Abstract
Although it is conceivable that cancer preventive isothiocyanates (ITCs), a family of compounds in cruciferous vegetables, induce cell cycle arrest and apoptosis through a mechanism involving oxidative stress, our study shows that binding to cellular proteins correlates with their potencies of apoptosis induction. More recently, we showed that ITCs bind selectively to tubulins. The differential binding affinities toward tubulin among benzyl isothiocyanate, phenethyl isothiocyanate, and sulforaphane correlate well with their potencies of inducing tubulin conformation changes, microtubule depolymerization, and eventual cell cycle arrest and apoptosis in human lung cancer A549 cells. These results support that tubulin binding by ITCs is an early event for cell growth inhibition. Here we demonstrate that ITCs can selectively induce degradation of both α- and β-tubulins in a variety of human cancer cell lines in a dose- and time-dependent manner. The onset of degradation, a rapid and irreversible process, is initiated by tubulin aggregation, and the degradation is proteasome-dependent. Results indicate that the degradation is triggered by ITC binding to tubulin and is irrelevant to oxidative stress. This is the first report that tubulin, a stable and abundant cytoskeleton protein required for cell cycle progression, can be selectively degraded by a small molecule.
Microtubules as a major cytoskeleton component in all eukaryotic cells play essential roles such as maintenance of cell polarity, intracellular traffic, organization, and cell motility (1–4). During cell division, the microtubule-formed mitotic spindle ensures the replicated chromosomes separate evenly at the end of the mitotic phase to the two daughter cells (1). It is because of its essential roles in cell growth that microtubules become a valid target for the development of anti-microtubule drugs against the rapidly growing cancer cells (2), as interference of microtubule dynamics arrests cell cycle progression and induces apoptosis (3). Therefore, microtubules have been considered one of the best targets to date for cancer chemotherapy (4).
Isothiocyanates (ITCs)3 are among the best studied chemopreventive small molecules (5). The three most studied ITCs, including benzyl-ITC (BITC; abundant in garden cress), phenethyl-ITC (PEITC; in watercress), and sulforaphane (SFN; in broccoli sprouts), have been shown to induce apoptosis and cell cycle arrest (5–8). Although it is believed that oxidative stress plays a role in cell cycle arrest and apoptosis induced by ITCs (6–12), we found that binding to proteins is a predominant intracellular chemical reaction of ITCs, and their protein binding affinities correlate well with inhibition of cell proliferation and induction of apoptosis (13). Recently, we identified tubulin, the microtubule constituent, as an in vivo target of ITCs by two-dimensional gel electrophoresis and mass spectrometry (14). The growth inhibition of human non-small lung cancer A549 cells by ITCs followed the order of BITC > PEITC > SFN. The same order of potency was seen in their binding affinities toward tubulin, induction of its conformational changes, and inhibition of its polymerization. The study provides the first evidence of an in vivo ITC-tubulin binding adduct, indicating that direct modification of cysteines in tubulin by ITCs, rather than oxidative stress, may trigger cell cycle arrest and apoptosis.
Here we report an unexpected novel finding that tubulin is selectively degraded in a variety of human cancer cells treated with ITCs. We provide evidence that the degradation is initiated by its binding with ITCs and mediated by the ubiquitin-proteasome pathway. Tubulin has long been viewed as a stable and abundant protein, and its levels in cells are tightly regulated (15). In the literature, the only studies on cellular tubulin level change are related to “the auto-regulation theory,” i.e. when microtubules collapse, the presence of a massive amount of tubulin monomers would selectively destabilize tubulin mRNA and subsequently decrease tubulin protein synthesis (16–18). To our knowledge, there is no report on tubulin degradation as a result of treatment with any agents. Our studies provide strong evidence that supports tubulin as a target of ITCs for cell growth inhibition, pointing to a new mechanism for the anti-microtubule or anti-mitosis effects of ITCs through covalent binding to tubulin and presenting a platform to study protein stability by modification with small molecules.
MATERIALS AND METHODS
Chemicals
SFN was a gift from Dr. Stephen Hecht (University of Minnesota). 4-Hydroxynonenal was a gift from Dr. Shantu Amin (Pennsylvania State College of Medicine, Hershey). BITC, PEITC, 5,5′-dithiobis(2-nitrobenzoic acid), N-methylphenethylamine (NMPEA), hydrogen peroxide, aminotriazole (ATZ), colchicine, vinblastine, taxol, cycloheximide (CHX), polyethylene glycol (PEG)-linked catalase, ATP, 2-deoxyglucose (2DG), arsenic trioxide, hydroquinone, cadmium chloride, tert-butylhydroquinone, menadione, cumene hydroperoxide, β-naphthoflavone, 1-nitro-1-cyclohexene, 1,2-ethanedithiol, acrolein, and all other reagents were the highest grade available from Sigma unless otherwise noted. Proteasome inhibitors MG132, proteasome inhibitor I, epoxomicin, and clasto-lactacystin β-lactone were purchased from Calbiochem. 3-Methyladenine and Z-Val-Ala-Asp-fmk (Z-VAD-fmk) were purchased from Biomol (Plymouth, PA). Pure porcine tubulin (>99%, T240-B) was purchased from Cytoskeleton Inc. (Boulder, CO).
Antibodies
Mouse monoclonal anti-α-tubulin (clone DM1A and B-5-1-2) antibodies, mouse monoclonal anti-β-tubulin (clone D66 and TUB 2.1) antibody, mouse monoclonal anti-ubiquitin (clone 6C1), mouse monoclonal anti-vimentin (clone V9), rabbit polyclonal anti-FLAG (F7425), rabbit IgG, and mouse monoclonal anti-β-actin (clone AC-15) antibodies were purchased from Sigma. Mouse monoclonal anti-γ-tubulin (clone TU-30) antibody, mouse monoclonal anti-IκBα (clone H-4) antibody, and protein G-conjugated agarose beads were purchased from Santa Cruz Biotechnology. Rabbit monoclonal anti-α-tubulin (clone 11H10) and rabbit monoclonal anti-β-tubulin (clone 9F3) antibodies were purchased from Cell Signaling (Danvers, MA).
Cell Culture and Treatments
Human cervical cancer HeLa, non-small lung cancer A549, human breast cancer MCF7, human colon carcinoma HCT-116, and human prostate cancer PC-3 were all from ATCC (Manassas, VA) and cultured in Dulbecco's modified Eagle's medium (Invitrogen) supplemented with 10% fetal bovine serum (Invitrogen), 1% penicillin/streptomycin (Invitrogen) at 37 °C, and 5% CO2. Cells (∼60% confluency) were treated with BITC, PEITC, and SFN at various concentrations for up to 24 h.
Lysis Buffers
Multiple lysis buffers were used to fractionate the soluble and insoluble fractions as follows: 1) M-PER mammalian protein extraction reagent (Pierce); 2) RIPA lysis buffer consisting of 50 mm phosphate, 150 mm NaCl, pH 7.4, 1% Nonidet P-40, 10 mm dithiothreitol, 1 mm EGTA, 1 mm EDTA, and 1 mm phenylmethylsulfonyl fluoride; 3) 20 mm Tris-HCl, 1% Triton X-100, 1 mm EDTA, 0.5 mm EGTA, pH 8.0; and 4) PBS with 1% Tween 20. Harvested cells were lysed on ice for 20 min before centrifugation at 13,200 × g for 10 min. The supernatant was defined as the soluble fraction and the pellet as the insoluble fraction. The whole cell lysate was obtained by lysing cells in 65 mm Tris-HCl, 150 mm NaCl, 2% SDS, 50 mm dithiothreitol, pH 8.0. The insoluble fraction was also dissolved in this SDS lysis buffer.
Cell Transfection
HeLa cells were seeded in 6-well plates with 50–60% confluence, cultured overnight, and transfected with either pcDNA3 vector or pcDNA3-FLAG-ubiquitin plasmid. All transient transfections were done using Lipofectamine 2000 reagent (Invitrogen).
Immunoblotting
The experiments were performed according to a previously published method (14). Briefly, cell lysate fractions or the whole cell lysates containing 20 μg of protein were resolved on 4–12% BisTris gels (Invitrogen), transferred to polyvinylidene difluoride membranes (Millipore, Billerica, MA), and detected by ECL reagents (GE Healthcare) after immunoblotting using antibodies.
Immunoprecipitation (IP)
Because tubulin forms aggregates after ITC treatment, the IP for tubulin was performed after the cell lysate was denatured. Briefly, cells were transfected with pcDNA3-FLAG-ubiquitin plasmid for 24 h before being treated with 10 μm BITC for 1 h. Harvested cells were lysed in 5 pellet volumes of lysis buffer containing 10 mm Tris, pH 8.0, 25 mm EDTA, and 1% SDS. The whole cell lysate was boiled for 5 min before diluting with 10 volumes of IP buffer containing 10 mm Tris, pH 8.0, 150 mm NaCl, 0.1 mm EDTA, 0.5% Tween 20. Samples were incubated overnight at 4 °C with rabbit polyclonal anti-FLAG or rabbit IgG (both 1 μg) followed by incubation with protein G-agarose beads at room temperature for 1 h. The beads were washed three times with the IP buffer before being loaded for SDS-PAGE. The transferred membrane was blotted with mouse monoclonal anti-α-tubulin.
Immunofluorescent Staining of Tubulin
The assay was performed according to a published method (14). Briefly, 104 cells were cultured on a microscope cover glass (12 mm diameter; Fisher) placed in individual wells of a 24-well tissue culture plate (BD Biosciences) for 24 h before being treated with 5 μm colchicine, taxol for 5 h or pretreated with 5 μm colchicine, taxol for 1 h and followed by 5 μm BITC for 4 h. The cells were then fixed sequentially with 3% paraformaldehyde in PBS for 20 min, quenched with 50 mm NH4Cl in PBS, and permeabilized with 1% bovine serum albumin, 0.075% saponin in PBS for 30 min. Cells were incubated at 4 °C overnight with mouse monoclonal anti-α-tubulin IgG1 (1:400; Sigma, clone DM1A), rinsed three times with 0.1% bovine serum albumin, 0.075% saponin in PBS, and then incubated with Alexa Fluor 488-conjugated goat anti-mouse IgG (1:800; Invitrogen) for 1 h at room temperature. Once the labeling was complete, the cover glasses were rinsed twice with PBS and mounted onto microscope slides with a small drop of ProLong AntiFade reagent (Molecular Probes). The fluorescent images were taken with a Fluoview-FV300 laser scanning confocal system (Olympus, Tokyo, Japan).
RT-PCR Analysis
Total RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's instruction. RNA samples were DNase-treated before RT-PCR was conducted using a SuperScript one-step RT-PCR kit (Invitrogen). PCR products were analyzed on a 1.5% agarose gel containing ethidium bromide. RT-PCR product of β-actin transcripts was used as an internal control. The following primers were used: α-tubulin, 5′-CTCCAGGGCTTCTTGGTTTTCC-3′ and 5′-TTTCACCATCTGGTTGGCTGGC-3′; β-tubulin, 5′-CCATACATACCTTGAGGCGA-3′ and 5′-GCCAAAAGGACCTGAGCGAA-3′; and β-actin, 5′-AAATCTGGCACCACACCTTC-3′ and 5′-TGATCTGGGTCATCTTCTCG-3′.
Ellman Assay
The measurement of free thiols in tubulin was similar to that described previously (14). In brief, 250 μl of 8 μm purified tubulin was pretreated with colchicine, vinblastine at 1 and 10 nm and 1, 10, and 40 μm, and taxol at 1 and 100 nm and 1 and 10 μm at room temperature for 15 min before being treated with 40 μm BITC (stoichiometric ratio of BITC to thiol is 1:2) for another 30 min. The reaction was stopped by adding 700 μl of 8 m guanidine hydrochloride and 50 μl of 20 mm 5,5′-dithiobis(2-nitrobenzoic acid), and the absorbance at 412 nm was measured by a UV-1700 UV-visible spectrophotometer (Shimadzu, Columbia, MD). A standard curve was generated using N-acetylcysteine.
Circular Dichroism Spectroscopy
The assay was performed according to a published method (14). CD spectra were recorded on a Jasco J-810 spectropolarimeter (Jasco, Easton, MD) at room temperature over the wavelength range of 250 to 200 nm using a 10-mm path length cell and averaging over three scans. For measurement of spectra of the drug-tubulin complex, the free drugs were removed from the complex by rapid gel filtration using Micro Bio-spin 30 columns (Bio-Rad).
Measurement of ROS
Cellular ROS generation was determined using hydrogen peroxide-sensitive fluorogenic precursor carboxy-H2-dichlorodihydrofluorescein diacetate (Invitrogen) as described before (13).
Flow Cytometry for Tubulin Level and Cell Cycle Determination
HeLa cells were treated with 10 μm BITC for 20 h before being fixed with 80% ice-cold ethanol. Cells were subsequently permeabilized for 5 min with PBS, 0.5% Triton X-100, blocked with 1% bovine serum albumin in PBS, 0.1% Triton X-100 for 2 h, incubated overnight at 4 °C with mouse monoclonal anti-tubulin (1:400; Sigma, clone DM1A) for 1 h, and incubated with Alexa Fluor 488-conjugated goat anti-mouse (1:400; Invitrogen) for 1 h. Once the tubulin labeling was complete, cells were further incubated with propidium iodide (250 mg/ml in PBS) and RNase A (1 mg/ml) for 30 min at room temperature. Dual label cell sorting was performed by flow cytometry using a FACSCalibur analyzer (BD Biosciences).
Measurement of Cellular ATP Levels
The amount of ATP was measured by an Enliten luciferin/rluciferase kit (Promega, Madison, WI). Briefly, HeLa cells were pretreated with 25 and 50 mm 2-deoxyglucose (2-DG) with or without 10 mm sodium azide for 2 h before 10 μm BITC was added. BITC treatment lasted another 6 h. Harvested cells were lysed with 2.5% trichloroacetic acid on ice for 20 min before centrifugation at 13,200 × g for 5 min. The supernatant was diluted with 100 volumes of 200 mm Tris acetate buffer, pH 7.75. The reaction was initiated by mixing 100 μl of the extract with 100 μl of the luciferin-luciferase luminous reagent. The luminescence was integrated for 5 s using a Tecan Ultrafluorescence microplate reader (Durham, NC).
Caspase-3 Activity Assay
The assay was performed as described previously (13). Cells were treated with 10 μm BITC, PEITC, or SFN for 1, 2, 4, 8, and 24 h before lysing in assay buffer containing 50 mm HEPES, 100 mm NaCl, 10 mm dithiothreitol, 1 mm EDTA, 0.1% CHAPS, pH 7.4. Ten microliters of cell lysate supernatant was mixed with 70 μl of assay buffer and 20 μl of 50 μm Ac-DEVD-rhodamine 110 substrate (Roche Applied Science). The mixture was incubated at 37 °C in the dark for 1 h. Release of rhodamine from the substrate was monitored with excitation at 485 nm and emission at 528 nm every 10 min with a Synergy HT fluorescent microplate reader (Bio-Tek, Winooski, VT).
Sample Preparation with iTRAQ Reagents
Human non-small lung cancer cells H460 and H596 were collected after 6 and 24 h treatment with 15 μm PEITC. Both cytoplasmic and nuclear fractions were separated using a commercial kit (catalog number K266, BioVision, Mountain View, CA). A total of 100 μg of proteins were processed using iTRAQ® reagents application kit (Applied Biosystems). Briefly, proteins were digested with trypsin solution at 37 °C overnight and labeled with the iTRAQ tags as follows: untreated, iTRAQ114.1; 6-h sample, iTRAQ115.1, and 24-h sample, iTRAQ 116.1. The labeled samples were mixed and fractionated using strong cation exchange chromatography using Macrospin SCX columns (Nest Group, Inc., Southborough, MA). Fractions were eluted using stepwise salt plugs of 12 different molar concentrations of 100–800 mm sodium chloride. Each fraction was desalted using reverse phase C18 spin columns (Nest Group, Inc.), as per the manufacturer's protocol, and evaporated to dryness.
Nano-liquid Chromatography-MS/MS Analysis
The analysis was performed on a TEMPO MDLC nanoflow liquid chromatography system interfaced with a QSTAR® Elite mass spectrometer (Applied Biosystems). The samples were reconstituted in Solvent A (98% acetonitrile, 2% water, and 0.1% formic acid). The nanoflow high pressure liquid chromatography system was used to deliver a flow rate of 300 nl/min. Chromatographic separation was accomplished by loading peptide samples onto a Vydac MS 300 C18 column (Alltech). Sequential elution of peptides was accomplished using a linear gradient from 5% Solvent A to 60% Solvent B (98% acetonitrile, 2% water, and 0.1% formic acid) in 150 min. The mass spectrometer was operated in positive ion mode with a resolution of 10,000–12,000 at full width half-maximum for the QSTAR Elite using a source temperature of 200 °C. Altogether, 12 runs were performed to finish one experiment. For MS/MS analysis, survey scans were acquired from m/z 300 to 1500 with up to two precursors selected for MS/MS from m/z 100 to 2000 using dynamic exclusion, and the rolling collision energy was used to promote fragmentation. To gain statistical evidence for differential expression of proteins, another two separate experiments were performed as described above.
iTRAQ Data Analysis
Relative abundance quantitation and peptide and protein identification were performed using Protein Pilot software 2.0 (Applied Biosystems). Each MS/MS spectrum was searched for species of Homo sapiens against the NCBI data base. The searches were run using the following parameters: fixed modification of methylmethanethiosulfate-labeled cysteine; fixed iTRAQ modification of free amine in the amino terminus and lysine; variable iTRAQ modifications of tyrosine; and allowing serine and threonine residues to undergo side reaction with the iTRAQ reagent. Relative quantification of proteins in the case of iTRAQ was performed on the MS/MS scans and the ratio of the areas under the peaks at 114.1, 115.1, and 116.1 Da, which were the masses of the tags that correspond to the iTRAQ reagents. The relative amount of a peptide in each sample was calculated by dividing the peak areas observed at 115.1 and 116.1 m/z by that observed at 114.1 m/z. The following criteria were required to consider a protein for further statistical analysis: two or more high confidence (95%) unique peptides had to be identified; the p value in the protein quantitation had to be p = 0.05; and the fold difference had to be greater than 1.2. The peptides without any modification of free amine in the amino terminus or without iTRAQ modification of free amine in the lysine were excluded from calculation of the protein ratios.
RESULTS
Both α- and β-Tubulins Are Depleted by ITC Treatment in a Variety of Cancer Cells
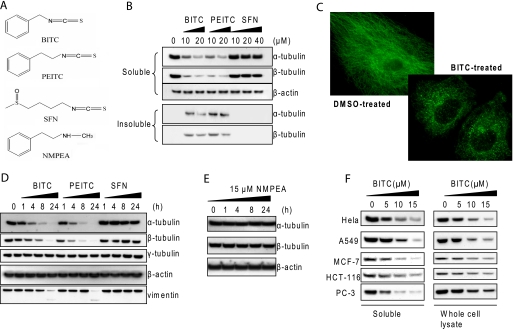
The structures of ITCs and an analog are shown in Fig. 1A. Previously, we showed that both α- and β-tubulins aggregated in human non-small lung cancer A549 cells treated with BITC and PEITC (14). Similar results were observed in BITC- and PEITC-treated HeLa cells, revealing a rapid decrease of both α- and β-tubulins in the (non-ionic detergent) soluble fraction of the cell lysate (Fig. 1B), whereas both α- and β-tubulins increased in the insoluble fraction. The aggregation of tubulin after ITC treatment was verified by using multiple lysis buffers containing different non-ionic detergents, including Nonidet P-40, Triton X-100, and Tween 20. The consistent immunoblot results were obtained using multiple antibodies that recognize various epitopes of α- and β-tubulin sequences. It is noted that none of the epitopes recognized by these antibodies contains cysteine residue, which may be modified by ITCs. The changes of α- and β-tubulin levels are comparable. Among the ITCs, BITC and PEITC at 10 μm were able to induce aggregation of most of the tubulin within 4 h; however, SFN failed to do so even at 40 μm. The potency of inducing tubulin aggregation followed the order BITC ≈ PEITC > SFN. Tubulin aggregation induced by ITCs was also observed by immunofluorescence microscopy. Fig. 1C shows that microtubules collapsed and tubulin formed isolated punctates in cytoplasm shortly (1 h) after BITC treatment (5 μm). The results also indicate that α- and β-tubulins did not translocate into the nucleus.
FIGURE 1.
ITC treatment induces tubulin aggregation and depletion. A, structures of BITC, PEITC, SFN, and NMPEA, a structural analog of PEITC. B, tubulins aggregate in cells treated with ITCs. HeLa cells were treated with various concentrations of BITC, PEITC, and SFN for 4 h. The soluble and insoluble fractions were extracted and immunoblotted for α- and β-tubulins. C, microtubules collapse and tubulin forms aggregates. HeLa cells were treated with dimethyl sulfoxide (DMSO, left) as vehicle control and 5 μm BITC (right) for 1 h; α-tubulin was immunostained in green. D, levels of other cytoskeleton proteins are unaffected after ITC treatment. HeLa cells were treated with 10 μm BITC, PEITC, and SFN individually for up to 24 h. The whole cell lysate was extracted and immunoblotted for α-, β-, and γ-tubulins, as well as β-actin and vimentin. E, NMPEA has no effect on tubulin stability. HeLa cells were treated with 15 μm NMPEA for up to 24 h. The whole cell lysate was extracted and immunoblotted for α- and β-tubulins. F, tubulin aggregation and depletion are observed in a variety of human cell lines. HeLa, A549, MCF-7, HCT-116, and PC-3 cells were treated with 0, 5, 10, and 15 μm BITC for 8 h. Both the soluble fraction and the whole cell lysate were extracted and blotted for α-tubulin.
Next, we studied the overall tubulin protein levels in the whole cell lysate under the same treatment conditions. Both α- and β-tubulin levels showed a time-dependent decrease from treatments with BITC and PEITC but not with SFN (Fig. 1D). The decrease in tubulin levels in the whole cell lysate induced by ITCs also followed the order of BITC ≈ PEITC > SFN. In contrast to ITCs, NMPEA, a structural analog of PEITC without the ITC functionality, did not induce tubulin depletion under similar conditions (Fig. 1E), suggesting that the ITC functional group is essential, and the structure of the side chain moiety could dictate the potency. We found that the tubulin aggregation and depletion occurred not only in cervical cancer HeLa cells but also in a variety of other human cancer cells, including lung (A549), breast (MCF-7), colon (HCT-116), and prostate cancer cell lines (PC-3) (Fig. 1F).
ITC-induced Tubulin Depletion Is Highly Selective in Cellular Proteome
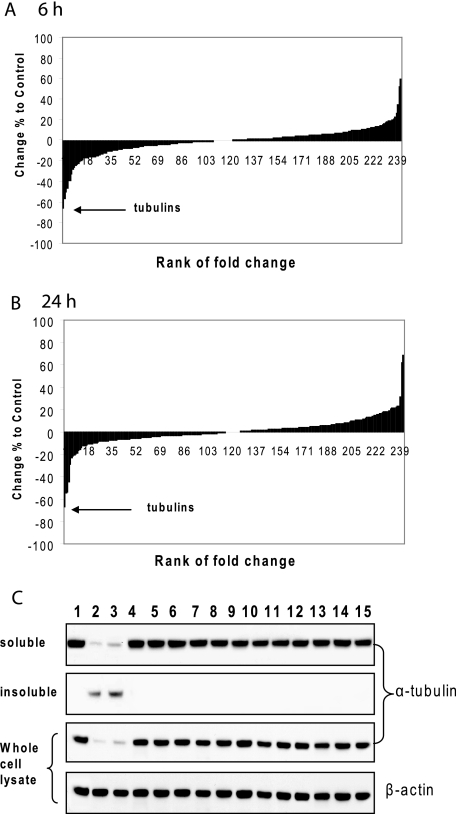
In contrast to level changes in α- and β-tubulins, the levels of other cytoskeleton proteins, such as γ-tubulin, actin, and vimentin, were unchanged under the same conditions. Vimentin showed cleavage after 24 h of ITC treatment (Fig. 1D). However, this is likely because of caspase cleavage as a result of apoptosis induced by ITCs (20), because the cleavage was completely blocked by pretreatment with Z-VAD-fmk, a pan-caspase inhibitor (supplemental Fig. 1). To further study whether ITC-induced tubulin degradation was selective, we employed a quantitative proteomic technique called iTRAQ, which labels cellular proteins of untreated control and treated cells with multiplexed, amine-specific, stable isotope reagents that enable simultaneous protein identification and expression level quantitation (19). A total of 242 and 335 proteins were identified from the cytoplasmic and nuclear fractions of human lung cancer H460 cells, respectively. In the cytoplasmic fraction, over 72 and 76% of proteins in the 6- and 24-h samples had the expression level changes within ±10% compared with untreated control, indicating that a majority of proteins were stable in expression level after PEITC treatment (Fig. 2, A and B). However, all identified α- and β-tubulin isoforms were found to have the most level decreases (Table 1). The extremely small p values (Table 1, column 3) indicate a high statistical significance in the protein quantitation. As a comparison, two isoforms of actin, also listed in Table 1, were much more stable. The results from both 6- and 24-h treatments indicated that tubulin level decreased rapidly, and the effects lasted for a long time. It should be noted that no tubulin was identified in the nuclear fraction confirming the previous observation that there was no tubulin translocation to the nucleus after ITC treatment. Taken together, it seems that ITC-induced tubulin depletion was highly selective among cellular proteins. Similar results were obtained by the same assay on H596, another non-small lung cancer cell line (data not shown).
FIGURE 2.
A and B, tubulins are selectively degraded in cells treated with PEITC. Proteomic quantitation was performed using iTRAQ on the cytoplasmic fraction of H460 cells after 6 h (A) and 24 h (B) of treatment with 15 μm PEITC. All α- and β-tubulin isoforms were identified among proteins with the most decreased levels. All 242 identified proteins were aligned according to the percentage of level change relative to the untreated control. A majority of proteins had a level change within ±10% in both time points. The arrows point to the identified tubulin isoforms. C, BITC and PEITC are unique in inducing cellular tubulin level decrease among 14 thiol-reactive compounds. After HeLa cells were treated with compounds at 10 μm for 8 h, the soluble fraction, insoluble fraction, and the whole cell lysate were extracted, purified, and immunoblotted for α-tubulin. Lanes 1–15 correspond to cells treated with DMSO, BITC, PEITC, SFN, arsenic trioxide, hydroquinone, cadmium chloride, tert-butylhydroquinone, menadione, cumene hydroperoxide, β-naphthoflavone, 1-nitro-1-cyclohexene, 1,2-ethanedithiol, 4-hydroxynonenal, and acrolein.
TABLE 1.
List of top five proteins with the most decreased levels in the cytoplasmic fraction of H460 cells after 6 and 24 h of treatment with 15 μm PEITC
Two isoforms of actin are also listed for comparison. The percentage of level change relative to the untreated control was ranked as indicated in Fig. 2, A and B. The p value was included to indicate the statistical significance of the protein quantitation. The unused value is confidence measurement of protein identification where a value of 1 indicates 90% confidence, and a value of >2 indicates 100% confidence. Sequence coverage was calculated by dividing the number of amino acids observed by the protein amino acid length.
| Level change ranking | Change in % of control | p value for quantitation | Protein name | NCBI accession number | Unused value | Sequence coverage |
|---|---|---|---|---|---|---|
| % | ||||||
| 6 h of treatment | ||||||
| 1 | −65.763 | 0.000494 | Tubulin β chain | P07437 | 13.08 | 79.7 |
| 2 | −56.687 | 0.013724 | Tubulin β-2C chain | P68371 | 1.73 | 78.8 |
| 3 | −49.941 | 9.88E-11 | Tubulin α-1B chain | P68363 | 18.14 | 66.5 |
| 4 | −46.716 | 0.084579 | Neuroblastoma breakpoint family member 3 | Q9H094 | 1.11 | 21.8 |
| 5 | −39.480 | 0.047385 | Tubulin α-1C chain | Q9BQE3 | 1.44 | 65.0 |
| 168 | 3.8304 | 0.188479 | Actin, cytoplasmic 1 | P60709 | 43.98 | 87.7 |
| 192 | 6.2065 | 0.136969 | Actin, cytoplasmic 2 | P63261 | 2.22 | 83.3 |
| 24 h of treatment | ||||||
| 1 | −67.135 | 0.000698 | Tubulin β chain | P07437 | 13.08 | 79.7 |
| 2 | −54.784 | 4.96E-11 | Tubulin α-1B chain | P68363 | 18.14 | 66.5 |
| 3 | −53.853 | 0.041881 | Tubulin β-2C chain | P68371 | 1.73 | 78.8 |
| 4 | −44.660 | 0.025489 | Tubulin α-1C chain | Q9BQE3 | 1.44 | 65.0 |
| 5 | −28.895 | 0.462542 | Importin-4 | Q8TEX9 | 2.09 | 23.9 |
| 69 | −4.1809 | 0.010398 | Actin, cytoplasmic 1 | P60709 | 43.98 | 87.7 |
| 170 | 5.0402 | 0.148074 | Actin, cytoplasmic 2 | P63261 | 2.22 | 83.3 |
BITC and PEITC Are Unique in Inducing Tubulin Depletion among a Variety of Thiol-reactive Compounds
To study the specificity of ITCs in inducing tubulin depletion, a total of 14 thiol-reactive compounds were used to treat HeLa cells at 10 μm. They were BITC, PEITC, SFN, arsenic trioxide, hydroquinone, cadmium chloride, tert-butylhydroquinone, menadione, cumene hydroperoxide, β-naphthoflavone, 1-nitro-1-cyclohexene, 1,2-ethanedithiol, 4-hydroxynonenal, and acrolein. Tubulin levels in the soluble and insoluble fractions and the whole cell lysate were checked after 8 h of treatment. Results in Fig. 2C indicate that only BITC and PEITC induced tubulin aggregation and depletion.
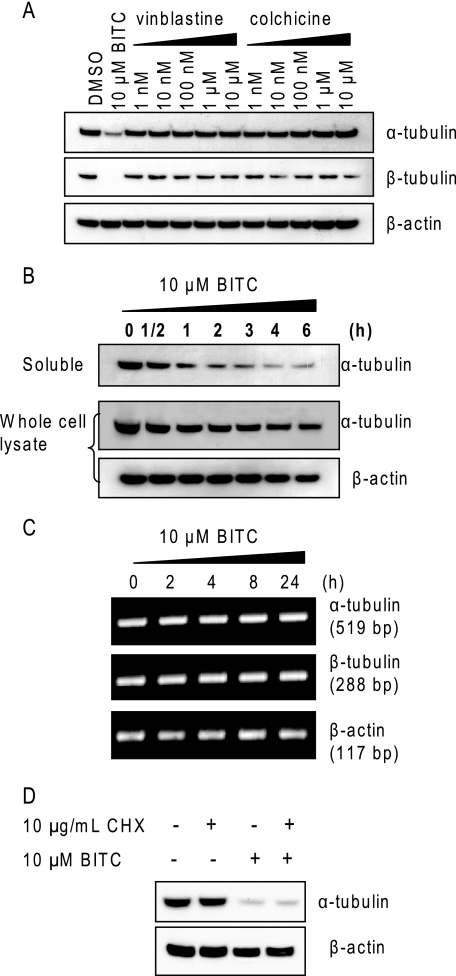
ITC-induced Tubulin Depletion Is Initiated by Aggregation and Followed by Protein Degradation
We reported previously (14) that ITCs are inhibitors of tubulin polymerization in vivo and in vitro (Fig. 1C). Therefore, the next question we asked was whether the tubulin level decrease is caused by the auto-regulation mechanism (16–18). To test this, we treated cells with two potent microtubule depolymerizers colchicine and vinblastine individually at their effective concentrations for up to 24 h. The results (Fig. 3A) show that colchicine and vinblastine alone did not have any substantial effects on tubulin levels, indicating that microtubule depolymerization may not cause tubulin depletion and suggesting that the ITC-induced tubulin depletion may not be mediated through the auto-regulation mechanism. To study whether the tubulin depletion is reversible, HeLa cells were initially treated with 10 μm BITC before culture medium containing BITC was completely replaced and cells were incubated with fresh medium for up to 6 h. The results (Fig. 3B) show that the onset of tubulin decrease in the soluble fraction was facile, observed only 30 min after ITC treatment, and the effect intensified as the treatment time prolonged, indicating that decrease of tubulin was an irreversible process and was initiated by tubulin aggregation. The rapid kinetics suggests that tubulin level changes may be due to post-synthetic modification. To confirm that, we examined if the tubulin transcript levels were affected by ITC treatment. The results of 25 cycles of RT-PCR (Fig. 3C) show that the transcript levels of both α- and β-tubulins in HeLa cells were not affected by BITC treatment, suggesting that the decrease in tubulin levels was caused by protein degradation. The conclusion that tubulin transcripts were unchanged by BITC treatment was confirmed by results from other RT-PCR cycle numbers (20 and 30) (data not shown). Further study (Fig. 3D) shows that BITC-induced tubulin degradation occurred with or without treatment with CHX, a protein translation inhibitor, indicating that the tubulin degradation is independent of protein translation and does not require de novo synthesis. Tubulin stability in the absence of BITC was confirmed by the fact that its level remains constant even after treatment with CHX for 8 h (Fig. 3D). Taken together, we conclude that a tubulin level decrease in the whole cell lysate is likely due to protein degradation.
FIGURE 3.
Tubulin degradation is induced by ITCs at post-synthesis level. A, tubulin-binding agents have no effects on tubulin levels in cells. HeLa cells were treated with 1, 10, and 100 nm and 1 and 10 μm vinblastine or colchicine for 8 h. The whole cell lysate was extracted and blotted for α- and β-tubulins. B, ITC-induced tubulin degradation is initiated by aggregation and is irreversible. HeLa cells were treated with 10 μm BITC for ½ and 1–4 h before medium containing BITC was replaced, and cells were grown in fresh medium up to 6 h. The soluble fraction and the whole cell lysate were extracted and blotted for α-tubulin. C, ITC treatment does not alter tubulin transcripts. HeLa cells were treated with 10 μm BITC for 2, 4, 8, and 24 h. Total RNA was isolated and used as template for one-step RT-PCR of both α- and β-tubulins. PCR products after 25 cycles were visualized on a 1.5% agarose gel containing ethidium bromide. The RT-PCR product of β-actin transcripts was used as an internal control. D, ITC-induced tubulin degradation is independent of protein translation and does not require de novo synthesis. HeLa cells were treated with 10 μg/ml cycloheximide (CHX) and/or 10 μm BITC for 8 h. The whole cell lysate was extracted and blotted for α-tubulin.
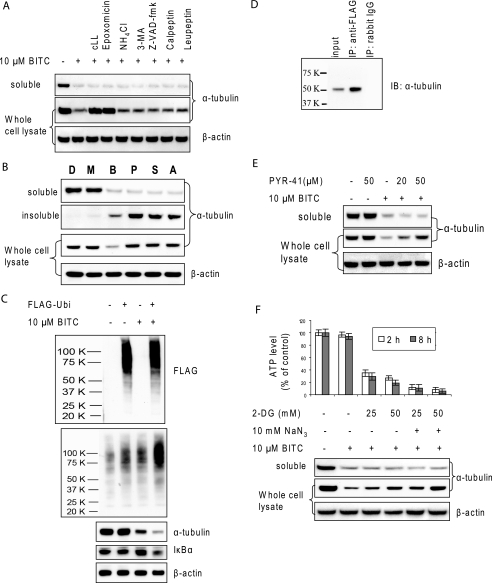
ITC-induced Tubulin Degradation Is Proteasome-dependent
To examine the proteolytic pathway of ITC-induced tubulin degradation, we pretreated HeLa cells with a variety of inhibitors, including proteasome inhibitors, clasto-lactacystin β-lactone and epoxomicin, lysosome inhibitor NH4Cl, autophagy inhibitor 3-methyladenine, pan-caspase inhibitor Z-VAD-fmk, calpain inhibitor calpeptin, and protease inhibitor leupeptin. The results (Fig. 4A) showed that only proteasome inhibitors completely blocked tubulin degradation in the whole cell lysate, whereas other compounds had little or no effect, indicating the specificity of proteasome as the pathway for tubulin degradation. However, it should also be noted that all inhibitors were unable to block tubulin aggregation as indicated by the tubulin level in the soluble fraction, once again suggesting that the aggregation is a separate process and may happen prior to degradation. Other protease inhibitors, including antipain, aprotinin, chymostatin, E-64, and lysosome inhibitors pepstatin A and chloroquine, were also tested and had no effect on either ITC-induced tubulin aggregation or degradation (data not shown).
FIGURE 4.
ITC-induced tubulin degradation is ubiquitin-proteasome-dependent. A, pretreatment of proteasome inhibitors blocks ITC-induced degradation. HeLa cells were treated with 10 μm BITC for 6 h with and without 1 h of pretreatment of 10 μm clasto-lactacystin β-lactone (cLL), 10 μm epoxomicin, 20 mm NH4Cl, 5 mm 3-methyladenine, 100 μm Z-VAD-fmk, 50 μm calpeptin, and 50 μm leupeptin, respectively. Both the soluble fraction and whole cell lysate were extracted and blotted for α-tubulin. B, pre-, co-, and post-treatment with MG132 blocks ITC-induced tubulin degradation but not aggregation. HeLa cells were treated with DMSO (D), 10 μm MG132 (M), 10 μm BITC (B), 10 μm MG132 30 min prior to addition of 10 μm BITC (P), 10 μm MG132 simultaneously with 10 μm BITC (S), and 10 μm MG132 30 min after addition of 10 μm BITC (A). After 6 h of treatment with BITC, the soluble fraction, insoluble fraction, and the whole cell lysate were extracted and blotted for α-tubulin. C, overexpression of ubiquitin promotes ITC-induced tubulin degradation. HeLa cells were transiently transfected with 1 μg of either pcDNA3 vector or pcDNA3-FLAG-ubiquitin for 20 h before treatment with either DMSO or 10 μm BITC for 6 h. The whole cell lysate was extracted and blotted for FLAG tag, ubiquitinated (Ubi) proteins, α-tubulin, IκBα, and β-actin. D, immunoprecipitated tubulin is ubiquitinated. HeLa cells were transfected with FLAG-ubiquitin for 24 h and then treated with 10 μm BITC for 1 h. IP products using anti-FLAG were blotted with for α-tubulin (middle lane). Positive control was the whole cell lysate before IP (left lane). Negative control was the whole cell lysate IPed with rabbit IgG (right lane). IB, immunoblot. E, inhibitor for ubiquitin-activating enzyme E1 blocks ITC-induced tubulin degradation but not aggregation. HeLa cells were pretreated with 20 or 50 μm PYR-41 before treatment with 10 μm BITC for 6 h. Both the soluble fraction and whole cell lysate were extracted and blotted for α-tubulin. F, ATP depletion blocks ITC-induced tubulin degradation but not aggregation. HeLa cells were pretreated with 2-DG and/or 10 mm NaN3 for 2 h before treatment with 10 μm BITC for another 6 h. ATP levels were measured before (empty bar) and after (solid bar) BITC treatment. The whole cell lysate was used for immunoblots for α-tubulin.
To further study the mechanism of ITC-induced tubulin degradation, we pre-, co-, and post-treated cells with proteasome inhibitor MG132 relative to the treatment with BITC. Tubulin aggregated in all cells treated with BITC with or without the presence of MG132. However, the presence of MG132 blocked ITC-induced tubulin degradation in the whole cell lysate and specifically in the insoluble fraction (Fig. 4B). Interestingly, the sample treated with MG132 30 min following the addition of BITC had less tubulin in the insoluble fraction, suggesting that the degradation was a rapid process. These results confirmed that ITC-induced tubulin degradation is proteasome-dependent.
ITC-induced Tubulin Degradation Is Ubiquitination- dependent
To study whether the ubiquitination pathway is involved in ITC-induced tubulin degradation, we transiently transfected control vector or a plasmid coding for FLAG-tagged ubiquitin into HeLa cells before BITC treatment (21). The immunoblots against FLAG tag (Fig. 4C) indicate that the transfection was successful, and the immunoblots against ubiquitinated proteins indicate that the transfection enhanced overall ubiquitination in cells. Cells with overexpressed ubiquitin had a faster ITC-induced tubulin degradation compared with control cells, suggesting that the degradation may involve ubiquitination. In contrast, overexpression of ubiquitin in the absence of BITC did not have any effect on tubulin levels. As a control, the level of IκBα, a known ubiquitin-proteasome dependent protein, also decreased in cells with overexpressed ubiquitin. Furthermore, tubulin was blotted in anti-FLAG immunoprecipitates, suggesting that tubulin was ubiquitinated (Fig. 4D). As a control, no tubulin band was observed in the IP products using rabbit IgG.
To further confirm the involvement of ubiquitination in ITC-induced tubulin degradation, we pretreated cells with PYR-41, a specific inhibitor for ubiquitin-activating enzyme E1 (22). The effects of PYR-41 were confirmed by the evidence that it blocked accumulation of protein ubiquitination in response to the proteasome inhibitor MG132 over a period of 1 h, even though it induced little decrease in the basal level (supplemental Fig. 2). Results (Fig. 4E) show that the pretreatment of PYR-41 almost completely blocked ITC-induced tubulin degradation and the blockage was dose-dependent, suggesting again that ITC-induced tubulin degradation is through the ubiquitination-dependent pathway. However, the presence of PYR-41 did not block tubulin aggregation, suggesting that the tubulin aggregation may be unrelated to the ubiquitination. It is noted that PYR-41 treatment alone had no effect on tubulin stability.
ITC-induced Tubulin Degradation Is ATP-dependent
The majority of damaged proteins in cells are degraded by 26 S proteasome through a ubiquitination- and ATP-dependent pathway (23–25). To examine whether ITC-induced tubulin degradation is ATP-dependent, we pretreated cells with 2-DG (a cellular ATP depletion agent) and/or sodium azide, a mitochondrial respiratory chain blocker, for 2 h before treatment with BITC. The results (Fig. 4F) indicate the following: BITC treatment for up to 8 h did not induce a substantial change of the ATP level. However, treatment with 2-DG and/or sodium azide significantly reduced the ATP level within 2 h. These pretreatments were all effective in blocking ITC-induced tubulin degradation, but not tubulin aggregation, suggesting that ITC-induced tubulin degradation, but not aggregation, is ATP-dependent. The reason that the blocking was not complete was probably because of incomplete ATP depletion.
ITC-induced Tubulin Degradation Is Independent of Oxidative Stress
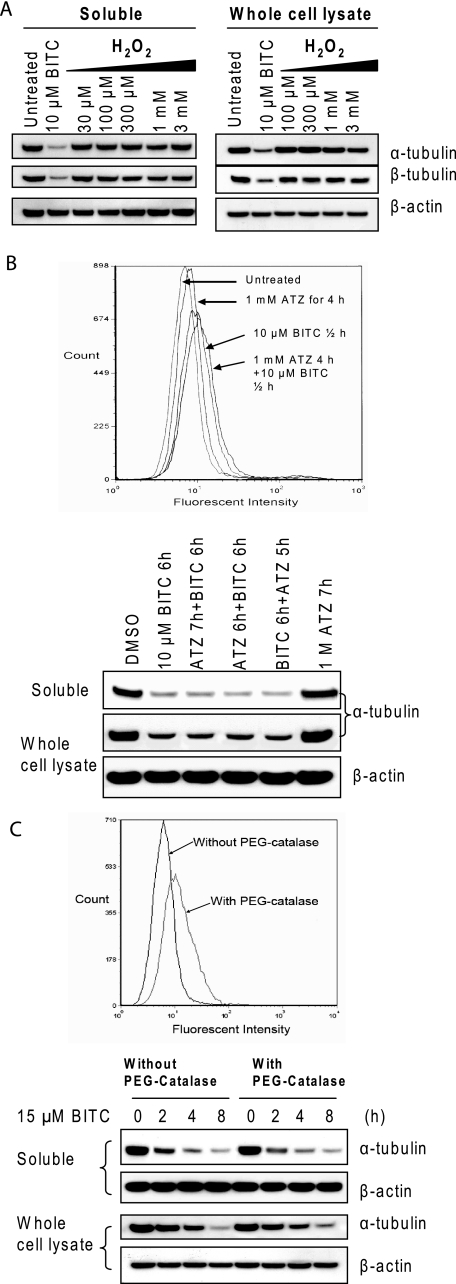
Because ITCs are known to induce ROS in cells (6–12), we asked the question whether oxidative stress is involved in tubulin degradation. HeLa cells were treated with H2O2 at a series of concentrations for 4 h. Results (Fig. 5A) show that treatments with up to 3 mm H2O2 failed to induce changes in tubulin level in either the soluble fraction or the whole cell lysate. In a separate experiment, we found that the peak levels of both superoxide radical and hydrogen peroxide generated in HeLa and A549 cells treated with 10 μm BITC or PEITC were much lower than those treated with 100 μm H2O2 (data not shown). Therefore, the results suggest that oxidative stress may not be responsible for ITC-induced tubulin aggregation and degradation. Next, we treated cells with ATZ, a known catalase-specific inhibitor. The results (Fig. 5B) show that the presence of ATZ had no effect on BITC-induced tubulin aggregation and degradation, even though it was effective in inducing endogenous oxidative stress. Furthermore, pretreating cells with cell-permeable PEG-catalase had no effect on tubulin aggregation and degradation, even though it is effective in relieving the oxidative stress induced by BITC (Fig. 5C). Because neither up- nor down-regulation of oxidative stress had an effect on ITC-induced tubulin aggregation and degradation, we conclude that ITC-induced tubulin aggregation and degradation may be irrelevant to oxidative stress.
FIGURE 5.
ITC-induced tubulin degradation is independent of oxidative stress. A, treatment with hydrogen peroxide does not induce tubulin aggregation and degradation. HeLa cells were treated with 100 and 300 μm and 1 and 3 mm H2O2 for 4 h. Both the soluble fraction and whole cell lysate were extracted and blotted for α- and β-tubulin. B, endogenously induced oxidative stress has no effects on tubulin stability. HeLa cells were treated with 10 μm BITC for 6 h, 1 mm ATZ (a catalase-specific inhibitor) for 7 h, 1 mm ATZ first for 1 h and followed by 10 μm BITC for another 6 h, both 1 mm ATZ and 10 μm BITC simultaneously for 6 h, and 10 μm BITC first for 1 h and followed by 1 mm ATZ for another 5 h. C, scavenging of ROS by cell-permeable catalase has no effect on tubulin stability. HeLa cells were treated with 15 μm BITC for 2, 4, and 8 h with or without pretreatment with PEG-catalase (500 units) for 2 h. Both the soluble fraction and whole cell lysate were extracted and blotted for α-tubulin.
ITC-induced Tubulin Degradation Is Triggered by ITC Covalent Binding
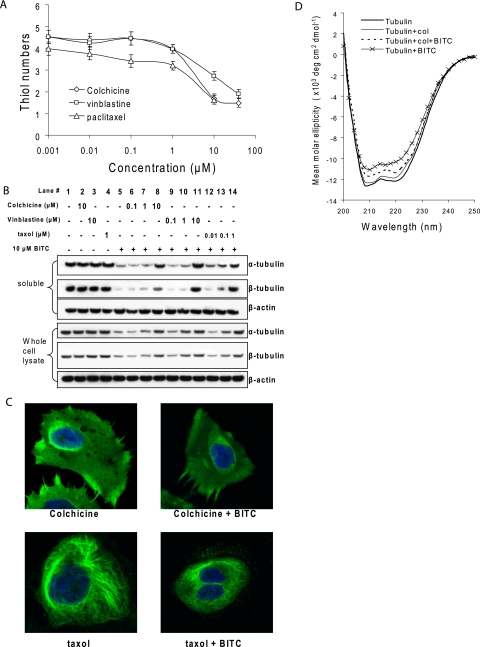
Colchicine, vinblastine, and taxol bind to the β-tubulin at various sites causing either disrupted (colchicine and vinblastine) or strengthened (taxol) microtubules (3, 4, 15). To study whether the tubulin-binding agents affect ITC binding, we pretreated purified tubulin with colchicine, vinblastine, and taxol before BITC treatment. The Ellman assay (Fig. 6A) shows that all three compounds significantly reduced BITC binding levels. For example, the number of tubulin cysteines modified by BITC (at the molar ratio of BITC:cysteine = 1:2) was reduced from 5.1 ± 0.4 (BITC treatment only) to 1.4 ± 0.2, 1.8 ± 0.2, and 1.7 ± 0.3 by the pretreatment of 10 μm colchicine and vinblastine and 1 μm taxol, respectively. To study the effects of blocking ITC binding on tubulin stability, we pretreated cells with colchicine, vinblastine, and taxol individually before treatment with BITC. The results (Fig. 6B) show that although these compounds alone had no effect on tubulin levels, at concentrations of 10 μm for colchicine or vinblastine and 1 μm for taxol, they effectively blocked BITC-induced tubulin aggregation and degradation. Confirming these results, the immunofluorescent staining assay (Fig. 6C) shows that the microtubules collapsed after colchicine treatment and were reinforced after taxol treatment; however, pretreatment with either colchicine or taxol completely blocked the formation of tubulin aggregates by BITC as shown in Fig. 1C. Together, these results suggest that ITC binding to tubulin may be an important early event for tubulin aggregation and degradation.
FIGURE 6.
Pretreatment with tubulin-binding agents blocks BITC binding to tubulin, BITC-induced tubulin conformational changes, and BITC-induced tubulin aggregation and degradation. A, tubulin-binding agents block ITC binding to tubulin. Purified tubulin (8 μm) was pretreated with colchicine, vinblastine at 1 and 10 nm and 1, 10, and 40 μm, and taxol at 1 and 100 nm and 1 μm and 10 μm at room temperature for 15 min before being treated with 40 μm BITC (the stoichiometric ratio of BITC to thiol is 1:2) for an additional 30 min. The number of thiols was measured by Ellman assay as described under “Materials and Methods.” The data were obtained from triplicate experiments. B, tubulin-binding agents block tubulin aggregation and degradation. HeLa cells were treated with the following: 10 μm colchicine (lane 2) for 7 h; 10 μm vinblastine (lane 3) for 7 h; 1 μm taxol (lane 4) for 7 h; 10 μm BITC (lane 5) for 6 h; 0.1 μm colchicine for 1 h followed by 10 μm BITC for 6 h (lane 6); 1 μm colchicine for 1 h followed by 10 μm BITC for 6 h (lane 7); 10 μm colchicine for 1 h followed by 10 μm BITC for 6 h (lane 8); 0.1 μm vinblastine for 1 h followed by 10 μm BITC for 6 h (lane 9); 1 μm vinblastine for 1 h followed by 10 μm BITC for 6 h (lane 10); 10 μm vinblastine for 1 h followed by 10 μm BITC for 6 h (lane 11); 10 nm taxol for 1 h followed by 10 μm BITC for 6 h (lane 12); 100 nm taxol for 1 h followed by 10 μm BITC for 6 h (lane 13); and 1 μm taxol for 1 h followed by 10 μm BITC for 6 h (lane 14). Both the soluble fraction and whole cell lysate were extracted and blotted for α- and β-tubulins. C, tubulin-binding agents block tubulin aggregate formation. HeLa cells were treated with 5 μm colchicine or taxol individually (left) or first with 5 μm colchicine or taxol for 30 min followed by 5 μm BITC for 2 h. Tubulin was immunostained in green and the nucleus in blue by 4′,6-diamidino-2-phenylindole. D, tubulin-binding agents block tubulin conformational changes. Purified tubulin (0.8 μm) was either incubated at room temperature for 45 min (bold solid line), or treated with 0.8 μm colchicine at room temperature for 45 min (thin solid line), or pretreated with 0.8 μm colchicine for 15 min followed by 8 μm BITC (stoichiometric ratio of BITC to thiol is 1:1) for another 30 min (dotted line), or treated with 8 μm BITC for 30 min (line with cross signs) before ellipticity was determined by CD under conditions described under the “Materials and Methods.”
ITCs can covalently bind to tubulin in vitro and in vivo via cysteines causing tubulin conformation changes (14). We studied whether pretreatment with a tubulin-binding agent has any effect on the structural stability of tubulin. The CD results (Fig. 6D) show that pretreatment with colchicine prevented tubulin ellipticity decay by BITC. Because purified tubulin is only stable for a few hours at room temperature, it is difficult to measure the equilibrium state of BITC-induced tubulin denaturation. Therefore, we measured conformational changes only 30 min after ITC treatment. During this time, thermal denaturation is negligible, whereas both the ITC-induced denaturation and the protective effects of colchicine against ITC-induced denaturations were demonstrated. Taken together, it seems that there is a cause-effect relationship in ITC binding to tubulin, ITC-induced tubulin conformation changes, and ITC-induced tubulin aggregation and degradation.
ITC-induced Tubulin Degradation Is Associated with G2/M Phase Arrest and Apoptosis Induction
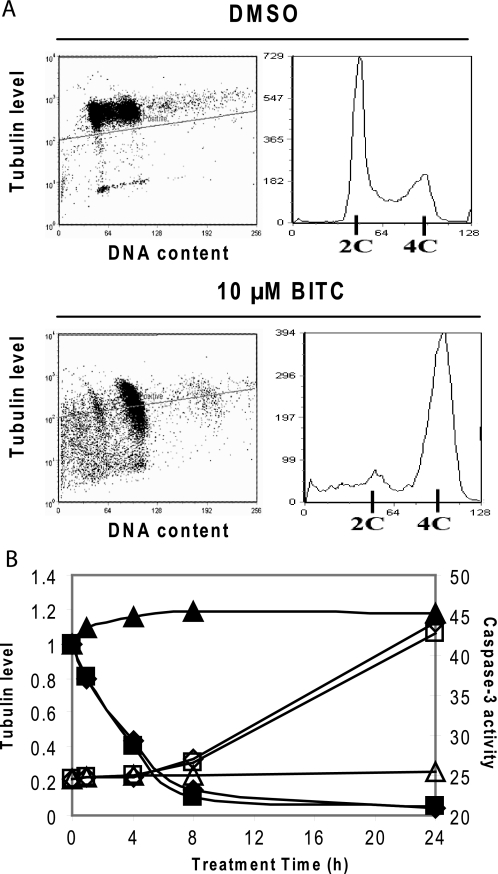
To study whether there is a relationship between ITC-induced tubulin degradation and cell growth inhibition, we treated cells with 10 μm BITC for 20 h. Cells were fixed, stained for both α-tubulin and DNA content, and sorted by flow cytometry. Fig. 7A (right panel) shows that G2/M and sub-G1 populations were significantly increased after BITC treatment, indicating G2/M phase arrest and apoptosis induction. Fluorescence intensity of immunostained tubulin (Fig. 7A, left panel) was greatly reduced in BITC-treated cells, indicating a depleted tubulin level. The correlation between changes of tubulin level and cell cycle distribution suggests that tubulin degradation may be involved in cell cycle arrest and apoptosis.
FIGURE 7.
Tubulin degradation correlates with ITC-induced G2/M phase arrest and apoptosis. A, decreased tubulin level is associated with increased G2/M and sub-G1 population. HeLa cells were treated with either DMSO or 10 μm BITC for 20 h before cells were fixed by 80% ethanol, immunostained for α-tubulin and DNA content, and sorted by flow cytometry. Left panel, DNA content is indicated by the x axis and immunofluorescence of α-tubulin is indicated by the y axis. Right panel, cell cycle distributions of the same samples according to DNA contents. B, correlation between kinetics of tubulin degradation and caspase-3 activation by ITC treatment. The immunoblots of α-tubulin in Fig. 1D was analyzed by ImageJ software. The tubulin band intensity (indicated by the left y axis and solid symbols) is plotted together with caspase-3 activity (indicated by the right y axis and open symbols) measured at various time points of ITC treatment. ♦ and ◇ indicate BITC treatment, ■ and □ indicate PEITC treatment, and ▴ and Δ indicate SFN treatment.
Furthermore, the depletion of tubulin in the whole cell lysate kinetically correlated with the induction of caspase-3 activity (Fig. 7B), as the time courses of tubulin degradation and caspase-3 activation were closely related. The induction of caspase-3 occurred right after the majority of tubulin was depleted. Furthermore, BITC and PEITC were stronger inducers of tubulin degradation than SFN, and both were also more effective in inducing caspase-3 activity than SFN (14).
DISCUSSION
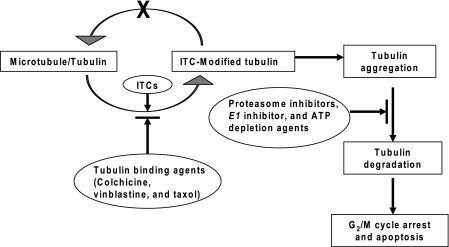
Previously, we have shown that binding to intracellular proteins by ITCs is a predominant event that correlates with their apoptosis activities (13). We have further demonstrated in human lung cancer A549 cells that tubulin is a potential protein target of ITCs by two-dimensional gel electrophoresis/MS, and BITC forms a cysteine adduct with tubulin in vivo (14). Moreover, we found that BITC and PEITC bind tubulin with high affinities. They are also potent in inducing tubulin conformational changes, disrupting microtubule networks, and eventually causing growth inhibition and apoptosis. In contrast, SFN and NMPEA, having little or no binding affinity to tubulin, are both devoid of much of the activities. The present study shows that BITC and PEITC can selectively induce aggregation and degradation of both α- and β-tubulins in a variety of cancer cells. Binding of tubulin to ITCs is potentially a triggering event. The potency of inducing tubulin degradation follows the order BITC ≈ PEITC > SFN, which correlates with their tubulin-binding affinities. Based on the in vitro and in vivo evidence, we propose a scheme of ITC-induced tubulin degradation (Scheme 1). Once inside cells, ITCs rapidly modify α- and β-tubulins in cytoplasm through covalent binding to key cysteines. The modification by ITCs triggers tubulin conformational changes, misfolding, and aggregate formation. The tubulin aggregates are further degraded by the ubiquitination-proteasome system. The tubulin-binding agents, including colchicine, vinblastine, and taxol, significantly reduced the BITC binding affinity to tubulin, prevented BITC-induced conformational changes, and blocked tubulin aggregation and degradation. In contrast, proteasome inhibitors, ubiquitin-activating enzyme E1 inhibitor, and ATP depletion agents can block ITC-induced tubulin degradation but not aggregation. This also suggests that aggregation and degradation are two independent but related events. ITC-induced tubulin degradation is likely indifferent of oxidative stress. It should be pointed out that similar results were obtained using both HeLa and A549 cells (not shown), indicating that the mechanism proposed here may not be cell type-specific. The binding-triggered tubulin degradation by small molecules is novel and could serve as a paradigm of future studies.
SCHEME 1.
Proposed mechanism of ITC-induced tubulin degradation.
Colchicine and vinblastine are known to disassemble the microtubules to α-β-tubulin dimer, whereas taxol is able to reverse the process and strengthen microtubules (4). The observation that both types of tubulin-binding agents significantly reduced the affinity of BITC binding to tubulin suggests that ITCs are able to bind to both α-β-tubulin dimer as well as microtubules. It is possible that binding to tubulin by colchicine, vinblastine, and taxol make the α-β-tubulin global structure rigid so that it prevents ITC binding. In fact, binding of these agents individually on three different sites of β-tubulin can physically block only one cysteine from ITC attack in β-tubulin (Cys241 for colchicine and Cys213 for vinblastine and taxol) (26, 27). However, multiple cysteines were found to be blocked from BITC attack by pretreatment of colchicine, vinblastine, or taxol. Our molecular dynamics simulations of tubulin also indicated that ITC binding causes local conformation distortion (14). It seems that ITC binding to tubulin may occur in a progressive manner; the local structural distortion induced by binding with one ITC molecule may lead to another. Therefore, stabilization of the tubulin global structure by tubulin-binding agents may block the cascade of ITC binding. Presently, we do not know why ITC-induced degradation is selective for α- and β-tubulins. Possible reasons are 1) certain proteins, like γ-tubulin, encapsulated in a protein complex, are inaccessible to ITCs; 2) some cysteines are less likely to be attacked by electrophiles because of their micro-environment, pocket spatial limits, etc.; and 3) binding of ITCs to proteins may occur, but it is unable to trigger structural change in the protein. It has been reported that proteins can be deactivated as a result of modification of active cysteines by ROS (28). Our results show that neither treatment of cells with hydrogen peroxide nor up- or down-regulation of oxidative stress in cells affect tubulin level or its degradation caused by ITCs. It could be explained by the fact that almost all cysteines in tubulin are buried in small hydrophobic pockets, inaccessible to hydrophilic ROS but accessible to the phenyl group containing BITC and PEITC. In contrast, SFN, a stronger inducer of oxidation in cells than PEITC (13) and a hydrophilic ITC, caused little tubulin aggregation and degradation.
It is known that when a cell senses misfolded proteins, it will activate two types of signals (29). First, it will dispatch a variety of chaperone proteins to assist the misfolded protein to retain its original conformation. However, if the misfolded protein is beyond repair, the cell will activate its degradation machinery (23). Another signature event of misfolded proteins is that they form aggregates to keep hydrophobic domains buried (30). The aggregation also indicates that the generation of misfolded proteins exceeds the capacity of degradation machinery of the cell or the failure of the proteasome system (31, 32). A novel finding in this study is that, like transfection of misfolded proteins (33, 34), ITCs may also induce the formation of protein aggregates. Our recent data indicate that ITC-induced aggresome-like complexes are enriched with, beside α- and β-tubulin, chaperone proteins, proteasome components, and ubiquitinated proteins.4 Unexpectedly, these purified complexes also showed enhanced proteasome activity, suggesting that aggregation, instead of a sign of the failure of the proteasome degradation mechanism, may set the stage for emergent “junk disposal,” and tubulin degradation is a result of the cooperative effort of aggregate components. Because ITC-induced tubulin degradation is ubiquitin- and ATP-dependent (Fig. 4), it is reasonable to postulate that 26 S proteasome is involved. It is noted that the most effective concentration range for both BITC and PEITC to induce tubulin degradation in cells was between 5 and 15 μm. Inhibition of tubulin degradation was observed when cells were treated with more than 25 μm BITC or PEITC, even though tubulin aggregation was aggravated with that dose (data not shown). The inhibition may be explained by the observation that both BITC and PEITC at high concentrations may suppress the ubiquitin-proteasome activities in cells.4 Another interesting finding in this study is that both autophagy and lysosome inhibitors failed to block tubulin degradation, indicating that ITC-induced tubulin degradation is not dependent on either of these two protein degradation machineries. Whether ITC-induced tubulin aggregation and degradation affect autophagy formation (35) requires further investigation. Nonetheless, our study offers a unique model system to study protein aggregation and degradation modified by small molecules and their downstream effects.
Because tubulin is essential for cell division, it is conceivable that fast-growing cells would be more sensitive to ITC-induced cell growth arrest and apoptosis. Indeed, it has been reported that c-Jun-transfected or 12-O-tetradecanoylphorbol-13-acetate-treated human lung cells and Ras-transformed human ovarian cells are more susceptible to PEITC-induced cell death than the corresponding control cells (12, 36). Previously, we reported that the anti-microtubule effect by ITC is at least partially responsible for cell cycle arrest at the mitosis phase and, ultimately, apoptosis (14). The present study suggests that tubulin degradation as a downstream effect of ITC binding may contribute to these effects. Although further investigation is needed, this study provides mechanism-based leads for the design and development of more efficacious ITC-related compounds for cancer prevention and therapy.
Supplementary Material
Acknowledgments
We thank Dr. Elizabeth S. Sztul (Department of Cell Biology, University of Alabama) and Dr. Changcheng Song (Laboratory of Cancer Prevention at NCI-Frederick) for fruitful discussion and suggestions. We also thank Drs. Susette Mueller and Karen Creswell for microscopy imaging and flow cytometry, Dr. Stephen Hecht (University of Minnesota) for providing SFN, and Dr. Ming You (Washington University) for providing FLAG-ubiquitin plasmid.
This work was supported, in whole or in part, by National Institutes of Health Grant CA100853 from NCI.
 The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1 and 2.
L. Mi, N. Gan, and F. L. Chung, unpublished results.
- ITC
- isothiocyanate
- BITC
- benzyl isothiocyanate
- PEITC
- phenethyl isothiocyanate
- SFN
- sulforaphane; N-methylphenethylamine
- CHAPS
- 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonic acid
- ATZ
- aminotriazole
- CHX
- cycloheximide
- PBS
- phosphate-buffered saline
- PEG
- polyethylene glycol
- RT
- reverse transcription
- 2-DG
- 2-deoxyglucose
- BisTris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol
- Z
- benzyloxycarbonyl
- fmk
- fluoromethyl ketone
- PSI
- proteasome inhibitor I
- ROS
- reactive oxygen species
- MS/MS
- tandem mass spectrometry
- IP
- immunoprecipitation.
REFERENCES
- 1.Hartwell L. H., Kastan M. B. ( 1994) Science 266, 1821– 1828 [DOI] [PubMed] [Google Scholar]
- 2.Lengauer C., Kinzler K. W., Vogelstein B. ( 1998) Nature 96, 643– 649 [DOI] [PubMed] [Google Scholar]
- 3.White E. ( 1996) Genes Dev. 10, 1– 15 [DOI] [PubMed] [Google Scholar]
- 4.Jordan M. A., Wilson L. ( 2004) Nat. Rev. Cancer 4, 253– 265 [DOI] [PubMed] [Google Scholar]
- 5.WHO ( 2004) IARC Handbook on Cancer Prevention, Vol. 9, pp. 43– 171, Lyon, France [Google Scholar]
- 6.Chen Y. R., Wang W., Kong A. N., Tan T. H. ( 1998) J. Biol. Chem. 273, 1769– 1775 [DOI] [PubMed] [Google Scholar]
- 7.Chiao J. W., Chung F. L., Kancherla R., Ahmed T., Mittelman A., Conaway C. C. ( 2002) Int. J. Oncol. 20, 631– 636 [DOI] [PubMed] [Google Scholar]
- 8.Xiao D., Choi S., Lee Y. J., Singh S. V. ( 2005) Mol. Carcinog. 43, 130– 140 [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y., Talalay P. ( 1998) Cancer Res. 58, 4632– 4639 [PubMed] [Google Scholar]
- 10.Zhang Y., Li J., Tang L. ( 2005) Free Radic. Biol. Med. 38, 70– 77 [DOI] [PubMed] [Google Scholar]
- 11.Singh S. V., Srivastava S. K., Choi S., Lew K. L., Antosiewicz J., Xiao D., Zeng Y., Watkins S. C., Johnson C. S., Trump D. L., Lee Y. J., Xiao H., Herman-Antosiewicz A. ( 2005) J. Biol. Chem. 280, 19911– 19924 [DOI] [PubMed] [Google Scholar]
- 12.Trachootham D., Zhou Y., Zhang H., Demizu Y., Chen Z., Pelicano H., Chiao P. J., Achanta G., Arlinghaus R. B., Liu J., Huang P. ( 2006) Cancer Cell 10, 241– 252 [DOI] [PubMed] [Google Scholar]
- 13.Mi L., Wang X., Govind S., Hood B. L., Veenstra T. D., Conrads T. P., Saha D. T., Goldman R., Chung F. L. ( 2007) Cancer Res. 67, 6409– 6416 [DOI] [PubMed] [Google Scholar]
- 14.Mi L., Xiao Z., Hood B. L., Dakshanamurthy S., Wang X., Govind S., Conrads T. P., Veenstra T. D., Chung F. L. ( 2008) J. Biol. Chem. 283, 22136– 22146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nogales E. ( 2000) Annu. Rev. Biochem. 69, 277– 302 [DOI] [PubMed] [Google Scholar]
- 16.Ben-Ze'ev A., Farmer S. R., Penman S. ( 1979) Cell 17, 319– 325 [DOI] [PubMed] [Google Scholar]
- 17.Cleveland D. W., Lopata M. A., Sherline P., Kirschner M. W. ( 1981) Cell 25, 537– 546 [DOI] [PubMed] [Google Scholar]
- 18.Cleveland D. W., Pittenger M. F., Feramisco J. R. ( 1983) Nature 305, 738– 740 [DOI] [PubMed] [Google Scholar]
- 19.Pierce A., Unwin R. D., Evans C. A., Griffiths S., Carney L., Zhang L., Jaworska E., Lee C. F., Blinco D., Okoniewski M. J., Miller C. J., Bitton D. A., Spooncer E., Whetton A. D. ( 2008) Mol. Cell. Proteomics 7, 853– 863 [DOI] [PubMed] [Google Scholar]
- 20.Xirodimas D., Saville M. K., Edling C., Lane D. P., Laín S. ( 2001) Oncogene 20, 4972– 4983 [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Vikis H. G., Yi Y., Futamura M., Wang Y., You M. ( 2007) Cancer Res. 67, 10207– 10213 [DOI] [PubMed] [Google Scholar]
- 22.Yang Y., Kitagaki J., Dai R. M., Tsai Y. C., Lorick K. L., Ludwig R. L., Pierre S. A., Jensen J. P., Davydov I. V., Oberoi P., Li C. C., Kenten J. H., Beutler J. A., Vousden K. H., Weissman A. M. ( 2007) Cancer Res. 67, 9472– 9481 [DOI] [PubMed] [Google Scholar]
- 23.Goldberg A. L. ( 2003) Nature 426, 895– 899 [DOI] [PubMed] [Google Scholar]
- 24.Ferrington D. A., Sun H., Murray K. K., Costa J., Williams T. D., Bigelow D. J., Squier T. C. ( 2001) J. Biol. Chem. 276, 937– 943 [DOI] [PubMed] [Google Scholar]
- 25.Shringarpure R., Grune T., Mehlhase J., Davies K. J. ( 2003) J. Biol. Chem. 278, 311– 318 [DOI] [PubMed] [Google Scholar]
- 26.Nogales E., Wolf S. G., Khan I. A., Luduena R. F., Downing K. H. ( 1995) Nature 375, 424– 427 [DOI] [PubMed] [Google Scholar]
- 27.Downing K. H., Nogales E. ( 1999) Cell Struct. Funct. 24, 269– 275 [DOI] [PubMed] [Google Scholar]
- 28.Salmeen A., Barford D. ( 2005) Antioxid. Redox. Signal. 7, 560– 577 [DOI] [PubMed] [Google Scholar]
- 29.Connell P., Ballinger C. A., Jiang J., Wu Y., Thompson L. J., Höhfeld J., Patterson C. ( 2001) Nat. Cell Biol. 3, 93– 96 [DOI] [PubMed] [Google Scholar]
- 30.Sherman M., Goldberg A. L. ( 2001) Neuron 29, 15– 32 [DOI] [PubMed] [Google Scholar]
- 31.Pandey U. B., Nie Z. P., Batlevi Y., McCray B. A., Ritson G. P., Nedelsky N. B., Schwartz S. L., DiProspero N. A., Knight M. A., Schuldiner O., Padmanabhan R., Hild M., Berry D. L., Garza D., Hubbert C. C., Yao T. P., Baehrecke E. H., Taylor J. P. ( 2007) Nature 447, 859– 863 [DOI] [PubMed] [Google Scholar]
- 32.Iwata A., Riley B. E., Johnston J. A., Kopito R. R. ( 2005) J. Biol. Chem. 280, 40282– 40292 [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Mata R., Gao Y. S., Sztul E. ( 2002) Traffic 3, 388– 396 [DOI] [PubMed] [Google Scholar]
- 34.Bence N. F., Sampat R. M., Kopito R. R. ( 2001) Science 292, 1552– 1555 [DOI] [PubMed] [Google Scholar]
- 35.Herman-Antosiewicz A., Johnson D. E., Singh S. V. ( 2006) Cancer Res. 66, 5828– 5835 [DOI] [PubMed] [Google Scholar]
- 36.Yang Y. M., Jhanwar-Uniyal M., Schwartz J., Conaway C. C., Halicka H. D., Traganos F., Chung F. L. ( 2005) Cancer Res. 65, 8538– 8547 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.