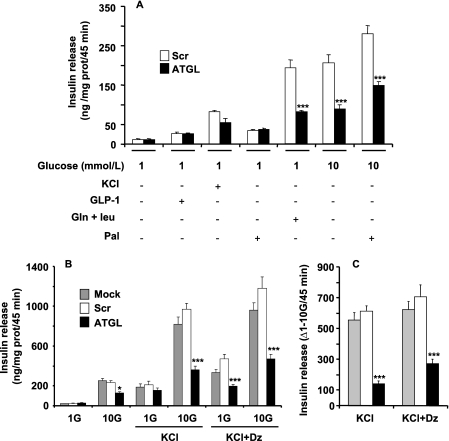
The effect of ATGL-KD on insulin secretion was measured in cells in response to glucose, amino acids (leucine plus glutamine), GLP-1, and palmitate. scrATGL-transfected cells had the same response to different secretagogues as mock-transfected cells (not shown for Fig. 5A; see Fig. 5B). Basal insulin release at 1 mm glucose was the same for scrATGL and shATGL cells. The response to KCl, GLP-1, and palmitate at low glucose was not affected by ATGL-KD (Fig. 5, A and B). In contrast, reduced ATGL expression decreased the effect of amino acids on insulin release by 60% and GSIS both in the absence and presence of palmitate by 50–55% (Fig. 5A). Total insulin content was decreased by 35% in shATGL-transfected cells (4.13 ± 0.15 and 2.73 ± 0.11 ng of insulin/μg of protein for scrATGL and shATGL, respectively, n = 7).
FIGURE 5.
ATGL knockdown in INS832/13 cells reduces fuel-induced insulin secretion. A, insulin release was measured in mock, scrATGL, and shATGL cells incubated as indicated for 45 min at 1 or 10 mm glucose with 0.5% d-BSA in the presence or absence of 0.25 mm palmitate (Pal), 10 nm GLP-1, 35 mm KCl, or 5 mm glutamine plus 5 mm leucine. B, effect of ATGL knockdown on the KATP-independent/amplification pathway(s) of insulin secretion was determined in cells incubated at 1 or 10 mm glucose (G) in the presence or absence of 35 mm KCl with or without 0.25 mm diazoxide (Dz). C shows the differences in insulin release between 1 and 10 mm glucose from data shown in B. Means ± S.E. are of 9–15 separate determinations from 3 to 5 independent experiments. *, p < 0.05; ***, p < 0.001 versus scrambled ATGL for the same incubation condition; one way-ANOVA, Bonferroni post hoc test.
To ascertain whether the reduction of GSIS in ATGL-KD cells involves KATP-independent/amplification mechanism(s) (43, 44), cells were incubated in the presence of 35 mm KCl ± diazoxide, a KATP channel opener. shATGL treatment of INS832/13 cells barely affected KCl-induced insulin secretion at low (1 mm) glucose (Fig. 5, A and B). However, in the presence of elevated KCl with or without diazoxide, GSIS was markedly curtailed in shATGL cells as compared with mock- or scrATGL-transfected cells (Fig. 5B). Thus, the activity of the “amplification” arm of GSIS, as evaluated by the difference in insulin secretion between 1 and 10 mm glucose in cells incubated with high KCl without or with diazoxide (45), was reduced by 60–75% in shATGL-treated cells (Fig. 5C). These results indicate that the defect of GSIS in ATGL-KD cells is largely due to altered KATP-independent/amplification of insulin secretion.
ATGL−/− Mice Are Hypoinsulinemic and Hypoglycemic and Show Decreased Plasma TG and FFA Levels
To confirm the importance of ATGL in the regulation of insulin secretion, ATGL−/− mice were used. Body weight and blood chemistry of ATGL−/− mice following an overnight fast are shown in Table 1. Fasted ATGL−/− mice were slightly heavier than ATGL+/+ mice. Circulating insulin levels were reduced by 70% in ATGL−/− mice, consistent with the 40% reduction in serum insulin reported in fed ATGL−/− mice on a mixed genetic background (50% C57BL/6 and 50% 129/Ola) (33). As expected, plasma TG and FFA were reduced (about 40%), and glucose levels were significantly (25%) decreased in ATGL−/− mice. Whole body fat mass was increased by 2-fold in ATGL−/− mice, as reported before on mice with a mixed genetic background (33).
TABLE 1.
Plasma insulin, glucose, FFA, TG levels and body weight in 10-week-old overnight fasted male ATGL+/+ and ATGL−/− mice
| ATGL+/+ | ATGL−/− | |
|---|---|---|
| Insulin (pmol/liter) | 111 ± 18 (n = 35) | 31 ± 7a (n = 31) |
| Glucose (mg/dl) | 167 ± 5 (n = 40) | 128 ± 7a (n = 36) |
| FFA (mmol/liter) | 0.32 ± 0.03 (n = 23) | 0.20 ± 0.01a (n = 22) |
| TG (mmol/liter) | 0.46 ± 0.02 (n = 24) | 0.28 ± 0.03a (n = 20) |
| BW (g) | 23.6 ± 0.3 (n = 85) | 24.6 ± 0.3b (n = 66) |
| BW (g)c | 26.0 ± 0.7 (n = 10) | 27.3 ± 0.8 (n = 7) |
| Body fat (g)c | 2.5 ± 0.3 (n = 10) | 4.9 ± 0.2a (n = 7) |
| Lean massc | 20.5 ± 0.9 (n = 10) | 19.4 ± 0.7 (n = 7) |
ap < 0.001 versus ATGL+/+ by unpaired, two-tailed Student's t test.
bp < 0.05.
c Body weight (BW), body fat, and lean mass in 9-week-old fed male mice are shown. Means ± S.E.
Reduced First Phase GSIS in Vivo in Insulino-sensitive Glucose-normotolerant ATGL−/− Mice
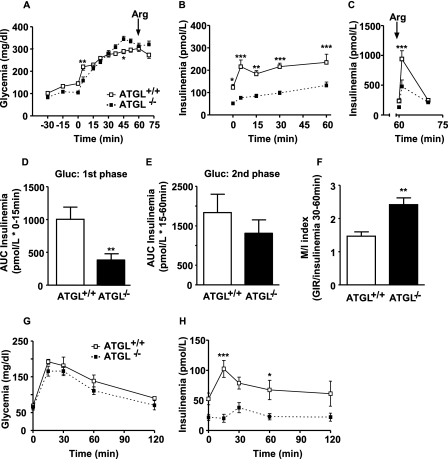
HGC were performed in 10-week-old overnight fasted conscious male mice to investigate the consequence of ATGL deficiency on insulin secretion in vivo. Conscious ATGL−/− mice had lower basal glycemia (105 ± 4 versus 145 ± 7 mg/dl) (Fig. 6A) and insulinemia (51.7 ± 7.7 versus 123.3 ± 13.6 pmol/liter) (Fig. 6B) than ATGL+/+ mice. During the clamp, the glucose infusion rate was adjusted to maintain blood glucose at ∼ 300 mg/dl (Fig. 6A). Insulin secretion in response to hyperglycemia was reduced in ATGL−/− mice (Fig. 6B). Calculation of the area under the curve (AUC) for the first 15 min of the clamp, subtracting basal insulinemia, indicated that first phase GSIS was reduced by 72% in ATGL−/− mice (Fig. 6D). However, the AUC for the second phase GSIS (15–60 min of the clamp) of ATGL−/− mice was not significantly different from that of ATGL+/+ mice (Fig. 6E). Consistent with reduced first phase GSIS, insulin secretion in response to an arginine bolus was reduced by 50% in ATGL−/− mice (Fig. 6C). The M/I index of insulin sensitivity (46) was increased in ATGL−/− mice (2.42 ± 0.21 versus 1.47 ± 0.13 μmol·kg−1·min−1 glucose infusion rate per pmol/liter insulin) indicating that ATGL−/− mice are more sensitive to insulin than wild type mice (Fig. 6F). In ATGL−/− mice, glucose tolerance was unchanged in comparison with ATGL+/+ mice (Fig. 6G). Normal glucose tolerance in ATGL−/− mice was maintained despite lower plasma insulin levels (Fig. 6H). These data confirm that ATGL−/− mice show improved insulin sensitivity in association with reduced insulin secretion, allowing unchanged glucose tolerance.
FIGURE 6.
ATGL−/− mice are insulino-sensitive and glucose-normotolerant and have a defect in glucose- and arginine-stimulated insulin secretion in vivo. A hyperglycemic clamp was performed in overnight fasted male wild type (ATGL+/+) and ATGL KO (ATGL−/−) mice. A, glucose levels during the clamp. B, insulin levels during the clamp and C, in response to an arginine (Arg) bolus (1 mmol/kg). D, first phase insulin secretion in response to elevated glucose (Gluc) expressed as AUC from 0 to 15 min. E, second phase insulin secretion (AUC) from 15–45 min. F, M/I index of insulin sensitivity calculated by dividing the glucose infusion rate (M) during the last 30 min of the clamp by circulating insulin levels (I) during the same period. M/I index is expressed as μmol·kg−1·min−1 glucose infusion per pmol/liter insulin. G and H, glycemia (G) and insulinemia (H) during an IPGTT in overnight fasted male ATGL+/+ and ATGL−/− mice. Mean ± S.E. are of 9 and 5 animals per group for HGC and IPGTT, respectively. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus ATGL+/+ for the same time; two-way ANOVA, Bonferroni post hoc test for A–C and G and H, and unpaired two-tailed Student's t test for D–F.
Impaired Insulin Secretion in Isolated Islets from ATGL−/− Mice
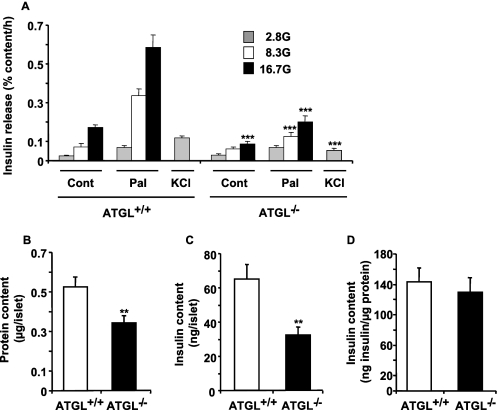
Insulin release was measured in isolated islets from overnight-fasted ATGL+/+ and ATGL−/− male mice. In ATGL+/+ mice, glucose increased insulin secretion by 3- and 7-fold at 8.3 and 16.7 mm glucose, respectively, in comparison with the value at 2.8 mm glucose (Fig. 7A). Exogenous palmitate induced a robust enhancement of insulin secretion in control islets at all glucose concentrations, and this enhancement was curtailed in ATGL−/− islets. Fig. 7A shows that GSIS either in the absence or presence of palmitate was dramatically reduced in islets deficient in ATGL, even when the data are expressed per total islet insulin content. Insulin secretion in response to a depolarizing concentration of KCl was also reduced by 50% in ATGL−/− islets. At low glucose, palmitate cause a 2–3-fold increase in insulin release in ATGL+/+ islets, and this secretory response remained unaltered in ATGL−/− islets (Fig. 7A). Altogether the data indicate that ATGL−/− islets show a marked reduction in insulin secretion in response to all classes of tested stimuli (glucose, palmitate in the presence of elevated glucose, and KCl).
FIGURE 7.
Isolated islets from ATGL−/− mice show reduced insulin release in response to glucose, palmitate, and KCl. A, insulin secretion in islets isolated from overnight fasted male ATGL−/− or ATGL+/+ mice incubated for 1 h in KRBH with 0.5% d-BSA at 2.8, 8.3, or 16.7 mm glucose (G) in the presence or absence of 0.4 mm palmitate (Pal) and at 2.8 mm glucose plus 35 mm KCl. Cont, control. Insulin release was normalized for the total islet insulin content shown in C. B, protein content per islet, and D, insulin content corrected by the protein content per islet. Means ± S.E. are of 15–20 separate determinations from islets of 5 ATGL−/− and 10 ATGL+/+ mice in five separate experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus ATGL+/+ for the same incubation condition; unpaired two-tailed Student's t test.
Total insulin content was reduced by 50% in ATGL−/− islets (Fig. 7C), largely due to the fact that ATGL−/− islets were smaller, as indicated by their protein content that was reduced by ∼35% (Fig. 7B). However, islet insulin content corrected for protein content per islet was similar in both islet groups (Fig. 7D).
Metabolic Correlates in ATGL−/− Islets
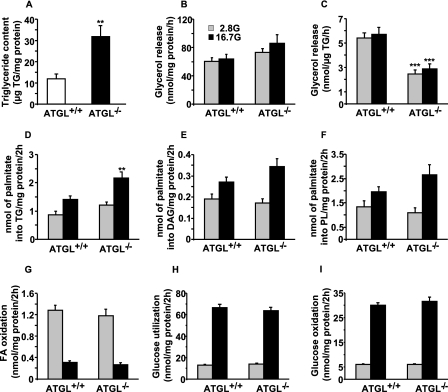
As observed in shATGL-KD cells, the deletion of ATGL led to a 2.6-fold increase in the islet TG content of ATGL−/− mice (Fig. 8A). Similar to shATGL-KD cells, lack of ATGL did not alter islet lipolysis when expressed per mg of protein (Fig. 8B). However, when normalized for TG content, lipolysis was reduced by 50% in ATGL−/− islets (Fig. 8C). There was no change in HSL transcript expression in islets from ATGL−/− mice (data not shown). Islets from ATGL−/− mice showed a 55% significant increase in FA esterification into TG at 16.7 mm glucose compared with ATGL+/+ islets (Fig. 8D). In contrast, FA esterification into DAG (Fig. 8E) and PL (Fig. 8F) was not significantly different in islets from both genotypes. FA oxidation (Fig. 8G), glucose utilization (Fig. 8H), and glucose oxidation (Fig. 8I) were similar at low and high glucose in isolated islets from ATGL−/− and ATGL+/+ mice, indicating that ATGL deficiency does not induce metabolic toxicity of islet tissue.
FIGURE 8.
Metabolic correlates of islets from ATGL+/+ and ATGL−/− mice. A, TG content in islets isolated from overnight fasted male mice. Means ± S.E. are of 13–14 separate determinations from islets of 8 ATGL−/− and 11 ATGL+/+ mice. Glycerol release, an index of lipolysis, expressed per protein (B) or TG content (C). Means ± S.E. are of 18–20 separate determinations from islets of 9 ATGL−/− and 15 ATGL+/+ mice in four separate experiments. Islets were incubated in KRBH at 2.8 or 16.7 mm glucose (G) with [9,10-3H]palmitate to assess FA esterification into TG (D), DAG (E), and PL (F), and FA oxidation (G). Means ± S.E. are of 12–14 separate determinations from islets of four ATGL−/− and four ATGL+/+ mice in three different experiments for FA oxidation, and means ± S.E. are of 19–20 separate determinations from islets of 10 ATGL−/− and 16 ATGL+/+ mice in five different experiments for FA esterification. Glucose utilization (H) and oxidation (I) were measured in islets incubated in KRBH at 2.8 or 16.7 mm glucose (G) with d-[U-14C]glucose and d-[5-3H]glucose. Means ± S.E. are of 27–31 separate determinations from islets of six ATGL−/− and six ATGL+/+ mice in three different experiments. **, p < 0.01; ***, p < 0.001 versus ATGL+/+ for the same incubation condition; unpaired two-tailed Student's t test.
DISCUSSION
The results show that ATGL is expressed in rat islets and INS832/13 cells, and that this key lipolytic enzyme plays a role in the regulation of fuel-induced insulin secretion, mainly via the KATP-independent/amplification arm of nutrient-induced insulin release. Thus, both ATGL-KD in INS cells or its deletion in mice resulted in defective GSIS and the same changes in lipid metabolism. The reduction in both GSIS and KCl-induced secretion was more prominent in isolated ATGL−/− islets mice than in INS cells, likely due to the fact that ATGL still remained expressed at appreciable levels in shATGL-treated cells.
Insulin release in response to glucose, and to palmitate or KCl at high glucose, was curtailed in ATGL-KD cells and ATGL−/− islets; insulin release in vivo promoted by glucose or arginine at high glucose was reduced. This is consistent with the view that intracellular lipid signaling is important in the secretory response of all classes of stimuli (3). Thus, increased expression of malonyl-CoA decarboxylase (47) in INS cells, or reduced lipolysis in vivo using nicotinic acid (48), impaired the secretory effects of both fuel and non-fuel stimuli. However, the exocytotic process per se was not altered by reduced ATGL expression because the rise in insulin release promoted by palmitate, GLP-1, and KCl at low glucose remained largely unchanged. As discussed before (3, 47, 49), we believe that the lipid amplification arm of glucose signaling that provides active molecules such as DAG synergizes with other “classical” pathways, e.g. Ca2+ and cAMP.
The decrease in circulating insulin levels in ATGL−/− mice can be explain in part by the increase in insulin sensitivity observed during the hyperglycemic clamp and as reported before in ATGL−/− mice on a mixed genetic background (33). Indeed, ATGL−/− mice need to secrete less insulin than ATGL+/+ mice to keep normal glycemia. However, the data obtained in isolated islets clearly demonstrate that ATGL−/− mice have a marked reduced fuel and non-fuel insulin secretion. Consequently, the decrease in first phase GSIS and in insulin secretion in response to arginine in vivo during the HGC and the very low level of insulin release in ATGL−/− mice after a glucose challenge during an IPGTT are related at least in part to a β-cell defect in insulin secretion independently of insulin action whose change is relatively modest in comparison with the alterations in insulin secretion.
The lack of ATGL in ATGL−/− mice or depletion of this enzyme in INS cells resulted in a 2.5–3-fold increase in TG content, and it might be argued that this might cause cell toxicity. We believe that such “lipotoxicity” can be discounted for the following reasons: glucose oxidation and the mitochondrial membrane potential remained unchanged in ATGL-KD cells; glucose usage, glucose oxidation, and FA oxidation were unaltered in ATGL−/− islets; basal insulin release and insulin secretion at low glucose in response to palmitate were unchanged; esterification of palmitate into DAG and PL remained constant as did total glycerol release; insulin content per mg of islet protein was unchanged; and finally, the rise in TG in ATGL-KD cells or ATGL−/− islets was relatively modest and TG accumulation in the β-cell has emerged as a protective mechanism against tissue lipotoxicity (50) rather than a cellular “offense” as thought previously (13).
The decreased circulating TG and FFA availability during fasting might contribute to defective GSIS in isolated islets from ATGL−/− mice. Thus, as discussed above, lowering of circulating FFA in fasted rats was shown to impair insulin secretion in response to all secretagogues both in vivo and ex vivo, an effect that was restored upon provision of exogenous FFA (48). However, the provision of exogenous FFA to “fasted” ATGL−/− islets did not restore GSIS. This favors the view, in accordance with data obtained in ATGL-KD cells, that lipolysis of endogenous lipid stores via ATGL plays an important role in GSIS. The results contrast with our previous data obtained in isolated islets from fasted male HSL−/− mice, which also have lowered plasma lipid levels, and in which the defect in GSIS was reversed by FFA supplementation (8). This suggests a more essential role of lipolysis via ATGL than HSL in GSIS as well as in response to other stimuli.
Glucose stimulated lipolysis in INS832/13 cells, whereas a modest but not significant trend to be higher was observed in mouse islets. Reported data in the literature indicate that glucose-stimulated glycerol release is largest in β-cell lines (51), intermediate in rat islets (10, 12, 52), and modest in mouse islets (8, 23, 51, 53). An excellent correlation between lipolysis and GSIS (51) was observed in INS-1 cells and mice islets, a finding that supports the concept that lipolysis is important for GSIS. Fex et al. (23) reported no effect of glucose on lipolysis in mouse islets, although we observed in overnight fasted male wild type and HSL−/− mice a 40 and 100% increase in glucose-induced lipolysis, respectively (8). For assay sensitivity reasons, lipolysis in vitro is measured over 2–3 h in KRBH medium (a rather long time for an incubation of a tissue in an incomplete medium without serum), whereas insulin secretion is determined over 45–60 min. A time course of lipolysis following glucose stimulation of rodent islet in vitro needs to be performed following sensitive assay development. Thus, enhanced lipolysis in response to glucose may be more prominent at early times following glucose stimulation. Alternatively and as discussed below, it is possible that only a specific fuel-sensitive TG pool is involved in the production of lipid signaling molecules for insulin secretion.
Surprisingly there was no change in the release of glycerol expressed per mg of protein in ATGL-KD cells and ATGL−/− islets, despite the fact that ATGL knockdown or its absence caused TG accumulation. This observation is consistent with the finding that the esterification of palmitate into DAG and the incorporation of labeled palmitate into NEFA were unchanged. A possible explanation for these observations is that shATGL cells or ATGL−/− islets readjusted their total levels of DAG and FFA as well as their esterification of FFA into DAG because these are well defended currencies that influence many biological processes. A first possibility is because cells display glycerolipid cycling processes (54) such that de novo synthesized sn1,2-DAG does not necessarily have to be esterified to TG before being hydrolyzed, but it can directly be hydrolyzed by HSL to MAG. In other words the β-cell may adapt to TG accumulation by redirecting newly formed DAG via a short cycle to MAG, thus bypassing TG formation. Another possibility is that increased TG lipolysis via other TG lipases compensated reduced flux via ATGL. Glycerol release results from the activity of ATGL, HSL, MAG lipase, acyltransferases in the TG synthesis pathways, DAG kinase, phosphatidate phosphohydrolase, glycerol kinase, and additional enzymes. Possibly, cells readjusted glycerol release by modulating flux of one or several of the mentioned enzymes. It should be pointed out that a similar observation has been reported in myotubes where ATGL-KD resulted in enhanced incorporation of labeled palmitate into TG with no difference in DAG and in NEFA (55).
How is it possible that ATGL may be important for insulin secretion if total glycerol release, an index of lipolysis, remained unaffected in ATGL-KD β-cells and ATGL−/− islets? We wish to propose the existence of fuel-insensitive and -sensitive TG pools in the β-cell, the latter linked to stimulus secretion coupling and regulation by ATGL. Recent work from our laboratory support this view.6 We speculate that the local production of lipid signaling molecules by ATGL and/or enzymes of glycerolipid/fatty acid cycling is important for the exocytotic release of insulin in response to various stimuli. In this context, HSL has been shown to associate with insulin secretory granules in the β-cell (22), and it will be of interest to assess the β-cell subcellular localization of ATGL.
Recently, Fex et al. (14) reported that β-cell specific HSL−/− mice had a reduced first phase GSIS and a diminution in insulin release in response to an arginine challenge, as we observed in ATGL−/− mice. They provided evidence that an altered exocytosis rate in the β-cell of HSL−/− mice that is not related to calcium influx is responsible for this defect in insulin secretion (14). Thus, the emerging evidence indicates that TG lipolysis via both ATGL and HSL play key role in the regulation of exocytosis and GSIS.
The nature of the “locally” produced lipid signaling molecule(s) provided by ATGL(s) and/or associated enzymes of TG/FFA cycling needs to be defined. The most likely candidates are DAG, FFA, and long chain fatty acyl-CoA. Long chain fatty acyl-CoA (56) and FFA (57) have been shown to promote exocytosis in permeabilized β-cells and might be used as substrates by enzymes that acylate exocytotic proteins, such as the synaptosomal associated protein-25 (SNAP-25) (58) and synaptotagmin (59), to enhance their association with the plasma membrane. Furthermore, phorbol esters, commonly used as stable and potent DAG mimics, via protein kinase C activation, cause SNAP-25 phosphorylation and stimulation of insulin exocytosis (60). DAG can also act on vesicle exocytosis via its binding to the C1 domain of the synaptic vesicle priming protein Munc13 (61), and we report that GSIS is defective in Munc13-1-deficient islets (62).
The cellular insulin content was reduced in islets of ATGL−/− mice and in shATGL-transfected INS cells. Recent studies have revealed a tight coupling between insulin secretion and biosynthesis. Islet cell autoantigen 512 (ICA512), an intrinsic tyrosine phosphatase-like protein of the insulin secretory granule membrane (63), is cleaved (64) following granule fusion to the plasma membrane. The resulting cleaved cytosolic fragment of this protein (ICA512-CCF) translocates to the nucleus where it prolongs the activity of STATs and thus insulin gene transcription and granule biogenesis (65). Possibly, the decreased exocytosis of insulin because of reduced ATGL expression in the β-cell leads to an adaptive reduced insulin biosynthesis and storage.
The role of lipolysis in human islets is not known, but we have observed both ATGL and HSL expression in human islets (data not shown). The possible importance of ATGL in human β-cell function and insulin secretion is supported by the identification of ATGL gene polymorphisms associated with type 2 diabetes (66). Furthermore, ATGL mutations leading to a truncated ATGL protein are responsible for a neutral lipid storage disease with myopathy that is characterized by systemic TG accumulation (67, 68). Recently, a novel mutation in the ATGL gene leading to a lack of the C-terminal region of the ATGL protein was identified in a patient with neutral lipid storage disease with myopathy (69). Interestingly, this patient showed a decrease in insulin secretory capacity with age. Whether ATGL participates in lipolysis and insulin secretion in human islets remains to be examined.
In conclusion, the results support the concept that β-cell lipolysis via ATGL is important for the provision of lipid-signaling molecules necessary for insulin secretion in response to fuel and non-fuel stimuli. Additional work is needed to conclusively identify these lipid signaling molecules, to understand how ATGL is regulated in the β-cell, and to determine whether this enzyme directly produces coupling factors (DAG and FFA) for insulin secretion or indirectly via glycerolipid/FFA cycling or other metabolic pathway(s) of lipid metabolism.
Supplementary Material
Acknowledgments
We thank Grace Ferguson and Mélanie Ethier for valuable technical help.
This work was supported, in whole or in part, by a National Institutes of Health grant (to N. R., V. P., and M. P.). This work was also supported by grants from the Canadian Diabetes Association and the Canadian Institute of Health Research (to M. P.).
 The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Table 1.
6 C. Nolan and M. Prentki, unpublished data.
- FFA
- free fatty acid
- ATGL
- adipose triglyceride lipase
- BSA
- bovine serum albumin
- d-BSA
- defatted BSA
- DAG
- diacylglycerol
- FA
- fatty acid
- GSIS
- glucose-stimulated insulin secretion
- HSL
- hormone-sensitive lipase
- KD
- knockdown
- KRBH
- Krebs-Ringer bicarbonate buffer containing HEPES
- MAG
- monoacylglycerol
- NEFA
- nonesterified fatty acid
- shRNA
- small hairpin RNA
- PBS
- phosphate-buffered saline
- PL
- phospholipid
- TG
- triglyceride
- TGL
- triglyceride lipase
- WAT
- white adipose tissue
- WT
- wild type
- ANOVA
- analysis of variance
- RT
- reverse transcription
- HGC
- hyperglycemic clamp
- IPGTT
- intraperitoneal glucose tolerance test
- Rh123
- rhodamine 123
- AUC
- area under the curve.
REFERENCES
- 1.Stein D. T., Esser V., Stevenson B. E., Lane K. E., Whiteside J. H., Daniels M. B., Chen S., McGarry J. D. ( 1996) J. Clin. Invest. 97, 2728– 2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stein D. T., Stevenson B. E., Chester M. W., Basit M., Daniels M. B., Turley S. D., McGarry J. D. ( 1997) J. Clin. Invest. 100, 398– 403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nolan C. J., Madiraju M. S., Delghingaro-Augusto V., Peyot M. L., Prentki M. ( 2006) Diabetes 55, Suppl. 2, S16– 23 [DOI] [PubMed] [Google Scholar]
- 4.Poitout V., Hagman D., Stein R., Artner I., Robertson R. P., Harmon J. S. ( 2006) J. Nutr. 136, 873– 876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prentki M., Joly E., El-Assaad W., Roduit R. ( 2002) Diabetes 51, Suppl. 3, S405– 413 [DOI] [PubMed] [Google Scholar]
- 6.El-Assaad W., Buteau J., Peyot M. L., Nolan C., Roduit R., Hardy S., Joly E., Dbaibo G., Rosenberg L., Prentki M. ( 2003) Endocrinology 144, 4154– 4163 [DOI] [PubMed] [Google Scholar]
- 7.Masiello P., Novelli M., Bombara M., Fierabracci V., Vittorini S., Prentki M., Bergamini E. ( 2002) Metabolism 51, 110– 114 [DOI] [PubMed] [Google Scholar]
- 8.Peyot M. L., Nolan C. J., Soni K., Joly E., Lussier R., Corkey B. E., Wang S. P., Mitchell G. A., Prentki M. ( 2004) Diabetes 53, 1733– 1742 [DOI] [PubMed] [Google Scholar]
- 9.Roduit R., Masiello P., Wang S. P., Li H., Mitchell G. A., Prentki M. ( 2001) Diabetes 50, 1970– 1975 [DOI] [PubMed] [Google Scholar]
- 10.Nolan C. J., Leahy J. L., Delghingaro-Augusto V., Moibi J., Soni K., Peyot M. L., Fortier M., Guay C., Lamontagne J., Barbeau A., Przybytkowski E., Joly E., Masiello P., Wang S., Mitchell G. A., Prentki M. ( 2006) Diabetologia 49, 2120– 2130 [DOI] [PubMed] [Google Scholar]
- 11.Yaney G. C., Civelek V. N., Richard A. M., Dillon J. S., Deeney J. T., Hamilton J. A., Korchak H. M., Tornheim K., Corkey B. E., Boyd A. E., 3rd ( 2001) Diabetes 50, 56– 62 [DOI] [PubMed] [Google Scholar]
- 12.Mulder H., Yang S., Winzell M. S., Holm C., Ahrén B. ( 2004) Diabetes 53, 122– 128 [DOI] [PubMed] [Google Scholar]
- 13.Koyama K., Chen G., Wang M. Y., Lee Y., Shimabukuro M., Newgard C. B., Unger R. H. ( 1997) Diabetes 46, 1276– 1280 [DOI] [PubMed] [Google Scholar]
- 14.Fex M., Haemmerle G., Wierup N., Dekker-Nitert M., Rehn M., Ristow M., Zechner R., Sundler F., Holm C., Eliasson L., Mulder H. ( 2009) Diabetologia 52, 271– 280 [DOI] [PubMed] [Google Scholar]
- 15.Jensen M. D., Ekberg K., Landau B. R. ( 2001) Am. J. Physiol. Endocrinol. Metab. 281, E789– 793 [DOI] [PubMed] [Google Scholar]
- 16.Vaughan M. ( 1962) J. Biol. Chem. 237, 3354– 3358 [PubMed] [Google Scholar]
- 17.Reshef L., Olswang Y., Cassuto H., Blum B., Croniger C. M., Kalhan S. C., Tilghman S. M., Hanson R. W. ( 2003) J. Biol. Chem. 278, 30413– 30416 [DOI] [PubMed] [Google Scholar]
- 18.Newsholme E. A., Crabtree B. ( 1976) Biochem. Soc. Symp. 61– 109 [PubMed] [Google Scholar]
- 19.Hahn P., Novak M. ( 1975) J. Lipid Res. 16, 79– 91 [PubMed] [Google Scholar]
- 20.Mulder H., Holst L. S., Svensson H., Degerman E., Sundler F., Ahrén B., Rorsman P., Holm C. ( 1999) Diabetes 48, 228– 232 [DOI] [PubMed] [Google Scholar]
- 21.Winzell M. S., Svensson H., Arner P., Ahrén B., Holm C. ( 2001) Diabetes 50, 2225– 2230 [DOI] [PubMed] [Google Scholar]
- 22.Lindvall H., Nevsten P., Ström K., Wallenberg R., Sundler F., Langin D., Winzell M. S., Holm C. ( 2004) J. Biol. Chem. 279, 3828– 3836 [DOI] [PubMed] [Google Scholar]
- 23.Fex M., Olofsson C. S., Fransson U., Bacos K., Lindvall H., Sörhede-Winzell M., Rorsman P., Holm C., Mulder H. ( 2004) Endocrinology 145, 3746– 3753 [DOI] [PubMed] [Google Scholar]
- 24.Jenkins C. M., Mancuso D. J., Yan W., Sims H. F., Gibson B., Gross R. W. ( 2004) J. Biol. Chem. 279, 48968– 48975 [DOI] [PubMed] [Google Scholar]
- 25.Villena J. A., Roy S., Sarkadi-Nagy E., Kim K. H., Sul H. S. ( 2004) J. Biol. Chem. 279, 47066– 47075 [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann R., Strauss J. G., Haemmerle G., Schoiswohl G., Birner-Gruenberger R., Riederer M., Lass A., Neuberger G., Eisenhaber F., Hermetter A., Zechner R. ( 2004) Science 306, 1383– 1386 [DOI] [PubMed] [Google Scholar]
- 27.Schweiger M., Schreiber R., Haemmerle G., Lass A., Fledelius C., Jacobsen P., Tornqvist H., Zechner R., Zimmermann R. ( 2006) J. Biol. Chem. 281, 40236– 40241 [DOI] [PubMed] [Google Scholar]
- 28.Soni K. G., Lehner R., Metalnikov P., O'Donnell P., Semache M., Gao W., Ashman K., Pshezhetsky A. V., Mitchell G. A. ( 2004) J. Biol. Chem. 279, 40683– 40689 [DOI] [PubMed] [Google Scholar]
- 29.Wei E., Gao W., Lehner R. ( 2007) J. Biol. Chem. 282, 8027– 8035 [DOI] [PubMed] [Google Scholar]
- 30.Haemmerle G., Zimmermann R., Hayn M., Theussl C., Waeg G., Wagner E., Sattler W., Magin T. M., Wagner E. F., Zechner R. ( 2002) J. Biol. Chem. 277, 4806– 4815 [DOI] [PubMed] [Google Scholar]
- 31.Fex M., Lucas S., Winsell M. S., Ahrén B., Holm C., Mulder H. ( 2006) Diabetes 55, Suppl. 2, S24– 31 [Google Scholar]
- 32.Hohmeier H. E., Mulder H., Chen G., Henkel-Rieger R., Prentki M., Newgard C. B. ( 2000) Diabetes 49, 424– 430 [DOI] [PubMed] [Google Scholar]
- 33.Haemmerle G., Lass A., Zimmermann R., Gorkiewicz G., Meyer C., Rozman J., Heldmaier G., Maier R., Theussl C., Eder S., Kratky D., Wagner E. F., Klingenspor M., Hoefler G., Zechner R. ( 2006) Science 312, 734– 737 [DOI] [PubMed] [Google Scholar]
- 34.Freeman H. C., Hugill A., Dear N. T., Ashcroft F. M., Cox R. D. ( 2006) Diabetes 55, 2153– 2156 [DOI] [PubMed] [Google Scholar]
- 35.Gotoh M., Maki T., Satomi S., Porter J., Bonner-Weir S., O'Hara C. J., Monaco A. P. ( 1987) Transplantation 43, 725– 730 [DOI] [PubMed] [Google Scholar]
- 36.Guay C., Madiraju S. R., Aumais A., Joly E., Prentki M. ( 2007) J. Biol. Chem. 282, 35657– 35665 [DOI] [PubMed] [Google Scholar]
- 37.Brun T., Duhamel D. L., Hu He K. H., Wollheim C. B., Gauthier B. R. ( 2007) Oncogene 26, 4261– 4271 [DOI] [PubMed] [Google Scholar]
- 38.Roduit R., Morin J., Massé F., Segall L., Roche E., Newgard C. B., Assimacopoulos-Jeannet F., Prentki M. ( 2000) J. Biol. Chem. 275, 35799– 35806 [DOI] [PubMed] [Google Scholar]
- 39.Massa M. L., Borelli M. I., Del Zotto H., Gagliardino J. J. ( 2001) J. Endocrinol. 171, 551– 556 [DOI] [PubMed] [Google Scholar]
- 40.Segall L., Lameloise N., Assimacopoulos-Jeannet F., Roche E., Corkey P., Thumelin S., Corkey B. E., Prentki M. ( 1999) Am. J. Physiol. 277, E521– E528 [DOI] [PubMed] [Google Scholar]
- 41.Saddik M., Lopaschuk G. D. ( 1991) J. Biol. Chem. 266, 8162– 8170 [PubMed] [Google Scholar]
- 42.Hutton J. C., Peshavaria M. ( 1983) Biochem. J. 210, 235– 242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Henquin J. C. ( 2000) Diabetes 49, 1751– 1760 [DOI] [PubMed] [Google Scholar]
- 44.Straub S. G., Sharp G. W. ( 2002) Diabetes Metab. Res. Rev. 18, 451– 463 [DOI] [PubMed] [Google Scholar]
- 45.Straub S. G., James R. F., Dunne M. J., Sharp G. W. ( 1998) Diabetes 47, 758– 763 [DOI] [PubMed] [Google Scholar]
- 46.DeFronzo R. A., Tobin J. D., Andres R. ( 1979) Am. J. Physiol. 237, E214– E223 [DOI] [PubMed] [Google Scholar]
- 47.Roduit R., Nolan C., Alarcon C., Moore P., Barbeau A., Delghingaro-Augusto V., Przybykowski E., Morin J., Massé F., Massie B., Ruderman N., Rhodes C., Poitout V., Prentki M. ( 2004) Diabetes 53, 1007– 1019 [DOI] [PubMed] [Google Scholar]
- 48.Dobbins R. L., Chester M. W., Stevenson B. E., Daniels M. B., Stein D. T., McGarry J. D. ( 1998) J. Clin. Invest. 101, 2370– 2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nolan C. J., Prentki M. ( 2008) Trends Endocrinol. Metab. 19, 285– 291 [DOI] [PubMed] [Google Scholar]
- 50.Cnop M., Hannaert J. C., Hoorens A., Eizirik D. L., Pipeleers D. G. ( 2001) Diabetes 50, 1771– 1777 [DOI] [PubMed] [Google Scholar]
- 51.Winzell M. S., Strom K., Holm C., Ahren B. ( 2006) Nutr. Metab. Cardiovasc. Dis. 16, Suppl. 1, S11– 16 [DOI] [PubMed] [Google Scholar]
- 52.Delghingaro-Augusto V., Nolan C. J., Gupta D., Jetton T. L., Latour M. G., Peshavaria M., Madiraju S. R., Joly E., Peyot M. L., Prentki M., Leahy J. ( 2009) Diabetologia 52, 1122– 1132 [DOI] [PubMed] [Google Scholar]
- 53.Sörhede Winzell M., Ahrén B. ( 2004) Horm. Metab. Res. 36, 795– 803 [DOI] [PubMed] [Google Scholar]
- 54.Prentki M., Madiraju S. R. ( 2008) Endocr. Rev. 29, 647– 676 [DOI] [PubMed] [Google Scholar]
- 55.Watt M. J., van Denderen B. J., Castelli L. A., Bruce C. R., Hoy A. J., Kraegen E. W., Macaulay L., Kemp B. E. ( 2008) Mol. Endocrinol. 22, 1200– 1212 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 56.Deeney J. T., Gromada J., H⊘y M., Olsen H. L., Rhodes C. J., Prentki M., Berggren P. O., Corkey B. E. ( 2000) J. Biol. Chem. 275, 9363– 9368 [DOI] [PubMed] [Google Scholar]
- 57.Olofsson C. S., Salehi A., Holm C., Rorsman P. ( 2004) J. Physiol. 557, 935– 948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gonzalo S., Linder M. E. ( 1998) Mol. Biol. Cell 9, 585– 597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chapman E. R., Blasi J., An S., Brose N., Johnston P. A., Südhof T. C., Jahn R. ( 1996) Biochem. Biophys. Res. Commun. 225, 326– 332 [DOI] [PubMed] [Google Scholar]
- 60.Shu Y., Liu X., Yang Y., Takahashi M., Gillis K. D. ( 2008) J. Neurosci. 28, 21– 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rhee J. S., Betz A., Pyott S., Reim K., Varoqueaux F., Augustin I., Hesse D., Südhof T. C., Takahashi M., Rosenmund C., Brose N. ( 2002) Cell 108, 121– 133 [DOI] [PubMed] [Google Scholar]
- 62.Kwan E. P., Xie L., Sheu L., Nolan C. J., Prentki M., Betz A., Brose N., Gaisano H. Y. ( 2006) Diabetes 55, 1421– 1429 [DOI] [PubMed] [Google Scholar]
- 63.Solimena M., Dirkx R., Jr., Hermel J. M., Pleasic-Williams S., Shapiro J. A., Caron L., Rabin D. U. ( 1996) EMBO J. 15, 2102– 2114 [PMC free article] [PubMed] [Google Scholar]
- 64.Trajkovski M., Mziaut H., Altkrüger A., Ouwendijk J., Knoch K. P., Müller S., Solimena M. ( 2004) J. Cell Biol. 167, 1063– 1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mziaut H., Trajkovski M., Kersting S., Ehninger A., Altkrüger A., Lemaitre R. P., Schmidt D., Saeger H. D., Lee M. S., Drechsel D. N., Müller S., Solimena M. ( 2006) Nat. Cell Biol. 8, 435– 445 [DOI] [PubMed] [Google Scholar]
- 66.Schoenborn V., Heid I. M., Vollmert C., Lingenhel A., Adams T. D., Hopkins P. N., Illig T., Zimmermann R., Zechner R., Hunt S. C., Kronenberg F. ( 2006) Diabetes 55, 1270– 1275 [DOI] [PubMed] [Google Scholar]
- 67.Akiyama M., Sakai K., Ogawa M., McMillan J. R., Sawamura D., Shimizu H. ( 2007) Muscle Nerve 36, 856– 859 [DOI] [PubMed] [Google Scholar]
- 68.Fischer J., Negre-Salvayre A., Salvayre R. ( 2007) Med. Sci. 23, 575– 578 [DOI] [PubMed] [Google Scholar]
- 69.Kobayashi K., Inoguchi T., Maeda Y., Nakashima N., Kuwano A., Eto E., Ueno N., Sasaki S., Sawada F., Fujii M., Matoba Y., Sumiyoshi S., Kawate H., Takayanagi R. ( 2008) J. Clin. Endocrinol. Metab. 93, 2877– 2884 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.