Abstract
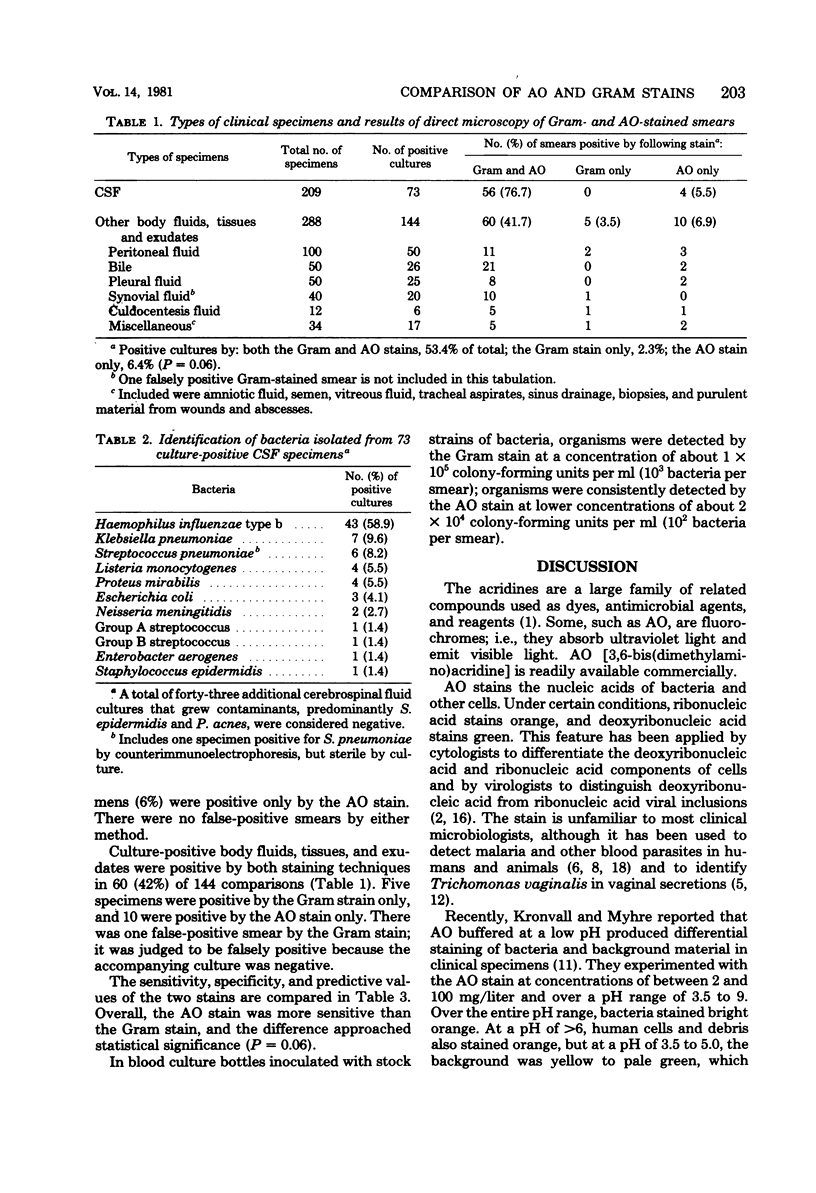
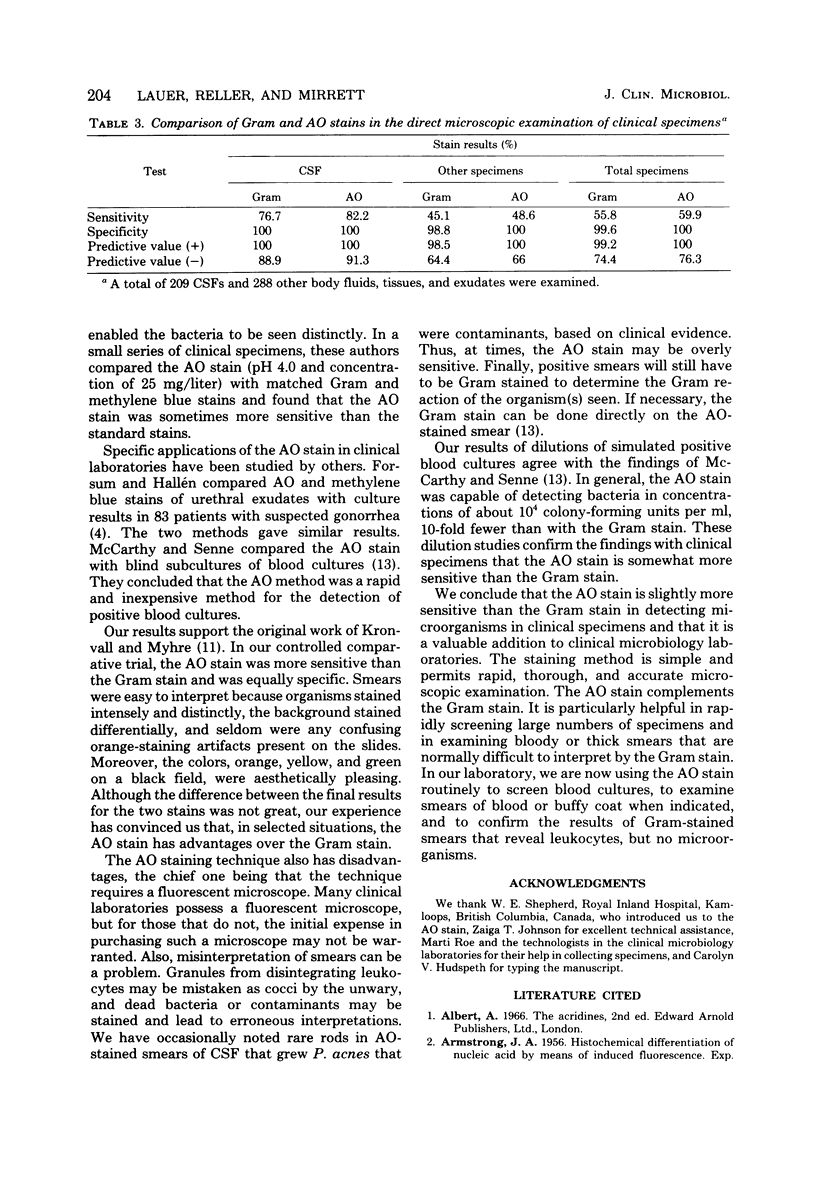
Acridine orange, a fluorochrome strain, is potentially superior to the Gram stain in the direct microscopic examination of clinical specimens because it gives striking differential staining between bacteria and background cells and debris. Its value in clinical laboratories was evaluated by testing 209 cerebrospinal fluids and 288 other body fluids, tissues, and exudates by both techniques. Smears were made in duplicate, fixed with methanol, stained, and examined without knowledge of the result of the companion smear or culture. Overall, acridine orange was slightly more sensitive than the Gram stain (acridine orange, 59.9%; Gram stain, 55.8%) and equally specific in detecting microorganisms. One smear was falsely positive by the Gram stain; none was falsely positive by the acridine orange stain. We conclude that acridine orange staining is a sensitive method for screening clinical specimens and reviewing selected specimens that are purulent, but negative by the Gram stain. Bloody fluids, thick exudates, and other normally difficult-to-read specimens were easily and quickly examined. We recommend, however, that positive smears be reexamined with the Gram stain to confirm the result and determine the Gram reaction of the microorganisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Feldman W. E. Relation of concentrations of bacteria and bacterial antigen in cerebrospinal fluid to prognosis in patients with bacterial meningitis. N Engl J Med. 1977 Feb 24;296(8):433–435. doi: 10.1056/NEJM197702242960806. [DOI] [PubMed] [Google Scholar]
- Forsum U., Hallén A. Acridine orange staining of urethral and cervical smears for the diagnosis of gonorrhea. Acta Derm Venereol. 1979;59(3):281–282. [PubMed] [Google Scholar]
- Fripp P. J., Mason P. R., Super H. A method for the diagnosis of Trichomonas vaginalis using acridine orange. J Parasitol. 1975 Oct;61(5):966–967. [PubMed] [Google Scholar]
- GAINER J. H. Demonstration of Anaplasma marginale with the fluorescent dye, acridine orange; comparisons with the complement-fixation test and Wright's stain. Am J Vet Res. 1961 Sep;22:882–886. [PubMed] [Google Scholar]
- Geiseler P. J., Nelson K. E., Levin S., Reddi K. T., Moses V. K. Community-acquired purulent meningitis: a review of 1,316 cases during the antibiotic era, 1954-1976. Rev Infect Dis. 1980 Sep-Oct;2(5):725–745. doi: 10.1093/clinids/2.5.725. [DOI] [PubMed] [Google Scholar]
- Hansen D. W., Hunter D. T., Richards D. F., Allred L. Acridine orange in the staining of blood parasites. J Parasitol. 1970 Apr;56(2):386–387. [PubMed] [Google Scholar]
- Hart G. Screening to control infectious diseases: evaluation of control programs for gonorrhea and syphilis. Rev Infect Dis. 1980 Sep-Oct;2(5):701–712. doi: 10.1093/clinids/2.5.701. [DOI] [PubMed] [Google Scholar]
- KASS E. H. Asymptomatic infections of the urinary tract. Trans Assoc Am Physicians. 1956;69:56–64. [PubMed] [Google Scholar]
- Kronvall G., Myhre E. Differential staining of bacteria in clinical specimens using acridine orange buffered at low pH. Acta Pathol Microbiol Scand B. 1977 Aug;85(4):249–254. doi: 10.1111/j.1699-0463.1977.tb01970.x. [DOI] [PubMed] [Google Scholar]
- Levett P. N. A comparison of five methods for the detection of Trichomonas vaginalis in clinical specimens. Med Lab Sci. 1980 Jan;37(1):85–88. [PubMed] [Google Scholar]
- McCarthy L. R., Senne J. E. Evaluation of acridine orange stain for detection of microorganisms in blood cultures. J Clin Microbiol. 1980 Mar;11(3):281–285. doi: 10.1128/jcm.11.3.281-285.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIVEN J. S. Fluorescence microscopy of nucleic acid changes in virus-infected cells. Ann N Y Acad Sci. 1959 Jul 21;81:84–88. doi: 10.1111/j.1749-6632.1959.tb49297.x. [DOI] [PubMed] [Google Scholar]
- Olcén P. Serological methods for rapid diagnosis of haemophilus influenzae, Neisseria meningitidis and Streptococcus pneumoniae in cerebrospinal fluid: a comparison of Co-agglutination, immunofluorescence and immunoelectroosmophoresis. Scand J Infect Dis. 1978;10(4):283–289. doi: 10.3109/inf.1978.10.issue-4.05. [DOI] [PubMed] [Google Scholar]
- Shute G. T., Sodeman T. M. Identification of malaria parasites by fluorescence microscopy and acridine orange staining. Bull World Health Organ. 1973 May;48(5):591–596. [PMC free article] [PubMed] [Google Scholar]


