Abstract
Uncontrolled activation of the alternative pathway of complement is thought to be associated with age-related macular degeneration (AMD). The alternative pathway is continuously activated in the fluid phase, and tissue surfaces require continuous complement inhibition to prevent spontaneous autologous tissue injury. Here, we examined the effects of oxidative stress on the ability of immortalized human retinal pigment epithelial cells (ARPE-19) to regulate complement activation on their cell surface. Combined treatment with H2O2 (to induce oxidative stress) and complement-sufficient serum was found to disrupt the barrier function of stable ARPE-19 monolayers as determined by transepithelial resistance (TER) measurements. Neither treatment alone had any effect. TER reduction was correlated with increased cell surface deposition of C3, and could be prevented by using C7-depleted serum, an essential component of the terminal complement pathway. Treatment with H2O2 reduced surface expression of the complement inhibitors DAF, CD55, and CD59, and impaired regulation at the cell surface by factor H present within the serum. Combined treatment of the monolayers with H2O2 and serum elicited polarized secretion of vascular epidermal growth factor (VEGF). Both, secretion of VEGF and TER reduction could be attenuated using either an alternative pathway inhibitor or by blocking VEGF receptor-1/2 signaling. Regarded together, these studies demonstrate that oxidative stress reduces regulation of complement on the surface of ARPE-19 cells, increasing complement activation. This sublytic activation results in VEGF release, which mediates disruption of the cell monolayer. These findings link oxidative stress, complement activation, and apical VEGF release, which have all been associated with the pathogenesis of AMD.
Age-related macular degeneration (AMD)6 is the leading cause of blindness in the elderly (1). Clinically, AMD is categorized as “dry” or “wet.” In the dry form of the disease, deposits (drusen) develop between the retinal pigment epithelium (RPE) and the underlying basement membrane (Bruch's membrane). The loss of photoreceptor function and vision observed in patients is attributed to atrophic changes in the RPE (1, 2). Wet AMD is characterized by choroidal neovascularization extending through Bruch's membrane and the RPE into the subretinal space. Subsequent leakage of exudative fluid and blood is thought to contribute to the eventual development of fibrosis characteristic of wet AMD. AMD is hypothesized to be a progressive disease, with the dry and wet forms likely representing different points on a spectrum of disease severity. Approximately 10–15% of patients with the less severe dry AMD go on to develop wet AMD (1).
Several observations suggest that uncontrolled activation of the complement cascade contributes to the development and progression of AMD. Polymorphisms in complement factor H, a circulating inhibitor of the alternative pathway of complement, are strongly associated with the development of AMD (3–6). Drusen-like lesions also develop in patients with dense deposit disease, a form of glomerulonephritis caused by dysregulation of the alternative pathway (7, 8). Analysis of the composition of drusen demonstrates that they contain important complement proteins, including C3, C5, membrane attack complex (MAC), and endogenous complement regulatory proteins (7, 8). Mice with a genetic deletion of factor H (cfh−/− mice) accumulate C3 throughout the RPE and the outer segment layer of the neuroretina, and lose visual function faster during aging than their wild type littermates (9). Furthermore, in a murine model of laser-induced choroidal neovascularization, blockade of signaling by C3a and C5a reduced the production of VEGF in the eye and reduced neovascularization (10). Taken together, these studies suggest that in AMD, inadequate control of the alternative pathway 1) contributes to the structural changes observed in RPE and Bruch's membrane, including drusen formation; and 2) is upstream of VEGF-mediated mechanisms.
The alternative pathway of complement is continually activated in the fluid phase, and inadequate inhibition of this pathway on tissue surfaces may permit spontaneous complement activation with rapid amplification and generation of pro-inflammatory activation fragments (11). In late-onset diseases such as AMD, local regulation of the alternative pathway may gradually be overwhelmed by cellular injury or the accumulation of debris (12, 13). Several environmental factors contribute to a high level of oxidative stress at the RPE layer, and oxidative injury of the RPE cells may be an important cause of AMD (14). Therefore, we hypothesized that oxidative stress may impair the ability of the RPE to regulate complement on its surface. In the intact adult human eye, only one cell surface complement inhibitor, membrane cofactor protein (MCP; CD46), has been identified on RPE cells (15). In the current study, we investigated whether ARPE-19 cells express the three cell surface complement inhibitors, CD46, decay accelerating factor (DAF; CD55), and CD59; and whether oxidative stress of RPE cells in culture alters surface expression of the complement inhibitory proteins or reduces inhibition of the alternative pathway on the surface of the cells by factor H. Second, we tested the hypothesis that rather than causing cell lysis, sublytic activation of complement on RPE cells induces VEGF release by these cells, which is known to compromise barrier function. The goal of these studies was to construct a model whereby oxidative stress in the eye could be linked to the inflammatory events that cause AMD, including uncontrolled activation of complement.
MATERIALS AND METHODS
Reagents
The reagents used in these studies included pooled normal human serum (NHS (Quidel)) as a source of complement proteins. To prevent formation of the terminal complement pathway, C7-depleted serum was used (Quidel), and purified C7 (250 μg/ml; Quidel) was added to the serum in some experiments. Primary antibodies to DAF (Chemicon International), CD59 (Chemicon International), MCP (Monosan), and C3 (ICN Pharmaceuticals) were used. Species-specific secondary antibodies were from Jackson ImmunoResearch and MP Biomedicals, Inc. To block the alternative pathway of complement activation, a targeted inhibitory protein (CR2-fH) was produced as previously described (16). This agent targets the inhibitory domain of factor H to sites of C3d deposition and effectively blocks alternative pathway activation. To block cell surface complement inhibition by factor H, we used a protein referred to as recombinant factor H domains 19–20 (rh-19–20) (17). This protein is a recombinant form of the 19th and 20th short consensus repeats of factor H and contains the polyanion and C3b-binding region, but not the N-terminal complement regulatory region of the full-length factor H protein. It competitively blocks interaction of native factor H with cell surfaces, thereby preventing regulation of complement activation and amplification by factor H on those surfaces. The VEGFR-1/2 inhibitor, SU5416 (Chemicon), was used to block the effects of VEGF. SU5416 (Z-3-[(2,4-dimethylpyrrol-5-yl)methylidenyl]-2-indolinone) is a lipophilic synthetic receptor tyrosine kinase inhibitor, which inhibits VEGFR-1/2 by binding to the ATP binding pocket within the kinase domain of the receptor. SU5416 has been shown to inhibit VEGF-dependent endothelial cell proliferation in vitro and in animal models.
Cell Culture System
These experiments were performed using ARPE-19 cells, a human retinal pigment epithelial cell line that displays the differentiated phenotype of RPE cells, and form a polarized monolayer on Transwell filters (Costar) (18, 19). These cells were grown in Dulbecco's modified Eagle's medium, F12 (Invitrogen) with 10% fetal bovine serum, and 1× penicillin/streptomycin. In some of the experiments the cells were grown as monolayers on Transwell filters. For those experiments, fetal bovine serum was removed completely for the final 5–7 days (2–3 media changes) prior to measurements, which we have previously shown does not alter survival or monolayer formation in these cells (20). Transepithelial resistance (TER) of the cell monolayer on the Transwell filters was determined by measuring the resistance across the monolayer with an EVOM volt-ohmmeter (World Precision Instruments). The value for cell monolayers was determined by subtracting the TER for filters without cells and then multiplying by the surface area of the filters. Cell monolayers were considered stable when TER was repeatedly measured as ∼40–45 Ω/cm2 (20). TER measurements, which are proportional to membrane permeability, are an accepted readout for the barrier function of an RPE monolayer (18, 20). In parallel experiments, cells were grown on plates or glass slides for ∼2 weeks after the cells reached confluence to mimic the conditions in the Transwell plates. Cells from these long-term cultures were used for flow cytometry (plates) or immunofluorescence microscopy (glass slides).
In Vitro Model of Oxidative Stress and Complement Activation
As a model of oxidative stress, stable ARPE-19 cell monolayers were treated with 0.5 mm of H2O2. It has previously been reported that doses of up to 1 mm are not cytotoxic, and do not lead to disruption of barrier function in these cells (21). After treatment with H2O2, monolayers were exposed to 25% NHS as a source of complement proteins. In some experiments NHS was replaced with C7-deficient HS, or the complement system in NHS was inhibited by heat inactivation of NHS (30 min at 52 °C) or by treatment with CR2-fH. VEGF effects through VEGFR-1/2 activation were inhibited by treating the cells with SU5416.
Flow Cytometry
Surface expression of the various complement regulatory proteins was examined by flow cytometry. ARPE-19 cells were grown in long-term cultures. The cells were released from the plates by treatment with Accutase (Innovative Cell Technologies, Inc.), washed in phosphate-buffered saline, and treated with H2O2 or rH-19–20 as described in the specific experiments. For complement activation experiments, the cells were then incubated in 10% NHS at 37 °C for 1 h. Staining of surface proteins was performed by incubating the cells with primary antibody at 4 °C for 1 h, followed by washing the cells in phosphate-buffered saline, and incubating them with appropriate secondary antibodies when necessary. Cells were then washed and resuspended in 500 μl of phosphate-buffered saline, run on a FACSCalibur machine (BD Biosciences), and analyzed with CellQuest Pro software (BD Biosciences). Fluorescence was expressed in relative fluorescence units.
Immunofluorescence Microscopy
Surface expression of DAF and CD59 on ARPE-19 cells was examined by immunofluorescence microscopy. Cells were plated on glass coverslips and grown for 2 weeks after reaching confluence to generate polarized monolayers. After washing with phosphate-buffered saline, the cells were fixed and permeabilized by immersion in cold acetone/methanol (1:1) for 5 min. Nonspecific binding was blocked by treatment of the cells with Superblock (ScyTek Laboratories), followed by incubation with anti-DAF, anti-MCP, anti-CD59, or anti-Na+K+-ATPase antibodies (1:400 for 1 h at 4 °C). The cells were washed, and then incubated with appropriate secondary antibody. As a negative control, primary antibodies were omitted. Staining of the cells was examined using a Nikon T-2000, inverted microscope equipped for confocal microscopy and analyzed with Slidebook 4.2 software (Intelligent Imaging Innovations). TUNEL (TdT-mediated dUTP nick-end labeling) staining of cells on Transwell filters was performed according to the protocol provided by the manufacturer (Roche Diagnostics) as published previously (22). In short, monolayers were fixed in 2% paraformaldehyde for 2 h at 4 °C followed by TUNEL labeling and DNA strand-break visualization with fluorescein.
ELISA for VEGF and C5b-9 Measurement
To measure production of VEGF by the cells, cell culture supernatants were concentrated (Amicon Ultra-4, 3000 Da cutoff; Millipore), solubilized in CellLytic MT (mammalian tissue lysis/extraction reagent; Sigma), and centrifuged at 20,000 × g for 5 min. Microplates were coated with the VEGF capture antibody (Antigenix America, Inc.) and 100 μl of the concentrated supernatant was added. The captured proteins were detected with the same VEGF-specific antibody conjugated to horseradish peroxidase, followed by development with the chromogenic substrate OPD (Sigma). Product development was assayed by measuring absorbance at 492 nm. Aliquots were assayed in duplicate, and values compared with a VEGF dose-response curve. To measure C5b-9 formed during complement activation, cell supernatants were diluted 1:10 and an ELISA for C5b-9 (Quidel) was performed according the manufacturer's instruction.
Western Blot Analysis
Cell culture supernatants were separated by electrophoresis on a 10% BisTris polyacrylamide gel (Invitrogen), and proteins were transferred to a nitrocellulose membrane. The membrane was probed with polyclonal antibody to C3 (ICN Pharmaceuticals) and antibody binding was visualized using a chemiluminescence detection kit (Amersham Biosciences).
Statistics
Data are expressed as mean ± S.D. Statview software was used for statistical analysis. The Fisher protected least significant difference (PLSD) was used to compare trends over time. Comparison of two conditions was performed using the Student's t test.
RESULTS
Oxidative Stress Sensitizes RPE to Injury upon Exposure to Serum
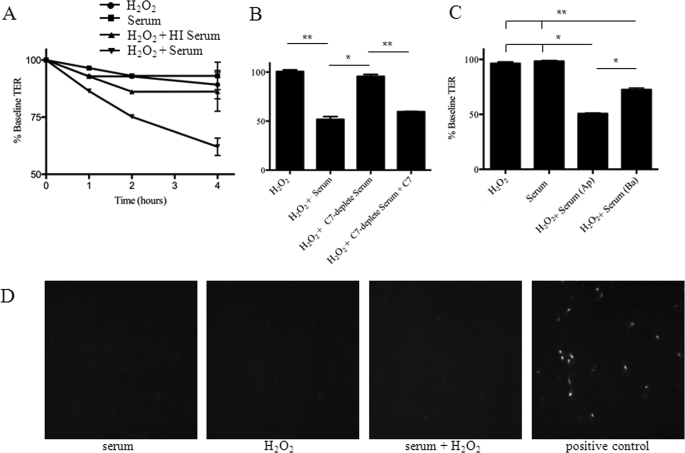
ARPE-19 cells were grown as monolayers on Transwell filters and TER was monitored until a stable value was achieved. TER of 40–45 Ω/cm2 is the result of the establishment of an epithelial barrier function based on the formation of tight junctions. Changes in TER measurements can then be used to probe for barrier function integrity as an early marker for RPE injury. Exposure of the cells to 25% NHS (as a source of complement proteins) did not alter TER (Fig. 1A). Likewise, exposure of the cells to 0.5 mm H2O2 to induce oxidative stress did not significantly alter TER, confirming previous results by Bailey and co-workers (21). However, treatment of the cells with 0.5 mm H2O2 and 25% NHS together did cause a significant reduction in TER in a time-dependent manner (p < 0.01). Heat inactivation of the serum to deplete complement activity significantly attenuated the drop in TER induced by exposure of oxidatively stressed cells to serum (p < 0.01). To confirm that the effect of serum was due to complement activation and not some other heat-labile component, C7-depleted serum was used in place of complement-sufficient NHS (Fig. 1B). As with heat inactivation, C7-depleted serum was found to be ineffective in reducing TER, indicating an important role for the membrane attack complex in reducing TER. Addition of purified C7 to the C7-depleted serum restored the ability of the serum to decrease TER, confirming that the lack of effect with the C7-depleted serum was due to the absence of this factor. Application of H2O2 and serum to the apical surface caused a greater change in TER than application to the basal surface (Fig. 1C; p = 0.02), indicating that the apical surface is more susceptible to complement-mediated injury after oxidative stress. Based on TUNEL staining, apoptotic cells were not observed with any of the treatment protocols, including the combined treatment of the cells with H2O2 and serum (Fig. 1D). To induce apoptosis in stable monolayers it was necessary to increase the H2O2 concentration 10-fold. Thus, exposure of oxidatively stressed cells to serum impairs the barrier function of the cellular monolayer, but in this system the drop in TER is not due to direct cytotoxicity.
FIGURE 1.
Oxidative stress in combination with normal human serum disrupts ARPE-19 monolayers due to complement activation. ARPE-19 cells were grown until a stable TER was obtained. A, cells were treated with H2O2, serum, H2O2 and heat-inactivated serum, or H2O2 and serum. Although treatment with H2O2 or serum alone did not cause a significant drop in the TER, combined treatment with H2O2 and serum did cause the TER to deteriorate, reaching maximum effects by 4 h. Treatment with H2O2 and serum caused a significant decline in TER compared with serum alone, H2O2 and HI serum, or H2O2 alone when analyzed by Fisher's protected least significant difference (PLSD). B, cells were treated with H2O2 and serum or H2O2 and C7-deficient serum for 4 h. C7-deficient serum, which cannot form the MAC, did not cause a decline in TER. Reconstitution of C7 restored the ability of the serum to decrease TER. C, when cells were exposed to H2O2 and serum for 4 h on their apical (Ap) surface there was a greater decline in TER than when they were exposed on their basal (Ba) surface. D, TUNEL-positive staining of the ARPE-19 cells was not seen with any of the treatment conditions, demonstrating that the decline in TER in response to these treatments was not due to cytotoxicity. Exposure to 5 mm H2O2 for 24 h was used as a positive control for the TUNEL. Data are expressed as mean ± S.D. (error bars) for panels A–C (n = 3–6 per condition); *, p < 0.05; **, p < 0.01.
Oxidative Stress of RPE Permits Complement Activation on the Surface of the Cells When Exposed to Serum
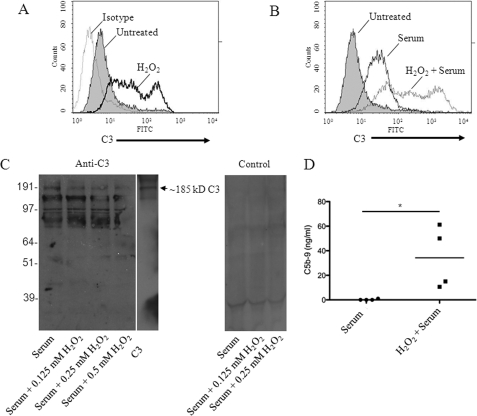
We next sought to determine whether oxidative stress impairs the ability of ARPE-19 cells to regulate complement activation on their surface by performing flow cytometry to measure fixation of C3 activation fragments (such as C3b and C3d) to the cell surface as a marker of complement activation. We found that treatment of ARPE-19 cells with H2O2 caused increased surface C3b/d staining even in the absence of exogenous complement proteins (Fig. 2A). The production and release of complement components by stressed or injured epithelial cells is well described (23). Next, we examined the effects of oxidative stress on complement regulation by cells that are exposed to NHS as a source of complement. Exposure of the cells to 10% NHS for 1 h caused fixation of C3b/d on the cells, but levels of surface C3b/d were higher in cells treated with H2O2 prior to exposure to serum (Fig. 2B). This increase in surface C3b/d was concomitant with a drop in intact C3 levels in the supernatant (Fig. 2C). Levels of intact (∼185 kDa) C3 were reduced by contact with cells previously treated with H2O2 in a dose-dependent manner. In other words, oxidative stress of the cells caused increased consumption of intact C3 later added to the supernatant. An ELISA for C5b-9 in cell supernatants confirmed that detectable levels of MAC were formed when serum was exposed to oxidatively stressed cells, but not when the serum was exposed to unmanipulated cells (Fig. 2D).
FIGURE 2.
Oxidative stress increases complement activation on the surface of ARPE-19 cells. Polarized ARPE-19 cells were treated for 1 h with 1 mm H2O2 to induce oxidative stress and surface C3b/d was detected by flow cytometry. A, staining of untreated cells with a polyclonal antibody to C3 (filled) was higher than that obtained with an isotype control (dotted line). Cells treated with H2O2 demonstrated higher surface levels of C3b/d (solid line) than untreated cells. B, ARPE-19 cells exposed to serum (solid line) demonstrated greater surface C3b/d than unmanipulated controls (filled), but oxidatively stressed ARPE cells (dotted line) showed greater surface C3b/d after exposure to serum than cells not exposed to H2O2. Representative results from at least three independent experiments are shown, and data are gated to show the results for viable cells only. C, cells were treated for 1 h with varying doses of H2O2, washed, and then exposed to serum. Western blot analysis of C3 in the supernatant confirmed that intact C3 (∼185 kDa) in the serum was consumed to a greater degree after exposure to the oxidatively stressed cells. The lower molecular weight bands were not seen when probed with control serum, and may represent C3 cleavage fragments or nonspecific binding of the anti-C3 antibody to other proteins in the supernatant. D, ELISA of C5b-9 levels in cell supernatants confirmed that complement was activated and MAC was formed when oxidatively stressed cells were exposed to serum, but not when unmanipulated cells were exposed to serum. *, p < 0.05.
It has previously been shown that the expression of complement regulatory proteins may be reduced on apoptotic and necrotic cells (13), which could account for the increase in C3b/d binding seen here. C3b/d deposition was compared in viable, apoptotic and necrotic populations identified by forward-scatter and side-scatter, as well as by staining with Annexin V and propidium iodide (data not shown). Increased C3b/d deposition after combined treatment with H2O2 and serum was seen on a population of apoptotic and necrotic cells always present after release of the cells using enzymatic treatment. However, more importantly, surface C3b/d was increased on viable cells, indicating that complement deposition on oxidatively stressed ARPE-19 cells is a result of non-cytotoxic changes to the surface of the cells. The data in Fig. 2 are gated to show C3b/d deposition on viable cells.
Expression of Surface Complement Regulatory Proteins Is Reduced during Oxidative Stress
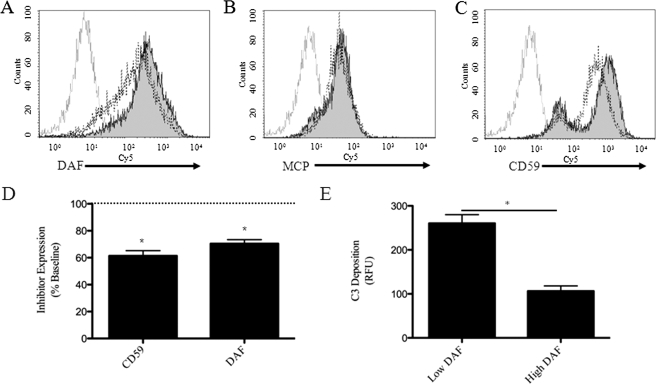
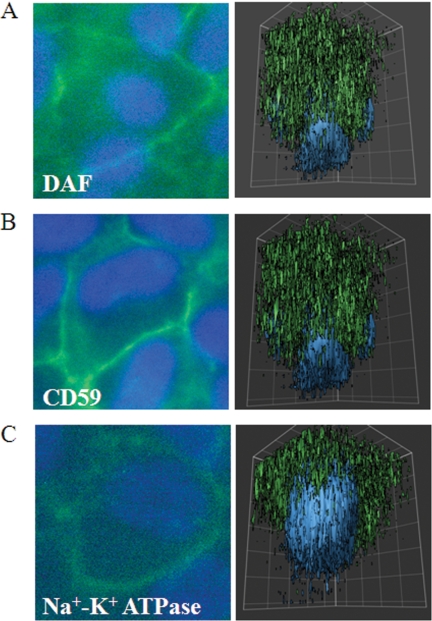
It has previously been reported, based on immunohistochemistry, that human RPE cells express MCP, whereas DAF and CD59 could not be detected (15). Here, we identified the presence of all three membrane-bound inhibitors on human ARPE-19 cells, using FACS analysis (Fig. 3). Immunofluorescence microscopy was performed together with three-dimensional reconstruction to identify whether surface expression of DAF and CD59 are polarized (Fig. 4). The apical side was verified by labeling with the apical marker Na+K+-ATPase. In unmanipulated, polarized monolayers, DAF and CD59 expression was concentrated on both the apical and basolateral sides; the antibody against CD46 was found to be unsuitable for immunofluorescence.
FIGURE 3.
Oxidative stress reduces the surface expression of DAF and CD59 by ARPE-19 cells. Staining of ARPE-19 cells for DAF, MCP, and CD59 (filled) demonstrated surface expression of all three membrane-bound inhibitors when compared with staining with isotype control (gray line). Gating was used to examine viable cells only. A, surface expression of DAF decreased after treatment of the cells for 1 h with 1 mm H2O2. B, surface expression of MCP did not change. C, surface expression of CD59 showed a bimodal distribution in unmanipulated cells, and surface levels in the higher expressing cells decreased after treatment with H2O2. Representative results from at least three independent experiments are shown. D, compared with surface levels on unmanipulated cells, the levels of CD59 and DAF on oxidatively stressed cells fell to 61 ± 4 and 70 ± 3%, respectively. E, cells were treated with H2O2 and serum, and surface DAF and C3b/d were stained. Cells with low levels of surface DAF had greater C3b/d deposition than those with high levels of DAF (n = 3 for D and E); *, p < 0.001.
FIGURE 4.
DAF and CD59 are expressed on the apical and basolateral surface of ARPE-19 cells. Cells were grown to confluence on coverslips. They were then permeabilized and stained for DAF (A), CD59 (B), and Na+K+-ATPase (C). Best focus views of DAF and CD59 demonstrated apical and basolateral localization in the plasma membrane, and three-dimensional reconstructions confirmed apical and basolateral concentration of DAF and CD59 compared with Na+K+-ATPase, which is known to be only apically concentrated.
Complement activation on oxidatively stressed ARPE-19 cells could be a result of increased local concentrations of complement proteins or activating proteases (11). Alternatively, we hypothesized that reduced surface levels of complement regulatory proteins could account for increased complement activation on the cells after treatment with H2O2. Surface levels of DAF decreased after treatment with H2O2 (Fig. 3, A and D) to ∼70% of those seen on unmanipulated cells (p < 0.001); whereas surface levels of MCP did not detectably change on the cells after treatment with H2O2 (Fig. 3B). Measurement of CD59 on unmanipulated cells revealed high and low expressing populations of cells (Fig. 3C). Treatment with H2O2 caused a downward shift in surface CD59 in the high-, but not the low-expressing population of cells. Mean CD59 expression for all cells fell to ∼60% of those on unmanipulated cells (Fig. 3D; p < 0.001). Mechanistically, DAF accelerates the decay of C3 convertases and reduces cleavage of C3 by the classical and alternative pathway of C3 convertases, so reduced levels of DAF are consistent with the observed increases in surface C3b/d deposition (Fig. 2). To further explore this relationship, cells were treated with H2O2 and exposed to serum, stained for both DAF and C3b/d, and examined by FACS analysis. The cells were gated on whether they expressed high or low levels of DAF. C3b/d deposition on the low DAF cells was ∼2.5-fold higher than that on the high DAF cells (Fig. 3E). CD59 inhibits formation of MAC on cell surfaces, but does not inhibit cleavage of C3. As shown in Fig. 1B, MAC formation is necessary for the drop in TER observed after treatment with H2O2 and serum. Hence, reduction in DAF or CD59 would make the RPE cells potentially more vulnerable to complement attack.
Contribution of Factor H in Protecting ARPE-19 Cells from Complement-mediated Damage Is Reduced in Cells Subjected to Oxidative Stress
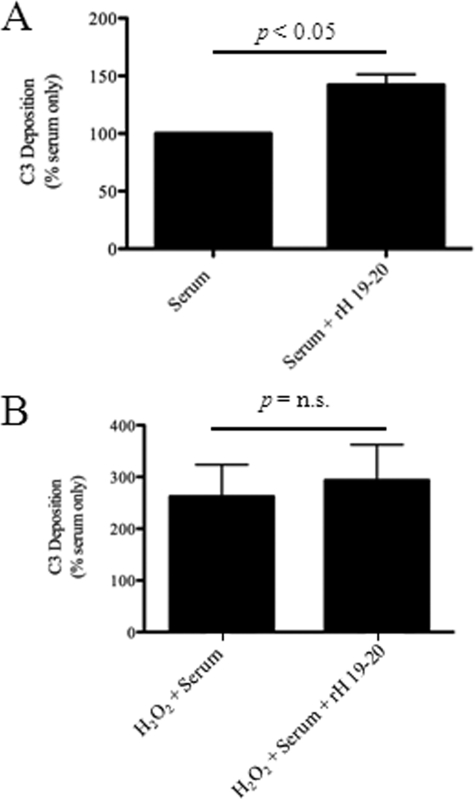
In addition to the cell surface complement inhibitors, serum factor H may regulate the alternative pathway on ARPE-19 cells (24). To determine whether factor H contributes to regulation of complement activation on the surface of ARPE-19 cells, we incubated the cells with rH-19–20, a recombinant protein that inhibits factor H activity on cell surfaces by blocking its binding. As shown in Fig. 2, exposure of cells to 10% serum alone leads to C3b/d deposition on the cell surface. The addition of 50 μg of rH-19–20 per ml of serum caused an increase in the amount of C3b/d deposited on the surface of the cells (Fig. 5A). The mean fluorescence with rH-19–20 was on average 40% higher than that in cells without rH-19–20 (n = 3, p < 0.05). These results indicate that under normal conditions, serum-derived factor H limits complement activation on the cell surface of ARPE-19 cells.
FIGURE 5.
Factor H in serum limits complement activation on the surface of unmanipulated ARPE-19 cells, but not on oxidatively stressed cells. ARPE-19 cells were exposed to 10% serum with or without the addition of 50 μg/ml of a dominant-negative form of factor H (rH-19–20). Factor H is an alternative pathway inhibitor found in high concentrations in normal serum. A, the addition of rH-19–20 to the cells caused greater deposition on their surface when normalized to values obtained with serum alone, indicating that factor H limits complement activation on the cell surface when the cells are exposed to an intact complement system. B, when cells were treated with H2O2 exposure to serum caused more than a 2-fold increase in C3b/d deposition compared with serum alone, but the addition of rH-19–20 to the reaction did not cause a significant further increase in surface C3b/d staining. Data are expressed as mean ± S.D. (error bars), n = 3 for each condition.
As indicated above, treatment of the cells with H2O2 prior to exposing the cells to serum caused increased C3b/d deposition. In the cells that were pretreated with H2O2, however, addition of rH-19–20 did not cause a significant further increase in C3b/d deposition on the cells upon serum exposure (the increase was 11%, n = 3, p = n.s.; Fig. 5B). Therefore, factor H, which controls complement activation and amplification on the surface of unmanipulated cells (Fig. 5), appears to not be able to compensate for the reduction of membrane-bound regulators that was observed in the cells subjected to oxidative stress (Fig. 3).
Complement Activation on Oxidatively Stressed ARPE-19 Cells Induces Secretion of VEGF, and VEGF Contributes to Disruption of the ARPE-19 Monolayer
VEGF-mediated choroidal neovascularization contributes to injury in AMD, and the RPE layer has been identified as an important site of VEGF production and secretion (25). Previous work has demonstrated that biologically active complement activation fragments induce the production of VEGF in an animal model of AMD (10). A previous study using t-butyl hydrogen peroxide as a source of oxidative stress demonstrated that this oxidant by itself induces a modest increase in the production and secretion of VEGF by ARPE-19 cells (26).
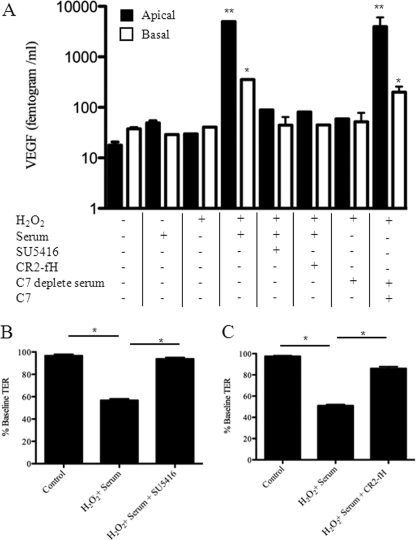
To determine the effect of oxidative stress and complement activation on VEGF secretion, VEGF levels were measured in the supernatants of ARPE-19 cells by ELISA. Consistent with previous reports by Kannan and co-workers (26) for ARPE-19 cells and by Blaauwgeers and co-workers (27) for differentiated human RPE cells, basal secretion of VEGF by unmanipulated RPE monolayers was higher than apical secretion (Fig. 6A). VEGF secretion was not altered consistently by H2O2 or NHS when applied individually to the apical chamber. However, co-administering H2O2 and NHS to the apical surface resulted in an ∼100-fold increase in apical VEGF secretion and a ∼50-fold increase in basal secretion. The maximum apical secretion in response to apical stimulation with H2O2 and NHS was ∼500 pg/ml of VEGF (Fig. 6A). Addition of CR2-fH or SU5416 prevented the H2O2 and NHS-induced VEGF release. When C7-depleted serum was used in this experiment the cells did not secrete VEGF. If C7 was added to the C7-depleted serum prior to exposure to the cells, however, the cells did secrete VEGF, confirming that formation of MAC on the oxidatively stressed cells is necessary for VEGF production in this system.
FIGURE 6.
Complement activation on ARPE-19 cells induces the secretion of VEGF, which in turn disrupts barrier function. ARPE-19 cells were grown as a monolayer until a stable TER was obtained. A, following 4 h of apical treatment, both apical and basal supernatants were removed and analyzed for VEGF content. At baseline, VEGF secretion is polarized, with increased secretion into the basal compartment. H2O2 and serum alone had no consistent effect on VEGF secretion, whereas H2O2 + serum together resulted in a significant increase in VEGF release. This increase showed a reversed polarity in comparison to the basal secretion, with increased secretion into the apical compartment. H2O2 + serum-mediated VEGF release can be inhibited by blocking either the alternative pathway of complement (CR2-fH) or VEGF receptor signaling (SU5416). VEGF was not secreted when C7-depleted serum was applied to oxidatively stressed cells, but was secreted when the C7-depleted serum was reconstituted with purified C7, demonstrating that MAC formation is involved with VEGF secretion by these cells. Data are expressed as mean ± S.D. (n = 3–6 per condition). B and C, treatment with H2O2 and serum caused a reduction in TER after 4 h. Co-administration of CR2-fH (an alternative pathway inhibitor) prevented the decline in TER, as did the addition of a VEGF-receptor-1/2 antagonist (SU5416). Statistical comparisons refer to no treatment versus H2O2 + serum; and C7-depleted serum versus C7-depleted serum plus purified C7. *, p < 0.05; **, p < 0.01.
If the drop in TER by oxidatively stressed ARPE-19 cell monolayers exposed to NHS was dependent on complement-mediated release of VEGF, then inhibiting the alternative pathway of complement with a recombinant targeted form of factor H (CR2-fH) or blocking VEGF receptor signaling with SU5416 should prevent the drop in TER. Both inhibitors were found to significantly reduce the combined effect of H2O2 and NHS on TER (Fig. 6, panels B and C), suggesting that alternative pathway activation and VEGF receptor signaling both contribute to disruption of the cell monolayer.
Together these results indicate that complement activation on RPE cells induces secretion of VEGF by the cells. The experiments utilizing the SU5416 inhibitor revealed that VEGF triggers an autocrine feedback-loop resulting in VEGF-mediated VEGF secretion. In vivo, this feedback-loop would allow sustained VEGF release to occur at the site of inflammation. This released VEGF could promote subsequent loss of RPE barrier property, choroidal angiogenesis, and the development of CNV. Complement activation and downstream VEGF production appear to be critical to the disruption of the ARPE-19 monolayer.
DISCUSSION
Our results demonstrate that oxidative stress impedes the ability of RPE cells to control activation of the complement system on their surface. ARPE-19 cells express membrane-associated complement inhibitors DAF, MCP, and CD59. Using rH-19–20, we also demonstrated that the fluid phase complement inhibitor factor H is functionally important for controlling complement activation on the surface of ARPE-19 cells. In oxidatively stressed cells, the surface levels of DAF and CD59 were reduced and the contribution of factor H to complement inhibition was less evident. These changes were associated with increased complement activation on the cell surface, as documented by increased C3b/d deposition, and resulted in the impairment of the barrier function of the RPE monolayer. Reduction in barrier function was not associated with cell loss due to cytotoxicity, but rather with secretion of VEGF, a cytokine that has been implicated in the disruption of RPE barrier function. Impairment of the barrier function was equivalently prevented by treating the cells with an alternative pathway of complement inhibitor or with an inhibitor of VEGF signaling. This suggests that, in the RPE monolayers, complement activation is not in itself cytolytic, but that complement-induced VEGF acts in an autocrine fashion to disrupt the epithelial tight junctions.
The alternative pathway of complement is continuously activated in the fluid phase, and tissue surfaces require continuous complement inhibition to prevent spontaneous autologous cell injury (11). The observed decrease in DAF surface expression, the relative decrease in surface inhibition by factor H, and release of C3 by the oxidatively stressed ARPE-19 cells may all foster complement activation on the cell surface. In addition, the reduction in CD59 would allow increased MAC assembly, which was found to be essential for the H2O2 and serum-induced drop in TER. Treatment of the cells with H2O2 appeared to cause a relative and an absolute decrease in the ability of factor H in the serum to control C3 conversion at the cell surface. This may be due to reduced interaction of factor H with the surface of oxidatively stressed cells, or factor H may not be able to compensate for reduced inhibition by DAF and increased C3 production. Finally, it may also be possible that factor H interacts with the oxidatively stressed cells through an additional domain other than short consensus repeats 19–20, which would affect the efficiency of rH-19–20 as a factor H cell surface competitor.
Polymorphisms in complement regulatory proteins, specifically factor H, are strongly associated with the development of AMD (3–6), and interruption of the complement system ameliorates injury in animal models of AMD (28). Structure-function analysis of a common AMD-associated polymorphism of factor H has demonstrated that the protein product of the disease-associated polymorphism shows reduced binding to glycosaminoglycans (29). It is not clear why the retina is the specific site of injury in patients carrying these polymorphisms or what acquired events contribute to retinal complement activation. However, as the complement cascade is continuously activated at a low level in the eye (30), perhaps there is a heightened need for tight regulation by complement inhibitors to prevent autologous tissue injury at this location. Any impairment in complement regulation could thus produce complement-mediated inflammation. Based upon our findings, we propose that the continual oxidative stress in the RPE layer may promote complement activation by reducing expression of DAF and CD59 on affected cells, by reducing surface inhibition by factor H, and by stimulating the cells to produce C3. Furthermore, lipofuscin products of RPE cell photooxidation may also activate complement directly (31). Thus, oxidative stress of the RPE layer may create a microenvironment that is rich in activating surfaces, but has a relative paucity of complement inhibitory proteins.
In addition, our results suggest that defects in complement inhibitors other than factor H may also be involved in AMD. Polymorphisms in DAF and CD59 have not yet been reported as risk factors for this disease, but our data would indicate that defective surface DAF or CD59 function would exacerbate complement-mediated inflammation on the RPE surface. However, the expression of multiple inhibitory proteins by RPE cells does seem to offer some redundancy. A detailed understanding of how this system of complement inhibition is gradually overwhelmed during the development of AMD will require further studies of animal models and human tissues at various stages of the disease. Such studies should help to confirm whether the mechanisms we have identified are engaged in the intact eye during disease development.
Other published studies with this same cell type have demonstrated that chemically induced oxidative stress by itself causes the ARPE-19 cell monolayers to increase the release of VEGF both apically and basally by ∼8- and 6-fold, respectively (26). However, as those experiments included 1% fetal bovine serum, it is plausible that in addition to the said authors' proposed mechanism involving hypoxia-inducible factor-1 signaling, the t-butyl hydrogen peroxide used in their protocol might result in complement activation. Complement activation fragments, in turn, may have then mediated VEGF secretion by the cells. In our experiments oxidative stress induced by H2O2 was sufficient by itself to cause C3 deposition on the cells, but it did not consistently increase VEGF secretion. On the other hand, oxidative stress together with exposure to complement-sufficient serum increased basal and apical VEGF secretion ∼50- and 100-fold, respectively. The apical concentration after 4 h was found to be ∼500 pg/ml of VEGF, which we have shown corresponds to the EC50 for TER reduction after apical VEGF application for these cells (20). It is unlikely that products in the serum, other than complement proteins, provided signals necessary for VEGF secretion by the cells under the conditions presented, as VEGF secretion could be completely prevented by alternative pathway inhibition. VEGF secretion, itself, mediated further VEGF production by the cells in an autocrine fashion. A similar autocrine effect, which would allow for sustained VEGF release, has previously been observed for VEGF-189 (32).
Our data lend further support to the idea that complement activation induces production of VEGF, and that complement inhibition may prevent VEGF-mediated injury as seen in choroidal neovascularization. Several therapeutic complement inhibitors are being developed for clinical use. Furthermore, reduced expression of complement regulatory proteins may be a general response of the RPE to various types of mechanical, chemical, and infectious stress. It is possible, therefore, that similar events may occur in many types of retinal injury, and that complement inhibition may be an effective therapeutic option for retinal diseases other than AMD.
Culture models are attractive for their simplicity, but are limited by their lack of tissue complexity. Our experiments revealed that apical exposure of RPE monolayers to oxidative stress and serum was more effective in compromising RPE barrier function than basal exposure. However, the application of serum to the cells exposes them to the entire complement system. In vivo, it is possible that the passage of large molecular weight complement proteins from the circulation toward the RPE layer will be unequal or that some of the complement components may be generated by the RPE or retina (33). Similarly, complement-mediated injury of oxidatively stressed RPE cells might only occur after other insults permit access of the large complement proteins into the restricted environment of the apical RPE. Thus to date, it is unclear which complement components are required for AMD pathogenesis, or whether the components are systemically derived or locally produced. Histological experiments have not provided insight, as complement proteins can be found to accumulate either on the basal side of the RPE in drusen (1) or the apical side in the outer segment layer (cfh−/− mouse (9)). Finally, whereas our experiments do not address the issue of chronic exposure to oxidative stress and complement activation as probably experienced in AMD, we carefully selected sublytic, non-cytotoxic levels of stimulation. Indeed, in preliminary studies we have shown that daily treatment of ARPE-19 cell monolayers with this low level of oxidative stress together with complement activation does not induce cell death over a 2-week time course, but permanently reduced TER, arguably representing a good model for chronic stress.7
In conclusion, a growing body of evidence indicates that uncontrolled activation of the alternative pathway of complement contributes to the development and progression of AMD. Our findings provide a mechanism whereby oxidative stress of RPE cells, phenotypically alters them such that their regulation of the complement system is reduced. Once activated, the complement system generates several biologically active fragments and induces the RPE cells to secrete VEGF, another molecule implicated in the pathogenesis of AMD. These findings provide a link between aseptic insults that occur in the eye and a pathogenic immune response. Our findings also support recent findings that suggest that complement activation is a proximal event in the development of AMD, occurring upstream of pathologic VEGF production. Therapeutic complement inhibitors may, therefore, prevent the formation of several pathogenic factors and slow the progression of AMD.
This work was supported, in whole or in part, by National Institutes of Health Grants DK077661 and DK076690 (to J. M. T.), DK035081 (to M. K. P.), EY13520 and EY017465 (to B. R.), and HL082485 (to S. T.). This work was also supported by Vision Core Grant EY014793, the Foundation Fighting Blindness, American Heart Association Grant 0735101N (to V. P. F.), and an unrestricted grant to the Medical University of South Carolina from Research to Prevent Blindness, Inc., New York.
7 K. Kunchithapautham and B. Rohrer, unpublished results.
- AMD
- age-related macular degeneration
- RPE
- retinal pigment epithelium
- TER
- transepithelial resistance
- DAF
- decay accelerating factor
- VEGF
- vascular epidermal growth factor
- MCP
- membrane cofactor protein
- MAC
- membrane attack complex
- TUNEL
- TdT-mediated dUTP nick-end labeling
- FACS
- fluorescence-activated cell sorter
- NHS
- normal human serum
- ELISA
- enzyme-linked immunosorbent assay
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- rh
- recombinant factor H.
REFERENCES
- 1.Gehrs K. M., Anderson D. H., Johnson L. V., Hageman G. S. ( 2006) Ann. Med. 38, 450– 471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogan M. J. ( 1972) Trans. Am. Acad. Ophthalmol. Otolaryngol. 76, 64– 80 [PubMed] [Google Scholar]
- 3.Edwards A. O., Ritter R., 3rd, Abel K. J., Manning A., Panhuysen C., Farrer L. A. ( 2005) Science 308, 421– 424 [DOI] [PubMed] [Google Scholar]
- 4.Hageman G. S., Anderson D. H., Johnson L. V., Hancox L. S., Taiber A. J., Hardisty L. I., Hageman J. L., Stockman H. A., Borchardt J. D., Gehrs K. M., Smith R. J., Silvestri G., Russell S. R., Klaver C. C., Barbazetto I., Chang S., Yannuzzi L. A., Barile G. R., Merriam J. C., Smith R. T., Olsh A. K., Bergeron J., Zernant J., Merriam J. E., Gold B., Dean M., Allikmets R. ( 2005) Proc. Natl. Acad. Sci. U. S. A. 102, 7227– 7232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haines J. L., Hauser M. A., Schmidt S., Scott W. K., Olson L. M., Gallins P., Spencer K. L., Kwan S. Y., Noureddine M., Gilbert J. R., Schnetz-Boutaud N., Agarwal A., Postel E. A., Pericak-Vance M. A. ( 2005) Science 308, 419– 421 [DOI] [PubMed] [Google Scholar]
- 6.Klein R. J., Zeiss C., Chew E. Y., Tsai J. Y., Sackler R. S., Haynes C., Henning A. K., SanGiovanni J. P., Mane S. M., Mayne S. T., Bracken M. B., Ferris F. L., Ott J., Barnstable C., Hoh J. ( 2005) Science 308, 385– 389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duvall-Young J., MacDonald M. K., McKechnie N. M. ( 1989) Br. J. Ophthalmol. 73, 297– 302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leys A., Vanrenterghem Y., Van Damme B., Snyers B., Pirson Y., Leys M. ( 1991) Graefes Arch. Clin. Exp. Ophthalmol. 229, 406– 410 [DOI] [PubMed] [Google Scholar]
- 9.Coffey P. J., Gias C., McDermott C. J., Lundh P., Pickering M. C., Sethi C., Bird A., Fitzke F. W., Maass A., Chen L. L., Holder G. E., Luthert P. J., Salt T. E., Moss S. E., Greenwood J. ( 2007) Proc. Natl. Acad. Sci. U. S. A. 104, 16651– 16656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nozaki M., Raisler B. J., Sakurai E., Sarma J. V., Barnum S. R., Lambris J. D., Chen Y., Zhang K., Ambati B. K., Baffi J. Z., Ambati J. ( 2006) Proc. Natl. Acad. Sci. U. S. A. 103, 2328– 2333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thurman J. M., Holers V. M. ( 2006) J. Immunol. 176, 1305– 1310 [DOI] [PubMed] [Google Scholar]
- 12.Atkinson J. P., Goodship T. H. ( 2007) J. Exp. Med. 204, 1245– 1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elward K., Griffiths M., Mizuno M., Harris C. L., Neal J. W., Morgan B. P., Gasque P. ( 2005) J. Biol. Chem. 280, 36342– 36354 [DOI] [PubMed] [Google Scholar]
- 14.Cai J., Nelson K. C., Wu M., Sternberg P., Jr., Jones D. P. ( 2000) Prog. Retin. Eye Res. 19, 205– 221 [DOI] [PubMed] [Google Scholar]
- 15.Vogt S. D., Barnum S. R., Curcio C. A., Read R. W. ( 2006) Exp. Eye Res. 83, 834– 840 [DOI] [PubMed] [Google Scholar]
- 16.Huang Y., Qiao F., Atkinson C., Holers V. M., Tomlinson S. ( 2008) J. Immunol. 181, 8068– 8076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira V. P., Herbert A. P., Hocking H. G., Barlow P. N., Pangburn M. K. ( 2006) J. Immunol. 177, 6308– 6316 [DOI] [PubMed] [Google Scholar]
- 18.Dunn K. C., Aotaki-Keen A. E., Putkey F. R., Hjelmeland L. M. ( 1996) Exp. Eye Res. 62, 155– 169 [DOI] [PubMed] [Google Scholar]
- 19.Dunn K. C., Marmorstein A. D., Bonilha V. L., Rodriguez-Boulan E., Giordano F., Hjelmeland L. M. ( 1998) Invest. Ophthalmol. Vis. Sci. 39, 2744– 2749 [PubMed] [Google Scholar]
- 20.Ablonczy Z., Crosson C. E. ( 2007) Exp. Eye Res. 85, 762– 771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bailey T. A., Kanuga N., Romero I. A., Greenwood J., Luthert P. J., Cheetham M. E. ( 2004) Invest. Ophthalmol. Vis. Sci. 45, 675– 684 [DOI] [PubMed] [Google Scholar]
- 22.Kunchithapautham K., Rohrer B. ( 2007) Autophagy 3, 433– 441 [DOI] [PubMed] [Google Scholar]
- 23.Pratt J. R., Basheer S. A., Sacks S. H. ( 2002) Nat. Med. 8, 582– 587 [DOI] [PubMed] [Google Scholar]
- 24.Pangburn M. K. ( 2000) Immunopharmacology 49, 149– 157 [DOI] [PubMed] [Google Scholar]
- 25.Lopez P. F., Sippy B. D., Lambert H. M., Thach A. B., Hinton D. R. ( 1996) Invest. Ophthalmol. Vis. Sci. 37, 855– 868 [PubMed] [Google Scholar]
- 26.Kannan R., Zhang N., Sreekumar P. G., Spee C. K., Rodriguez A., Barron E., Hinton D. R. ( 2006) Mol. Vis. 12, 1649– 1659 [PubMed] [Google Scholar]
- 27.Blaauwgeers H. G., Holtkamp G. M., Rutten H., Witmer A. N., Koolwijk P., Partanen T. A., Alitalo K., Kroon M. E., Kijlstra A., van Hinsbergh V. W., Schlingemann R. O. ( 1999) Am. J. Pathol. 155, 421– 428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bora P. S., Sohn J. H., Cruz J. M., Jha P., Nishihori H., Wang Y., Kaliappan S., Kaplan H. J., Bora N. S. ( 2005) J. Immunol. 174, 491– 497 [DOI] [PubMed] [Google Scholar]
- 29.Prosser B. E., Johnson S., Roversi P., Herbert A. P., Blaum B. S., Tyrrell J., Jowitt T. A., Clark S. J., Tarelli E., Uhrín D., Barlow P. N., Sim R. B., Day A. J., Lea S. M. ( 2007) J. Exp. Med. 204, 2277– 2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sohn J. H., Kaplan H. J., Suk H. J., Bora P. S., Bora N. S. ( 2000) Invest. Ophthalmol. Vis. Sci. 41, 3492– 3502 [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J., Jang Y. P., Kim S. R., Sparrow J. R. ( 2006) Proc. Natl. Acad. Sci. U. S. A. 103, 16182– 16187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ancelin M., Chollet-Martin S., Hervé M. A., Legrand C., El Benna J., Perrot-Applanat M. ( 2004) Lab. Invest. 84, 502– 512 [DOI] [PubMed] [Google Scholar]
- 33.Hageman G. S., Luthert P. J., Victor Chong N. H., Johnson L. V., Anderson D. H., Mullins R. F. ( 2001) Prog. Retin. Eye Res. 20, 705– 732 [DOI] [PubMed] [Google Scholar]